Abstract
Background and Aims:
Hypotension following spinal anaesthesia for caesarean section is common in spite of adequate fluid loading. Phenylephrine is the recommended drug to treat spinal hypotension during caesarean section. Recently, norepinephrine boluses are being suggested as an alternative to phenylephrine boluses. The aim of our study was to compare the effectiveness of bolus doses of norepinephrine with phenylephrine to treat spinal hypotension during caesarean section.
Methods:
Fifty patients undergoing elective caesarean section under spinal anaesthesia were randomly assigned into two groups. Group P patients received phenylephrine 50 μg as an intravenous bolus and group N received 4 μg of norepinephrine as intravenous bolus to treat spinal hypotension. The primary objective of our study was to compare the number of bolus doses of norepinephrine or phenylephrine required to treat spinal hypotension. The secondary objectives were to compare the incidence of bradycardia, hypertension, nausea and vomiting in mother and foetal outcomes.
Results:
The number of boluses of vasopressors required to treat hypotension was significantly lower in group N (1.40 ± 0.577 vs. 2.28 ± 1.061, P = 0.001). The frequency of bradycardia was high in group P, but this difference was not statistically significant (4%vs. 20%, P = 0.192). Maternal complications such as nausea and vomiting and shivering were comparable between the groups. The foetal parameters were also comparable between the two groups.
Conclusion:
Intermittent boluses of norepinephrine are effective in the management of spinal-induced hypotension during caesarean section. The neonatal outcomes were similar in both the groups. Norepinephrine boluses can be considered as an alternative to phenylephrine boluses.
Key words: Caesarean section, hypotension norepinephrine, phenylephrine, spinal, vasopressors
INTRODUCTION
Nowadays, caesarean sections are commonly performed under spinal anaesthesia to avoid the risk of airway complications and to limit the neonatal drug transfer associated with general anaesthesia.[1] But in spite of adequate fluid loading, maternal hypotension is a common complication after spinal anaesthesia. Hypotension can lead to dizziness, nausea and vomiting in the mother, decreased uterine blood flow and foetal hypoxia and acidosis. Prompt treatment of hypotension with intravenous fluids or vasopressor is necessary to avoid these detrimental maternal and neonatal effects.[2] Phenylephrine is considered as the first-line agent for the treatment of hypotension in caesarean section as it causes less of foetal acidosis than ephedrine.[3] But the drawback with this drug is the reduction in heart rate (HR) and cardiac output, which may adversely affect the outcomes of both the mother and the fetus. Norepinephrine is a potent vasopressor with β-adrenergic properties. Recently, norepinephrine infusion is being suggested as an alternative to phenylephrine to treat spinal-induced hypotension for caesarean delivery.[4,5] It could be more advantageous than phenylephrine as it causes less reduction in HR and cardiac output.[6] Phenylephrine 100 μg is found to be equipotent to norepinephrine 8 μg.[7] The aim of our study was to compare the effectiveness of bolus doses of norepinephrine with phenylephrine to treat spinal hypotension during caesarean section.
METHODS
This prospective double-blinded randomised control trial was conducted in a tertiary care teaching hospital after approval from the hospital ethics committee (IEC-AIMS-2018-ANES-094, 29-05-2018), Clinical Trial Registry of India (CTRI) registration (CTRI/2018/08/015364) and written informed consent from patients between September 2018 and March 2019. The study was conducted as per consort guidelines and followed ethical guidelines of the Declaration of Helsinki. Fifty term parturients between 18 and 50 years of age with singleton pregnancy belonging to the American Society of Anesthesiologists (ASA) physical class I and II posted for elective caesarean section under spinal anaesthesia were included in the study. Parturients with allergy or hypersensitivity to phenylephrine or norepinephrine, height <140 or >180 cm, any hypertensive disorders of pregnancy, cardiovascular or cerebrovascular disease and foetal abnormalities were excluded from the study. All parturients were premedicated with oral metoclopramide 10 mg and ranitidine 150 mg on the night prior and the morning of surgery. In the theatre, 18-gauge intravenous cannula was inserted, and standard monitoring with non-invasive arterial pressure, electrocardiography and pulse oximetry was established. The baseline vitals were noted. They were then loaded with 15 mL/kg of lactated Ringer's solution. Subarachnoid block (at L3–L4 or L4–L5 level using standard technique) with 1.8 mL of 0.5% hyperbaric bupivacaine plus 0.2 mL of fentanyl was given using 25-G Whitacre needle in the left lateral position. The patients were then turned supine with a wedge under the right buttock. Supplemental oxygen was given through facemask at a flow rate of 5 L/min. The highest level of sensory blockade achieved was assessed with ice cubes 5 min after intrathecal injection. The parturients were randomised into groupP and group N by computer-generated random sequence of numbers and concealed by closed envelope technique. The anaesthetist posted in the recovery loaded the drugs. Norepinephrine and phenylephrine were diluted and loaded in an identical coded 10-mL syringe to give norepinephrine 4 μg/mL (Aficard, Aesmira, Mumbai, India) and phenylephrine 50 μg/mL (Frenin, Samarth Life Sciences Pvt. Ltd., Mumbai, India). An anaesthetist who was posted in the theatre used vasopressor-labeled syringe to treat hypotension and collected the data for analysis. The patient and the investigator were blinded to the vasopressor used. Blood pressure and HR were monitored every 2 min till 10 min, and thereafter every 5 min till the end of surgery. Group P patients received phenylephrine 50μg as an intravenous bolus and group N patients received 4μg of norepinephrine intravenous bolus whenever the systolic arterial pressure dropped below 20% of baseline. After delivery of baby, 10 U of oxytocin was given as a slow infusion. Incidences of hypotension, bradycardia, tachycardia, hypertension and the total dose of vasopressor and intravenous fluid infused intraoperatively were noted. Bradycardia was defined as a HR less than 50 beats/min (bpm) and was treated with intravenous atropine 0.6 mg. Tachycardia was defined as HR >120 bpm. Hypertension was defined as a 20% increase in systolic blood pressure from baseline and its incidence as a result of norepinephrine or phenylephrine boluses was noted. A paediatrician who was not aware of the vasopressor used noted Apgar score at 1 and 5 min. Umbilical vein sample at the time of birth for blood gas analysis was collected, and pH, PCO2, bicarbonate and base excess were analyzed. Foetal acidosis was defined as pH <7. The time taken from skin incision to delivery of baby and the time taken from uterine incision to delivery of the baby and the total duration of surgery were noted. Incidences of dizziness, nausea or vomiting due to maternal hypotension were also noted.
The primary objective of our study was to compare the number of intravenous bolus doses of norepinephrine or phenylephrine required to treat spinal hypotension in caesarean patients. The secondary objectives were to compare the incidence of bradycardia, hypertension, nausea and vomiting in mother and foetal outcomes such as Apgar score andumbilical vein blood gases.
Because no study could be located in the existing literature with the same dosage at the time of study period, with respect to the primary objective of the total number of doses given in two groups, a pilot study was conducted with 10 patients in each group. A minimum sample size based on the mean and standard deviation (1.40 ± 0.548 vs. 2.2 ± 1.304) with 95% confidence and 80% power was calculated as 25 in each group. All the statistical analysis was done in IBM SPSS 20.0 (SPSS Inc, Chicago, USA). The results are given as mean ± standard deviation for all the continuous variables and frequency for categorical variables. Pearson's Chi-square test with continuity correction was used for finding the association between two categorical variables. Independent sample t-test was applied for comparing the mean of continuous parameters between two groups. Paired sample t-test was used to compare the average Apgar score at 1 and 5 min within the groups. P value of <0.05 was considered as statistically significant difference.
RESULTS
The study included 50 patients who were randomly allocated into two equal groups [Figure 1]. The patient demographics with respect to age, height, weight and ASA physical status were comparable between the two groups. All patients achieved adequate spinal block height above T5 at 5 min, and the level of dermatomal height achieved was comparable between the groups. The surgical times required were also comparable between the groups [Table 1]. Intraoperative blood loss and total intravenous fluids transfused were comparable between the two groups. The number of boluses of vasopressors required to treat hypotension was significantly lower in group N patients (1.40 ± 0.577 vs. 2.28 ± 1.061, P = 0.001). The frequency of bradycardia was high in group P, but the difference was not statistically significant (4% vs. 20% P = 0.192) [Table 2]. Maternal complications such as nausea, vomiting and shivering were comparable between the groups. The foetal parameters were comparable between the two groups, and no statistical difference was noted [Table 3]. There were no episodes of tachycardia or hypertension in both the groups [Figures 2 and 3].
Figure 1.
Consort flow diagram
Table 1.
Demographic data, block characteristics and surgical data
| Group N (n=25) | Group P (n=25) | P | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 29.96±4.046 | 29.04±4.748 | 0.102 |
| Weight (kg) | 75.92±14.387 | 73.88±10.62 | 0.571 |
| Height (cm) | 155.84±6.479 | 156.00±5.51 | 0.925 |
| ASA1 | 14 (56%) | 16 (64%) | 0.773 |
| ASA2 | 11 (44%) | 9 (36%) | |
| Dermatomal block | |||
| T3 | 1 (4%) | 2 (8%) | 0.788 |
| T4 | 20 (80%) | 20 (80%) | |
| T5 | 4 (16%) | 3 (12%) | |
| Surgical timein min | |||
| Induction to delivery | 10.76±2.862 | 10±2.363 | 0.311 |
| Skin incision to delivery | 5.84±1.700 | 5.44±1.685 | 0.408 |
| Uterine incision to delivery | 2.12±0.600 | 1.92±1.038 | 0.408 |
| Duration of surgery | 69.60±12.493 | 70.12±10.856 | 0.876 |
Independent sample t-test, Chi-square test with continuity correction
Table 2.
Haemodynamic variables and maternal complications
| Group N (n=25) | Group P (n=25) | P | |
|---|---|---|---|
| No. of boluses of vasopressors | 1.40±0.577 | 2.28±1.061 | 0.001 |
| Incidence of bradycardia | 1 (4%) | 5 (20%) | 0.192 |
| Maternal complications | |||
| Nausea/vomiting | 2 (8%) | 2 (8%) | 0.695 |
| Shivering | 4 (16%) | 1 (4%) | 0.174 |
Chi-square test with continuity correction
Table 3.
Foetal parameters
| Group N (n=25) | Group P (n=25) | P | |
|---|---|---|---|
| Umbilical pH | 7.320±0.038 | 7.318±0.476 | 0.850 |
| PCO2 | 43.864±5.864 | 46.700±1.172 | 0.108 |
| PO2 | 28.180±9.421 | 25.672±5.879 | 0.264 |
| Lactates | 1.929±0.433 | 2.308±1.494 | 0.238 |
| Apgar1 | 8±0.000 | 7.92±0.640 | 0.538 |
| Apgar5 | 8.92±0.277 | 8.88±0.332 | 0.646 |
Independent sample t-test, paired sample t-test
Figure 2.
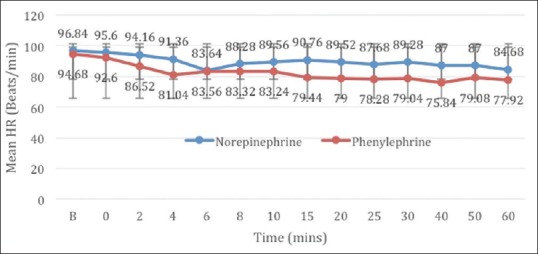
Mean heart rate
Figure 3.
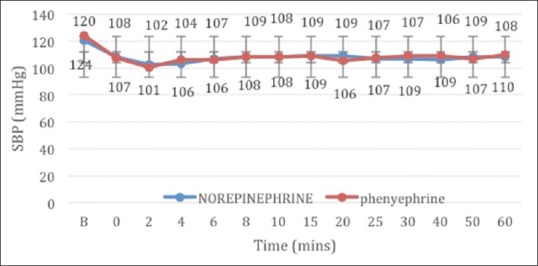
Mean systolic blood pressure
DISCUSSION
The study compared the effects of intermittent bolus doses of norepinephrine and phenylephrine in the treatment of spinal-induced hypotension during caesarean section. The results of the study showed that intermittent boluses of intravenous norepinephrine are effective in managing spinal hypotension with no detrimental effects on the neonatal and maternal outcome. The number of norepinephrine boluses required to maintain blood pressure was significantly less than when phenylephrine boluses were used. There was less incidence of bradycardia in the norepinephrine group.
Various vasopressors have been used to treat spinal hypotension. Phenylephrine is considered to be the drug of choice in obstetric patients.[8] To treat spinal hypotension, the vasopressor may be given as intermittent boluses or as an infusion. Infusions allow tighter blood pressure control with less intervention required by the anaesthetist.[9] The use of intermittent boluses of the drug may be feasible in poor-resource settings where infusion pumps are not available or are only available in limited numbers, and hence cannot be available to all parturients who undergo a caesarean section. Hence, bolus doses of phenylephrine are used in many centres to treat spinal-induced hypotension though phenylephrine infusions are found to be better than boluses. Another advantage of the use of norepinephrine is that it is cheaper than phenylephrine. There was a concern regarding the use of norepinephine in the peripheral vein. But no signs of ischaemic complications in the limbs were reported by its use through a peripheral vein.[10,11]
Studies comparing the use of ephedrine and phenylephrine for spinal hypotension in obstetric patients had shown that the use of ephedrine was associated with neonatal acidosis.[12] W. Mon et al. in their randomized control trial on cardiac output changes with phenylephrine and ephedrine found that even though cardiac output and HR were maintained better with ephedrine, less neonatal acidosis was noted with the use of phenylephrine.[13] Hence, now phenylephrine, a short-acting α adrenergic agonist, is considered as the first-line agent for the treatment of hypotension in caesarean section. But it is associated with a reduction in cardiac output and HR which may be undesirable to the mother and the fetus.[14] Recently, norepinephrine boluses are being suggested as an alternative to phenylephrine boluses to treat spinal-induced hypotension for caesarean delivery.[15,16] Norepinephrine is a potent vasoconstrictor with β adrenergic properties in addition to the α adrenergic action. Thus, it is associated with less incidence of bradycardia or decrease in cardiac output.[13,17]
Various studies were done to find the equivalent doses of norepinephrine and phenylephrine. The ED 90 of an intermittent bolus dose of norepinephrine is 6 μg.[17] Based on the results of the study by Ngan Kee et al.,[7] we used 4 μg of norepinephrine to treat hypotension as 4 μg of norepinehrine was found to be equipotent to 50 μg of phenylephrine. In a study by Mohta et al.,[18] norepinephrine was found to be 11 times more potent than phenylephrine and100μg phenylephrine was approximately equivalent to 9 μg of norepinephrine. Sharkey AM et al. compared bolus doses of phenylephrine 100 μg with norepinephrine 6 μg and found haemodynamic control during caesarean section to be better with norepinephrine due to less fluctuations in HR.[19] Intermittent bolus dose of norepinephrine was compared with ephedrine and phenylephrine, and it was reported to be a potent drug to treat spinal hypotension.[20,21] Xu et al. in a systematic review and meta-analysis showed norepinephrine to have similar efficacy in managing maternal hypotension compared with phenylephrine.[22]
Ngan Kee et al. used a norepinephine infusion of 5 μg/mL and found it to be effective in maintaining blood pressure with no detrimental effect on neonatal outcome.[6] Prophylactic infusions of norepinephrine were also used to maintain maternal blood pressure without any adverse neonatal outcomes.[23]
There are controversies regarding the use of norepinephrine through peripheral veins, but we did not encounter side effects with its use in any of our patients. The major limitation of the present study was that we used vasopressor to maintain the systolic pressure without monitoring the cardiac output. We could have used noninvasive cardiac output monitor. Furthermore, a larger sample size could have provided a wider perspective on maternal and foetal effects. In our study, we found that intermittent boluses of norepinephrine are effective in the management of spinal-induced hypotension during caesarean section. The study can be extended to a larger number of patients with intermittent or continuous infusions of norepinephrine.
CONCLUSION
Intermittent boluses of norepinephrine are effective in the management of spinal-induced hypotension during caesarean section. The neonatal arterial blood gases and Apgar scores are also comparable with phenylephrine. Norepinephrine boluses can be considered as an alternative to phenylephrine boluses.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kulkarni KR, Naik AG, Deshpande SG. Evaluation of antihypotensive techniques for cesarean section under spinal anesthesia: Rapid crystalloid hydration versus intravenous ephedrine. Anesth Essays Res. 2016;10:637–42. doi: 10.4103/0259-1162.191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasanin A, Mokhtar AM, Badawy AA, Fouad R. Post-spinal anesthesia hypotension during cesarean delivery, a review article. Egypt J Anaesth. 2017;33:189–93. [Google Scholar]
- 3.Xu C, Liu S, Huang YZ, Guo XW, Xiao HB, Qi DY. Phenylephrinevs ephedrinein cesarean delivery under spinal anesthesia: A systematic literature review and meta-analysis. Int J Surg. 2018;60:48–59. doi: 10.1016/j.ijsu.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho B, Dyer RA. Norepinephrine for spinal hypotension during cesarean delivery: Another paradigm shift? Anaesthesiology. 2015;122:728–30. doi: 10.1097/ALN.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 5.Mets B. Should norepinephrine, rather than phenylephrine, be considered the primary vasopressor in anesthetic practice? Anesth Analg. 2016;122:1707–14. doi: 10.1213/ANE.0000000000001239. [DOI] [PubMed] [Google Scholar]
- 6.Kee WD, Lee SWY, Ng FF, Tan PE, Khaw KS. Randomized double blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–45. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 7.Kee WD. A random allocation graded dose-response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology. 2017;127:934–41. doi: 10.1097/ALN.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 8.Kee WD. The use of vasopressors during spinal anaesthesia for caesarean section. Curr Opin Anesthesiol. 2017;30:319–25. doi: 10.1097/ACO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary M, Bajaj JK. Study coparing phenylephrine bolus and infusion for maternal hypotension and neonatal outcome during cesarean section under spinal anesthesia. Anesth Essays Res. 2018;12:446–51. doi: 10.4103/aer.AER_23_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medlej K, Kazzi AA, Chehade AE, Eldine MS, Chami A, Bachir R, et al. Complications from administration of vasopressors through peripheral venous catheters: An observational study. J Emeg Med. 2018;54:47–53. doi: 10.1016/j.jemermed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Kee WD. Norepinephrine for maintaining blood pressure during spinal anaesthesia for caesarean section: A 12-month review of individual use. Int J Obstet Anesth. 2017;30:73–84. doi: 10.1016/j.ijoa.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Prakash S, Pramanik V, Chellani H, Salhan S, Gogia AR. Maternal and neonatal effects of bolus administration of ephedrine and phenylephrine during spinal anaesthesia for caesarean delivery: A randomized study. Int J Obstet Anesth. 2010;19:24–30. doi: 10.1016/j.ijoa.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Mon W, Stewart A, Fernando R, Ashpole K, El-Wahab N, Macdonald S, et al. Cardiac output changes with phenylephrine and ephedrine infusions during spinal anaesthesia for cesarean section: A randomized, double blind trial. J Clin Anaesth. 2017;37:43–8. doi: 10.1016/j.jclinane.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Nag DS, Samaddar DP, Chatterjee A, Kumar H, Dembla A. Vasopressors in obstetric anesthesia: A current perspective. World J Clin Cases. 2015;3:58–64. doi: 10.12998/wjcc.v3.i1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xian W, Shen X, Liu S, Yang J, Xu S. The efficacy and safety of norepinephrine and its feasibility as a replacement for phenylephrine to manage maternal hypotension during elective cesarean delivery under spinal anesthesia. Biomed Res Int. 2018:1869189. doi: 10.1155/2018/1869189. doi: 10.1155/2018/1869189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling D, Qian D, Xiumei S, Yang L, Yuelan W. Comparison of prophylactic bolus norepinephrine and phenylephrine on hypotension during spinal anesthesia for cesarean section. Int J Clin Exp Med. 2017;10:12315–21. [Google Scholar]
- 17.Onwochei DN, Ngan KW, Fung L, Downey K, Xiang YY, Carvalho JC. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: A sequential allocation dose finding study. Anesth Analg. 2017;125:212–8. doi: 10.1213/ANE.0000000000001846. [DOI] [PubMed] [Google Scholar]
- 18.Mohta M, Dubey M, Malhotra RK, Tyagi A. Comparison of the potency of elective caesarean section. Int J Obstet Anesth. 2019;38:25–31. doi: 10.1016/j.ijoa.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Sharkey AM, Siddiqui N, Downey K, Ye XY, Guevara J, Carvalho JC. Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat spinal-induced hypotension in cesarean deliveries: Randomized controlled trial. Anesth Analg. 2018 doi: 10.1213/ANE.0000000000003704. doi: 10.1213/ANE.0000000000003704. [DOI] [PubMed] [Google Scholar]
- 20.Elnabtity AM, Selim MF. Norepinephrine versus ephedrine to maintain arterial blood pressure during spinal anesthesia for cesarean delivery: A prospective double-blinded trial. Anesth Essays Res. 2018;12:92–7. doi: 10.4103/aer.AER_204_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Mao M, Liu S, Xu S, Yang J. A comparative study of bolus norepinephrine, phenylephrine, and ephedrine for the treatment of maternal hypotension in parturients with preeclampsia during cesarean delivery under spinal anesthesia. Med Sci Monit. 2019;25:1093–101. doi: 10.12659/MSM.914143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S, Shen X, Liu S, Yang J, Wang X. Efficacy and safety of norepinephrine versus phenylephrine for the management of maternal hypotension during cesarean delivery with spinal anesthesia: A systematic review and meta-analysis. Medicine. 2019;98:e14331. doi: 10.1097/MD.0000000000014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kee WN, Lee SW, Ng FF, Khaw KS. Prophylactic norepinephrine infusion for preventing hypotension during spinal anaesthesia for cesarean delivery. Anesth Analg. 2018;126:1989–94. doi: 10.1213/ANE.0000000000002243. [DOI] [PubMed] [Google Scholar]