Abstract
Presented is an ultra-high-pressure liquid chromatographic tandem mass spectrometry (UPLC–MS/MS) method developed for the detection of propylene glycol, glycerol, ethylene glycol and diethylene glycol using isotopically labeled standards in urine as part of ongoing studies to evaluate whether urinary propylene glycol and/or vegetable glycerin concentration are indicators of recent use. Propylene glycol and vegetable glycerol are found in many products that are consumed and used including electronic cigarettes (e-cigarettes). E-cigarettes are battery-powered devices used as an alternative to traditional cigarettes. The liquid formulations aerosolized in these devices largely consist of propylene glycol and/or vegetable glycerol. Published reports regarding the ratio of propylene glycol to glycerol content in these formulations ranged from 50:50 to 100 percent of either. For the analysis of urine specimens from both users and non-users of e-cigarettes, calibrators, controls and specimens were derivatized using benzoyl chloride prior to analysis. They were analyzed using a Waters AcQuity Xevo TQ-S Micro UPLC–MS/MS. Chromatographic separation was performed on an AcQuity UPLC BEH C18 1.7 um, 2.1 × 50 mm, column using a 20 mM ammonium formate in water and 20 mM ammonium formate in methanol as the mobile phase. The method was validated using SWGTOX guidelines for linearity, precision and accuracy, stability, carryover and limit of detection. The linear range was determined using a seven-point calibration curve ranging between 0.5 and 100 mcg/mL. The bias for all validation controls was determined to be ±20% of the expected concentrations with CVs of <15%. A total of 124 urine specimens analyzed collected with 50 specimens collected from self-reported non-smokers (cigarettes/e-cigarettes) confirmed cotinine free using the DRI® Cotinine Assay (Thermo Scientific, Waltham, MA) and 74 specimens collected before and after 12 hours self-reported e-cigarettes abstinence e-cigarette users. Propylene glycol and glycerol were determined to have concentration ranges of “none detected” to 1470 and “none detected” to 2950 mcg/mL, respectively.
Introduction
Presented is an ultra-high-pressure liquid chromatographic tandem mass spectrometry (UPLC–MS/MS) method for the detection of diethylene glycol, ethylene glycol, glycerol and propylene glycol in urine as part of ongoing studies to evaluate urine propylene glycol and/or vegetable glycerin/glycerol urinary concentration in recent electronic cigarette (e-cigarettes) users. E-cigarettes are battery-powered devices used as an alternative to traditional cigarettes. These devices are especially popular with smokers wishing to quit or reduce their habit (1). They deliver nicotine and/or flavors in aerosols generated using an e-cigarette liquid formulation (e-liquids) that consist largely of propylene glycol and/or vegetable glycerin. (2). A large variety of devices and flavors, and active drug(s), vitamins or natural products formulations are currently in the marketplace. The process of inhaling the vapors (or aerosols) produced by e-cigarettes is known as “vaping”. In 2018, the e-cigarette market was valued at $11.5 billion and by 2023 it is projected to reach $44.6 billion (3).
Propylene glycol and vegetable glycerol are “generally recognized as safe” in the food/pharmaceutical industry (4). Propylene glycol, a synthetic organic compound, is found in a large number of food products, drug formulations and cosmetics (5). Vegetable glycerin, a naturally occurring compound, is used as a food additive to help reduce water loss and prolongs shelf life, as a laxative and expectorant in medical and pharmaceutical preparations and in mouthwashes, skin and hair care products, shaving cream, toothpastes and soaps in personal care products (5, 6).
Published reports found the ratio of propylene glycol to vegetable glycerin content in e-liquids could range from 50:50 to 100 percent of either compound (7, 8). E-cigarettes have been added to the Federal Food, Drug and Cosmetic Act by the Federal Drug Administration (FDA) and are now in the process of being regulated; alerts have been issued regarding diethylene glycol contamination of e-cigarettes/e-liquids entering the US market, in particular those from China (7). Ethylene glycol, propylene glycol and diethylene glycol are widely used as components of antifreeze for automobiles. Ethylene glycol and diethylene glycol are not authorized as ingredients in pharmaceutical products and food but are allowed as residuals and can be found as contaminants in various consumer products. Concentrations of 0.1% of diethylene glycol or ethylene glycol in e-liquids are considered acceptable and safe.
For the analysis of relatively low molecular weight compounds such as glycols and vegetable glycerol poor atmospheric pressure ionization using either electrospray ionization or atmospheric pressure chemical ionization is to be expected (9, 10). Pre-column derivatization using the Schotten–Baumann reaction (11) to form the benzoyl ester has been shown to improve ionization and detection limits of glycols in several published tandem mass spectrometry methods (12–17). The presented UPLC–MS/MS method to quantitate diethylene glycol, ethylene glycol, glycerol and propylene glycol in urine was developed to assess urinary propylene glycol and/or glycerol urine concentrations in specimens from e-cigarette users and non-smokers. The analysis was performed using derivatization with benzoyl chloride and liquid/liquid extraction. The corresponding deuterated internal standards (ISTDs) were used for all analytes except for diethylene glycol, which used ethylene glycol-D4. A total of 124 urine specimens submitted for the analysis of propylene glycol, vegetable glycerin, ethylene glycol and diethylene glycol from the Center for the Study of Tobacco Products at Virginia Commonwealth University were evaluated. Specimens included 50 urines from self-reported non-smokers (cigarettes/e-cigarettes), confirmed cotinine free using the DRI® Cotinine Assay, (Thermo Scientific, Waltham, MA) and 74 urines from self-reported e-cigarette users after 12 hours abstinence. Propylene glycol and vegetable glycerin were determined to have concentration ranges of “none detected” to 1470 and “none detected” to 2950 ng/mL, respectively.
Method
Reagents and chemicals
Ammonium formate and benzoyl chloride were purchased from Acros Organics, (Fairlawn, NJ). N-heptane, saline (0.9% NaCl, unbuffered) and sodium hydroxide pellets were purchased from Fisher Scientific (Fairlawn, NJ). Glycine was obtained from Sigma Chemicals (St. Louis, MO). Diethylene glycol, ethylene glycol, vegetable glycerin and propylene glycol were all HPLC grade and purchased from Sigma-Aldrich (Burlington, MA). The ISTDs, ethylene glycol-D4, vegetable glycerin-D5 and propylene glycol-D6 were purchased from CDN Isotope (Pointe Claire, Quebec, Canada). Methanol and water were LCMS grade.
Separate stock solutions for the preparation of calibrators and controls containing each diethylene glycol, ethylene glycol, glycerol and propylene glycol at 1000 mcg/mL were prepared in water. The ISTD mixture containing 1000 mcg/mL of ethylene glycol-D4, vegetable glycerin-D5 and propylene glycol-D6 was prepared by adding 10 μL of each analyte stock to 10 mL of water. Working ISTD (10 mcg/mL) was prepared fresh with each analytical run as the vegetable glycerin-D5 was noted to degrade after approximately 1 week.
Calibrator and QC preparation
Calibrators were prepared by fortifying 1.0 mL aliquots of saline. Prior to each analytical run, fresh six-point calibration curves containing diethylene glycol, ethylene glycol, vegetable glycerin and propylene glycol were prepared in duplicate at concentrations of 0.5, 1, 10, 25, 50 and 100 mcg/mL. Quality control (QC) specimens containing diethylene glycol, ethylene glycol, glycerol and propylene glycol were prepared at the start of the method validation and analyzed at the following concentrations: limit of quantitation (LOQ), target concentration of 0.5 mcg/mL; low (LQC), target concentration 3 mcg/mL, medium (MQC), target concentration 30 mcg/mL and high QC (HQC), target concentration 80 mg/mL. The dilution QC (Dil), target concentration 200 mcg/mL, was analyzed at 1:1 dilution to ensure accurate quantification if the specimen concentrations exceeded the highest calibrator or the specimen volume was insufficient for testing. A negative control (glycol-free) containing ISTDs only and a matrix blank (glycol-free without ISTD) were included in each analytical run. Two different stock solutions were prepared. One was used to prepare the controls, and the other was used to prepare the calibrators. All QC lots and standards were stored at 5°C for the duration of the method validation.
Procedure
Specimens were analyzed on a Waters AcQuity Xevo TQ-S Micro UPLC–MS/MS (Milford, MA). Chromatographic separation was performed on an AcQuity UPLC BEH C18 1.7 um, 2.1 × 50 mm, column (Milford, MA; Figure 1). Multiple reaction monitoring and transition ions were monitored in positive ion mode with the cone voltage (V) and collision energy (eV) listed in Table I. The mobile phase consisted of (A) 20 mm ammonium formate in water and (B) 20 mm ammonium formate in methanol. The mobile phase gradient started with 50% B ratio increasing to 100% B over 3 minutes and held for 0.5 minutes, then re-equilibrated to 50% B for 1 minute. The total runtime was 4.5 minutes. The flow rate was set at 0.3 mL/minute, the column temperature was 40°C and the injection volume was 0.5 μL.
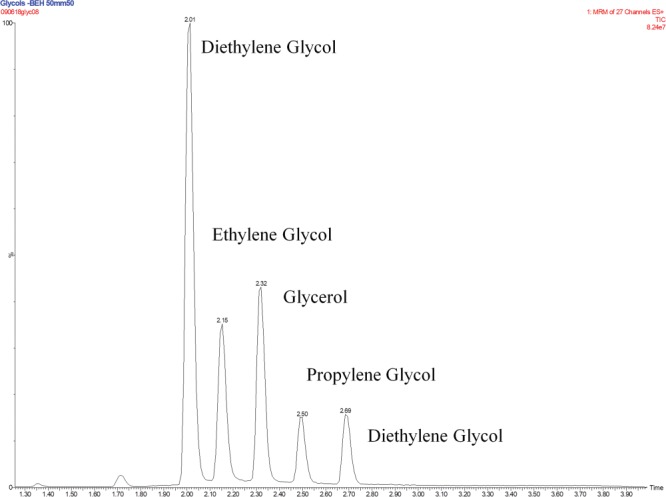
Figure 1.
The chromatographic separation of propylene glycol, glycerol, ethylene glycol and diethylene glycol.
Table I.
Transition Ions, Cone Voltages and Collision Energy for the Glycols and Glycerol
| Analyte | (m/z) | CV | CE (eV) |
|---|---|---|---|
| Diethylene glycol | 315 > 77,105,149 | 28 | 56,32,14 |
| Ethylene glycol | 271 > 77,105,149 | 34 | 54,34,14 |
| Ethylene glycol-D4 | 275 > 77,105,153 | 38 | 44,28,12 |
| Glycerol | 405 > 77,105,283 | 46 | 54,30,10 |
| Glycerol-D5 | 409 > 77,105,288 | 46 | 60,26,10 |
| Propylene glycol | 285 > 77,105,163 | 36 | 46,26,10 |
| Propylene glycol-D6 | 292 > 77,105,153 | 10 | 56,34,10 |
Sample preparation
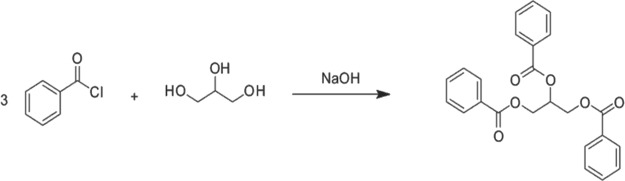
The extraction protocol follows the Schotten–Baumann reaction (Figure 2). In microcentrifuge tubes, 50 μL of ISTD mix (10 mcg/mL) was added to 50 μL of specimen followed by 100 μL 4 N NaOH and 25 μL benzoyl chloride, the derivatizing reagent. Samples were vortex mixed and allowed to stand for 5 minutes for the reaction to occur. To terminate the reaction, 50 μL 10% glycine solution was added. Samples were vortex mixed and allowed to stand for 3 minutes. The analytes were extracted into 1 mL n-heptane. Tubes were capped, vortex mixed and then centrifuged. Fifty microliters of the top organic layer were transferred into a 96-well plate or test tube and dried under a gentle stream of nitrogen at 40°C. Samples were then reconstituted with 500 μL mobile phase and placed on the UPLC–MS/MS for analysis.
Figure 2.
Schotten–Baumann reaction.
Method validation
The method was validated using SWGTOX guidelines as a basis for linearity, accuracy and precision, stability, carryover and limit of detection studies. Calibrators and controls were prepared in saline. Using the described UPLC–MS/MS method, linearity was assessed with standard curves ranging from 0.5 to 100 mcg/mL over 5 days in duplicate. Peak height ratios were calculated for each analyte and its corresponding ISTD, and then used in constructing the standard curve by plotting the peak height ratio vs the concentration of the standards. Unknown concentrations of each glycol and glycerol were calculated using linear regression. The lower LOQ was administratively set at 0.5 mcg/mL. Accuracy and precision were determined from the prepared QC samples. QC samples were analyzed in triplicate each run over five different analytical runs. Acceptable bias did not exceed ±20% at each concentration with a coefficient of variation (% CV) of ≤15% except at the LOD of ≤20%. Two types of precision were assessed during the validation: intra-day and inter-day. Stability was determined under several specific conditions and time intervals, using two of the control specimens, LQC and HQC. All studies included three replicate analyses of each QC specimen. Refrigeration to room temperature stability was assessed using QC . QC specimens stored at 5°C were put through three cycles where they were brought to room temperature with the last cycle and analysis preformed 24 hours after the last cycle. They were then prepared and quantitated against freshly prepared calibrators. The “bench-top” stability was assessed to evaluate the possible effects of specimen transportation and processing in the laboratory by having the QC specimens sit at room temperature for 72 hours. They were then extracted and quantitated against freshly prepared calibrators. The “post-preparative” stability of the analytes was evaluated by having extracts sit in the UPLC–MS/MS’s auto-sampler. A batch of the extracted LQC and HQC were quantitated against a freshly prepared calibration curve. The extracted controls were then allowed to sit in the auto-sampler for 24 and 48 hours at 4°C after which they were re-injected and quantitated from the initial calibration. The results of the initial analysis were compared to those of the re-injected samples. Sample carryover was evaluated in each of the five validation runs by immediately following the injection of the highest calibrator with a negative control (drug-free) was injected. A total of 124 urine specimens were collected and analyzed as part of an IRB-approved study. This included 50 specimens collected from individuals who reported no use of any nicotine-containing product or e-cigarettes and 74 urine specimens from self-reported e-cigarette users that used ≥1 mL e-liquid daily for more than 1 month. Urine specimens were collected before and 12 hours post last reported self-use. These participants used their own e-liquids that contained various ratios of propylene glycol and glycerin.
Results
For all glycols and vegetable glycerin, the calibration curves were determined to be linear from 0.5 to 100 mcg/mL (r2 = 0.9990). LOQC samples were used to verify that the LOQ was within ±20% of the target value and had a response at least 10 times greater than the signal to noise ratio of the blank. The bias of the controls was within the ±20 range and had intra and inter-run precision CV ≤ 15%, except at the LOD which had CV of ≤ 20% for each of the analytes (Table II). All analytes were stable under the refrigeration to room temperature cycles and bench-top stability conditions. Analytes were stable for 48 hours post preparation with the exception of diethylene glycol which required analysis within a few hours of preparation (Table 3). No carryover was observed in the negative control.
Table II.
Intra- and Inter-day Precision
| Control Mean (%CV) | Propylene glycol | Glycerin | Ethylene glycol | Diethylene glycol |
| Intra-day precision (n = 3) | ||||
| LOQ (1 mcg/mL) | 1.2 (20) | 1.1 (13) | 1.0 (6) | 0.8 (13) |
| Low (3 mcg/mL) | 3.0 (10) | 2.4 (15) | 3.1 (3) | 2.8 (13) |
| Mid (20 mcg/mL) | 21 (14) | 24 (6) | 21 (9) | 23 (9) |
| Hi (80 mcg/mL) | 78(12) | 90 (4) | 86 (2) | 82 (11) |
| Dil (200 mcg/mL) | 184 (10) | 225 (4) | 220 (4) | 190 (7) |
| Inter-day precision (n = 15) | ||||
| LOQ (1 mcg/mL) | 1.0 (17) | 1.0 (12) | 1.0 (5) | 0.9 (20) |
| Low (3 mcg/mL) | 2.9 (4) | 2.4 (15) | 3.1 (4) | 2.8 (11) |
| Mid (20 mcg/mL) | 21 (11) | 23 (3) | 22 (9) | 22 (10) |
| Hi (80 mcg/mL) | 80 (10) | 85 (15) | 83 (7) | 83 (9) |
| Dil (200 mcg/mL) | 193 (12) | 223 (15) | 204 (9) | 216 (9) |
Table III.
Stability Precision for Under Various Conditions
| Control Mean (%CV) | Propylene glycol | Glycerin | Ethylene glycol | Diethylene glycol |
| Fridge/room temp (n = 3) | ||||
| Low (3 mcg/mL) | 3.0 (10) | 3.6 (11) | 3.1 (7) | 3.1 (14) |
| Hi (80 mcg/mL) | 67 (2) | 93 (2) | 87 (4) | 79 (3) |
| Bench top (n = 3) | ||||
| Low (3 mcg/mL) | 3.3 (6) | 2.4 (15) | 3.1 (4) | 2.7 (4) |
| Hi (80 mcg/mL) | 75 (9) | 73 (1) | 79 (1) | 79 (2) |
| Post-preparative 48 hours (n = 3) | ||||
| Low (3 mcg/mL) | 2.8 (8) | 3.4 (12) | 2.9 (4) | 1.0 (49) |
| Hi (80 mcg/mL) | 90 (6) | 94 (12) | 91 (10) | 86 (12) |
Application to the method
Urine collected from 50 different individuals who self-reported no e-cigarette use/cigarette smoking resulted in the detection of propylene glycol in 43 of the specimens with a mean ± standard deviation concentration of 41 ± 53 mcg/mL. Vegetable glycerin was detected in 34 of the specimens with determined mean ± standard deviation concentration of 59 ± 81 mcg/mL Ethylene glycol and diethylene glycol (DEG) were not detected in any of the specimens. Detectable concentrations of any of the glycols or vegetable glycerin were absent in two of the specimens. The 74 urine specimens collected from e-cigarette users before and after 12 hours abstinence resulted in propylene glycol detection in all of the samples with a mean ± standard deviation concentration of 211 ± 281 mcg/mL. Vegetable glycerin was detected in 70 of the specimens with a mean ± standard deviation concentration of 111 ± 118 mcg/mL. Ethylene glycol and diethylene glycol (DEG) were not detected in any of the specimens.
Discussion
As a result of the increase in e-cigarette usage, analytical methods for the detection of the major aerosolized components generated, propylene glycol and vegetable glycerin in biological specimens are needed. There are several methods for the analysis of glycols with LC–MS/MS after a derivation step using the Schotten–Baumann method. These methods have analyzed ethylene glycol, triethylene glycol, 4-butanediol, 2-butanediol, 2,3-butanediol, 1 3-propanediol and/or propylene glycol (12–17). The presented method is for the analysis of diethylene glycol, ethylene glycol and propylene glycol as well as vegetable glycerin. The presented method was able to improve ionization and detection of these glycols and vegetable glycerin after pre-column derivatization using the benzoyl chloride as the derivatizing reagent, Schotten–Baumann reaction. This method used corresponding deuterated internal standards except for diethylene glycol which used ethylene glycol-D4. The method was determined to be accurate and precise for the analysis of urine specimens. The lack of stability of the vegetable glycerin-D5 in the internal working standard preparation required it be prepared fresh for each analysis. The lack of stability of the diethylene glycol in the extracted specimens required that the samples be analyzed on the day of derivatization. The analytical method was successfully applied to genuine urine specimens which confirmed that the linear range of the presented assay was acceptable for both propylene glycol and vegetable glycerin concentrations in both e-cigarette users and non e-cigarette users. These compounds were detected in both e-cigarette users and non e-cigarette users. The presented data in mcg/mL resulted in averages that were greater for the e-cigarette user but were not normalized using the creatinine so further analysis would be needed to determine whether or not propylene glycol and/or vegetable glycerin would make suitable biomarkers for identification of e-cigarette use. Neither diethylene glycol nor ethylene glycol were detected in any of the genuine urine specimens. As both of these glycols are not authorized as ingredients in pharmaceutical products and food, their detection in genuine urine specimens would be unexpected.
Conclusion
A UPLC–MS/MS method for the determination of propylene glycol, vegetable glycerin, ethylene glycol and diethylene glycol benzoyl chloride derivatives in urine was developed. It utilized Schotten–Baumann reaction procedure prior to ionization and chromatographic analysis and was particularly suited for analysis of urine specimens. Further, the assay may be easily adapted for the analysis of glycols in clinical and/or forensic urine specimens and could be applied to other biological matrixes.
Funding
This project was funded in part by the National Institute of Health (DA033934, DA036105), the National Institute on Drug Abuse of the National Institutes of Health under award numbers P50DA036105 and U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. All authors contributed significantly to the study, and all authors have read and approved the final manuscript. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. Dr Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent for a device that measures the puffing behavior of ECIG users.
References
- 1. Patel D., Davis K.C., Cox S., Bradfield B., King B.A., Shafer P. et al. (2016) Reasons for current e-cigarette use among U.S. adults. Preventive Medicine, 93, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breland A., Soule E., Lopez A., Ramôa C., El-Hellani A., Eissenberg T. (2016) Electronic cigarettes: What are they and what do they do? Annals of the New York Academy of Sciences, 1394, 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(2018) E-cigarette market by product, vaporizer, vape mod, distribution channel, aftermarket products, and geography – Global size, share, development, growth, and demand forecast: 2013–2023. Prescient and Strategic Intelligence Private, Report SE10521. [Google Scholar]
- 4. Flavor and Extract Manufacturers Association Safety assessment and regulatory authority to use flavors—Focus on e-cigarettes, 2014; http://www.femaflavor.org/safety-assessment-and-regulatory-authority-use-flavors-focus-e-cigarettes (accessed Mar 15, 2017).
- 5. Wexler P. Encyclopedia of Toxicology, 3rd edition. Academic Press/Elsevier: London, UK, 2014; pp754, 1113–756, 1116. [Google Scholar]
- 6. Pagliaro M., Rossi M.. Future of Glycerol, 2nd edition. Kraus Royal Society of Chemistry: Thomas Graham House, Science Park, Milton Road Cambridge, UK, 2010; pp6–9. [Google Scholar]
- 7. Peace M.R., Baird T.R., Smith N.S., Wolf C.E., Poklis J.L., Poklis A. (2016) Concentration of nicotine and glycols in 27 electronic cigarette formulations. Journal of Analytical Toxicology, 40, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dong Y., Yan K., Ma Y., Yang Z., Zhao J., Ding J. (2013) Analysis of refill liquids for electronic cigarettes. Addiction, 237, 1671–1679. [DOI] [PubMed] [Google Scholar]
- 9. Bayer E., Gfrorer P., Rentel C. (1999) Coordination-ionspray-MS (CIS-MS), a universal detection and characterization method for direct coupling with separation techniques. Angewandte Chemie, 38, 992–995. [DOI] [PubMed] [Google Scholar]
- 10. Van Berkel G.J., Quirke J.M.E., Tigani R.A., Dilley A.S., Covey T.R. (1998) Derivatization for electrospray ionization mass spectrometry. 3. Electrochemically ionizable derivatives. Analytical Chemistry, 70, 1544–1554. [DOI] [PubMed] [Google Scholar]
- 11. Grinias J.P., Wong J.T., Nesbitt K.M. (2017) Using benzoyl chloride derivatization to improve small-molecule analysis in biological samples by LC–MS/MS. LCGC North America, 35, 760–768. [Google Scholar]
- 12. Holčapek M., Virelizier H., Chamot-Rooke J., Jandera P., Moulin C. (1999) Trace determination of glycols by HPLC with UV and electrospray ionization mass spectrometric detections. Analytical Chemistry, 71, 2288–2293. [DOI] [PubMed] [Google Scholar]
- 13. Gao S., Wilson D.M., Edinboro L.E., Mcguire G.M., Williams S.G.P., Karnes H.T. (2003) Improvement of sensitivity for the determination of propylene glycol in rat plasma and lung tissue using HPLC/tandem MS and derivatization with benzoyl chloride. Journal of Liquid Chromatography and Related Technologies, 26, 3413–3431. [Google Scholar]
- 14. Sørensen L.K., Hasselstrøm J.B. (2012) A hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry method for the simultaneous determination of g-hydroxybutyrate and its precursors in forensic whole blood. Forensic Science International, 222, 352–359. [DOI] [PubMed] [Google Scholar]
- 15. Johansen S.S., Windberg C.N. (2011) Simultaneous determination of g-hydroxybutyrate (GHB) and its analogues (GBL, 1.4-BD, GVL) in whole blood and urine by liquid chromatography coupled to tandem mass spectrometry. Journal of Analytical Toxicology, 12, 8–14. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez F., Ibanez M., Sancho J.V. (2008) Fast determination of toxic diethylene glycol in toothpaste by ultra-performance liquid chromatography-time of flight mass spectrometry. Analytical and Bioanalytical Chemistry, 391, 1021–1027. [DOI] [PubMed] [Google Scholar]
- 17. Imbert L., Saussereau E., Lacroix C. (2014) Analysis of eight glycols in serum using LC-ESI–MS-MS. Journal of Analytical Toxicology, 38, 676–680. [DOI] [PubMed] [Google Scholar]


