Abstract
Background
Tigilanol tiglate, a short-chain diterpene ester, is being developed as intratumoral treatment of a broad range of cancers. We conducted the first-in-human study of intratumoral tigilanol tiglate in patients with solid tumors.
Methods
Tigilanol tiglate was administered in a multicentre, non randomized, single-arm study, with escalating doses beginning with 0·06 mg/m2 in tumors estimated to be at least twice the volume of injection (dose-escalation cohorts). Patients with smaller tumors were assigned to the local effects cohort and received the appropriate dose for tumor size.
Findings
Twenty-two patients were enrolled. The maximum dose was 3·6 mg/m2 and the maximum tolerated dose was not reached. There was one report of dose-limiting toxicity (upper airway obstruction), two serious adverse events (upper airway obstruction and septicemia), 160 treatment-emergent adverse events, and no deaths. Injection site reactions in all tumors and tumor types occurred even at the lowest dose. Six of the 22 patients experienced a treatment response, with four of the six patients achieving complete response.
Interpretation
Intratumoral tigilanol tiglate was generally well tolerated, the maximum tolerated dose was not reached, and clinical activity was observed in 9 tumor types including complete response in four patients. These results support the continued development of tigilanol tiglate for intratumoral administration.
Funding
QBiotics Group Limited Brisbane, Queensland, Australia was the sponsor of the study.
Keywords: Diterpene ester, EBC-46, Intratumoral, Protein kinase C, Tigilanol tiglate
Research in Context.
Evidence before this study
Intratumoral injection therapy for cancer currently remains a topic of intense interest, even though current clinical research is heavily focused on immunotherapy. As of January 2019, the PubMed database lists 3671 references under the search term "intratumoral injection”. Injection of antineoplastic agents directly into a tumor not only reduces systemic exposure, minimizes off-target toxicity, and limits the total amount of drug used but also induces robust antitumor activity in the injected lesion and, potentially, in noncontiguous non-injected lesions. Tigilanol tiglate possesses antitumor activity and appears to be effective and well tolerated when injected intralesionally as an alternative to surgery for canine mast cell tumors and soft tissue sarcomas in veterinary settings. Studies in syngeneic and xenograft mouse models showed that intratumoral injection of tigilanol tiglate into subcutaneous tumors resulted in PKC-dependent hemorrhagic necrosis within 24 h, complete loss of viable tumor cells, and marked vascular disruption at 24 h after treatment.
Added value of this study
Although surgery and radiotherapy constitute the great majority of local therapies for tumors, their application and effectiveness can be limited by many factors such as the overall status of the patient, proximity and/or infiltration of tumors into adjacent tissues, tumor inaccessibility, large tumour volume, intolerance of normal tissue to repeated courses of treatment, the presence of metastases and the availability of local facilities in developing nations. As a consequence, better local therapies are still needed for a wide range of tumors to reach the expanding network of infiltrating malignant cells that can be missed by surgery, to spare nearby normal tissue that would be damaged by radiation, and to control tumors that are otherwise untreatable. This study contributes to the body of knowledge supporting the utility of intratumoral injection as a component of local anticancer therapy and specifically to the role of PKC activation in solid tumor treatment.
Implications of all the available evidence
This first-in-human, dose-escalation, clinical study of the novel small molecule tigilanol tiglate administered intratumorally to patients with a range of cutaneous, subcutaneous, or nodal tumors showed that intratumoral administration of tigilanol tiglate is generally well tolerated, a maximum tolerated dose was not declared, and there were preliminary signs of efficacy. These results support the continued development of tigilanol tiglate for intratumoral administration. Future studies could include dosing levels based on target tumor volume ranges. To elucidate further the potential clinical usefulness of intratumoral therapy generally and tigilanol tiglate particularly, longer follow-up periods (for wound healing and efficacy assessments) and volumetric dosing assessments on treatment day (baseline) instead of at screening for RECIST response evaluations should be considered.
Alt-text: Unlabelled box
1. Introduction
Surgery and radiotherapy remain the conventional approaches to local treatment of malignant tumors, but these modalities can be limited by the location, accessibility, and size of the tumor and availability of medical facilities. Intratumoral (IT) administration of anti-neoplastic agents has been a treatment option for many years [1] and represents an alternative to surgery and radiotherapy in patients with localized accessible tumors, providing high drug concentrations at the tumor site with minimal exposure of non-target tissues [2]. A number of agents across different drug classes are being studied in the setting of IT administration [3], [4], [5].
Tigilanol tiglate (EBC-46) is a novel short-chain diterpene ester derived from the seeds of the native Australian blushwood tree (Fontainea picrosperma) [6] and is currently in development for the local treatment of a broad range of tumors [[7], [8], [9]].
Tigilanol tiglate induces a respiratory burst from human polymorphonuclear leukocytes [7] and when injected directly into a tumor, increases vascular endothelial permeability, provokes mitochondrial swelling and plasma membrane destruction in tumor cells, inhibits the growth and induces cell death of a number of human tumor cell lines [9]. It also induces a transcriptional profile with the characteristics of a Th1 immune response, suggesting an immunomodulatory effect that may play a role in tumor regression [10].
Tigilanol tiglate is a potent activator of protein kinase C (PKC) [9], which comprises a family of enzymes that induce changes in signal transduction pathways modulating diverse cellular responses including cell replication [[11], [12], [13], [14]]. Recent clinical data support a tumor suppressive effect for PKC [15,16], although earlier studies suggested an oncogenic role [17].
Preclinical studies employing murine xenograft models showed that a single IT injection of tigilanol tiglate produced vascular disruption, hemorrhagic necrosis, an acute highly localized inflammatory response, rapid tumor cell death and regression of solid tumors [7,9,10,18]. In addition, numerous veterinary clinical studies have demonstrated that tigilanol tiglate administered IT was effective against neoplasms such as cutaneous mast cell tumors and soft tissue sarcomas [8,18,19]. Animal toxicity studies established that tigilanol tiglate has an acceptable safety profile, producing significantly greater local responses (erythema, edema, eschar formation) following IT injection compared with injection into normal skin.
We report the first-in-human study of IT tigilanol tiglate in patients with solid tumors. The results underpin the next phase in the development of tigilanol tiglate for the intratumoral treatment of solid tumors.
2. Patients and methods
2.1. Patient population
This was a Phase I, open-label, multicenter (four sites in Australia), single-arm, non-randomized, dose-escalation study of IT tigilanol tiglate in patients with accessible cutaneous, subcutaneous or nodal tumors refractory to conventional therapy. Eligible patients were >18 years with an ECOG performance status of 0 to 2 [20], a life expectancy >12 weeks, and measurable disease. Patients were not enrolled if they received any treatment within 3 weeks (or 6 weeks for nitrosoureas or mitomycin C) of study treatment, had uncontrolled CNS metastases, or were at increased risk for bleeding, including patients on anticoagulation. Pregnant or nursing females and patients considered to be inappropriate candidates for the study also were excluded. The study was approved by the Institutional Review Boards and Independent Ethics Committees at each participating site, and written consent by patients was required.
2.2. Study design
The primary objective was to establish the safety, tolerability and maximum tolerated dose (MTD) of IT tigilanol tiglate. Secondary objectives were to evaluate the preliminary efficacy of tigilanol tiglate and determine its pharmacokinetics (PK). The exploratory objective was to characterize the pharmacodynamics of tigilanol tiglate through analysis of post-administration blood and tumor tissue. Tigilanol tiglate was dissolved in 100% propylene glycol and mixed 4:6 with 30 mM sodium acetate buffer (pH 4·2) to provide stability and solubility and was provided in 2 mL vials containing 1·5 mg/mL or 2 mg/mL. Vials of diluent (40% propylene glycol in 30 mM acetate buffer) were supplied to allow preparation of the appropriate concentration.
Patients received tigilanol tiglate via direct bolus injection(s) into no more than 3 selected superficial tumors on Day 1. The total administered volume of the solution was determined by body surface area (BSA) using the formula Volume = (BSA x Dose Level)/Concentration of Drug, where Volume is in mL, BSA is in m2, Dose Level is in mg/m2, and Concentration of Drug is in mg/mL [21]. The solution was injected into a volume of tumor estimated to be twice the volume of the injected solution (e.g., 1 mL tigilanol tiglate into 2 cm3 of tumor). Where tumors were larger than that required for the dose, a section of the tumor was injected. When multiple tumors were treated, the dose was divided in proportion to the target volume of each tumor. The dose was administered using a minimal number of injections in a fanning manner to spread the dose evenly throughout the tumor. After assessments over 24 h, patients were discharged from the study site on Day 2 and returned for follow-up on Days 3, 5, 8, 15, and 22 and, if wound healing or stabilization did not occur by Day 22, every 7 days thereafter until full healing or stabilization was achieved.
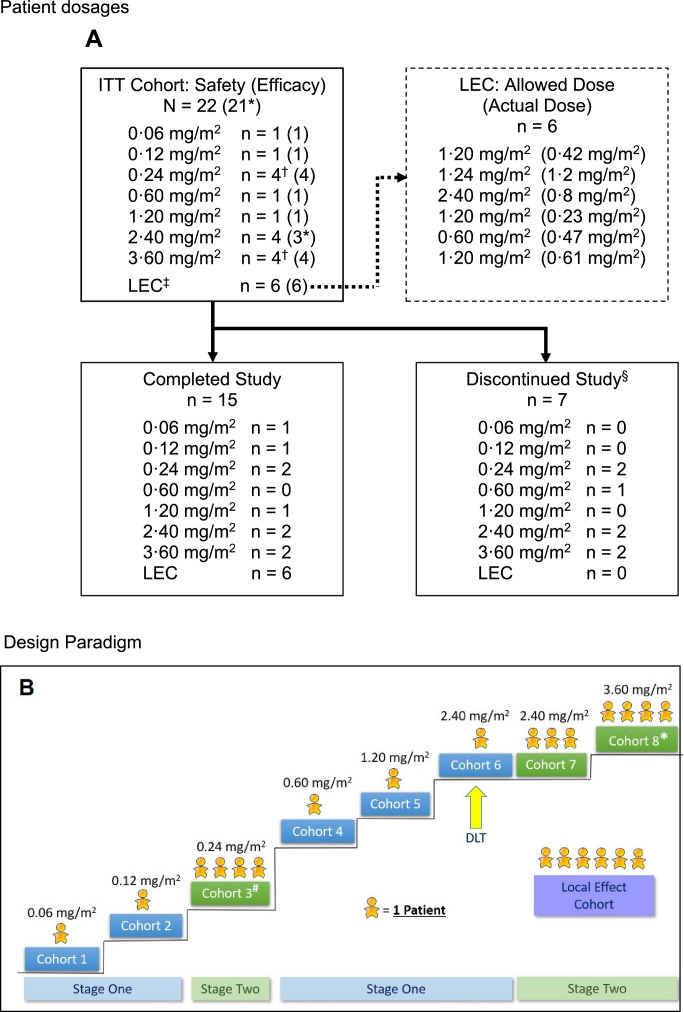
The study was divided into Stages 1 and 2 (Fig. 1:A and B). Stage 1 was conducted to establish the safety and tolerability of escalating doses and concentrations of tigilanol tiglate in single-patient cohorts until a severe treatment-emergent adverse event (TEAE) or dose-limiting toxicity (DLT) occurred, or the Safety Review Committee (SRC) determined the next stage should be commenced. TEAEs were defined as AEs that commenced at or after the start of tigilanol tiglate administration. Transition from Stage 1 to Stage 2 was to occur after Cohort 2. Stage 2 was conducted to determine the safety and tolerability of dose levels in cohorts of at least three patients using the conventional 3 + 3 design (minimum of three patients per dose cohort, with the potential to add an additional three patients to a cohort based on the incidence of DLTs) [22]. Following Cohort 3, the protocol was amended to revert the enrollment back to single-patient cohorts (Stage 1) to reduce the number of patients exposed to doses that were not likely to be therapeutically relevant. Escalation to the next dose was then planned to continue until the MTD was reached or the SRC or the sponsor determined that dose escalation should be terminated. A maximum tigilanol tiglate concentration of 1·5 mg/mL was used for Stage 2, with any subsequent dose escalation achieved by increases in tigilanol tiglate volume. The maximum dose used in this study was 3·60 mg/m2.
Fig. 1.
A: Patient dosages.
EC = local effect cohort; RECIST = Response Evaluation Criteria in Solid Tumors.
*One patient at dose level 2·40 mg/m2 was excluded from the efficacy population because a post-baseline RECIST assessment of the target tumors was not performed.
†In these cohorts, one patient experienced leakage of tigilanol tiglate, so an additional patient was added to the cohort.
‡Six patients had insufficient tumor mass to be enrolled in the dose- escalation stages and were enrolled in the LEC. These patients received the highest concentration of tigilanol tiglate that had been shown to be tolerated, a total dose no higher than the dose tested in the most recent dose-escalation cohort, and a minimum volume of 0·1 mL per tumor. This cohort followed the same assessment schedule as the dose-escalation cohorts.
§Six patients discontinued participation due to disease progression and one due to unintentional lack of follow-up. The mean time to discontinuation was 25·4 days (range: 18 to 43 days).
B: Design Paradigm.
# A patient in this cohort experienced leakage following dosing, therefore a total of four were enrolled to satisfy dose escalation rules
* A patient in this cohort was under-dosed, therefore a total of four were enrolled to satisfy dose escalation rules.
2.3. Safety and efficacy evaluations
The safety population comprised all enrolled patients who received any dose of tigilanol tiglate, including those patients who did not complete the study. Routine clinical and laboratory assessments including hematology and biochemistry, and assessment of other safety variables including injection site reactions, wound healing, and AEs were performed on Days 1 and 2 and at each follow-up visit through Day 22 with the exceptions of hematology and biochemistry (Days 1, 2, 8, 15 and 22) and wound healing (from Day 8 onwards). The injection site reaction assessed pain, erythema and swelling of the skin or mucosa limited to the injection site and was graded as 1 if ≤ 3 cm adjacent to the tumor borders, grade 2 if > 3 cm and ≤ 6 cm, grade 3 if > 6 cm and grade 4 if it was life threatening, chronically disabling or a hemorrhage. The efficacy population was defined as all enrolled patients that received any dose of tigilanol tiglate and had at least one post-baseline tumor response assessment. To assess efficacy, target tumors were measured at baseline and on Day 22 using both calipers and computed tomographic (CT) scans, and RECIST 1·123 criteria then were applied to assess anti-tumor responses. Some patients had efficacy assessments beyond Day 22.
2.4. Pharmacokinetics
The PK population consisted of all enrolled patients who received any dose of tigilanol tiglate and had an evaluable plasma concentration profile. Blood for PK and biomarker analysis was collected within 30 min prior to dosing and then 5, 15, and 30 min and 1 h, 2 h, 4 h, 6 h, 8 h and 24 h after dosing. Plasma samples were assayed for tigilanol tiglate maximum observed concentration (Cmax), time of Cmax (Tmax), area under the plasma concentration-time curve from time zero to the last quantifiable sampling point post-dose, area under the plasma concentration-time curve extrapolated to infinity (AUC0-∞), elimination half-life, and systemic clearance in accordance with CPR analytical laboratory method ALM-084. PK parameters were determined using Phoenix WinNonlin version 7·0 (Pharsight Corporation, USA).
2.5. Statistical analysis
The currently supported version of SAS Software (Version 9·4) was used to perform all data analyses.
Continuous variables were summarized using the statistical mean, median, standard deviation, minimum and maximum. Mean with standard deviation, and median with interquartile range, were presented to one more decimal place. Categorical variables were summarized with frequency counts and percentages. Percentages were rounded to one decimal place, with the relevant patient population being the denominator. Only basic descriptive statistics were performed.
The sample size for this study had been selected without performing a power calculation to provide descriptive information on safety, tolerability, and PK following administration of tigilanol tiglate and was done to minimize the number of patients exposed to potentially sub-therapeutic levels of the drug.
3. Results
3.1. Patient characteristics
Of the 22 patients enrolled in the study, 15 (68%) were male and seven (32%) were female; the median age was 64 years (range: 31 to 86 years); and 21 were Caucasian and one was Asian. At enrolment, seven patients had AJCC stage IV disease (32%), four had stage III (18%), five had stage II (23%) and two had stage I (9%); disease stage was not known and/or not recorded for four patients (18%). The first patient was consented and screened on 13 Feb 2015 and the last patient consented and screened on 30 May 2017. The last follow-up assessment (Patient 206 – Day 64) was on 30 Jun 2017. A total of 29 tumors representing nine tumor types were treated in the 22 patients; squamous cell carcinoma was present in ten patients, followed by melanoma in three, basal cell carcinoma and breast adenocarcinoma in two each, and single cases of atypical fibroxanthoma, atypical myxoid fibrosarcoma, metastatic colorectal adenocarcinoma, adenoid cystic carcinoma and angiosarcoma. Seventeen patients had single tumors treated, three had two tumors treated, and two had three tumors treated. Thirteen patients (59%) had at least one antecedent oncologic surgical procedure and 17 (77%) had received chemotherapy or radiotherapy. Fifteen patients completed the study and seven were deemed to have discontinued (6 after Day 22) as all of their injection site wounds had not healed or stabilized and the investigators felt the patients’ disease progressed to the point where they should be taken off study.
3.2. Dose escalation
Single-patient cohorts 1 and 2 of Stage 1 received 0·06 and 0·12 mg/m2 of tigilanol tiglate, respectively, and no DLTs were observed (Table 1). Per protocol, transition to Stage 2 then proceeded and four patients were dosed in Cohort 3 at 0⋅24 mg/m2; the fourth patient was enrolled because leakage of study drug out of the tumor following IT injection occurred in one patient. Given the satisfactory tolerability of IT tigilanol tiglate in Cohort 3, the protocol was amended to allow resumption of Stage 1 (single-patient cohorts), and this was continued through three dose levels to 2·4 mg/m2. At this dose, a DLT of airway swelling was encountered, which led to transition to a second Stage 2 and expansion of the cohort to a total of four patients (Cohorts 6 and 7). Escalation then proceeded to the 3·6 mg/m2 dose level, which was completed without DLT. Although MTD was not reached in the study, dose escalation in the second Stage 2 was discontinued at the Cohort 8 dose level of 3·6 mg/m2, which was deemed by the sponsor to have provided an appropriate balance of safety and potential efficacy.
Table 1.
Tigilanol tiglate dose level by cohort.
| Stage | Cohort* | Cohort dose level (mg/m2) | Tigilanol tiglate concentration (mg/mL) | No. of patients |
|---|---|---|---|---|
| 1 | 1 | 0·06 | 0·25 | 1 |
| 1 | 2 | 0·12 | 0·50 | 1 |
| 2 | 3 | 0·24 | 1·00 | 4† |
| 1 | 4 | 0·60 | 1·50 | 1 |
| 1 | 5 | 1·20 | 1·50 | 1 |
| 1 | 6 | 2·40 | 1·50 | 1 |
| 2 | 7 | 2·40 | 1·50 | 3 |
| 2 | 8 | 3·60 | 1·50 | 4† |
| N/A | LEC | Various | 1·50 | 6‡ |
N/A = not applicable.
If no dose-limiting toxicity occurred, the subsequent cohort received the next highest dose.
One patient experienced leakage of tigilanol tiglate, so an additional patient was included in the cohort.
LEC (local effect cohort) included nominal doses of 0·6 mg/m2 (n = 1), 1·2 mg/m2 (n = 4), and 2·4 mg/m2 (n = 1).
3.3. Safety and tolerability
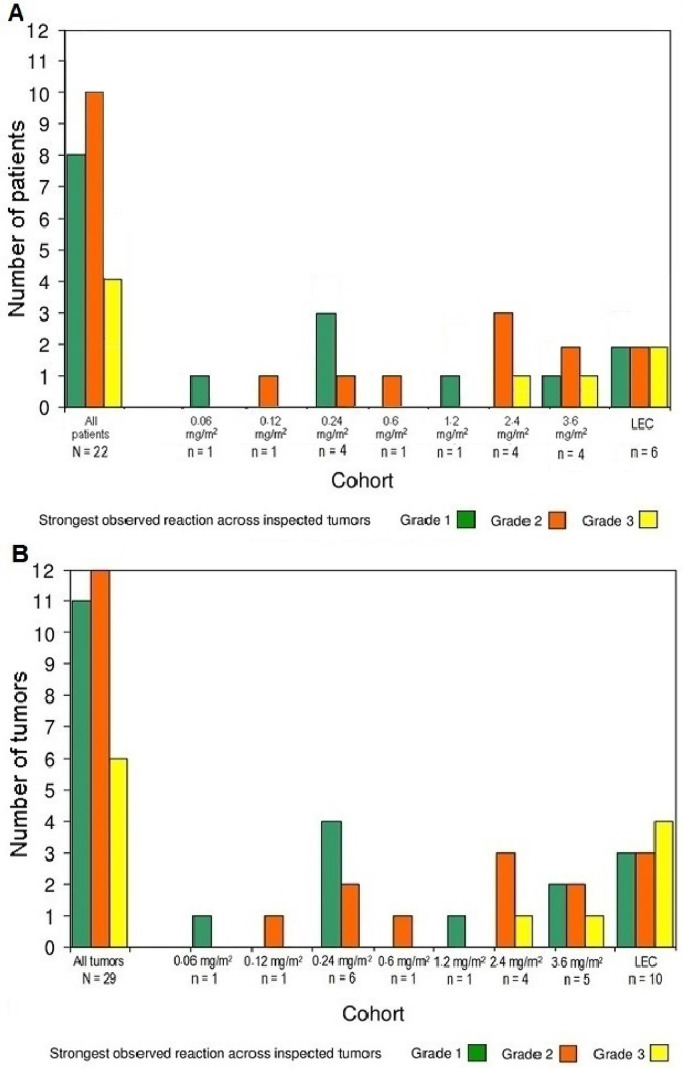
The vast majority of AEs (96%) were mild to moderate: 135 events were assessed as Grade 1, 81 as Grade 2, six as Grade 3 (four reports of injection site pain and single reports of abdominal pain and stridor), and two as Grade 4 (life-threatening upper airway obstruction and sepsis, respectively). There was one DLT and there were two serious AEs, 160 TEAEs, and no deaths. The DLT (upper airway obstruction) occurred in a patient who was treated with 2·4 mg/m2. The two serious AEs were sepsis and upper airway obstruction (also the above DLT). Sepsis due to Streptococcus pyogenes, which was considered possibly related to IT tigilanol tiglate, developed 6 weeks after IT injection and subsequent tumor ulceration in an elderly male with chronic venous insufficiency and an atypical fibroxanthoma on his leg, which eventually healed without evidence of residual tumor. Upper airway obstruction, which developed after subcutaneous infra-auricular/upper neck IT injection, was considered probably related to IT tigilanol tiglate because of altered anatomy including lymphatic drainage after surgery (neck dissection) and radiotherapy (undertaken prior to recruitment to this study) and subsequent parapharyngeal edema necessitating a precautionary tracheostomy. Abdominal pain was considered “not related” to IT tigilanol tiglate. The most common AE was injection site reaction in 12 patients, representing 46% of all treatment-related AEs and 33% of all TEAEs. The observed injection site reactions were Grade 1 for eight patients, Grade 2 for ten patients, and Grade 3 for four patients, and there was an observed dose-response relationship with respect to the frequency and intensity of injection site reactions. Injection site reactions are shown in Fig. 2. TEAEs by nominal dose and toxicity grade are listed in Table 2.
Fig. 2.
Number of patients with injection site reactions by dose cohort and grade of reaction (A). Number of tumors with injection site reactions by dose cohort and grade of reaction (B). LEC = Local effect cohort
Table 2.
Number of TEAEs* by nominal dose level, toxicity grade and CTCAE.
| Nominal dose level | 0·06 mg/m2 |
0·12 mg/m2 |
0·24 mg/m2 |
0·6 mg/m2 |
1·2 mg/m2 |
2·4 mg/m2 |
3·6 mg/m2 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 1 |
1 |
4 |
2 |
5 |
5 |
4 |
||||||||||||||
| Toxicity grade | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 |
| TEAE | |||||||||||||||||||||
| Abdominal discomfort | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Agitation | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Anxiety | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Cellulitis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – |
| Chills | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Cold sweat | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Conjunctivitis | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| ↑ C-reactive protein | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Dyspnea | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | – |
| Erythema | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | 1 | – | – |
| Eye irritation | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – |
| Eye pain | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| Eye swelling | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| Feeling hot | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – |
| Headache | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | – | 1 | – | – |
| Hot flush | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Hypercalcemia | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hyperglycemia | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hypertension | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Hyperventilation | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Injection site edema | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Injection site pain | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | 5 | – | – | – | 1 | – |
| Injection site reaction | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3 | – | – | – | – | – |
| Injection site swelling | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| ↑ Lacrimation | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| Nasal discomfort | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| Neck pain | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Neoplasm progression | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Obstructive airway disorder§ | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – |
| Edema peripheral | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| ↓ Oxygen saturation | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Pain | – | – | – | – | – | – | – | – | – | – | – | – | 3 | – | – | 2 | – | – | – | – | – |
| Pyrexia | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| ↑ Respiratory rate | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Sepsis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – |
| Skin abrasion | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| Skin exfoliation | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Skin ulcer | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 1 | – | – |
| Stridor§ | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – |
| Swelling face | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – |
| Tachycardia | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Tremor | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – |
| Tumor ulceration | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – |
| Vascular disorders | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – |
| Wound secretion | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – |
CTCAE = Common Terminology Criteria for Adverse Events [24]
There were 53 events in 12 of the 22 patients (54·5%) considered possibly related to tigilanol tiglate and 107 events in 12 of the 22 patients (54·5%) considered probably related to tigilanol tiglate (160 events in total).
Obstructive airway disorder and stridor represent the same event in one patient.
3.4. Efficacy
No clear dose-response relationship with respect to efficacy was observed with increasing dose in the dose-escalation cohorts. Best target tumor responses by RECIST 1·1 criteria using caliper measurements from Day 1 are shown in Table 3. Six out of 22 patients had a treated tumor whose injected area responded according to RECIST 1·1. One of 16 patients in the dose-escalation cohort treated with 2·4 mg/m2 experienced a complete response (disappearance of the injected target lesion), clinically confirmed after eventual healing of the injection site ulceration (patient 406); three of the 16 patients experienced partial response, one of whom was treated with 0·6 mg/m2, one with 0·24 mg/m2, and one with 2·4 mg/m2. Ten patients experienced stable disease, one experienced progressive disease, and response was not evaluable in one patient. Review of efficacy data from patients in the local effect cohort (LEC) who received an appropriate dose for tumor size (based on animal data) revealed three of six patients (50%) achieving complete response, three of six (50%) with stable disease, and none with progressive disease
Table 3.
Efficacy treatment response.
| Cohort | Patient No. | Tumor (location) | Nominal/ actual dose (mg/m2) | Estimated tumor volume (cm2) | Estimated percentage tumor treated | Best RECIST (area of tumor injected) response by calipers from Day 1 |
|---|---|---|---|---|---|---|
| Dose-escalation cohorts | ||||||
| 1 | 401 | SCC (back) | 0·06/0·06 | 501·0 | 1% | SD |
| 2 | 201 | SCC (shin) | 0·12/0·12 | 0·9 | 87% | SD |
| 3 | 101 | BCC (nose) | 0·24/0·22 | 2·1 | 37% | SD |
| 3 | 202 | SCC (lateral to eyes, behind ear) | 0·24/0·18 | 0·2 | 100% | PR |
| 0·3 | 100% | |||||
| 0·5 | 100% | |||||
| 3 | 301 | Breast AC (chest) | 0·24/0·24 | 2.4 | 47% | PD |
| 3 | 402 | SCC (zygoma) | 0·24/0·15 | 7·2 | 10% | SD |
| 4 | 403 | SCC (medial canthus) | 0·6/0·59 | 1·4 | 100% | PR |
| 5 | 203 | SCC (inner cheek/palate) | 1·2/1·19 | 12·0 | 21% | SD |
| 6 | 405 | SCC (infra-auricular) | 2·4/2·32 | 58·2 | 10% | Not assessable |
| 7 | 406 | Atypical fibroxanthoma (leg) | 2·4/2·38 | 13·3 | 48% | CR |
| 7 | 408 | SCC (scalp) | 2·4/2·39 | 29·5 | 21% | SD |
| 7 | 409 | SCC (back) | 2·4/2·4 | 27·5 | 21% | PR |
| 8 | 103 | Metastatic melanoma (leg) | 3·6/1·76 | 16·1 | 35% | SD |
| 8 | 206 | Metastatic colorectal AC (abdomen) | 3·6/3·6 | 39·0 | 25% | SD* |
| 8 | 303 | Myxoid fibrosarcoma (cheek) | 3·6/3·61 | 4·7 | 100% | SD |
| 10·4 | 60% | |||||
| 8 | 410 | SCC (temporal) | 3·6/3·47 | 31·9 | 26% | SD |
| Local effect cohort | ||||||
| LEC | 404 | Melanoma (axilla) | 0·6/0·47 | 0·5 | 100% | CR followed by PD† |
| 0·5 | 89% | |||||
| LEC | 102 | Metastatic melanoma (arm) | 1·2/0·42 | 0·7 | 100% | CR† |
| 0·4 | 76% | |||||
| 0·2 | 100% | |||||
| LEC | 407 | Angiosarcoma (nose) | 1·2/0·61 | 2·1 | 100% | CR |
| LEC | 204 | ACC (hard palate) | 1·2/1·2 | 4·4 | 77% | SD |
| LEC | 302 | Breast AC (breast) | 1·2/0·23 | 0·2 | 100% | SD |
| 0·3 | 100% | |||||
| LEC | 205 | BCC (nose) | 2·4/0·84 | 3·1 | 100% | SD |
SCC = squamous cell carcinoma; AC = adenocarcinoma; ACC = adenoid cystic carcinoma; BCC = basal cell carcinoma; CR = complete response; PD = progressive disease; PR = partial response; SD = stable disease.
Patient 206 had no caliper measurements for RECIST so CT scan assessment is provided.
Anenestic responses: 1) Distant effect for patient 404 on fine needle aspiration-proven contralateral parotid nodal deposit and clinically suspicious leg melanoma. Patient clinically and ultrasound clear at 33 months post-treatment followed by systemic metastases although axillary and parotid nodes remained clear. 2) Local effect for patient 102 on most distal untreated arm lesion. See Fig. 3.
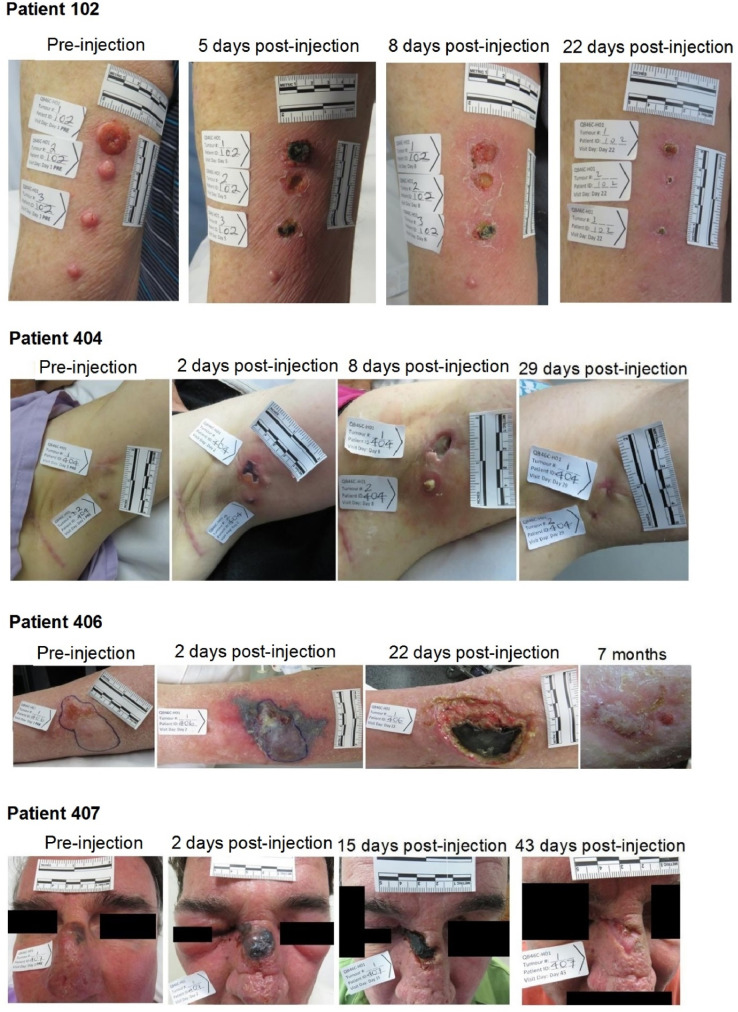
Two patients in the LEC experienced anenestic tumor responses. One of these patients (Patient 404), who had metastatic melanoma in axillary nodal disease, fine needle aspiration-proven contralateral parotid nodal deposit, and a clinically suspicious leg deposit, remained clinically and ultrasonographically clear for 33 months post-treatment but subsequently developed widespread metastatic disease although axillary and parotid nodes remained clear. The other patient (Patient 102) had melanoma with dermal, nodal and pleural metastases. This patient had an earlier local response to radiation therapy for chest wall metastases but developed progressive disease despite receiving four doses of pembrolizumab. Patient 102 experienced a complete response in the three injected cutaneous melanoma metastases on the right upper extremity. Significantly, a fourth cutaneous tumor, which was not injected with study drug (seen inferior to the injected lesions), experienced an anenestic response and completely resolved macroscopically during follow-up. Approximately 4 weeks after injection of the upper extremity lesions, a superficial sternal lesion (biopsy-proven metastatic melanoma) was injected, which also showed a complete response. Of note, CT scans showed anenestic responses in non-injected lymph node and pleural lesions, with complete resolution of an involved 24-mm left axillary node and a 29-mm right pleural nodule, and a reduction in size of an involved right inguinal node. The patient remained well and off treatment until a CT scan performed 14 months after the second tigilanol tiglate injection revealed progressive tumor involving bone and lymph nodes. Patient 407, who had biopsy-proven angiosarcoma of the nasal bridge, had been recommended for total rhinectomy. He achieved a complete response from a single injection of tigilanol tiglate. Three punch biopsies at 12 weeks revealed no residual tumor, and the patient remains disease free on CT scan at 25 months and clinically at 30·5 months post-injection. Fig. 3 shows the changes in tumor appearance with treatment for these four patients.
Fig. 3.
Patient 102 Tumor response: Four melanomas of the right arm. The estimated tumor volume treated was 100% for tumors 1 and 3 and 76% for tumor 2; tumor 4 was not treated. All target tumors had length, width, depth and volume of zero at Days 22, 29 and 36. The figure shows the progression from immediately pre-injection and 5, 8, and 22 days post-injection.
Patient 404 Tumor response: Melanoma of the axilla. The figure shows the progression from immediately pre-injection and 2, 8, and 29 days post-injection. The patient was assessed as a complete response through 33 months with an anenestic response, followed by systemic metastases.
Patient 406 Tumor response: An atypical fibroxanthoma of the leg and experienced stable disease. The figure shows the progression from immediately pre-injection, at 2, and 22 days post-injection, and at final assessment at 7 months.
Patient 407 Tumor response: An angiosarcoma of the nose with a recommendation for total rhinectomy. The figure shows the progression from immediately pre-injection and 2, 15, and 43 days post-injection. The patient was assessed as a complete response and remains clinically disease free 30.5 months post-injection.
The median volume of tumors treated in the dose-escalation cohorts was 37% of the total target tumor volume (range: 1% to 100%; average: 50%) compared with a median of 100% (range: 76% to 100%; average: 94%) in the LEC. The average size of tumors in the dose-escalation cohorts was very large (~40 cm3, maximum ~500 cm3) compared with tumors in the LEC (~1·2 cm3, maximum ~4·4 cm3). Leakage from ulcerating tumors occurred following ten injections, with a mean estimated loss of 10% to 20% of the administered volume. Median wound healing time of the treatment site was approximately 30 days post-injection.
3.5. Pharmacokinetics
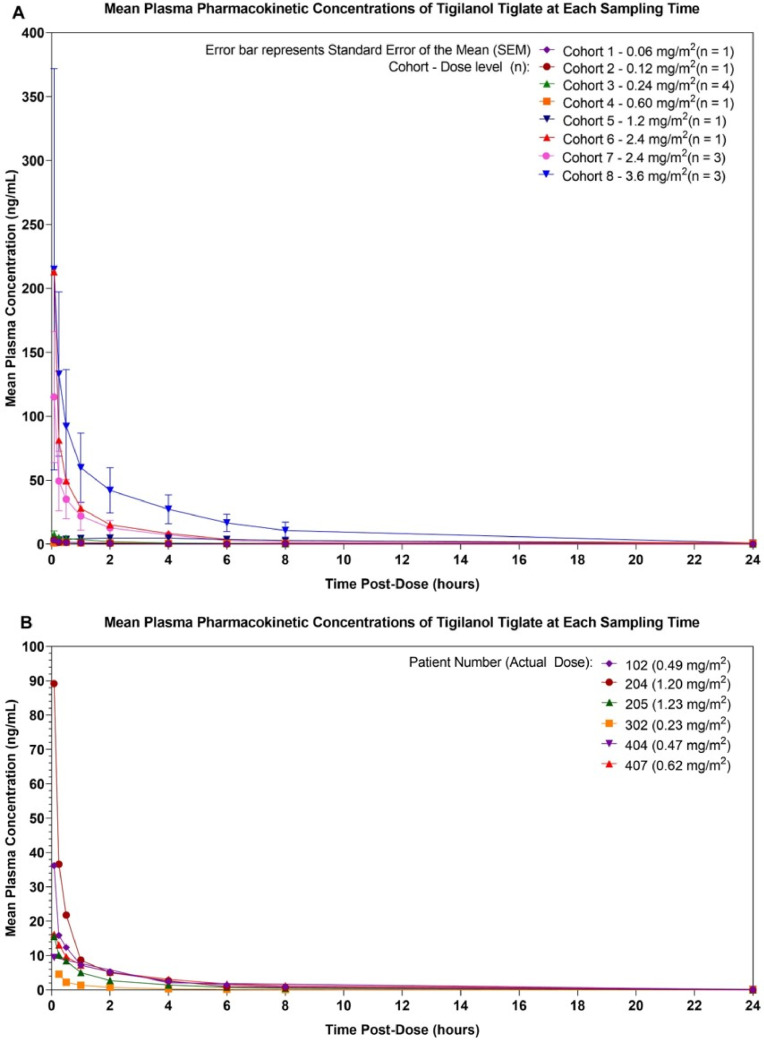
Individual PK profiles for dose-escalation cohorts and the LEC are shown in Fig. 4. Review of these plots indicates that, within a few minutes of injection, tigilanol tiglate was detected in the plasma (median Tmax = 5 min; range: 0·07 to 2·0 h). Plasma concentration then declined rapidly to low levels within 2 to 4 h post-injection, suggesting that larger tumors could be treated with staged injections. There was no apparent trend for increased half-life with increased dose, with an overall median of 3·64 h (range: 1·55 to 9·42 h). Review of data across the cohorts demonstrated a dose-proportional increase in systemic exposure of tigilanol tiglate, as measured by Cmax, AUC0-t, and AUC0-∞ on Day 1 for doses of 0·06 to 3·6 mg/m2. These PK parameters exhibited an approximately linear relationship with dose across the dosing range using a power model approach.
Fig. 4.
Plasma concentration of tigilanol tiglate by dose level shown as individual PK profiles for dose-escalation cohorts (A) and LEC (B).
4. Discussion
This was the first-in-human study to assess the safety and tolerability, efficacy and PK of tigilanol tiglate administered via IT injection to subcutaneous or nodal metastatic lesions in patients with advanced primary or metastatic tumors, using a dose-escalation design. As a first-in-human study, dose levels for the escalation cohorts were necessarily determined by BSA rather than by target tumor volume. As tigilanol tiglate is an IT treatment and efficacious dosing is based on tumor volume and not BSA, efficacy results and factors such as dosing trend have the potential to be confounded.
Although MTD was not reached in the study, the dose escalation in Stage 2 ceased at the Cohort 8 dose level of 3·60 mg/m2, which was deemed to have provided an appropriate balance between safety and potential efficacy. Most TEAEs were related to the local effects and mechanism of action of tigilanol tiglate. TEAEs generally were managed with symptomatic therapy such as analgesics. Some systemic exposure to tigilanol tiglate occurred and, within the range of doses used, the AEs related to systemic exposure appeared to be mild to moderate, self-limited and of short duration. Future studies will consider multiple administration of lower doses.
This study used the RECIST 1·1 guidelines [23] for tumor assessment (by calipers and CT scan), which require tumors to be at least 10 mm in one dimension, limiting the ability to include smaller tumors. The LEC allowed patients with insufficient tumor volumes to qualify for dose escalation to be treated with a tigilanol tiglate dose that had been tolerated in a dose-escalation cohort. The median volume of target tumors treated in the dose-escalation cohort was 37% (range: 1% to 100%, average 50%) of the BSA. In contrast, the median volume of target tumors treated in the LEC was 100% (range: 76% to 100%, average 94%) of the BSA. In addition, the range of tumor volumes in the dose-escalation cohort was very large (up to ~500 cm3), whereas the LEC had a much smaller range (up to 4·5 cm3). These factors help explain the better responses in the LEC, which may reflect more accurately the tumor responses anticipated when tigilanol tiglate is dosed according to tumor volume in later phase trials. Three patients in the LEC, one with three treated metastatic melanomas on the arm, one with axillary node recurrence in a previously dissected area, and one with angiosarcoma on the nose, experienced complete response; two experienced complete response within the study period and one eventually outside this period. Both melanomas demonstrated an anenestic effect. Possible mechanisms for the regression of a tumor outside the scope of localized treatment could include exposure to tigilanol tiglate by lymphatic drainage, bystander inflammatory response, or systemic activation of the immune system. The responses in the LEC are especially encouraging, as signals of clinical efficacy were identified despite the many inter- and intra-patient variables including; a variety of tumors and tumor volumes, different anatomic sites and a predominantly late stage setting.
With respect to PK outcomes, systemic exposure following IT dosing might be explained by the known high vascularity of tumors. However, the study did not measure IT concentrations, although animal data have demonstrated that tigilanol tiglate levels achieved within the tumor were significantly higher than those reached in plasma [7].
5. Conclusion
In this first-in-human Phase I study, IT administration of tigilanol tiglate was generally well tolerated, the MTD was not reached, and signals of clinical efficacy were identified across nine tumor types. Four patients- two with metastatic melanoma, one with atypical fibroxanthoma and one with angiosarcoma -achieved complete response in the treated lesions, with the two melanoma patients demonstrating an anenestic response. These results support the continued development of tigilanol tiglate for IT administration into a Phase II efficacy trial and provide evidence of the potential role of PKC activation in the treatment of solid tumors.
Data sharing
QBiotics Group will make available to qualified scientific and medical researchers, upon signing a data access agreement, de-identified data that underlie the results of the study reported in this Article including text, tables, figures and appendices. Email requests for the data should be made to qbioticspublications@qbiotics.com. Provision of data will be completed without external investigator support.
Declaration of Competing Interest
BP reports consulting fees received from QBiotics for the development of future trials with EBC-46. PDS reports personal fees from BioSceptre Australia Pty Ltd, outside the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the patients and families, who participated in the Tigilanol Tiglate (EBC-46) trial. We would also like to thank all the study teams involved at the participating sites.
Funding source
QBiotics sponsored and funded the study and were involved in study design and study management support. QBiotics funded; INC Research and EMR Associates (independent project management and study monitoring) and Wolff Medical Communications (manuscript writing and editorial support). QBiotics had no input into data acquisition, data analysis, data interpretation or manuscript preparation, but assisted with minor adjustments to the final draft. All authors had access to the raw data and had final responsibility for the submitted paper.
References
- 1.Muller J.H., Held E. Direct injection under operation of malignant tumors and areas of neoplastic infiltration with colloidal radioactive gold, Au198. Gynaecologia. 1951;131:389−94. [PubMed] [Google Scholar]
- 2.Goldberg E.P., Hadba A.R., Almond B.A., Marotta J.S. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. J Pharm Pharmacol. 2002;54:159−80. doi: 10.1211/0022357021778268. [DOI] [PubMed] [Google Scholar]
- 3.Ellmark P., Mangsbo S.M., Furebring C., Norlén C., Tötterman T.H. Tumor-directed immunotherapy can generate tumor-specific T-cell responses through localized co-stimulation. Cancer Immunol Immunother. 2017;66:1−7. doi: 10.1007/s00262-016-1909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwala S.S. Intralesional therapy for advanced melanoma: promise and limitation. Curr Opin Oncol. 2015;27:151−56. doi: 10.1097/CCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson D., Fisher S., Robinson B. The “Trojan Horse” approach to tumor immunotherapy: targeting the tumor microenvironment. J Immunol Res. 2014;2014 doi: 10.1155/2014/789069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant E.L., Wallace H.M., Trueman S.J., Reddell P.W., Ogbourne S.M. Floral and reproductive biology of the medicinally significant rainforest tree, fontainea picrospermia (Ephorbiaceae) Ind Crop Prod. 2018;108:416−22. [Google Scholar]
- 7.Barnett C.M.E., Broit N., Yap P.-.Y. Optimizing intratumoral treatment of squamous head and neck tumoral models with the diterpine ester Tigilanol tiglate. Invest New Drugs. 2018 doi: 10.1007/s10637-018-0604-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Campbell J., Poulos C., Lowden S. Using triamcinolone in combination with the investigational anticancer agent EBC-46 (tigilanol tiglate) in the local treatment of a canine subcutaneous mast cell tumour. CVE Control Therapy Ser. 2014;286:11−7. [Google Scholar]
- 9.Boyle GM, D’Souza MMA, Pierce CJ. Intra-lesional injection of the novel pkc activator EBC-46 rapidly ablates tumors in mouse models. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen J., Boyle G., D'Souza M. Investigating a naturally occurring small molecule, EBC-46, as an immunotherapeutic agent to help treat cancer. Eur J Cancer. 2016;69(Suppl 1):S153. [Google Scholar]
- 11.Cooke M., Magimaidas A., Cesado-Medrano V., Kazanietz M.G. Protein kinase c in cancer: the top five unanswered questions. Mol Carcinog. 2017;56:1531−42. doi: 10.1002/mc.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond M.L., Prehoda K.E. Molecular control of atypical protein kinase C: tipping the balance between self-renewal and differentiation. J Mol Biol. 2016;428:1455−64. doi: 10.1016/j.jmb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington E.O., Löffler J., Nelson P.R., Kent K.G., Simons M., Ware J.A. Enhancement of migration by protein kinase Cα and inhibition of proliferation and cell cycle progression by protein kinase Cδ in capillary endothelial cells. J Biol Chem. 1997;272:7390−7. doi: 10.1074/jbc.272.11.7390. [DOI] [PubMed] [Google Scholar]
- 14.Newton A.C. Protein kinase C: structure, function and regulation. J Biol Chem. 1995;270:28495−8. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 15.Newton A.C., Brognard J. Reversing the paradigm: protein kinase c as a tumor suppressor. Trends Pharmacol Sci. 2017;38:438−47. doi: 10.1016/j.tips.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowling CM, Phelan J, Callender JA. Protein kinase C beta II suppresses colorectal cancer by regulating IGF-1 mediated cell survival. Oncotarget. 2016;7:20919−33. doi: 10.18632/oncotarget.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidaki M., Nakakuma H., Kawaguchi T. Altered expression of protein kinase c in adult T-cell leukemia cells. Int J Hematol. 1992;56:135−41. [PubMed] [Google Scholar]
- 18.Melo S.R., Januário E.V., Zanutto E., Lowden S., Matera J.A. Proceedings of theveterinary cancer society annual conference 2017, Portland, OR; October 26-28. 2017. Time-assessed infra-red thermal characterization of canine cutaneous mast cell tumors (cMCT) treated intratumorally with the investigational anticancer agent Tigilanol Tiglate (EBC-46) [Google Scholar]
- 19.Miller J., Campbell J., Blum A. Dose characterization of the investigational anticancer drug tigilanol tiglate (EBC-46) in the local treatment of canine mast cell tumours. Front Vet Sci. 2019;6:1–10. doi: 10.3389/fvets.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649−55. [PubMed] [Google Scholar]
- 21.Du Bois D., Du Bois E.F. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med. 1916;17:863−71. [PubMed] [Google Scholar]
- 22.Le Tourneau C., Lee J.J., Siu L.L. Dose escalation methods in phase i cancer clinical trials. J Natl Cancer Inst. 2009;101:708−20. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised Recist guideline (version 1.1) Eur J Cancer. 2009;45:228−47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health. Common terminology criteria for adverse events (CTCAE) v4.03; June 14, 2010