Abstract
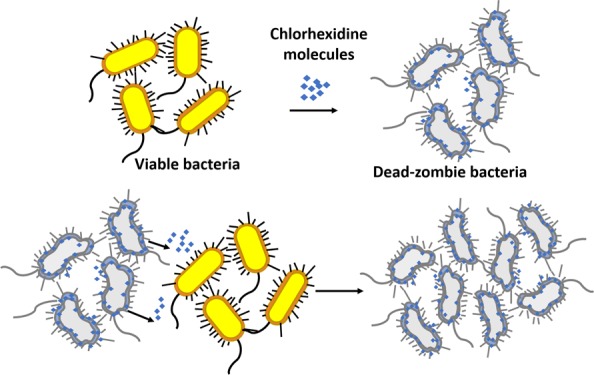
We report a biocidal zombie effect of chlorhexidine, a wide-scope biocidal agent commonly used in disinfectant and antiseptic formulations. The zombie effect refers to the ability of dead bacteria killed by a biocidal agent to act as efficient biocidal agents toward a new generation of viable bacteria. The killed bacteria serve as a reservoir for the antibacterial agent incorporated within them; and the new viable population of bacteria acts as a trap of the bioactive agent, shifting the equilibrium of this agent between the reservoir in the dead cells and their aqueous environment. This report is a major generalization of the zombie phenomenon reported previously for silver from the points of view of extending to organic antibacterial agents; extending the effect to both Gram-negative—Pseudomonas aeruginosa PAO1—and Gram positive—Staphylococcus aureus—representative bacteria; showing that the zombie effect is maintained in the second and third generations; showing the effect to operate in an environment of growth media, which extends it to life-supporting environments; and proving that cross-killing is possible, that is, killed S. aureus cells fully inactivated viable P. aeruginosa.
Introduction
In a previous report,1 we have introduced a new mechanism of the activity of antimicrobial agents, which we denoted as the “zombie effect”, wherein biocidally killed bacteria are capable of inactivating viable bacterial cells by serving as active reservoirs of the accumulated biocidal agent that originally inactivated them. Specifically, the concept was demonstrated by showing the high bactericidal action of silver nitrate-killed Pseudomonas aeruginosa cells toward a viable culture of the same strain. In the underling mechanism of the effect, we proposed that the silver ions undergo complexation and partial reduction to metallic silver within the bacterial cells. When new viable bacterial cells are introduced into the system, an equilibrium shift occurs, which causes silver ions to be released from the killed bacteria and inactivate the viable cells. Here, we further develop this new concept in five aspects, which greatly extend the silver zombie report: first, we show the generality of the phenomenon by testing the zombie effect with an entirely different antibacterial agent—the organic molecule chlorhexidine digluconate (CH). Second, we show that representatives of both Gram-negative and Gram-positive bacteria, P. aeruginosa PAO1 and Staphylococcus aureus ATTC 29213, which were killed by this agent, revealed strong zombie biocidal activities. Third, we examine and prove the feasibility of the effect in growth media, which potentially extends the effect to life supporting environments. Fourth, we show that the zombie effect is carried to a second and third “generation” of zombies. And fifth, we prove that cross-killing is possible, that is, CH-killed S. aureus cells fully inactivate viable P. aeruginosa.
The zombie cell effect was induced as described below in Experimental Section. In brief, the deadly zombie cells were produced by inactivating a ca. 108 colony forming unit (CFU)/mL of either P. aeruginosa or S. aureus with 20.0 ppm CH in a HEPES buffered solution for 3 h. Then, the zombie cells were carefully removed from the solution by centrifugation and resuspended in a solution to which a viable cell population of bacteria was added, after which the bacterial viability was evaluated. The CH concentration for the preparation of the zombie cells was chosen as the minimal concentration, exerting a kill of at least 99.999% in the bacterial population. In practical perspective, clinical formulations of CH are 25 times more concentrated than the solution used in the zombie cell experiment described here.
Results and Discussion
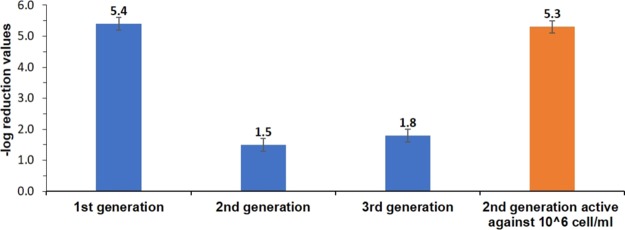
The antibacterial effect of CH-killed Gram-negative P. aeruginosa toward a viable population of the same bacterium is presented in Figure 1. It is seen that starting off with ca. 108 CFU/mL, the CH-killed cells are capable of reducing 5.4 orders of magnitude of the new bacterial population (i.e., 99.999% kill). This demonstrates that the zombie effect is not limited to inorganic silver for which it was first discovered1 but has more general features, encompassing even organic biocidal molecules. Figure 1 also shows that the zombie antibacterial effect can be transmitted from one dead generation to the next: separating the generation of the zombie-killed bacteria cells from the solution and applying them to a freshly prepared 108 CFU/mL population resulted in a reduction of approximately 2 orders of magnitude in viability; and similarly, repeating the process with the second generation zombies and applying them onto a third population results in a similar viability reduction of ∼2 orders of magnitude. It should be noted, though, that the potency of the antibacterial action of the second and the third generation of zombie is more pronounced when testing them against a lower inoculum of 106 CFU/mL (Figure 1, orange column), an inoculum typically used in antibacterial efficacy evaluations, which led to total eradication of the bacteria. These results show that the antibacterial activity of the CH molecules is retained after the inactivation process, and that CH can accumulate in the dead cells, which in turn provides an available source for further bactericidal action against viable cells. Bearing in mind that the amount of CH in the system is limited, there should be a limit to the number of times that the process can be repeated. Indeed, after the third cycle, the killing effect is terminated.
Figure 1.
Antibacterial effect of chlorhexidine-killed P. aeruginsoa toward 108 cell/mL suspension of P. aeruginosa in HEPES buffer. The activity of three zombie-cell generations is shown in blue. Orange: The zombie bactericidal effect of CH-killed P. aeruginosa second generation zombies against 106 cell/mL suspension of P. aeruginosa in HEPES buffer.
Applying the same methodology on a different bacterium—Gram-positive S. aureus ATTC 29213 bacteria—resulted in a similar antibacterial effect (Figure 2, blue column): CH-killed S. aureus zombies were capable of reducing 3.6 orders of magnitudes in the bacterial viability. This implies that the zombie phenomenon is a general trait at least in regard to bacterial Gram types. In fact, having CH-P. aeruginosa zombies more active than CH-S. aureus zombies is consistent with the relative potency of CH toward the two strains examined separately, wherein S. aureus showed more tolerance toward CH (a reduction of 6.8 out of 8.0 orders of magnitude in the viability) in comparison to a complete (8 orders of magnitude) eradication of P. aeruginosa. Next, obtaining two different bacterial strains of CH-zombies led to the possibility of testing cross-killing, in which one bacterial strain is inactivated by zombie cells of a second strain. And so, CH-killed S. aureus cells were added to a suspension of 106 viable P. aeruginosa cells: full inactivation of bacterial viability was observed (Figure 2, orange column), which strengthens the notion that the zombies effect is not limited to cells of one particular bacterial strain but is most probably relevant to any type of bacteria. It is worthwhile mentioning that the cell morphology and its mode of grouping are important parameters influencing on the interaction of CH with it and its adsorption characteristics. The bacteria for which the zombie effect is shown here differ greatly in these parameters: P. aeruginosa is characterized with rode-shaped and singled cells whereas S. aureus is characterized with spherical clustered cell.
Figure 2.
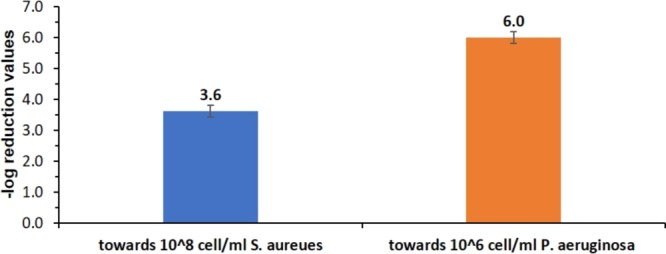
Blue: Antibacterial effect of CH-killed S. aureus toward 108 cell/mL suspension of S. aureus (blue). Orange: Cross-killing: antibacterial effect of CH-killed S. aureus toward 106 cell/mL suspension of P. aeruginosa.
It is also of relevance to examine the zombie effect in life-supporting environments that promote bacterial proliferation. This was carried out by resuspending the CH-killed P. aeruginosa bacteria in a Luria–Bertani (LB) broth, a standard growth medium which was used also to cultivate a viable culture of the same bacterium (Figure 3). As can be seen, the bacterial growth is completely inhibited from time zero. This observation points to the possibility of having an active zombie effect in wound environments treated with CH;2,3 in fact, it may account for its known prolonged antimicrobial activity including in the presence of blood and bio-organic components.4,5
Figure 3.
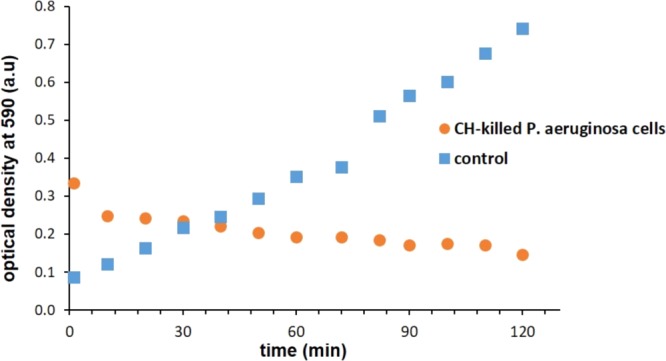
Inhibition effect of CH-killed P. aeruginsoa cells toward the logarithmic growth phase of P. aeruginosa in LB broth. Control: Uninhibited growth.
For the zombie effect to operate, the biocidal agent should be such that its killing action is not accompanied by deactivation (metabolic or physicochemical), or that such deactivation is slow; and that its molecules should adsorb and chemisorb to the bacterial cell components. This is crucial for the transfer of CH from the zombie reservoir upon exposure to an adsorptive target which is empty, that is, the next generation of living bacteria to be eradicated; the adsorption equilibrium established in the dead bacteria will be shifted toward the adsorption sites of the living bacteria, following Le-Chatelier’s principle of equilibria shifts. We, therefore, investigated the adsorption characteristics of CH onto the bacterial cells which determine the basic conditions for the attachment/release of CH to and from the bacterial cells. CH is a cationic molecule (Figure 4 inset) exerting a bacteriostatic effect at low concentrations and bactericidal effect at higher concentrations, by targeting various anionic sites such as phosphate groups present on the surface of the bacterial cell, specifically in the peptidoglycan layers of the bacterial cell wall, thus disrupting the cell envelope permeability, which then leads to inactivation.6,7Figure 3 plots the adsorption isotherm of CH onto bacterial cells of P. aeruginosa in HEPES buffer, by monitoring the CH concentration which remains in the solution at equilibrium with the adsorbed CH. The experimental data showed an excellent fit (r2 = 0.97) with Langmuir’s adsorption isotherm equation (eq 1)
| 1 |
Here, [CH]ads is the concentration of the adsorbed CH at equilibrium, [CH]max is the maximum coverage concentration at equilibrium, K is Langmuir’s constant (the adsorption equilibrium constant), and Ceq is the measured concentration of CH in the solution at equilibrium. The fitting provided a maximum loading concentration of 15.2 ± 0.5 ppm per 109 cells and a Langmuir constant of 0.83 ± 0.20 ppm–1. The fit to Langmuir’s model indicates indeed that the antibacterial effect of the zombie cell stems from strongly adsorbed CH molecules, which in our case results from the interaction of the dicationic sites of CH with negative moieties on the surface of bacteria (such as phosphate groups) thus forming strong ionic bonds. Note that this maximal loading value, translates to ∼1.8 × 108 molecules per one cell. It should be noted that because the adsorption sites are heterogeneous, the Langmuir constant represents an apparent average adsorption equilibrium constant.8 In summary then, the zombie effect has three main stages
| 2 |
| 3 |
| 4 |
Here, reaction 2 is the formation of the CH reservoir within the dead bacteria; in 3, a Langmuir-type adsorption equilibrium is established; in 4 the equilibrium 3 is shifted to the new batch of viable cells, killing it; and as shown above, the second generation of zombies, and even the third one, can repeat this cycle.
Figure 4.
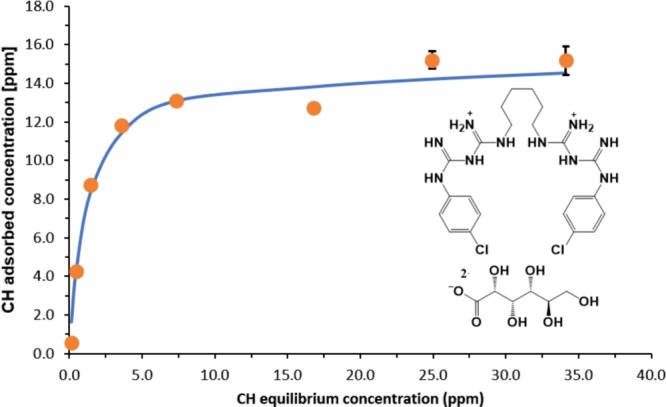
CH adsorption isotherm on P. aeruginosa cells in HEPES buffer and the fit to Langmuir’s equation. Inset: Molecular structure of chlorhexidine digluconate.
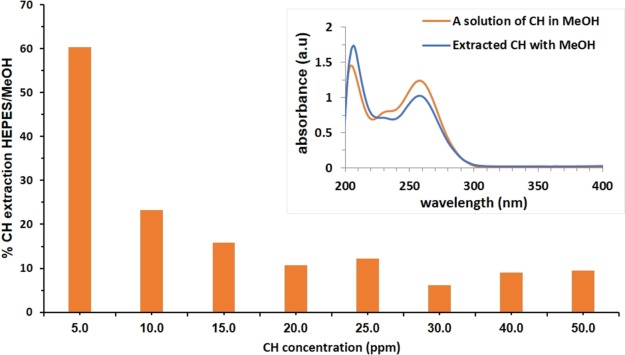
Whereas the adsorption isotherm experiment represents equilibrium points between the CH in the solution and the adsorbed CH, it was also of interest to examine the total extraction of CH from the cells. This can be achieved with methanol, which is able to efficiently release all the CH initially applied to the bacterial population. Figure 5 summarizes the extraction experiment and shows the % of CH extracted with HEPES buffered solution from the total amount extracted with MeOH as a function of the CH exposure concentration of the viable cells. It is seen that, at all exposure concentrations, the buffered HEPES solution is capable of extracting only a portion of the adsorbed CH, thus creating the equilibrium conditions, while the release into MeOH represents the potential available amount of CH to be released by the zombie cells in open systems such as a fresh wound. This extraction also provides an important proof that, under the zombie experiment conditions, the CH molecules remain intact upon interaction with the bacterial cells—the spectrum of the extract is identical to that of pure CH solution (Figure 5, inset). That is, when the equilibrium is shifted and the CH molecules detach from the dead cells and interact with new viable cells, their biocidal activity is, in principle, unchanged.
Figure 5.
Extraction of CH from CH-killed P. aeruginosa: extraction percentage with HEPES from the total extraction with MeOH as a function of the CH exposure concentration of the viable cells. Inset: CH remains intact after its killing action, ready to kill by its release from the dead cells.
We have thus shown the generality of the recently introduced zombies’ phenomenon wherein an equilibrium shift leads to a release of an antibacterial agent from the killed bacterial cells. The equilibrium shift is the result of the presence of adsorptive target sites in the new viable cells of the same bacterial strain, or of a different bacterial strain. It is important to note that the effect is probably relevant in general to persistent antibacterial agents of which activity is not affected by the killing action or by the environment within which they operate. As the effect was shown to exist in both static nonreplicating and in growing bacteria, it should, in principle, play a role in the activity of high-persistent, strongly attached antimicrobial agents applied to living tissues, including local and systemic antibiotics.
Finally, an outlook: the observation of the cross-killing zombie effect leads to a more general concept of utilizing inactivated safe bacterial cells as inert biological carriers for bioactive molecules and for their sustained release. Whereas drug delivery systems are constantly being developed for the benefit of improving product efficacy, safety, and patient compliance, the use of inactivated bacterial cells as biological delivery systems, to best of our knowledge, have not been described yet. Zombie cells of safe bacteria should not, in principle, impose a toxic reaction on human cells as they are fully inactivated and do not produce toxins. Likewise, they should not accumulate in the body as they are bio-degradable. Related studies on “bacterial-ghosts”,9 which are bacterial cell envelopes devoid of any cytoplasmic content that are derived from Gram-negative bacteria, showed that non-living bacterial envelopes can be used as efficient delivery systems for drugs,10 nucleic acids,11 pesticides, enzymes, and as intrinsic adjuvant antigen carrier vaccines;12 we believe that dead cells have a similar potential, with the advantage that various cell components can be used for a wide range of interactions with various useful medical payloads.
Experimental Section
Chemicals
Chlorhexidine digluconate solution, 20% w/w, was purchased from Sigma. Nutrient agar, tryptic soy agar (TSA), tryptic soy broth (TSB), and LB broth were purchased from DIFCO. Sodium thioglycolate, sodium thiosulfate, lecithin, and HEPES were purchased from Acros Organics. Tween 80 (poly(ethylene glycol)sorbitan monooleate) was purchased from Fluka.
Bacteria
Wild-type P. aeruginosa PAO1 strain and S. aureus ATCC 29213 were kindly provided by Prof. E. Banin, Bar-Ilan University, Israel and by Prof. M. Reches, The Hebrew University of Jerusalem, respectively.
Preparation of CH-Killed Bacterial Cells
An overnight grown culture of P. aeruginosa PAO 1 (90 mL) in LB medium at 37 °C was harvested by centrifugation (10 min, 4500 rpm, at RT), washed three times with 30 mL of HEPES buffer (30 mL, 40.0 mM, pH 7.4), and diluted with the same medium to a suspension of 1 × 109 cells/mL. The appropriate dilution factor was determined by diluting the bacterial culture to an optical density (OD590) of 0.3, corresponding to 1 × 108 cells/mL. Bacterial suspension (1.0 mL) and 1.0 mL of CH 200.0 mM solution were added to 8.0 mL HEPES buffered solution, resulting in 1 × 108 cells/mL and CH concentration of 20.0 ppm. The bacterial solution was mixed in an incubated shaker at 30 °C for 3 h. Then, the CH-killed bacterial suspension (of which the kill was ensured following the procedure described below) was centrifuged (10 min, 4800 rpm, RT), and the pellets were carefully removed from the solution. To ensure 99.9999% kill in the original bacterial suspension, a 0.8 mL sample of the solution was diluted 1:1 with a neutralizing solution (0.2% w/w sodium thioglycolate, 1.9% w/w sodium thiosulfate, 1% w/w Tween 80, 1.4% w/w lecithin) for 5 min. After neutralization, the samples were serially diluted (10 fold) with saline solution (8.5% w/w NaCl) and spread-plated on nutrient agar Petri plates. The plates were incubated at 37 °C for 48 h, and the bacterial colonies were enumerated. CH-killed S. aureus ATCC 29213 cells were obtained in similar manner as described for P. aeruginosa, by replacing the nutrient broth, and the nutrient agar with TSB and TSA, respectively.
Evaluating the Bactericidal Efficacy of the CH-Killed Bacteria
(I) The general test: A pellet of the CH-killed cells was suspended in 9.0 mL of HEPES. 1.0 mL sample of a fresh 1 × 109 cell/mL bacterial suspension, (obtained by the procedure described above), was added to the CH-killed cells, and the obtained suspension was mixed in an incubated shaker at 30 °C for 24 h. Then, 0.8 mL aliquots from the bacterial suspensions were neutralized, serially diluted, spread-plated, incubated, and enumerated as described above. The bactericidal experiments were repeated two to three times, and the results are presented as mean log reduction values, log(Nt/N0), where Nt is the bacterial concentration at time t and N0 is the bacterial concentration at time zero.
(II) Bactericidal tests of the second and the third generation killed bacteria. These were carried out by separating the formally killed cells from the solution by centrifugation (10 min, 4500 rpm at RT), resuspension of the resulted pellet in HEPES buffer to which a new bacterial suspension of either 1 × 108 or 1 × 106 viable cells/mL, and incubating and enumerating as described in above.
(III) Cross-killing of P. aeruginosa by CH-killed S. aureus bacteria. The cross-killing experiment was carried out in a similar procedure by applying the CH-killed S. aureus cells to a viable P. aeruginosa suspension cell of 1 × 106 cells/mL.
Evaluating the Bactericidal Efficacy of the CH-Killed Bacteria in a Growth Medium
A CH-killed P. aeruginosa cell pellet was resuspended in 250 μL of LB broth and transferred to a plastic cuvette. A 0.3 mL aliquot of an overnight culture P. aeruginosa in LB was harvested into 30 mL of fresh LB and was grown to OD590 of 0.1. 3.0 mL of the growing cell suspension was added to the cuvette, and the optical density of the mix was followed at 590 nm. Readings were taken every 10 min for a total of 2 h. As a control, 250 μL of LB broth was added to 3.0 mL of the growing cells suspension, and the OD of the suspension was monitored accordingly.
Adsorption of CH onto Bacterial Cells
A viable bacterial suspension of P. aeruginosa in HEPES buffer at OD of 3.0 was obtained from overnight cultures as described above. 1.0 mL of the bacterial suspension and 1.0 mL of a CH solution at increasing concentrations (50.0, 100.0, 150.0, 200.0, 250.0, 300.0, 400.0, and 500.0 ppm) were added to 8.0 mL of HEPES buffered solution. The resulting suspensions were mixed at 30 °C for 3 h in an incubated shaker. Then, the cells were separated from the solutions by centrifugation, and the supernatants were filtered by 0.2 μm syringe filters. The concentrations of CH in the filtered supernatant were measured spectrophotometrically through its maximum absorbance at 254 nm. The adsorbed concentrations were obtained by subtracting the measured concentration in the cells supernatants from these in the control CH solutions.
Extraction of CH from CH-Killed Bacteria
Extraction experiments with HEPES and with methanol were performed by suspending a pellet of CH-killed P. aeruginosa cells obtained as described above, in 10.0 mL of the extracting solvent and stirring the suspension at 30 °C for 24 h in a shaker incubator. Then, the suspension was centrifuged, the supernatant was removed and filtered with a 0.2 μm syringe filter, and the CH concentration was measured spectroscopically through its absorption peak in the extracting solvent (254 nm in HEPES buffer and 260 nm in methanol).
The authors declare no competing financial interest.
References
- Ben-Knaz Wakshlak R.; Pedahzur R.; Avnir D. Antibacterial Activity of Silver-Killed Bacteria: The “Zombies” Effect. Sci. Rep. 2015, 5, 9555. 10.1038/srep09555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak J.; Cleland H.; Campbell F. Dressings for Superficial and Partial Thickness Burns. Cochrane database Syst. Rev. 2008, 4, CD002106. 10.1002/14651858.CD002106.pub3. [DOI] [PubMed] [Google Scholar]
- Lim K.-S.; Kam P. C. A. Chlorhexidine--Pharmacology and Clinical Applications. Anaesth. Intensive Care 2008, 36, 502–512. 10.1177/0310057x0803600404. [DOI] [PubMed] [Google Scholar]
- Laufman H. Current Use of Skin and Wound Cleansers and Antiseptics. Am. J. Surg. 1989, 157, 359–365. 10.1016/0002-9610(89)90570-9. [DOI] [PubMed] [Google Scholar]
- Leonardo M. R.; Tanomaru Filho M.; Silva L. A. B.; Nelson Filho P.; Bonifácio K. C.; Ito I. Y. In Vivo Antimicrobial Activity of 2% Chlorhexidine Used as a Root Canal Irrigating Solution. J. Endod. 1999, 25, 167–171. 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- McDonnell G.; Russell A. D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. G. Chlorhexidine: Is It Still the Gold Standard?. Periodontol. 2000 1997, 15, 55–62. 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Fireman-Shoresh S.; Hüsing N.; Avnir D. Adsorption / Desorption Characteristics of Cis -Platin on Mercapto-Silylated Silica Surfaces. Langmuir 2001, 17, 5958–5963. 10.1021/la010513z. [DOI] [Google Scholar]
- Tabrizi C. A.; Walcher P.; Mayr U. B.; Stiedl T.; Binder M.; McGrath J.; Lubitz W. Bacterial Ghosts - Biological Particles as Delivery Systems for Antigens, Nucleic Acids and Drugs. Curr. Opin. Biotechnol. 2004, 15, 530–537. 10.1016/j.copbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Paukner S.; Kohl G.; Lubitz W. Bacterial Ghosts as Novel Advanced Drug Delivery Systems: Antiproliferative Activity of Loaded Doxorubicin in Human Caco-2 Cells. J. Control. Release 2004, 94, 63–74. 10.1016/j.jconrel.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Ebensen T.; Paukner S.; Link C.; Kudela P.; de Domenico C.; Lubitz W.; Guzmán C. A. Bacterial Ghosts Are an Efficient Delivery System for DNA Vaccines. J. Immunol. 2004, 172, 6858–6865. 10.4049/jimmunol.172.11.6858. [DOI] [PubMed] [Google Scholar]
- Hajam I. A.; Dar P. A.; Won G.; Lee J. H. Bacterial Ghosts as Adjuvants: Mechanisms and Potential. Vet. Res. 2017, 48, 37. 10.1186/s13567-017-0442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]