Abstract
We herein report a non-smoking 81-year-old man with advanced synchronous multiple primary lung cancers (SMPLC), containing squamous cell carcinoma with strong programmed death-ligand 1 expression in the middle lobe and adenocarcinoma with epidermal growth factor receptor (EGFR) exon 19 deletion in the lower lobe. Programmed death-1 (PD-1) inhibitors were administered as first-line chemotherapy; however, treatment response was poor response. There have been no reported SMPLC cases similar to this. During treatment, his non-smoking status and EGFR deletion might have been the cause of the patient's poor response to first-line PD-1 inhibitor treatment.
Keywords: Adenocarcinoma, Epidermal growth factor receptor, Programmed death-ligand 1, Programmed death-1 inhibitors, Squamous cell carcinoma, Synchronous multiple primary lung cancers
1. Introduction
Multiple primary cancers are usually defined as malignant tumors simultaneously at more than two sites (multicentricity) or heterochrony in the same organ. Martini et al. were the first to recognize and categorize patients with multiple lung cancers in 1975 [1]. According to several studies, synchronous multiple primary lung cancer (SMPLC) occurs in 0.26–1.74% of all lung cancer patients [[1], [2], [3], [4]].
At present, immune checkpoint inhibitors (ICI), including programmed death-1 (PD-1) inhibitors and programmed death-ligand 1 (PD-L1), have been used as standard therapy for non-small cell lung cancers (NSCLCs) [[5], [6], [7], [8]]. Immunohistochemical examination of tumor PD-L1 expression is the only available clinical test to predict the efficacy of PD-1/PD-L1 inhibitors. Higher PD-L1 expression is also associated with better outcomes in non-small cell lung cancer (NSCLC) patients treated with PD-1/PD-L1 inhibitors [5,8].
Here, we report a rare case, wherein the patient had SMPLC including squamous cell carcinoma (SQCC) with strong PD-L1 expression and adenocarcinoma with epidermal growth factor receptor (EGFR) exon 19 deletion. PD-1 inhibitors were administered as first-line chemotherapy, but poor response was observed.
2. Case report
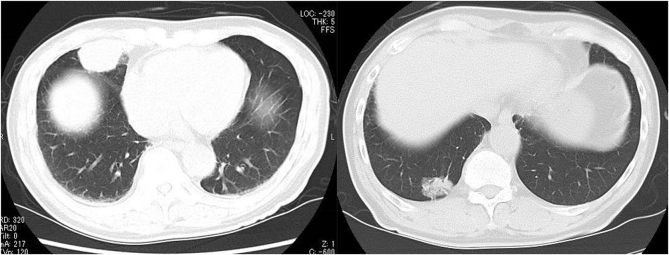
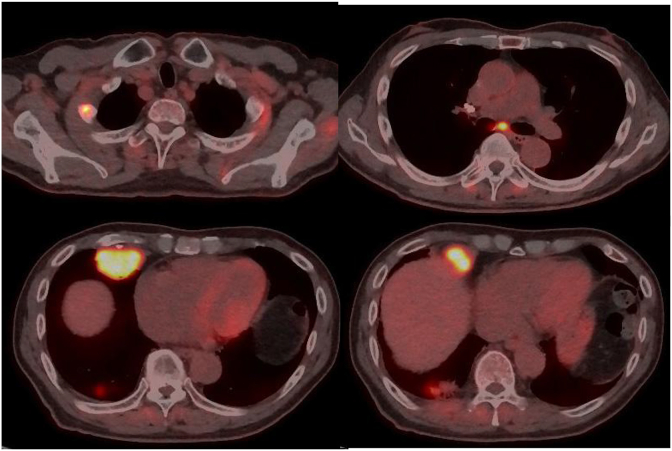
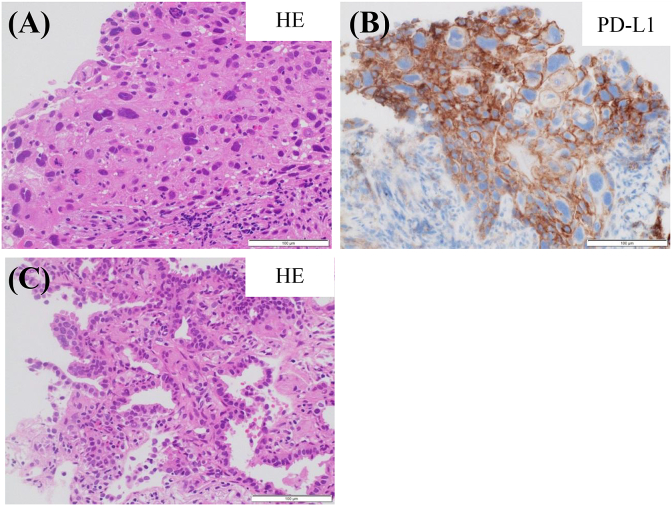
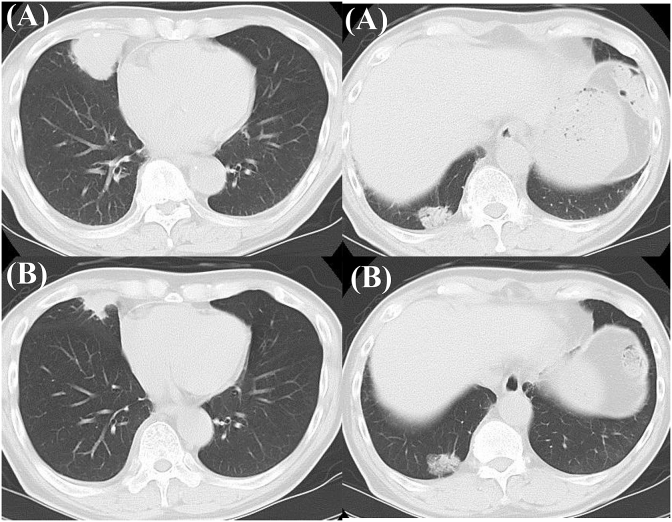
In February 2019, a non-smoking 81-year-old man was admitted to the psychiatry department of our hospital for aggravation of the depression symptom. He was then referred to our department, after a nodular shadow was noted on the right side of his chest X-ray during a medical checkup conducted on admission (Fig. 1). The patient had been under treatment for depression from 2003. His Eastern Cooperative Oncology Group performance status was 0. Levels of carcinoembryonic antigen and cytokeratin 19-fragments were elevated to 6.6 ng/mL (normal range, 0–5.0 ng/mL) and 5.3 ng/mL (normal range, 0–3.5 ng/mL), respectively. Chest computed tomography (CT) revealed a 3.3 cm solid pulmonary mass in the right middle lobe and a 2.7 cm pulmonary nodule surrounded by ground glass opacity in the right lower lobe (Fig. 2). Examination of 18 fluorine fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) images revealed intense FDG accumulation in the right lung tumors, mediastinal lymph nodes, and second right rib (Fig. 3). Histological examination of transbronchial lung biopsy specimens from the right middle pulmonary mass and CT-guided biopsy specimens from the right lower pulmonary mass revealed poorly differentiated SQCC and well-differentiated adenocarcinoma, respectively (Fig. 4A and C). SQCC of the right middle lobe showed 100% tumor proportion score (TPS) for PD-L1 (Agilent Dako IHC 22C3 platform) (Fig. 4B) and no expression of EGFR mutations (Roche cobas® EGFR Mutation Test v2) and anaplastic lymphoma kinase (ALK) rearrangements (Histofine ALK iAEP® Kit). Adenocarcinoma of the right lower lobe showed exon 19 deletion and no expression of PD-L1. Although the tumors that caused the rib and mediastinal lymph nodes metastasis were unidentified, the patient was diagnosed with synchronous double primary lung cancers containing SQCC and adenocarcinoma, stage IV. The patient did not prefer stressful cytotoxic chemotherapy. As SQCC had already moved to the pleura and couldt easily lead to direct invasion of the chest wall, we decided to treat the SQCC first. Pembrolizumab (200 mg, once every 3 weeks) was administered as first-line chemotherapy in March 2019. After six courses of pembrolizumab, the patient exhibited progressive disease (Fig. 5A) and was transitioned to secondary treatment with TS-1 (40 mg twice daily, after breakfast and after the evening meal, for 28 consecutive days, followed by a 14-day rest). After two courses with TS-1 therapy, chest CT images revealed reductions in the size of both tumors (Fig. 5B).
Fig. 1.
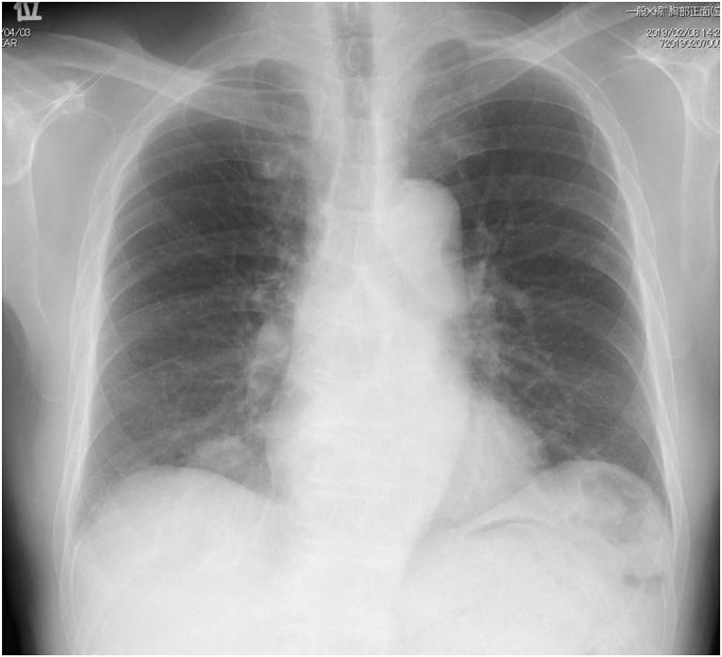
Chest radiograph at first presentation showing a tumor in the right lower lung field.
Fig. 2.
Computed tomography images at first presentation showing a 3.3 cm pulmonary tumor in the right middle lobe and a 2.7 cm pulmonary nodule surrounded by ground glass opacity in the right lower lobe.
Fig. 3.
Eighteen fluorine fluorodeoxyglucose positron emission tomography/computed tomography showing intense fluorodeoxyglucose accumulation in the right lung tumors, mediastinal lymph nodes, and second right rib.
Fig. 4.
(A) Histological examination of transbronchial lung biopsy specimens obtained from the right middle pulmonary mass revealing poorly differentiated squamous cell carcinoma.
(B) Immunohistochemical staining of PD-L1 for squamous cell carcinoma revealing higher PD-L1 expression (100% tumor proportion score).
(C) Histological examination of CT-guided biopsy specimens from the right lower pulmonary mass revealing well-differentiated adenocarcinoma.
Fig. 5.
(A) Computed tomography (CT) images after six courses of pembrolizumab showing enlargement of squamous cell carcinoma in the right middle lobe and no change in adenocarcinoma in the lower lobe, compared with pretreatment images.
(B) CT images after two courses with TS-1 therapy as second-line chemotherapy showing reductions in the size of both tumors.
3. Discussion
Martini et al. (1975) suggested that to be diagnosed as SMPLC, the tumors should be physically distinct from each other and should be malignant with a different histologies. If the histology is the same, the tumor should be located in a different segment, lobe, or lung, with the absence of carcinoma in common lymphatics and the exclusion of extrapulmonary metastasis [1]. With regard to the histological types of SMPLC, a single histological type was most commonly found [[1], [2], [3], [4]]. The true incidence of SMPLC, presently ranging from 0.26% to 1.74%, may be increasing because of the widespread use of early detection tools, such as multislice spiral CT and FDG-PET/CT scanning [[1], [2], [3], [4]]. Yu et al. reported that the incidence of adenocarcinoma is increasing and there is no significant difference in overall survival (OS) between SMPLC patients and solitary primary lung cancer patients after surgical treatment [10]. Further, smoking history is highly associated with SMPLC onset [4,9]. However, the prognosis of SMPLC remains unclear due to its rarity and may be associated with its histologic type.
In this report, the patient was a non-smoker, and had SQCC and adenocarcinoma in different lobes with different histological types. Additionally, the SQCC had PD-L1 TPS of 100% without EGFR gene mutations and the adenocarcinoma had EGFR exon 19 deletion with no expression of PD-L1. To the best of our knowledge, there have been no such reported cases.
In the KEYNOTE-024 trial, compared with chemotherapy, use of pembrolizumab, which is a PD-1 inhibitor, as first-line treatment exhibited a median progression-free survival (PFS) of 10.3 months versus 6.0 months and response rate of 44.8% versus 27.8% in advanced NSCLC patients with PD-L1 expression on at least 50% of tumor cells, with no sensitizing EGFR mutations or ALK rearrangements [5]. Higher PD-L1 expression is associated with better outcomes in NSCLC patients treated with PD-1/PD-L1 inhibitors [11]. However, we found that PD-1 inhibitor did not show any effect on this patient's SQCC, which showed high PD-L1 expression. Recent meta-analysis indicated that in non-smokers, PD-1/PD-L1 inhibitors failed to improve OS (HR, 0.8, 95% CI, 0.54–1.06) and PFS (HR 0.90, 95% CI, 0.11–7.59) when compared to chemotherapy. Smoking status may be a predictive marker for better survival as PD-1/PD-L1 inhibitors may be effective in smokers [12]. The mechanism underlying the difference in effectiveness of PD-1/PD-L1 inhibitors between smokers and non-smokers is still unexplained. It is well known that various carcinogens in tobacco smoke are responsible for much of the mutagenesis in lung cancer. Smoking is linked to the expression of neoantigens and increase in somatic mutations [13]. The average mutation frequency in non-smokers in NSCLC is 10-fold less than that in smokers with NSCLC [14]. Although Carbone et al. reported that there was no significant association between mutation burden and PD-L1 expression [15], since cancer types with relatively high mutation burden tended to show better outcomes with PD-1/PD-L1 inhibitors [13,16], it is possible that mutational landscape of a given tumor is an important predictive marker of the response to PD-1/PD-L1 inhibitors [17].
Here, the PD-1 inhibitor did not show any effect on our patient's adenocarcinoma with EGFR exon 19 deletion with no expression of PD-L1. In a retrospective analysis of 58 patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors, only 3.6% of patients harboring EGFR mutations or ALK rearrangements responded to PD-1/PD-L1 inhibitors, while 23.3% of patients with wild-type EGFR and ALK-negative or unknown tumors were responders [18]. A retrospective study showed that treatment with PD-1/PD-L1 inhibitors resulted in PFS as short as 1.2–2.1 months in patients with EGFR mutations [19]. Recent meta-analysis showed that when compared to chemotherapy, PD-1/PD-L1 inhibitors failed to improve OS for patients with EGFR mutations in comparison to patients with wild-type EGFR (HR, 1.05, 95% CI, 0.70–1.55 versus HR, 0.66, 95% CI, 0.58–0.76) [20]. In NSCLC patients, non-smokers exhibited higher EGFR mutation rate [21]. Tumor mutation burden in NSCLC patients with EGFR mutation is lower than wild type [22]. This is one of the main reasons for decreased efficacy of PD-1/PD-L1 inhibitors in treating EGFR-mutant NSCLC. Furthermore, several studies reported that the density of CD8-positive T cell infiltration in tumor tissues was associated with the effectiveness of PD-1/PD-L1 inhibitors [23,24]. Gainor et al. reported that patients with EGFR mutation did not show an inflamed tumor microenvironment, resulting from a lack of CD8-positive T cell infiltration [18]. Thus, EGFR-mutant NSCLC patients likely generate fewer neoantigens, leading to less inflamed tumor microenvironments.
In conclusion, this rare case showed SMPLC including SQCC with high expression of PD-L1 and adenocarcinoma harboring EGFR exon 19 deletion. That the patient was a non-smoker and had EGFR mutations might have caused his poor response to first-line PD-1 inhibitor treatment.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
References
- 1.Martini N., Melamed M. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 2.Hiraki A., Ueoka H., Yoshino T. Synchronous primary lung cancer presenting with small cell carcinoma and non-small cell carcinoma: diagnosis and treatment. Oncol. Rep. 1999;6:75–80. [PubMed] [Google Scholar]
- 3.Ferguson M.K., DeMeester T.R., DesLauriers J. Diagnosis and management of synchronous lung cancers. J. Thorac. Cardiovasc. Surg. 1985;89:378–385. [PubMed] [Google Scholar]
- 4.Sugimura H., Watanabe S., Tsugane S., Morinaga S., Yoneyame T. Case-control study on histologically determined multiple primary lung cancer. J. Natl. Cancer. Inst. 1987;79:435–441. [PubMed] [Google Scholar]
- 5.Reck M., Rodriguez-Abreu D., Robinson A.G. KEYNOTE-024 investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 6.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rittmeyer A., Barlesi F., Waterkamp D. OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohwedder J.J., Weatherbee L. Multiple primary bronchogenic carcinoma with a review of the literature. Am. Rev. Respir. Dis. 1974;109:435–445. doi: 10.1164/arrd.1974.109.4.435. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y.C., Hsu P.K., Yeh Y.C. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann. Thorac. Surg. 2013;96 doi: 10.1016/j.athoracsur.2013.04.142. 1966–74. [DOI] [PubMed] [Google Scholar]
- 11.Hecht S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer. Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 12.Li B., Huang X., Fu L. Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer patients: a meta-analysis. OncoTargets Ther. 2018;11:3691–3696. doi: 10.2147/OTT.S156421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi N.A., Hellmann M.D., Snyder A. Cancer Immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindan R., Ding L., Griffith M. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbone D.P., Reck M., Paz-Ares L. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champiat S., Ferté C., Lebel-Binay S., Eggermont A., Soria J.C. Exomics and immunogenics: bridging mutational load and immune checkpoints efficacy. OncoImmunology. 2014;3 doi: 10.4161/onci.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann M., Rizvi N., Wolchok J.D., Chan T.A. Genomic profile, smoking, and response to anti-PD-1 therapy in non-small lung carcinoma. Mol. Cell Oncol. 2015;3 doi: 10.1080/23723556.2015.1048929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainor J.F., Shaw A.T., Sequist L.V. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin. Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blicki O., Paleiron N., Margery J. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target. Oncol. 2017;12:563–569. doi: 10.1007/s11523-017-0510-9. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.K., Man J., Lord S. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J. Thorac. Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Rosel R., Moran T., Queralt C. Screening for epidermal growth factor receptor mutatrions in lung cancer. N. Engl. J. Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 22.Spigel D.R., Schrock A.B., Fabrizio D. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J. Clin. Oncol. 2016;34(Suppl 15):S907. [Google Scholar]
- 23.Tumeh P.C., Harview C.L., Yearley J.H. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S.P., Liao R.Q., Wang W.J. Stromal PD-L1-positive regulatory T cells and PD-1-positive CD8-positive T cells define the response of different subsets of non-small cell lung cancer to PD-1/PD-L1 blockade immunotherapy. J. Thorac. Oncol. 2018;13:521–532. doi: 10.1016/j.jtho.2017.11.132. [DOI] [PubMed] [Google Scholar]