Abstract
Background
Metabolic syndrome is a cluster of metabolic risk factors. The clear causes of its development are not known yet and there is no comprehensive treatment of this disease. There is a trend to use natural substances in the treatment of various diseases, but their effects need to be well explored. We decided to test effect of rutin compared to the effect of the standard drug atorvastatin.
Methods
As a model of metabolic syndrome we used males of hypertriacylglycerolemic rats in combination with high-fat-high-fructose diet. Rutin (100 mg/kg) and atorvastatin (50 mg/kg) were administered orally daily for 5 weeks.
Results
We determined biochemical parameters from blood: HDL-cholesterol, LDL-cholesterol, total cholesterol, triacylglycerols. Relaxation and contraction response of aorta was measured to determine vessel dysfunctions and possible predisposition to cardiovascular disease. The negative influence on cognitive functions could be associated with the development of metabolic cognitive syndrome. Therefore we aimed to monitor spatial memory by Morris water maze test. Both rutin and atorvastatin had a tendency to decrease levels of serum triacylglycerols, but only atorvastatin significantly reduced levels od LDL-cholesterol and increased HDL-cholesterol levels. Both compounds significantly reduced the phenylephrine-induced contractile response of the aorta and improved the relaxation response. Further, treated animals learned better compared to untreated rats in the Morris water maze.
Conclusion
Based on our results we can assume that atorvastatin and rutin had positive effect on spatial memory and vessel reactivity. Atorvastatin optimized lipid profile of blood serum.
Keywords: Metabolic syndrome, Rutin, Atorvastatin, Dyslipidemia, Aorta, Spatial memory
Abbreviations: AD, Alzheimer disease; ACh, acetylcholine; ANOVA, one-way analysis of variance; cAMP, cyclic adenosine monophosphate; eNOS, endothelial NO synthase; Glc, glucose; GLUT-4, glucose transporter 4; HDL-cholesterol, high density lipoprotein cholesterol; HFFD, high-fat-high-fructose diet; HMG-CoA, β-hydroxy β-methylglutaryl-CoA; HTG, hypertriacylglycerolemic; HTG-HFFD, hypertriacylglycerolemic rat with high-fat-high-fructose diet; HTG-HFFD-A, hypertriacylglycerolemic rat with high-fat-high-fructose diet with atorvastatin; HTG-HFFD-R, hypertriacylglycerolemic rat with high-fat-high-fructose diet with rutin; IRS-1, insulin receptor substrate 1; LDL-cholesterol, low density lipoprotein cholesterol; MetS, metabolic syndrome; MWM, Morris water maze; NOS, NO synthase; O, superoxide anion; OGTT, oral glucose tolerance test; PKC, proteinkinase C; PXR, pregnane X receptor; ROS, reactive oxygen species; SEM, standard error of the mean; TG, triacylglycerols
1. Introduction
Metabolic syndrome (MetS) belongs to civilization diseases that are becoming increasingly common. MetS is a cluster of risk factors including dyslipidemia, hypertension, high glucose levels or insulin resistance, obesity and inflammation. We do not recognize the clear cause of this disease. Its development is caused by environmental factors in conjunction with the genetic background. The reason why metabolic syndrome is so prevalent is the recent modern way of life, which means sedentary work, poor diet habits and no physical activity (Alberti and Zimmet, 2005). This disease may further lead to the development of serious medical conditions such as type 2 diabetes mellitus, cardiovascular and cerebrovascular diseases even with fatal consequences (Grundy, 2006). In addition to these diseases, MetS can cause cognitive decline (Kim and Feldman, 2015). The first step in the treatment of MetS is preventive measures. It is necessary to change the lifestyle, improve eating habits, increase physical activity and lose weight. If the preventive measures are not sufficient and the condition does not improve, the use of pharmacological treatment is needed. It is very difficult to find suitable drugs for treatment because there is no drug that would treat the metabolic syndrome as a whole. The only option is to treat individual risk factors (Wong, 2005). Because of drug interactions and multiple side effects, it is not possible to treat all components of the metabolic syndrome. However, it is not known which of the risk factors is essential in the development of this disease and therefore it is unclear treatment of which factor should be prescribed as major treatment (O’Neil and O’Driscoll, 2015). Nowadays, it is well known that oxidative stress plays an important role in metabolic syndrome and endothelial dysfunction. The mechanism of vessel damage is primarily caused by a defect in the protective function of nitric oxide on the endothelium. We assumed that antioxidants could help maintain normal endothelial function and could also contribute to the treatment of other MetS abnormalities. For this reason, natural substance with antioxidant effects – rutin – have been used to treat the effects of MetS. We compared the effect of the rutin with the standard drug atorvastatin, which belongs to the group of statins.
2. Methods
2.1. Animal model and design of experiment
Prague hereditary hypertriacylglycerolemic rats (HTG, n = 30) from the Department of Toxicology and Breeding of Laboratory Animals, Dobrá Voda of the Institute of Pharmacology and Toxicology, Centre of Experimental Medicine, Slovak Academy of Sciences (IEPT CEM SAS) were used for experiments. At the beginning of the experiment, the animals were 12 weeks old. The experiments were approved by the State Veterinary and Food Administration of the Slovak Republic and the Ethical Committee of IEPT CEM SAS. The animals had food and water ad libitum and light/dark cycle 12/12 (12 h of dark, 12 h of light). Animals were divided into three experimental groups (n = 10 rats/group) and fed 13 weeks with modified diet (high-fat-high-fructose diet; HFFD). Pellets of modified diet contained 1% cholesterol, 7.5% pork lard and 10% fructose. After 13 weeks, the animals in all three groups had changed the HFFD diet to standard diet and two groups of them received the drug treatment for next 5 weeks. Atorvastatin (Atorvastatin calcium, Sigma-Aldrich) was administered at the dose of 50 mg/kg (HTG-HFFD-A, n = 10 rats) and rutin (Rutin hydrate, Sigma-Aldrich) at the dose of 100 mg/kg rutin (HTG-HFFD-R, n = 10 rats). Drugs were dissolved in methylcellulose and administered once a day by gavage in volume 0.2 ml/100 g of rat body weight. Untreated group received the same amount of methylcellulose.
2.2. Determination of lipid profile
Erba Lachema Ltd (Brno, Czech Republic) kits were used to determine the lipid profile from the blood serum. We measured levels of total cholesterol, LDL-cholesterol, HDL-cholesterol and triacylglycerols. Absorbance of the resulting colored compound was measured spectrophotometrically.
2.3. Glucose tolerance test
In the glucose tolerance test, the basal glucose (Glc) level was measured in a drop of blood taken from the tail vein after 14 h of starvation. Glc levels were measured with a glucose meter (Contour, Bayer, Germany). Then Glc solution (50%) was given intraperitoneally to animals in a volume of 0.4 ml/100 g of rat body weight, i.e. 2 g Glc/kg of rat body weight. Glucose was repeatedly measured in a drop of blood with the glucose meter at 30, 60, 90, and 120 min after intraperitoneal administration of 50% Glc.
2.4. Functional measurement of the aorta
The thoracic aorta was selected after the opening of the abdominal and thoracic cavity. The individual aorta was placed in a modified Krebs solution. It was cut into about 2 mm-long circles. The rings were clamped between two hooks of a triangular shape and immersed in a Krebs solution contained (mmol/l) NaCl (122.0), KCl (5.9), NaHCO3 (15.0), D-glucose (11.0), MgCl2 (1.25), CaCl2 (1.25). The solution was gassed with (95% O2 and 5% CO2), pH 7.4 at 37° C. The rings were mounted on a holder that was connected to a tensiometric sensor and stabilized for 60 min at an optimal tension of 10 mN. During the stabilization, the rings were rinsed several times with Krebs solution. The isometric contraction force was measured using a static – dynamic apparatus (M 1101, Czech Republic) and recorded on the Kutesz 185 (Hungary). After stabilization, the rings were contracted by using depolarizing solution (Krebs solution in which NaCl was equimolarly replaced with in 100 mM KCl). After washing the reservoir with Krebs solution and stabilizing the vessels, phenylephrine (FE, 10−6 mol/l) was added to induce the contraction and, after reaching the plateau response, acetylcholine (ACh) was cumulatively administered in concentrations of 10−8, 3 × 10−8, 10−7, 3 × 10−7, 10−6, 3 × 10−6, 10−5 mol/l and the endothelium-dependent relaxation response was monitored.
2.5. Morris water maze
It is the method where rats are learned to find the hidden platform under water (0.5 cm deep-seated under water level) in the pool (diameter of 180 cm, depth of 50 cm, water temperature of about 23 °C). The inner wall of the pool and the island were black color. The pool was virtually divided into four quadrants and to a central and a peripheral zone. The camera was attached above to record movements of rats. These movements were later analyzed by Software ANY-maze (Stoelting, USA). On the day zero, the platform was up the level of water, with the contrast object on it, so the rats could see the platform. On the first time, the rat was placed only into the opposite quadrant of the pool. The rat could orient itself due to the marked figures localized around the pool. If the animal did not reach the island within 60 s, it was gently navigated by hand onto the platform and it was allowed them to stay there for 20 s. On the testing days 1–4, platform was submerged 0.5 cm under water. Four training trials were carried out daily, thus the rat was placed subsequently into each of the four quadrants. After the final trial each animal was carefully dried with a towel and placed under a lamp to dry. We recorded the duration (in seconds) for which animals were able to find a platform.
2.6. Statistical evaluation
The data were statistically evaluated using GraphPad Prism 6 Software (La Jolla,& usaň. &data were expressed as means ± SEM. One-way analysis of variance (ANOVA) was used to evaluate the difference among all experimental groups (using the Bonferroni multiple comparison test). The limit of p < 0.05 was considered as statistically significant difference.
3. Results
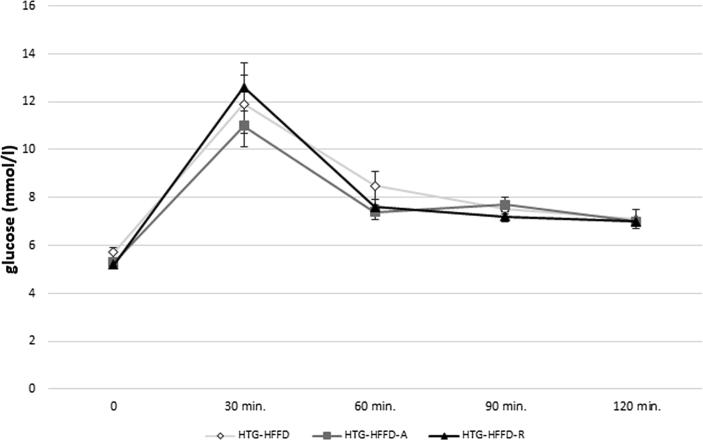
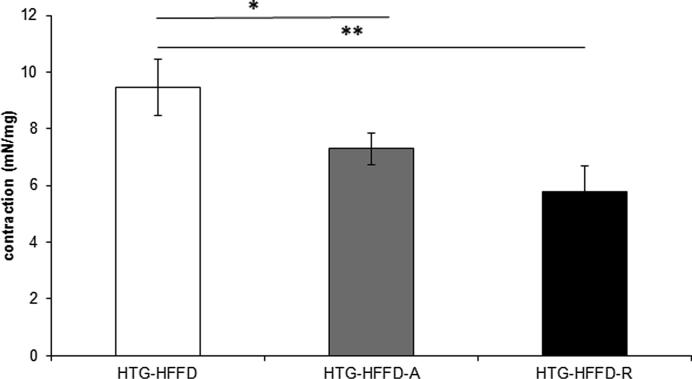
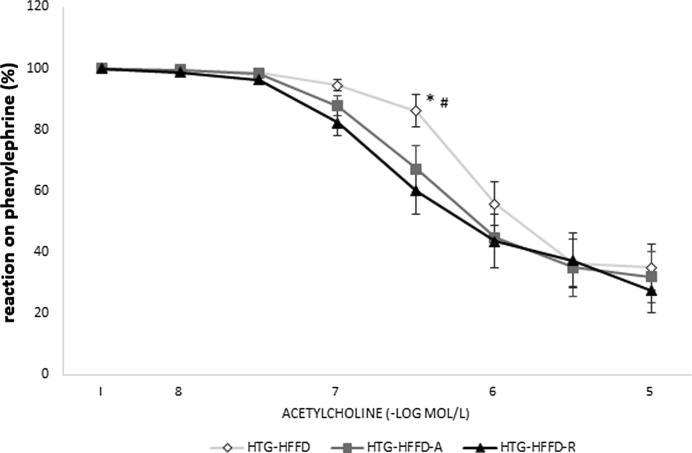
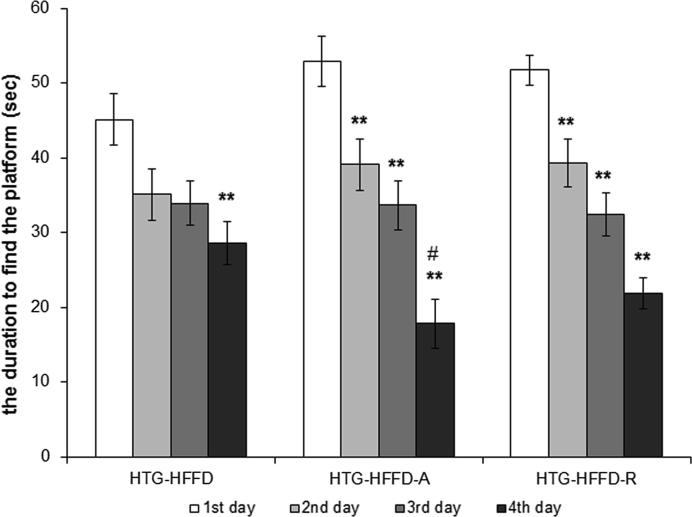
Rutin had a tendency (p < 0.08) to decrease levels of serum TG compared to untreated group. Atorvastatin had the same, but this reduction was less intense than the effect of the rutin. Rutin and atorvastatin did not statistically significantly affect serum total cholesterol levels compared to the untreated HTG-HFFD group. While rutin did not affect LDL cholesterol, atorvastatin significantly reduced these levels. HTG-HFFD-A group of animals have the lowest LDL-cholesterol level compared to untreated HTG-HFFD (p < 0.05) and HTG-HFFD-R (p < 0.01). Atorvastatin increased the HDL-cholesterol level and it was statistically significantly higher than at the rutin treated group (Table 1). Rutin or atorvastatin did not cause significant changes during the glucose tolerance test (Fig. 1). The antioxidant rutin significantly reduced the phenylephrine-induced contractile response (p < 0.01) of the aorta compared to HTG-HFFD group. We also observed similar results with HTG-HFFD vs. HTG-HFD-A, but with less significance (p < 0.05) (Fig. 2). Administration of rutin and atorvastatin resulted in a significant improvement in the relaxation response at a concentration of 3 × 10−7 mol/l of acetylcholine compared to the HTG-HFFD group (both p < 0.05) (Fig. 3). Maximum of the relaxation was not affected. HTG-HFFD animals treated with atorvastatin or rutin needed a significantly shorter time to find the islet even on the 2nd and 3rd day of testing compared to their outputs on the day 1. At the end of the test (Day 4), atorvastatin-treated animals had a significantly shorter time to find the islet compared to the untreated HTG-HFFD group (Fig. 4).
Table 1.
The effect of 5 weeks daily treatment with atorvastatin (50 mg/kg) and rutin (100 mg/kg) on serum lipid profile (mmol/l) including triacylglycerols, total cholesterol, HDL-cholesterol and LDL-cholesterol. The rats were divided into three groups; HTG-HFFD – hypetriacylglycerolemic rats fed with high-fat-high-fructose diet (HFFD), HTG-HFFD-A – hypertriacylglycerolemic rats fed with HFFD receiving atorvastatin, HTG-HFFD-R – hypertriacylglycerolemic rats fed with HFFD treated with rutin. HDL-cholesterol and LDL-cholesterol. HDL-cholesterol – high density lipoprotein cholesterol; LDL-cholesterol – low density lipoprotein cholesterol, n = 10 rats/group. Data are expressed as means ± SEM. *p < 0.05 HTG-HFFD vs HTG-HFFD-A, ##p < 0.01 HTG-HFFD-A vs HTG-HFFD-R.
| Triacylglycerols after treatment (mmol/l) | Total cholesterol after treatment (mmol/l) | HDL-cholesterol after treatment (mmol/l) | LDL-cholesterol after treatment (mmol/l) | |
|---|---|---|---|---|
| Control group HTG + HFFD |
3.65 ± 0.53 | 1.82 ± 0.09 | 0.52 ± 0.05 | 0.54 ± 0.04 |
| HTG-HFFD + A | 3.18 ± 0.62 | 1.67 ± 0.06 | 0.67 ± 0.07* | 0.42 ± 0.04* |
| HTG-HFFD + R | 2.78 ± 0.46 | 1.92 ± 0.10 | 0.36 ± 0.05## | 0.69 ± 0.07## |
Fig. 1.
The effect of 5 weeks daily treatment with atorvastatin (50 mg/kg) and rutin (100 mg/kg) on course of glucose tolerance test. Rutin or atorvastatin did not change course of glucose tolerance test. The rats were divided into three groups: HTG-HFFD – hypetriacylglycerolemic rats fed with high-fat-high-fructose diet (HFFD), HTG-HFFD-A – hypertriacylglycerolemic rats fed with HFFD receiving atorvastatin, HTG-HFFD-R – hypertriacylglycerolemic rats fed with HFFD treated with rutin, n = 5 rats/group. Data are expressed as means ± SEM. No significant difference.
Fig. 2.
The effect of 5 weeks daily treatment with atorvastatin (50 mg/kg) and rutin (100 mg/kg) on phenylephrine induced contraction of aorta (mN/mg). The atorvastatin and rutin significantly reduced contractile response (p < 0.01) of the aorta. The rats were divided into three groups: HTG-HFFD – hypetriacylglycerolemic rats fed with high-fat-high-fructose diet (HFFD), HTG-HFFD-A – hypertriacylglycerolemic rats fed with HFFD receiving atorvastatin, HTG-HFFD-R – hypertriacylglycerolemic rats fed with HFFD treated with rutin, n = 10 rats/group. Data are expressed as means ± SEM. *p < 0.05 HTG-HFFD vs HTG-HFFD-A, **p < 0.01 HTG-HFFD vs HTG-HFFD-R.
Fig. 3.
The effect of 5 weeks daily treatment with atorvastatin (50 mg/kg) and rutin (100 mg/kg) on the relaxation response of the aorta induced by cumulative application of acetylcholine. Rutin and atorvastatin improved the relaxation response at a concentration of 3 × 10−7 mol/l of acetylcholine. The rats were divided into three groups: HTG-HFFD – hypetriacylglycerolemic rats fed with high-fat-high-fructose diet (HFFD), HTG-HFFD-A – hypertriacylglycerolemic rats fed with HFFD receiving atorvastatin, HTG-HFFD-R – hypertriacylglycerolemic rats fed with HFFD treated with rutin, n = 10 rats/group. Data are expressed as means ± SEM. *p < 0.05 HTG-HFFD vs HTG-HFFD-A, #p < 0.05 HTG-HFFD vs HTG-HFFD-R.
Fig. 4.
The effect of 5 weeks daily treatment with atorvastatin (50 mg/kg) and rutin (100 mg/kg) on the duration to find the hidden platform (sec) at the Morris water maze. HTG-HFFD animals treated with atorvastatin or rutin needed a significantly shorter time to find the islet even on the 2nd and 3rd day of testing compared to their outputs on the day 1. At the end of the test (Day 4), atorvastatin-treated animals had a significantly shorter time to find the islet. The rats were divided into three groups: HTG-HFFD – hypetriacylglycerolemic rats fed with high-fat-high-fructose diet (HFFD), HTG-HFFD-A – hypertriacylglycerolemic rats fed with HFFD receiving atorvastatin, HTG-HFFD-R – hypertriacylglycerolemic rats fed with HFFD treated with rutin, n = 10 rats/group. Data are expressed as means ± SEM. **p < 0.01 vs 1st day in the given group, #p < 0.05 vs HTG-HFFD untreated group at 4th day.
Based on our results we can assume that atorvastatin and rutin had positive effect on spatial memory and vessel reactivity. Moreover, atorvastatin decreased levels of LDL-cholesterol.
4. Discussion
MetS is a worldwide health problem that, in addition to metabolic disorders, can lead to the development of cardiovascular and cerebrovascular diseases, which are currently one of the most common causes of death. Therefore, a great effort is made to reduce the manifestation and development of MetS in a pharmacological and non-pharmacological ways. In our work we studied the effect of natural substance rutin and chemical standard atorvastatin on a condition similar to MetS induced by HFFD in HTG rats. Our goal was to compare effect of rutin and atorvastatin on MetS parameters. Our results confirmed our hypothesis that administration of rutin can improve endothelium-dependent relaxation and decreased contraction. Rutin and atorvastatin also improved course of MWM that can mean improvement in executive function. Atorvastatin (50 mg/kg) lowered LDL-cholesterol, increased HDL-cholesterol and tended to lower total cholesterol but rutin had no effect on lipid profile. Neither of tested substances affected course of glucose tolerance test.
It is known that statins significantly reduce cardiovascular morbidity and mortality. In addition to the reduction of lipid levels, they also have pleiotropic effects – they protect endothelial function (Liao and Laufs, 2005) and normalize increased systolic blood pressure (Török et al., 2007). However, they have very serious side effects, hepatotoxicity, muscle toxicity, which may result in rhabdomyolysis. Therefore, it would be appropriate to find drugs with similar effect, but with a lower risk. As expected, the protective effect of atorvastatin resulting in a significant reduction in LDL levels and an increase in the HDL level was fond under MetS-like conditions in our results. The mechanism of decrease serum cholesterol and lipoprotein concentrations of atorvastatin is based on inhibition of HMG-CoA reductase and subsequent inhibition of cholesterol biosynthesis in the liver as well as on the increase in the number of LDL receptors in the liver. This mechanism accelerates the absorption and catabolism of LDL and increases HDL levels (Rang et al., 2007). In our experiments, atorvastatin tended to lower total cholesterol levels.
The rutin is rapidly absorbed after oral administration and rapidly excreted by the stool. Katsube et al. (2006) found that rutin is a significant LDL antioxidant in in vitro studies. Our results showed that rutin had a tendency to decrease TG compared to untreated HTG-HFFD group, it did not alter the total cholesterol or HDL cholesterol levels. These results correspond to Prince and Kamalakkannan, 2006, Wang et al., 2015, who also did not detect the effect of rutin on total cholesterol levels, but reported lowered levels of TG after administration, especially in animal models of diabetes. However, data of the influence of rutin on serum lipid levels vary. Several authors have found a reduction in total cholesterol levels after rutin administration (Jadhav and Puchchakayala, 2012, Niture et al., 2014). The mechanism of action is likely by the ability of the rutin to reduce dietary cholesterol absorption, as demonstrated by the amount of sterols in rat faeces. This leads to a decrease in both plasma and hepatic cholesterol (Ziaee et al., 2009). Explanation for the controversial results may lie in differences in diet and treatment duration, route of administration, and daily dose size. The relatively minor effects of the rutin may be due to the imbalance between time duration of HFFD administration and time duration of treatment in our experimental conditions.
Elevated blood glucose levels are one of the feature in metabolic syndrome. We tested the effect of atorvastatin and rutin on this variable as well. We did not detect any significant improvement in intraperitoneal glucose tolerance test, which is consistent with the results of Costa et al. (2003), who found that atorvastatin treatment did not produce any change in oral glucose tolerance test (OGTT) or induce any change in glucose and insulin response in OGTT (Costa et al., 2003). Our 5-week treatment was probably short to see an effect as indicate the results of Yu et al. (2008) who found that atorvastatin 6-week treatment increased glucose tolerance in mice with dyslipidemia without affecting insulin sensitivity. These authors did not find effect after 3-week treatment. Statins may even aggravate the metabolism and usage of Glc. In addition, simvastatin suppresses glucose-induced insulin release from rat islet β-cells (Yada et al., 1999) and increases glucose levels in serum via induction of gluconeogenesis (Gotoh and Negishi, 2014). Atorvastatin may cause reduction in insulin-induced tyrosine phosphorylation of insulin receptor substrat-1 (IRS-1) and serine/threonine phosphorylation of protein kinase B or decrease in glucose uptake by adipocytes. Statins also activate the pregnane X receptor (PXR) (Hoffart et al., 2012, Yamasaki et al., 2009). Recent studies have demonstrated that PXR activation mediates drug-induced development of hyperglycemia (Gotoh and Negishi, 2014, Rysa et al., 2013). However, we did not notice such deterioration in our results. Oral administration of rutin in a same dose as we administered to diabetic rats for a period of 45 days resulted in decrease of plasma glucose, further an increase in insulin levels were observed along with the restoration of glycogen content and the activities of carbohydrate metabolic enzymes (Prince and Kamalakkannan, 2006). It has been demonstrated that rutin can modulate glucose homeostasis. In skeletal muscle, an increase in intracellular calcium concentration may induce glucose transporter-4 (GLUT-4) translocation with consequent glucose uptake (Cazarolli et al., 2008).
Among the pleiotropic effects of atorvastatin, there is an improvement in vascular reactivity. We have found a significant improvement in endothelial-dependent relaxation following administration of this substance, a common feature of statin effects. They have a positive effect on vascular smooth muscle – improving of relaxation not only dependent but also independent on endothelium (Sotomayor et al., 2005). In precontracted vessels, simvastatin was able to induce relaxation even in the absence of endothelium (Perez-Guerrero et al., 2005). Mechanism of this effect of statins plays an important role in oxidative stress because they reduce the level of superoxide anion radical (O) and increase activity of NO synthase (NOS), thus endothelial function returns to normal (Wagner et al., 2000). In addition, they increase the activity and expression of endothelial NOS (eNOS) (Török et al., 2007).
Hyperglycemia and glucose intolerance lead to excessive formation of reactive oxygen species (ROS) and glucose conversion to sorbitol by the action of the enzyme aldose-reductase. These mechanisms cause activation of protein kinase C (PKC), decrease in NADPH, which can lead to endothelial dysfunction (Geraldes and King, 2010). Furthermore, excessive formation of the superoxide anion radical (O) causes a reduction in the bioavailability of NO. On the one hand, there is a reduction in NO formation from eNOS and NO degradation (Sotníková and Bauer, 2010). In our experiments, we have found a significant improvement in aorta relaxation in HTG-HFFD-R as compared to the HTG-HFFD group. This is consistent with the results of Ajay et al. (2003) who found that rutin released NO from the endothelium, which may improve acetylcholine-induced relaxation. Flavones and flavonols induce relaxation and reduce contraction due to calcium entry into the cell or phenylephrine-induced Ca2+ release from endoplasmic reticulum. Similarly, Panchal et al. (2011) observed improved endothelial function of the aorta after rutin therapy. Improving vascular endothelium function at high glucose levels is probably due to the ability of rutin to inhibit aldose-reductase and to reduce sorbitol concentration, indicating the potential of the rutin to maintain a normal intracellular NADPH level (Reddy et al., 2011). It is likely that mechanism was also responsible for significantly reduced contraction in the HTG-HFFD-R group. Ajay et al. (2003) explained this reduction by the relaxing effect of the rutin and its strong antioxidant activity, which can increase the bioavailability of NO. Since we have not observed any differences in the oxidative stress markers, we do not expect this action in our experimental design.
There is suggestion that MetS and MetS components play role in the development of age-related cognitive decline, mild cognitive impairment, vascular dementia and Alzheimer's disease (AD). MetS along with cognitive impairment of degenerative or vascular origin start to be marked as “a metabolic-cognitive syndrome”. Mechanisms by which HFFD and MetS could lead to worsening of responses in the brain are in particular oxidative stress (Thiels et al., 2010), abnormal lipid metabolism in the brain and impaired vascular reactivity, damage of insulin-sensitive processes responsible for neuronal survival, learning and memory (Yates et al., 2012, Liu et al., 2009). Because of this, we have focused on testing the effect of antioxidant and statin on impaired spatial memory of the rats with MetS-like conditions. We found that both rutin and atorvastatin have gretly improved the spatial memory tested in the Morris Water Maze already on the second day of testing. This effect was also present in all subsequent days, which is consistent with the results of Xu et al. (2014) when rutin attenuated spatial memory in Alzheimer’s disease transgenic mice. Rutin improves spatial memory tested in MWM, what could be caused by its reduction of the expression of glial fibrillary acidic protein, cyclooxygenase-2, interleukin-8, inducible NOS, nuclear factor-κB and preventing inflammation and changes in hippocampus (Javed et al., 2012). Further it was found that rutin significantly increased extracellular signal-regulated protein kinase 1, cAMP response element-binding protein and brain-derived neurotrophic factor gene expression in the hippocampus of rats, which are genes involved in memory and learning (Moghbelinejad et al., 2014). Unlike statins, where controversial results were found. In some studies, such as Posvar et al., 1996, Wagstaff et al., 2003 it was found that statins can cause a reversible cognitive decline, mild transient restlessness, euphoria, and mental confusion. On the other hand, it has been reported that statins have been associated with a reduced risk of dementia and slow progression of AD (Ostrowski et al., 2007). Statins act by restoration of cholesterol homeostasis in those individuals who have congenital hyperlipidemia conditions. An optimal local cholesterol level may be necessary for normal physiologic processing. Increased amounts of local cholesterol may result in the formation of Aβ plaques, while decreased amounts may prevent normal physiologic functioning and cause the reversible cognitive impairment (Shepardson et al., 2011).
5. Conclusion
Based on our results we can assume that administration of rutin (100 mg/kg) results primarily in an improvement of vascular function in both contraction and endothelium-dependent relaxation. Rutin did not significantly affect MetS-induced dyslipidemia. As expected, atorvastatin (50 mg/kg) lowered LDL-cholesterol, increased HDL-cholesterol and tended to lower total cholesterol. In addition, atorvastatin reduced contraction and improved endothelial vascular relaxation. Both, rutin and atorvastatin improved course of MWM that can mean improvement in spatial memory mechanisms in the brain. Neither antioxidant or statin affected glucose levels in glucose tolerance test. There is suggestion that not only atorvastatin but also natural substance rutin could apply in the reduction of MetS components. Treatment with atorvastatin combined with rutin supplementation may be considered.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgement
This work was supported by the Slovak national grants VEGA 2/0054/15 and VEGA 2/0120/19.
Authors' contribution statement
MD – main author of the manuscript, Morris water maze, glucose tolerance test, analysis and statistics, contribution to conception and design.
TKB – functional measurement of aorta, Morris water maze, glucose tolerance test – She has made substantial contribution to conception and design and acquisition of date, she also has made contribution to analysis. She has been involved in drafting of manuscript. She has given final approval of final version of manuscript and she agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
LB – Morris water maze. He has made substantial contribution to conception and design and acquisition of date, he has been involved in drafting of manuscript. He has given final approval of final version of manuscript and he agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
ŠK – blood sample taking. He has made substantial contribution to conception and design. He has been involved in drafting of manuscript. He has given final approval of final version of manuscript and he agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
SL – blood sample taking. He has made substantial contribution to conception and design. He has been involved in drafting of manuscript. He has given final approval of final version of manuscript and he agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
SR – functional measurement of aorta. She has made substantial contribution to conception and design and acquisition of date, she also has made contribution to analysis. She has been involved in drafting of manuscript. She has given final approval of final version of manuscript and she agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
KV – design of experiment. He has made substantial contribution to conception and design. He has been involved in drafting of manuscript. He has given final approval of final version of manuscript and he agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
GZ – interpretation of data, head of the experimental group. She has made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. She has been involved in drafting of manuscript. She has given final approval of final version of manuscript and she agreed to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriate investigated and resolved.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alberti K.G.M.M., Zimmet P. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Ajay M., Gilani A.H., Mustafa M.R. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003;74:603–612. doi: 10.1016/j.lfs.2003.06.039. [DOI] [PubMed] [Google Scholar]
- Cazarolli L.H., Zanatta L., Alberton E.H. Flavonoids: cellular and molecular mechanism of action in glucose homeostasis. Mini. Rev. Med. Chem. 2008;8:1032–1038. doi: 10.2174/138955708785740580. [DOI] [PubMed] [Google Scholar]
- Costa A., Casamitjana R., Casals E. Effects of atorvastatin on glucose homeostasis, postprandial triglyceride response and C-reactive protein in subjects with impaired fasting glucose. Diabet. Med. 2003;20:743–745. doi: 10.1046/j.1464-5491.2003.00993.x. [DOI] [PubMed] [Google Scholar]
- Geraldes P., King G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh S., Negishi M. Serum- and glucocorticoid-regulated kinase 2 determines drug-activated pregnane X receptor to induce gluconeogenesis in human liver cells. J. Pharmacol. Exp. Ther. 2014;348:131–140. doi: 10.1124/jpet.113.209379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy M. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 2006;47:1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Hoffart E., Ghebreghiorghis L., Nussler A.K. Effects of atorvastatin metabolites on induction of drug-metabolizing enzymes and membrane transporters through human pregnane X receptor. Br. J. Pharmacol. 2012;165:1595–1608. doi: 10.1111/j.1476-5381.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav R., Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int. J. Pharm. Pharm. Sci. 2012;4:251–256. [Google Scholar]
- Javed H., Khan M.M., Ahmad A. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;17(210):340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- Katsube T., Imawaka N., Kawano Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006;97:25–31. [Google Scholar]
- Kim B., Feldman E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015;47(1–10) doi: 10.1038/emm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J.H., Laufs U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Patil I.Y., Jiang T. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. Am. J. Pathol. 2009;175:355–364. doi: 10.1371/journal.pone.0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghbelinejad S., Nassiri-Asl M., Farivar T.N. Rutin activates the MAPK pathway and BDNF gene expression onbeta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 2014;224:108–113. doi: 10.1016/j.toxlet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Niture N.T., Ansari A.A., Naik S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: an effect mediated through cytokines, antioxidants and lipid biomarkers. Ind. J. Exp. Biol. 2014;52:720–727. [PubMed] [Google Scholar]
- O’Neil S., O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- Ostrowski S.M., Wilkinson B.L., Golde T.E. Statins reduce amyloid-?? Production through inhibition of protein isoprenylation. J. Biol. Chem. 2007;282:26832–26844. doi: 10.1074/jbc.M702640200. [DOI] [PubMed] [Google Scholar]
- Panchal S.K., Poudyal H.V., Arumugam T. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011;141:1062–1069. doi: 10.3945/jn.111.137877. [DOI] [PubMed] [Google Scholar]
- Perez-Guerrero C., Marquez-Martin A., Herrera M.D. Regulation of vascular tone from spontaneously hypertensive rats by the HMG-CoA reductase inhibitor, simvastatin. Pharmacology. 2005;74:209–215. doi: 10.1159/000085957. [DOI] [PubMed] [Google Scholar]
- Posvar E.L., Radulovic L.L., Cilla D.D. Tolerance and pharmacokinetics of single-dose atorvastatin, a potent inhibitor of HMGCoA reductase, in healthy subjects. J. Clin. Pharmacol. 1996;36:728–731. doi: 10.1002/j.1552-4604.1996.tb04242.x. [DOI] [PubMed] [Google Scholar]
- Prince P., Kamalakkannan N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006;20:96–102. doi: 10.1002/jbt.20117. [DOI] [PubMed] [Google Scholar]
- Rang H.P., Dale M.M., Ritter J.M. Rang and Dale's Pharmacology. sixth ed. Churchill Livingstone; Edinburg: 2007. Atheroscelrosis and lipoprotein metabolism; p. 289. [Google Scholar]
- Reddy G.B., Muthenna P., Akileshwari C. Inhibition of aldose reductase and sorbitol accumulation by dietary rutin. Curr. Sci. 2011;101:1191–1197. https://pdfs.semanticscholar.org/139e/a1adb387da68d7541f602acbc7a1471dea1d.pdf?_ga=2.170035747.2041951483.1554288160-836850391.1554288160 [Google Scholar]
- Rysa J., Buler M., Savolainen M.J. Pregnane X receptor agonists impair postprandial glucose tolerance. Clin. Pharmacol. Ther. 2013;93:556–563. doi: 10.1038/clpt.2013.48. [DOI] [PubMed] [Google Scholar]
- Shepardson N., Shankar G., Selkoe D. Cholesterol level and statin use in Alzheimer disease. Arch. Neurol. 2011;68:1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotníková, R., Bauer, V., 2010. Nitric oxide production and its changes in diabetic endothelial dysfunction. In: Stefek, M. (Ed.), Advances in molecular mechanisms and pharmacology of diabetic complications. Transworld Research Network, Trivandrum, Kerala, India, pp. 129–151.
- Sotomayor D., Álvarez M., Pérez-Guerrero C. Improvement of age-related endothelial dysfunction by simvastatin: effect on NO and COX pathways. Br. J. Pharmacol. 2005;146:1130–1138. doi: 10.1038/sj.bjp.0706420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E., Urban N.N., Gonzalez-Burgos G.R. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2010;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török J., Ľupták I., Matúšková J. Comparison of the effect of simvastatin, spironolactone and L-arginine on endothelial function of aorta in hereditary hypertriacylglycerolemic rats. Physiol. Res. 2007;56:33–40. doi: 10.33549/physiolres.931395. [DOI] [PubMed] [Google Scholar]
- Wagner A.H., Kohler T., Ruckschloss U., Just I., Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- Wagstaff L.R., Mitton M.W., Arvik B.M. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–880. doi: 10.1592/phco.23.7.871.32720. [DOI] [PubMed] [Google Scholar]
- Wang Y.B., Ge Z.M., Kang W.Q. Rutin alleviates diabetic cardiomyopathy in a rat model of type 2 diabetes. Exp. Ther. Med. 2015;9:451–455. doi: 10.3892/etm.2014.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N.D. Intensified screening and treatment of the metabolic syndrome for cardiovascular risk reduction. Prev. Cardiol. 2005;8:47–54. doi: 10.1111/j.1520-037x.2005.4073.x. [DOI] [PubMed] [Google Scholar]
- Xu P.X., Wang S.W., Yu X.L. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing A oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014;264:173–180. doi: 10.1016/j.bbr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Yada T., Nakata M., Shiraishi T. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br. J. Pharmacol. 1999;126:1205–1213. doi: 10.1038/sj.bjp.0702397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki D., Nakamura T., Okamura N. Effects of acid and lactone forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on the induction of MDR1 expression and function in LS180 cells. Eur. J. Pharm. Sci. 2009;37:126–132. doi: 10.1016/j.ejps.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Yates K.F., Sweat V., Yau P.L. Impact of metabolic syndrome on cognition and brain. Arterioscler. Thromb. Vasc. Biol. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Wang F., Meng X. Short–term use of atorvastatin affects glucose homeostasis and suppresses the expression of LDL receptors in the pancreas of mice. Mol. Med. Rep. 2008;18:1791–2997. doi: 10.3892/mmr.2018.9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaee A., Zamansoltani F., Nassiri M. Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin. Pharmacol. Toxicol. 2009;104:253–258. doi: 10.1111/j.1742-7843.2008.00368.x. [DOI] [PubMed] [Google Scholar]