Abstract
Background and Aims:
Thoracic paravertebral block (TPVB) provides effective analgesia in breast surgery. Recently, use of erector spinae plane block (ESPB) in controlling post-operative pain has proved effective. This study aimed to compare the effect of ESPB with TPVB in post-mastectomy acute pain control.
Methods:
A prospective, randomised double-blinded study enrolled 70 adult female patients, scheduled for modified radical mastectomy. Patients were randomised into two groups, receiving 20 ml of 0.25% bupivacaine: group I (TPVB) and group II (ESPB). Post-operative 24 h morphine consumption, intra-operative fentanyl consumption, time of the first request for analgesia and post-operative visual analogue scale (VAS), heart rate (HR), mean blood pressure (MBP) and complications were recorded.
Results:
Post-operative 24 h morphine consumption and time of the first request for analgesia were comparable between both groups (P = 0.32 and 0.075, respectively). There was no significant difference in the intra-operative fentanyl consumption. There was also no significant difference in VAS between both groups over the 24 h of study. Four patients in group I developed pneumothorax with no significant differences between both groups (P = 0.114). Incidence of nausea and vomiting was comparable between both groups. All patients displayed a stable haemodynamic profile.
Conclusion:
Both TPVB and ESPB can be effectively used in controlling post-mastectomy pain and reduce intra-operative and post-operative opioid consumption.
Key words: Erector spinae plane block, modified radical mastectomy, paravertebral block, post-operative pain, ultrasound, visual analogue scale
INTRODUCTION
Post-mastectomy analgesia consists of many regional techniques.[1] Paravertebral block (PVB) is the most effective studied technique, but due to its anatomic proximity to pleura and central neuroaxial system, it is a challenging one.[2] Erector spinae plane block (ESPB) has been used successfully for post-operative analgesia in breast surgeries.[3,4] We hypothesised that ESPB could provide effective post-mastectomy pain control; hence, it could replace other regional techniques. Also, it is effective, safe and simple. We aimed to compare the analgesic effect of ESPB with TPVB in breast surgery regarding opioid consumption, duration of analgesia, haemodynamic profile and complications.
METHODS
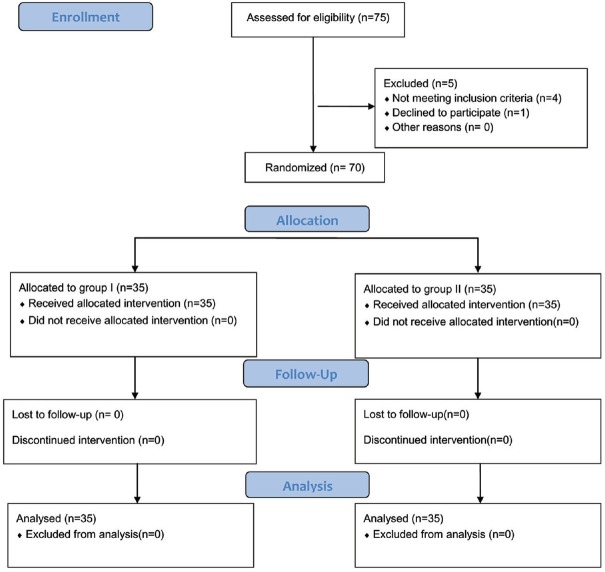
Seventy adult female patients were enrolledin this prospective, double-blinded randomised study. Approval was obtained from the institutional ethics committee (identification number: 32514/08/18) the study was conducted in accordance with the principles of the Declaration of Helsinki. and written informed consent was collected from all participants. Patients were scheduled for unilateral modified radical mastectomy in a teaching hospital from August 2018 to January 2019. They were 20 to 60 years old and belonged to the American Society of Anesthesiologists' (ASA) physical status I or II. The trial followed the CONSORT 2010 statement guidelines for conducting randomised controlled trials (RCTs) [Figure 1].
Figure 1.
CONSORT flow diagram
Exclusion criteria included having severe respiratory or cardiac disorders, hepatic or renal insufficiency, coagulopathy, local infection at the injection site, spine or chest wall deformity, allergy to any of the study drugs, opioid addiction or severe obesity (body mass index [BMI] >35 kg/m2). Un-cooperative patients and those who could not express pain intensity by visual analogue scale (VAS) were also excluded.
Patients were randomly assigned to two groups, 35 each, with 1:1 allocation ratio using computer-generated random numbers. The numbers were concealed in sealed opaque envelopes. A blinded nurse (not participating in the study or data collection) used the random numbers to assign patients to their groups. Group I included those to receive TPVB and group II were to receive ESPB.
One anaesthetist performed general anaesthesia (GA) and regional block, and another, blinded to the assignment of patients to the groups, collected the data. All operations were done by the same surgeon. A pre-operative visit was conducted to collect patient history. Clinical examination was performed, including complete blood count (CBC), coagulation profile, liver function tests, renal function tests, chest X-ray and electrocardiography. Patients were trained on how to assess pain using the 10 cm (0: no pain to 10: maximum imaginable pain) Visual Analogue Scale (VAS).
On arrival at the operating room (OR), devices were attached to the patients to monitor physical parameters (Cardiocaps/5; Datex Ohmeda, Helsinki, Finland). A five-lead electrocardiogram (ECG), a non-invasive blood pressure monitor (NIBP) and a pulse oximeter were attached to each patient. An intravenous (IV) line was established and patients received midazolam 0.05 mg/kg and ondansetron 4 mg IV (prophylactic anti-emetic) before administering the blocking agents.
Thoracic paravertebral block (TPVB) was performed at level T5 with patients in sitting position. A high-frequency transducer probe (6–12 MHz) connected to an ultrasound (US) machine (Philips® cx 50 extreme edition, USA) was positioned in a para-median sagittal plane, approximately 2–2.5 cm lateral to the spinous process at the ipsilateral side of surgery location. The skin was sterilised and the US probe covered with a sterile cap. A 22-gauge, 50 mm blunt insulated nerve block needle (B. Braun Medical Inc., Bethlehem, PA) was introduced in an in-plane direction. After perforating the costotransverse ligament and confirming negative aspiration of blood, the drug was injected. Anterior movement of the pleura indicated appropriate spread of local anaesthesia (LA) in the paravertebral space.
The ESPB was performed at level T5 with patients in sitting position. A transducer probe was positioned in a para-median sagittal plane approximately 3 cm lateral to the spinous process at the ipsilateral side of surgery. Following the same sterilisation procedure, the needle was introduced in an in-plane direction. The transverse process of the vertebrae, trapezius muscle, rhomboid major and erector spinae muscle was visualised, and the drug was injected after confirming negative aspiration of blood. The LA spread lifted the erector spinae muscle off the bony shadow of the transverse process [Figure 2].
Figure 2.
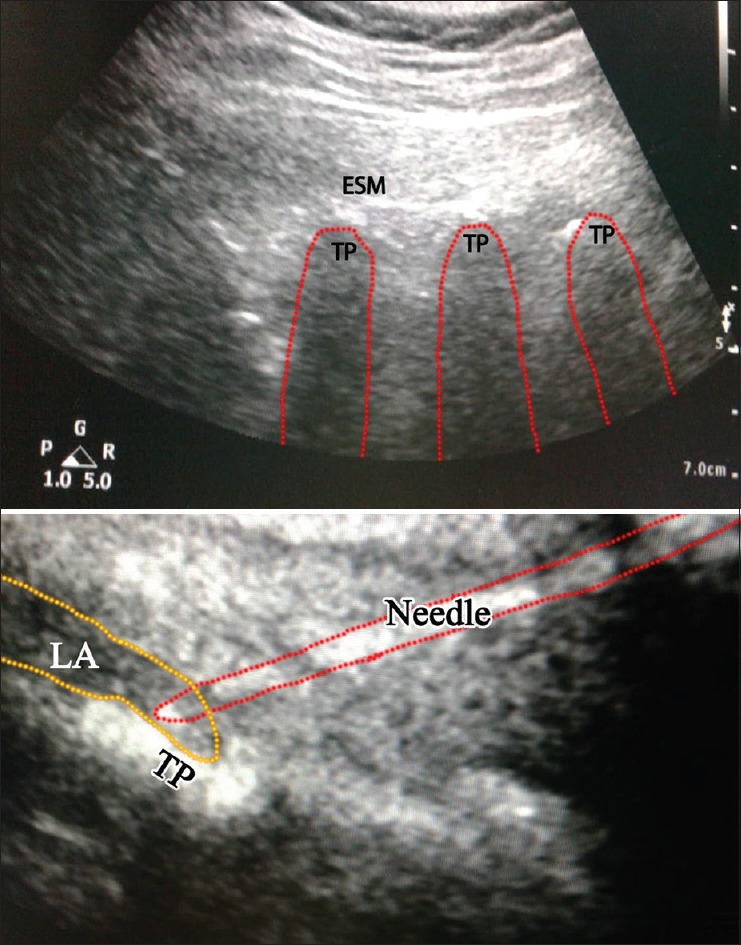
Sonoanatomy at the level of the fifth thoracic vertebrawith shadow of the needle advanced towards the transverse process and local anaesthetic injection between the tip of transverse process and the fascia of erector spinae muscle. (In this patient with thick adipose tissue at the site of the block, the curved probe was used) TP = Transverse process, ESM = Erector spinae muscle, LA = Local anaesthetic
Each patient received 20 ml of 0.25% bupivacaine. The success of the block and extension of sensory loss were evaluated using the pinprick test 20 min after the injection of drugs. The block failed if the loss of sensation was not attained within 30 min. Also, the ease of performance of blocks and number of attempts for performance of block were observed.
The same GA technique was used for all patients. Basic monitoring was done (adding capnography to previous monitoring techniques). Electrodes for monitoring the bispectral index (BIS) were attached (BISTM, model A-2000s; Aspect Medical Systems, Norwood, MA, USA). Intravenous induction was done using fentanyl 1 μg/kg, propofol 2 mg/kg and cisatracurium 0.15 mg/kg. Anaesthesia was maintained by administering isoflurane 1.5–2% in a mixture of oxygen and air and cisatracurium 0.03 mg/kg IV as required. The ventilator settings were adjusted to keep EtCO2 between 35 and 40 mmHg. Fentanyl 1 μg/kg IV was given when the heart rate (HR) or mean blood pressure (MBP) increased more than 20% above baseline.
After completion of the surgery, the neuromuscular block was antagonised using IV neostigmine 2.5 mg and atropine 1 mg. Following that, tracheal extubation was done after fulfilment of the extubation criteria. All patients were transferred to post-anaesthesia care (PACU) and kept there until achieving an Aldrete score ≥9. After that, they were transferred to the ward.[5]
The primary objective of the study was to measure total morphine consumption 24 h after surgery. Rescue analgesia in the form of morphine 0.1 mg/kg IV was given to patients with a VAS score >3. Secondary objectives included assessing total intra-operative fentanyl consumption; pain using the 10 cm VAS on arrival to PACU and then at 2, 4, 6, 8, 12, 18 and 24 h after surgery and time to first request for analgesia. MBP and HR were recorded at baseline (T0) before regional block; 5, 10 and 15 min after block (T1–T3); at skin incision and every 30 min till the end of surgery (T4–T8). Post-operative HR and MBP were recorded on arrival to PACU and after 2, 4, 6, 8, 12, 16, 20 and 24 h (T9–T17).
Complications included post-operative nausea and vomiting (PONV) (metoclopramide 10 mg IV was given when needed). Other complications related to the drug used or the techniques (e.g., pneumothorax, LA toxicity) were recorded up to 24 h after surgery.
Statistical analysis was done by SPSS 25 (SPSS Inc., Chicago, IL, USA). Normality of data was checked using the Shapiro–Wilk test. Numerical parametric data were presented as mean and standard deviation (SD) and compared between the two groups utilising Student's independent t-test for data, showing normal distribution. Non-parametric data (VAS) were presented as the median and inter-quartile range (IQR) and compared using the Mann–Whitney U test. Categorical variables were expressed as patients' number and percentage (%) and were analysed using the Chi-square test or Fisher's exact test when appropriate. P < 0.05 was considered significant.
Using Minitab 18 and based on the results of a previous study measuring post-operative opioid consumption,[4] a sample size ofminimum32 patients in each group was needed to detect a significant difference in means of post-operative morphine consumption (2.9 mg) between PVB group (considered to be the control group) and ESPB group, at α error of 0.05, SD of 3.8 and 85% power of study. So, in the current study, 35 cases were enrolled in each group to overcome possible dropouts.
RESULTS
Out of 75 female patients evaluated for eligibility, only 70 were chosen for analysis. Five patients were excluded from the study (onerefused to participate, one had chest wall deformity, two were ASA status III and one had coagulopathy). The remaining 70 patients were randomly divided into two groups (35 patients each) [Figure 1].
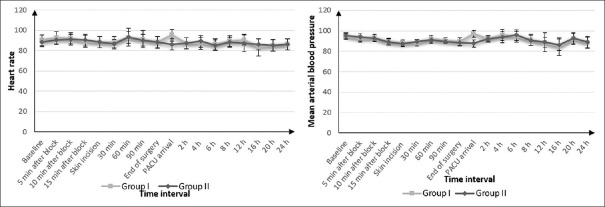
The demographic data, ASA statuses and duration of surgery in both groups were comparable [Table 1]. No significant differences were observed regarding intra-operative fentanyl and 24-h post-operative morphine consumption between both groups (P = 0.11 and 0.32, respectively). Time to first request for analgesia was also comparable (6.35 ± 0.42, 6.5 ± 0.60 h, respectively; P = 0.075) [Table 2]. Pain scores were not significantly different between both groups on admission to PACU and at 2,4 6, 8, 12, 18 and 24 h after surgery (P = 0.487, 0.927, 0.878, 0.316, 0.228, 0.628, 0.102 and 0.942, respectively). In both groups, VAS began to increase to >3 at 6 h after surgery. But, visual analogue scale (VAS) in both groups was significantly increased at 6 h (P = 0.001) [Figure 3]. Intra-operative and post-operative haemodynamic stability showed no significant differences between both the groups [Figure 4]. In addition, no significant differences were found regarding PONV (12 and 10 patients experienced nausea while four and three patients experienced vomiting in group I and group II, respectively) [Table 2]. Four patients developed pneumothorax in group I versus none in group II, with no significant difference between both groups (P = 0.114). One patient needed chest tube insertion while in three patients, pneumothorax resolved spontaneously [Table 2]. No other complications were noticed. No failure of the block was observed in both groups, but two attempts were needed for performance of PVB.
Table 1.
Demographic data, ASA classification and duration of surgery
| Variable | Group I | Group II | P |
|---|---|---|---|
| Age (year) | 41±11.8 | 37.7±12.9 | 0.279 |
| BMI (kg/m2) | 27.7±5.4 | 28.4±5.4 | 0.45 |
| ASA (%) | |||
| I | 20 (57.1%) | 22 (62.9%) | 0.62 |
| II | 15 (42.8%) | 13 (37.1%) | |
| Duration of surgery (min) | 173.2±8.7 | 170±8.2 | 0.11 |
Data presented as mean±SD or patient’s number. BMI – Body mass index, ASA – American Society of Anesthesiologists, SD – Standard deviation
Table 2.
Intra-operative and post-operative opioid consumption, time to first analgesic request and complications
| Variable | Group I | Group II | P |
|---|---|---|---|
| Intra-operative fentanyl consumption (µg) | 141.2±11.9 | 135.9±14.5 | 0.11 |
| Total post-operative morphine (mg) | 27.3±2.9 | 26.7±2.1 | 0.32 |
| Time to first analgesic request (h) | 6.35±0.42 | 6.58±0.60 | 0.075 |
| Nausea (%) | 12 (34.3%) | 10 (28.6%) | 0.60 |
| Vomiting (%) | 4 (11.4%) | 3 (8.6%) | 0.69 |
| Pneumothorax (%) | 4 (11.4%) | 0 (0.0%) | 0.114 |
Data presented as mean±SD or patient’s number. SD – Standard deviation
Figure 3.
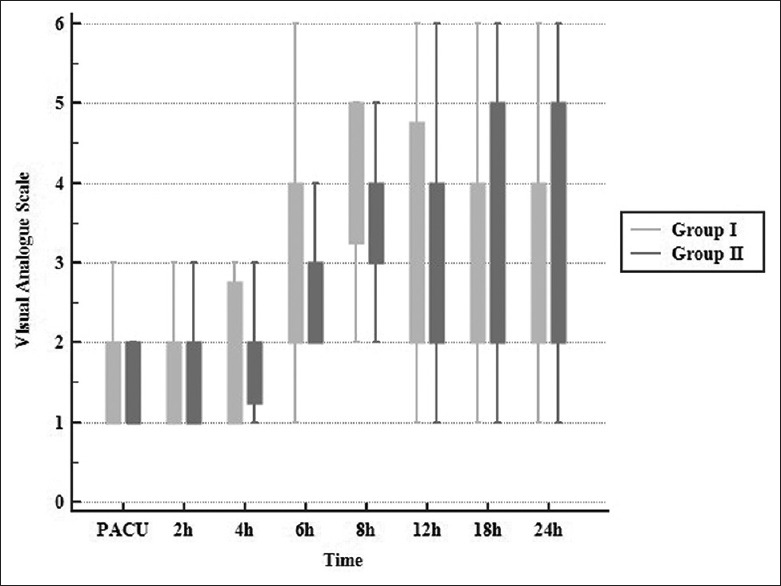
Visual analogue scale in two groups. Data presented as median (inter-quartile range)
Figure 4.
Heart rate (beat/min) and mean blood pressure (mmHg) changes in two groups. Data presented as mean ± standard deviation
DISCUSSION
Injecting LA into the paravertebral space resulted in an analgesic effect. This occurred through direct contact with the spinal nerve roots and the spread of LA into the epidural space. Thus, the TPVB can unilaterally cause both somatic and sympathetic nerve block.[6]
The ESPB causes the same effect. It blocks the dorsal and ventral rami of the spinal nerves as the LA diffuses anteriorly into the adjacent paravertebral and inter-costal spaces.[7] Therefore, it is considered a peri-paravertebral regional technique.[8]
In this study, TPVB and ESPB proved to be effective in the management of post-mastectomy pain. Both regional blocks reduced intra-operative and post-operative opioid consumption, showing the comparable duration of analgesic effect and stable haemodynamic profiles.
However, the incidence of complications was lower with the use of ESPB than TPVB.
There was no significant difference in PONV between both groups, which is still clinically significant. PONV is a common, distressing and multi-factorial complaint following breast surgeries, especially cancer surgeries. There was no significant difference as well between both groups in the development of pneumothorax, but it is also of major clinical concern.
In our study, pneumothorax developed due to certain factors related to the study of patients. One patient suddenly moved during the performance of TPVB while the other three required multiple injections due to poor visualisation of the needle tip. The reason for this might have been the presence of a thick adipose tissue layer. The use of ultrasound (US) also reduces the incidence of complications. The introduction of US-guided block did not guarantee 100% prevention of dangerous complications, which has been more in PVB group. However, US-guided ESPB, being a simple technique with superficial anatomical landmarks, may be a safe and effective alternative to TPVB.
Overall, the performance of ESPB was easier than PVB. The study results were consistent with a previous study conducted by Fallatah et al.[9] on patients with PVB. They administered 1 mg morphine intravenously (rescue analgesia) every 5 min until the pain score was ≤3. They found that PVB provided better post-operative analgesia after breast surgery than IV morphine patient-controlled analgesia (PCA), with higher haemodynamic stability and less adverse effects. Also, Wahba et al.[10] and Abdel-Halim J. M. K.[11] both reported reduction in post-operative morphine consumption in patients who received PVB. Moreover, a pooled review of 242 cases, who received ESPB, stated that the reduction in opioid use was observed in 76% of the cases.[12]
Gürkan et al.[4] found that total 24 h morphine consumption decreased by 65% in patients who received single-shot US-guided ESPB using 20 ml of 0.25% bupivacaine after breast surgery. However, there was no significant difference in pain scores between the ESPB group and the control group.
Melvin et al.[13] concluded in their case series study that pre-incision ESPB administered at the T10–T12 level provided effective perioperative opioid-sparing analgesia in patients undergoing lumbosacral spine surgery. Catheter insertion was used in more major surgeries and patients suffering complex pain to prolong the duration of analgesia and avoid opioid dose escalation.
Leyva et al.[14] found that ESPB provided extended, adequate and opioid-sparing pain control after minimally invasive mitral valve surgery, using a catheter for continuous infusion for 48 h, without reported complications.
El Mourad et al. reported that the time to first request for analgesia after TPVB in modified radical mastectomy was 5.3 ± 3.1 h.[15] On the other hand, Krishna et al.[16] reported a prolonged duration of analgesic effect with bilateral ESPB in cardiac surgery, up to 10 h. Prolonged duration of analgesia may be attributed to using a different dose of a different drug.
Singh et al.[17] injected 25 ml of 0.25% bupivacaine in ESP before modified radical mastectomy. Four out of five patients exhibited a pain score between two and four in the first 8 h while one patient exhibited a pain score of six after 4 h. Also, Takahashi et al.[18] reported a case with failed back surgery syndrome treated with ESPB. Pain relief lasted approximately 10 h after the initial block.
In contrast to the current study's findings, Law et al.[19], in a meta-analysis of RCTs, demonstrated that PVB with sedation for inguinal herniorrhaphy reduced PONV compared with GA and systemic analgesia. Also, it was associated with less PONV and urinary retention compared with spinal anaesthesia, but it needed a longer time to perform.
Davies et al.[20] also documented in a meta-analysis study that the incidence of PONV decreased in patients who received PVB. Furthermore, Fahy et al.[21] concluded that PVB resulted in a decreased need for post-operative anti-emetic medication in patients undergoing a mastectomy.
On the other hand, Aufforth et al.[22] documented that patients who received PVB for breast cancer surgery exhibited PONV in a similar manner to patients without PVB. The study suggested that PVB may have played a role in decreasing PONV in patients undergoing breast re-construction surgery, not cancer surgery. However, Gürkan et al.[4] demonstrated that although morphine consumption decreased in the ESPB group compared to the control group, this was insufficient to produce a significant difference in PONV.
Naja et al.[23] reported incidence of pleural puncture (0.8%) and pneumothorax (0.5%) after PVB. On the other hand, Pace et al., who conducted a study of 1427 patients receiving PVB, found no incidence of pneumothorax. This has been attributed to the use of an US-guided technique that is associated with very few complications.[24]
ESPB carries lower risk for serious complications because the injection is performed in the tissue plane, away from potentially problematic structures.[8] Only one case developed pneumothorax after ESP, reported by Ueshima,[25] without enough explanation of the type and length of the needle used and without exclusion of patients with bullous lung disease.
D'Ercole et al.[26] and Saito et al.[27] demonstrated that patients with either PVB or ESPB had a stable haemodynamic profile despite the sympathetic block. Also, there were no reports on systemic toxicity associated with bilateral PVB, despite the need for relatively large doses of LA.[26] In addition, in the study of Krishna et al.,[16] no LA toxicity was reported with bilateral ESPB for pain control in cardiac surgery.
Consistent with our results, Gürkan et al.,[28] compared ESPB and PVB to IV morphine in breast surgery. They found that both types of blocks provided better post-operative analgesia than IV morphine. They recommended that clinicians choose one of both based on their clinical experience and personal preference.
The current study was constrained by a few limitations. First, pain score was not evaluated during the patients' movement. Second, a single injection was used to detect the exact duration of the block, and a catheter can be used instead to extend the duration of analgesia.
In future studies, different additives, types and concentrations of LA should be used. ESPB should also be compared with other regional techniques to identify the optimal technique to be used in other chest surgeries.
CONCLUSION
Both TPVB and ESPB provide effective pain control after breast surgeries with a comparable duration of analgesic effect, reduction of intra-operative and post-operative opioid consumption and stable haemodynamic profile. US-guided ESPB could be considered a safe and effective alternative to TPVB as it is a simple technique with superficial anatomical landmarks.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given/her/their consent for/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hong B, Bang S, Chung W, Yoo S, Chung J, Kim S. Multimodal analgesia with multiple intermittent doses of erector spinae plane block through a catheter after total mastectomy: A retrospective observational study. Korean J Pain. 2019;32:206–14. doi: 10.3344/kjp.2019.32.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talawar P, Kumar A, Bhoi D, Singh A. Initial experience of erector spinae plane block in patients undergoing breast surgery: A case series. Saudi J Anaesth. 2019;13:72–4. doi: 10.4103/sja.SJA_560_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvi O, Tulgar S. Use of the ultrasound-guided erector spinae plane block in segmental mastectomy. Turk J Anaesthesiol Reanim. 2019;47:158–60. doi: 10.5152/TJAR.2019.50024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altıparmak B, Toker MK, Uysal Aİ, Demirbilek SG. Comparison of the efficacy of erector spinae plane block performed with different concentrations of bupivacaine on postoperative analgesia after mastectomy surgery: Randomized, prospective, double-blinded trial. BMC Anesthesiol. 2019;19:31. doi: 10.1186/s12871-019-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni KR. Single needle thoracic paravertebral block with ropivacaine and dexmeditomidine for radical mastectomy: Experience in 25 cases. Int J Anesth Pain Med. 2016;2:1. [Google Scholar]
- 7.Forero M, Rajarathinam M, Adhikary SD, Chin KJ. Erector spinae plane block for the management of chronic shoulder pain: A case report. Can J Anesth. 2018;65:288–93. doi: 10.1007/s12630-017-1010-1. [DOI] [PubMed] [Google Scholar]
- 8.López MB, Cadórniga ÁG, González JM, Suárez ED, Carballo CL, Sobrino FP. Erector spinae block. A narrative review. Cent Eur JClin Res. 2018;1:28–39. [Google Scholar]
- 9.Fallatah S, Mousa WF. Multiple levels paravertebral block versus morphine patient-controlled analgesia for postoperative analgesia following breast cancer surgery with unilateral lumpectomy, and axillary lymph nodes dissection. Saudi J Anaesth. 2016;10:13–7. doi: 10.4103/1658-354X.169468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth. 2014;30:129–35. [Google Scholar]
- 11.Abdel-Halim JM. Continuous thoracic paravertebral block: An adjunct to general anaesthesia in major breast surgery. Egypt J Anaesth. 2011;27:83–7. [Google Scholar]
- 12.Tsui BC, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: A pooled review of 242 cases. J Clin Anesth. 2019;53:29–34. doi: 10.1016/j.jclinane.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: A case series. Can J Anaesth. 2018;65:1057–65. doi: 10.1007/s12630-018-1145-8. [DOI] [PubMed] [Google Scholar]
- 14.Leyva FM, Mendiola WE, Bonilla AJ, Cubillos J, Moreno DA, Chin KJ. Continuous erector spinae plane (ESP) block for postoperative analgesia after minimally invasive mitral valve surgery. J Cardiothorac Vasc Anesth. 2018;32:2271–4. doi: 10.1053/j.jvca.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 15.El Mourad MB, Amer AF. Effects of adding dexamethasone or ketamine to bupivacaine for ultrasound-guided thoracic paravertebral block in patients undergoing modified radical mastectomy: A prospective randomized controlled study. Indian J Anaesth. 2018;62:285–91. doi: 10.4103/ija.IJA_791_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishna SN, Chauhan S, Bhoi D, Kaushal B, Hasija S, Sangdup T, et al. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: A randomized controlled trial. J Cardiothorac Vasc Anesth. 2019;33:368–75. doi: 10.1053/j.jvca.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Chowdhary N. Erector spinae plane block an effective block for postoperative analgesia in modified radical mastectomy. Indian J Anaesth. 2018;62:148–9. doi: 10.4103/ija.IJA_726_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi H, Suzuki T. Erector spinae plane block for low back pain in failed back surgery syndrome: A case report. J A Clin Rep. 2018;4:60. doi: 10.1186/s40981-018-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law LS, Tan M, Bai Y, Miller TE, Li YJ, Gan TJ. Paravertebral block for inguinal herniorrhaphy: A systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2015;121:556–69. doi: 10.1213/ANE.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 20.Davies R, Myles P, Graham J. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96:418–26. doi: 10.1093/bja/ael020. [DOI] [PubMed] [Google Scholar]
- 21.Fahy AS, Jakub JW, Dy BM, Eldin NS, Harmsen S, Sviggum H, et al. Paravertebral blocks in patients undergoing mastectomy with or without immediate reconstruction provides improved pain control and decreased postoperative nausea and vomiting. Ann Surg Oncol. 2014;21:3284–9. doi: 10.1245/s10434-014-3923-z. [DOI] [PubMed] [Google Scholar]
- 22.Aufforth R, Jain J, Morreale J, Baumgarten R, Falk J, Wesen C. Paravertebral blocks in breast cancer surgery: Is there a difference in postoperative pain, nausea, and vomiting? Ann Surg Oncol. 2012;19:548–52. doi: 10.1245/s10434-011-1899-5. [DOI] [PubMed] [Google Scholar]
- 23.Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade incidence of failed block and complications. Anaesthesia. 2001;56:1181–201. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]
- 24.Pace MM, Sharma B, Anderson-Dam J, Fleischmann K, Warren L, Stefanovich P. Ultrasound-guided thoracic paravertebral blockade: A retrospective study of the incidence of complications. Anesth Analg. 2016;122:1186–91. doi: 10.1213/ANE.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 25.Ueshima H. Pneumothorax after the erector spinae plane block. J Clin Anesth. 2018;48:12. doi: 10.1016/j.jclinane.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 26.D'Ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32:915–27. doi: 10.1053/j.jvca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Den S, Cheema S, Tanuma K, Carney E, Carlsson C, et al. A singleinjection, multisegmental paravertebral block–extension of somatosensory and sympathetic block in volunteers. Acta Anaesthesiol Scand. 2001;45:30–3. doi: 10.1034/j.1399-6576.2001.450105.x. [DOI] [PubMed] [Google Scholar]
- 28.Gürkan Y, Aksu C, Kuş A, Yörükoǧlu UH. Erector spinae plane block and thoracic paravertebral block for breast surgery compared to IV-morphine: A randomized controlled trial. J Clin Anesth. 2020;59:84–8. doi: 10.1016/j.jclinane.2019.06.036. [DOI] [PubMed] [Google Scholar]