Abstract
Background
BM32, a grass pollen allergy vaccine containing four recombinant fusion proteins consisting of hepatitis B-derived PreS and hypoallergenic peptides from the major timothy grass pollen allergens adsorbed on aluminium hydroxide has been shown to be safe and to improve clinical symptoms of grass pollen allergy upon allergen-specific immunotherapy (AIT). We have investigated the immune responses in patients from a two years double-blind, placebo-controlled AIT field trial with BM32.
Methods
Blood samples from patients treated with BM32 (n = 27) or placebo (Aluminium hydroxide) (n = 13) were obtained to study the effects of vaccination and natural allergen exposure on allergen-specific antibody, T cell and cytokine responses. Allergen-specific IgE, IgG, IgG1 and IgG4 levels were determined by ImmunoCAP and ELISA, respectively. Allergen-specific lymphocyte proliferation by 3H thymidine incorporation and multiple cytokine responses with a human 17-plex cytokine assay were studied in cultured peripheral blood mononuclear cells (PBMCs).
Findings
Two years AIT comprising two courses of 3 pre-seasonal injections of BM32 and a single booster after the first pollen season induced a continuously increasing (year 2 > year 1) allergen-specific IgG4 response without boosting allergen-specific IgE responses. Specific IgG4 responses were accompanied by low stimulation of allergen-specific PBMC responses. Increases of allergen-specific pro-inflammatory cytokine responses were absent. The rise of allergen-specific IgE induced by seasonal grass pollen exposure was partially blunted in BM32-treated patients.
Interpretation
AIT with BM32 is characterised by the induction of a non-inflammatory, continuously increasing allergen-specific IgG4 response (year 2 > year1) which may explain that clinical efficacy was higher in year 2 than in year 1. The good safety profile of BM32 may be explained by lack of IgE reactivity and low stimulation of allergen-specific T cell and cytokine responses.
Fundings
Grants F4605, F4613 and DK 1248-B13 of the Austrian Science Fund (FWF).
Keywords: Allergy, Allergen, Allergen-specific immunotherapy (AIT), Recombinant allergy vaccine, BM32, IgE, IgG subclass, T cell response, Cytokine response
Abbreviations: AIT, Allergen specific immunotherapy; BSA, Bovine serum albumin; HRP, Horse radish peroxidase; Ig, Immunoglobulin; PBMCs, Peripheral blood mononuclear cells; PBS, Phosphate buffered saline; SI, Stimulation Index; Th, T helper cell; Cpm, counts per minute
Research in context.
Evidence before this study
BM32 is a novel vaccine for treatment of grass pollen allergy. It comprises four recombinant fusion proteins consisting of hepatitis B virus-derived PreS fused to hypoallergenic peptides from the IgE binding sites of the four major timothy grass pollen allergens Phl p 1, 2, 5 and 6. The vaccine was designed to lack allergenic activity, reduce the presence of allergen-specific T cell epitopes and, upon immunization, induce allergen-specific IgG responses. These effects have been demonstrated in preclinical studies. BM32 has been evaluated regarding safety, dosing and immunological effects in a clinical AIT trial conducted outside the grass pollen season in an exposure chamber setting. In a recent two year, double-blind, placebo-controlled multicentre AIT field trial, it was found that treatment with BM32 was well tolerated and reduced symptoms of grass pollen allergy. This treatment effect was found to be more pronounced in the second year of treatment.
Added value of this study
Our sub-study is the first to investigate specific B cell and T cell as well as cytokine responses to the major grass pollen allergen molecules and synthetic allergen-derived peptides during the 2 years of field treatment and two seasons of natural grass pollen exposure. We found that AIT, with only few pre-seasonal injections with BM32 induced a continuously increasing allergen-specific IgG4 response, which could be strongly boosted with one single injection. The allergen-specific IgG4 response was achieved with only minor activation of allergen-specific T cell responses and allergen-specific pro-inflammatory cytokine responses were absent.
Implications of all the available evidence
Our study demonstrates that BM32 is a non-inflammatory form of AIT, which builds up a continuously increasing allergen-specific IgG4 response (year 2 > year1). Our immunological findings thus may explain the high safety profile of the vaccine and the higher clinical efficacy in year 2 compared to year 1 in the AIT field trial. Our findings also suggest that clinical efficacy of BM32 can be increased by applying vaccination schedules, which enhance the build-up of allergen-specific IgG4 responses and that clinical efficacy may further increase during prolonged treatment with only few booster injections.
Alt-text: Unlabelled box
1. Introduction
Almost 30% of the population are affected by Immunoglobulin-E (IgE)-associated allergy, the most common hypersensitivity disease [1,2]. Allergic patients suffer from a variety of allergic symptoms such as rhinoconjunctivitis (i.e., hayfever), asthma, food allergy, allergic skin inflammation and life-threatening anaphylactic shock [3, 4, 5]. The longitudinal determination of the evolution of allergen-specific IgE responses and the development of allergic symptoms in population-based birth cohorts indicates that allergy often starts in the form of a clinically silent IgE sensitization in early childhood which later in life progresses to symptoms of increasing intensity [6, 7]. While symptoms of allergy can be mitigated by anti-inflammatory drugs and biologics, only allergen-specific immunotherapy (AIT) seems to be able to modify the course of the disease as it was found to prevent the progression of mild forms of allergy to severe forms when given early in childhood [8, 9, 10, 11]. AIT may be considered as a therapeutic vaccination treatment which, upon administration of the disease-causing allergens, induces a protective IgG antibody response and immunological alterations in cellular immune responses towards a non-inflammatory phenotype [8,12,13]. The induction of allergen-specific IgG4 antibodies is considered as an important biomarker for clinically successful AIT as it prevents allergen-induced mast cell and basophil activation, IgE-facilitated allergen presentation to T cells and thus T cell activation and boost of IgE production upon natural allergen exposure by competing with IgE for the binding sites on the allergens [14,15].
However, several factors limit the broad application of AIT. Current forms of AIT are based on natural allergen extracts which, due to the fact that they are made from natural allergen sources, maybe concerned by safety problems and incomplete effectiveness [16,17]. Thus, several efforts have been made to increase safety, quality, efficacy and convenience of AIT by molecular approaches [18,19].
The recombinant grass pollen allergy vaccine BM32 has been constructed to induce blocking IgG antibodies against the IgE binding sites of the major grass pollen allergens and thus to prevent allergen-induced mast cell and basophil degranulation, IgE-facilitated allergen presentation and consecutive T cell activation as well as rises of allergen-specific IgE production induced by natural allergen contact [20]. The vaccine contains recombinant fusion proteins composed of hypoallergenic peptides from the IgE-binding sites of the four major timothy grass pollen allergens (Phl p 1, 2, 5 and 6) and the hepatitis B virus derived PreS protein [21,22]. The choice of these allergens for the vaccine was based on serological data confirming that most patients worldwide are sensitized to these respective allergens [23]. PreS was selected as a non-allergenic carrier molecule able to recruit T cell help for induction of allergen-specific blocking antibodies [24]. In contrast to previously developed recombinant hypoallergenic vaccines which showed only reduced IgE reactivity but maintained allergen-specific T cell epitopes, the presence of allergen-specific T cell epitopes in BM32 has been reduced to diminish side effects during AIT caused by T cell activation [25]. Immunization experiments showed that BM32 indeed induced the production of allergen-specific blocking IgG antibodies [26]. The reduced ability of the BM32 proteins to induce mast cell-mediated immediate and T cell-mediated late phase reactions was confirmed in a skin test study conducted in grass pollen allergic patients [27]. Thereafter, a safety and dose-finding study was conducted outside the grass pollen season in a pollen exposure chamber showing the safety of the vaccine [21]. This study also showed that vaccination with BM32 reduces symptoms of grass pollen allergy upon pollen exposure and demonstrated the induction of blocking allergen-specific IgG antibodies, reduced sensitivity of effector cells and the suppression of allergen-specific T cell reactivity in treated patients.
Recently, BM32 was evaluated for the first time in a double-blind, placebo-controlled multicentre field trial which showed that the vaccine reduces symptoms of grass pollen allergy during two years of seasonal grass pollen exposure [28]. Here we report the results of a sub-study which was conducted to analyse the immunological effects of treatment with BM32 in patients during natural grass pollen exposure over a period of two years. Specifically, allergen-specific IgE as well as IgG levels including IgG1 and IgG4 subclass measurements were performed. Furthermore, we studied allergen- as well as allergen-derived peptide-specific T cell proliferation and cytokine patterns induced by allergen stimulation in BM32 and placebo treated patients.
2. Material and methods
2.1. Sub-study design and subjects
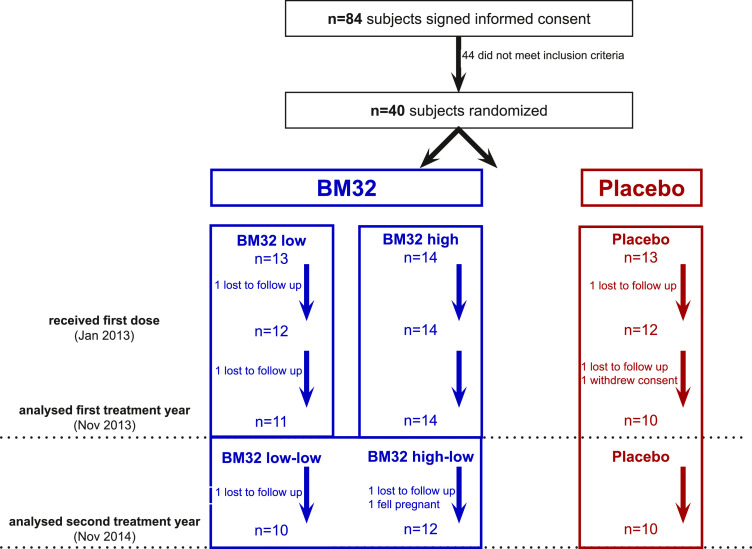
Study subjects were participants of a multicentre, double blind, placebo controlled parallel group trial to investigate safety and efficacy of the new grass pollen vaccine BM32 (EudraCT no. 2012-000442-35, ClinicalTrial.gov Identifier NCT01538979)(28). This sub-study conducted only in the Vienna centre was dedicated to investigating the immunological effects of treatment with BM32 during two years of in-field treatment. To that aim it included blood sampling during the grass pollen season (June) of both treatment years in addition to the blood sampling of the main study in January, April, September of both treatment years and November of the first treatment year. The sub-study was approved by the Ethics committee of the Medical University of Vienna EK Nr. 1757/2012. Fig. 1 provides an overview of subject recruitment, randomization and their allocation to the treatment arms. Of the 84 patients signing informed consent, 40 were randomized. For inclusion and exclusion criteria, please refer to table S1. A report of the clinical effects of the complete study and the study protocol are available online in Niederberger et al. [28].
Fig 1.
Sub-study population, randomisation and analysis in treatment year 1 and 2. Numbers of subjects signing informed consent, randomised into placebo, BM32-low, BM32-high treatment during first treatment year and receiving placebo and BM32-low in the second year are displayed. For in- and exclusion criteria, please refer to Table S1. Numbers of subjects who discontinued and reasons are shown.
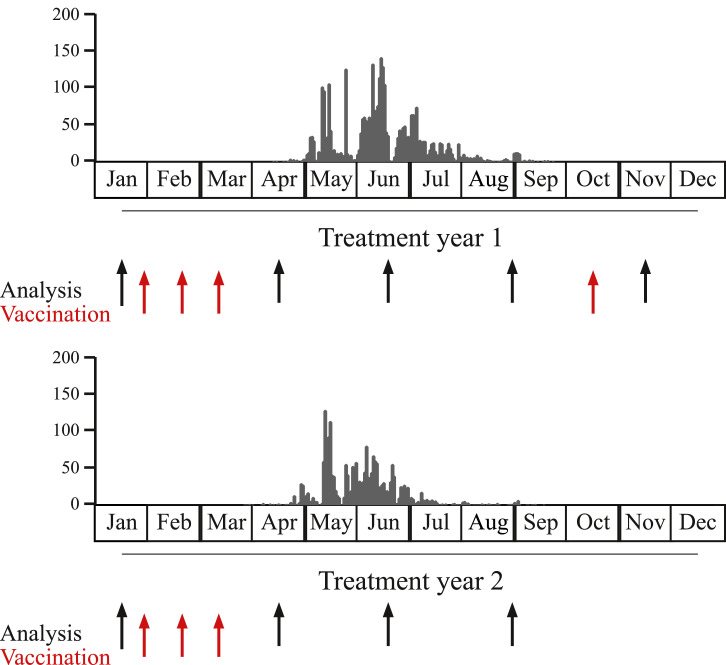
Fig. 2 provides a schematic overview of grass pollen exposure during the two years of treatment in the Vienna study centre as well as the time points of treatment and blood sampling for the immunological analysis reported in this study. Before the grass pollen season of the first year, 27 patients received three monthly subcutaneous injections of a mix of 20 microgram of each of the four BM32 fusion proteins adsorbed to aluminium hydroxide (i.e., BM32 low) or a mix of 40 microgram of each of the four proteins (i.e., BM32 high) before the pollen season and one booster injection in October, after the pollen season (Figs. 1 and 2). A blinded evaluation was performed by an independent data monitoring committee (IDMC) after the grass pollen season of year 1. This committee recommended to continue with the BM32 low dose in year 2, which was given in the form of three monthly pre-seasonal injections to 10 patients of the BM32 low and to 12 patients of the BM32 high group who had continued in year 2. From the 13 placebo patients receiving 3.0 mg/ml Aluminium hydroxide in a physiologic buffer containing 0.9% NaCl who had started in year one, 10 continued in year two. For this sub-study on immunological responses blood samples were obtained at five time points during treatment year 1 (January, April, June, August/September, November) and at four time points during treatment year 2 (January, April, June, August/September) (Fig. 2).
Fig 2.
Schematic overview of the study. Patients received three vaccinations (red arrows) before the season of each treatment year (1 and 2) and one booster vaccination in October of treatment year 1. Grass pollen counts (y-axes, grey bars, grains per m3 per day) are shown for year 1 (upper graph) and year 2 (lower graph). Time points for blood collection are shown by black arrows.
2.2. Sub-study design and immunological characterization of patients at baseline
In this sub-study, 40 grass pollen allergic patients from the Vienna study centre undergoing treatment with the grass pollen vaccine BM32 (n = 27) or placebo (n = 13) were analysed regarding allergen-specific immunological parameters. These patients were part of a multicentre double-blind placebo-controlled phase IIb trial (ClinicalTrials.gov identifier NCT01538979) [28]. Fig. 1 shows that 10 of the 13 patients who had started in year 1 with the low dose of BM32, continued treatment in year 2 with the low dose and 12 of the 14 subjects who had started with the high dose in year 1 continued in year 2 with the low dose. Of the 13 patients treated with placebo in year 1, 10 subjects continued during the second year with placebo. Patients received three monthly injections before the grass pollen season of year 1 and 2 and a booster injection after the grass pollen season of year 1 in October, amounting to a total of 7 vaccinations during the two years (Fig. 2, red arrows). The single booster injection in October of year 1 allowed us to study the influence of a single injection on immune responses. Blood samples were obtained always before the injections and approximately one month after the pre-seasonal treatment course of three injections and the single booster injection in October (i.e., November) (Fig. 2, black arrows). In addition, blood samples were collected in June (during grass pollen season) and in September (after grass pollen season) (Fig. 2, black arrows).
Demographic, immunological and serological data of the subjects at baseline before treatment are displayed in Table 1 indicating a balanced distribution of mean grass pollen-specific IgE and IgG levels as well as baseline allergen-specific T cell proliferation and cytokine levels in the placebo and in the treatment group.
Table 1.
Demographic, serological and immunological characterization of the sub-study population at baseline visit. Differences between BM32-treated and placebo group are indicated. (* = p < .05 and n.s. = not significant). Baseline values for Phl p 1, Phl p 2, Phl p 5 and Phl p 6 specific IgE levels and T cell proliferation are displayed only for subjects sensitized to the relevant allergen.
|
Patient characteristics at baseline visit | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Placebo (n = 10) |
BM32 (n = 25) |
|||||||
|
Sex |
Male | 4/10 (40%) | 13/25 (52%) | |||||
|
Female |
6/10 (60%) |
12/25 (48%) |
||||||
| Mean | Median | Range | Mean | Median | Range | p-value | ||
| Age | 27.3 | 24.8 | 21–45 | 27.3 | 26.0 | 19–46 | n.s. | |
| Proliferation (SI) | Recombinant mix-specific | 1.7 | 1.4 | 0.7–3.6 | 1.6 | 1.3 | 0.04–5.6 | n.s. |
| Phl p 1-specific | 1.3 | 1.3 | 0.7–22 | 1.4 | 1.3 | 0.7–2.7 | n.s. | |
| Phl p 2-specific | 1.1 | 1.1 | 0.5–1.9 | 1.2 | 1.2 | 0.6–2.1 | n.s. | |
| Phl p 5-specific | 1.1 | 1.0 | 0.7–1.9 | 1.3 | 1.3 | 0.6–2.3 | n.s. | |
| Phl p 6-specific | 1.4 | 1.3 | 0.4–3.7 | 1.3 | 1.1 | 0.6–2.8 | n.s. | |
| Pre S-specific | 0.04 | 0.03 | 0.01–0.16 | 0.40 | 0.04 | 0.01–5.1 | n.s. | |
| BM32-mix specific | 1.4 | 1.3 | 0.7–2.2 | 1.6 | 1.5 | 0.8–3.0 | n.s. | |
| Cytokines (pg/ml) in response to stimulation with a mix of Phl p 1, 2, 5, and 6 | IL-1 | 79.7 | 50.2 | 12.5–213.3 | 88.2 | 57.3 | 3.3–259.3 | n.s. |
| IL-2 | 58.8 | 20.9 | 8.4–368.5 | 38.9 | 18.0 | 0–245.2 | n.s. | |
| IL-4 | 3.6 | 3.1 | 0–7.8 | 4.1 | 3.1 | 0–13.9 | n.s. | |
| IL-5 | 34.7 | 34.4 | 13.2–57.7 | 33.7 | 34.0 | 7.6–66.2 | n.s. | |
| IL-6 | 583.1 | 521.0 | 104.1–1856.9 | 766.6 | 630.2 | 49.0–3672.0 | n.s. | |
| IL-7 | 14.6 | 0 | 0–145.9 | 0.9 | 0 | 0–9.7 | n.s. | |
| IL-10 | 139.8 | 7.7 | 0–1327.4 | 5.4 | 3.9 | 0–31.1 | n.s. | |
| IL-12 | 54.4 | 0 | 0–537.0 | 0.1 | 0 | 0–3.27 | n.s. | |
| IL-13 | 139.0 | 91.7 | 18.6–454.9 | 69.9 | 52.8 | 0–167.1 | n.s. | |
| IL-17 | 103.3 | 64.8 | 11.3–342.6 | 73.7 | 55.3 | 0–271.8 | n.s. | |
| GCSF | 363.2 | 146.7 | 63.9–1303.5 | 354.4 | 215.0 | 0–1534.5 | n.s. | |
| GMCSF | 47.5 | 26.1 | 8.6–197.1 | 30.7 | 24.2 | 0–117.4 | n.s. | |
| IFN-gamma | 245.8 | 265.2 | 110.2–387.0 | 211.0 | 159.8 | 24.2–468.3 | n.s. | |
| MCP | 2317.7 | 2227.2 | 755.4–3925.8 | 2204.1 | 2146.4 | 167.7–4023.4 | n.s. | |
| MIP-1 | 738.7 | 622.2 | 290.2–1913.3 | 880.1 | 950.2 | 68.1–2260.5 | n.s. | |
| TNF-alpha | 790.8 | 525.6 | 0–2226.0 | 1683.1 | 1217.0 | 61.0–6437.6 | n.s. | |
| IgE (kUA/L) | Total | 206.8 | 156.0 | 8.8–494.0 | 195.6 | 77.9 | 10.4–1035.0 | n.s. |
| Phl p 1-specific | 24.9 | 6.2 | 0.7–103.0 | 21.4 | 12.5 | 0.7–83.0 | n.s. | |
| Phl p 2-specific | 15.3 | 11.8 | 6.5–31.0 | 6.0 | 2.8 | 1.1–20.8 | n.s. | |
| Phl p 5-specific | 24.2 | 8.1 | 1.2–121.0 | 30.0 | 12.0 | 2.8–118.0 | n.s. | |
| Phl p 6-specific | 15.2 | 6.1 | 2.0–50.2 | 12.9 | 4.4 | 1.2–67.9 | n.s. | |
| IgG (OD405nm) | Phl p 1-specific | 0.4 | 0.3 | 0.2–0.6 | 0.4 | 0.3 | 0.1–0.6 | n.s. |
| Phl p 2-specific | 0.3 | 0.2 | 0.1–0.6 | 0.3 | 0.3 | 0.1–1.2 | n.s. | |
| Phl p 5-specific | 0.6 | 0.5 | 0.2–1.1 | 0.7 | 0.7 | 0.1–1.4 | n.s. | |
| Phl p 6-specific | 0.5 | 0.3 | 0.1–1.4 | 0.3 | 0.3 | 0.1–0.8 | n.s. | |
| IgG1 (OD405nm) | Phl p 1-specific | 0.02 | 0.02 | 0.01–0.03 | 0.02 | 0.01 | 0–0.07 | n.s. |
| Phl p 2-specific | 0 | 0 | 0–0.01 | 0.01 | 0 | 0–0.11 | n.s. | |
| Phl p 5-specific | 0.03 | 0.01 | 0–0.12 | 0.07 | 0.01 | 0–0.56 | n.s. | |
| Phl p 6-specific | 0.03 | 0.01 | 0–0.11 | 0 | 0 | 0–0.03 | n.s. | |
| IgG4 (OD405nm) | Phl p 1-specific | 0.02 | 0.01 | 0–0.05 | 0.02 | 0.01 | 0–0.09 | n.s. |
| Phl p 2-specific | 0 | 0 | 0–0.01 | 0.01 | 0 | 0–0.05 | n.s. | |
| Phl p 5-specific | 0.03 | 0.01 | 0–0.18 | 0.06 | 0.03 | 0–0.26 | n.s. | |
| Phl p 6-specific | 0 | 0 | 0–0.02 | 0 | 0 | 0–0.04 | n.s. | |
2.3. Assessment of IgE and IgG levels
IgE and IgG levels against grass pollen allergens Phl p 1, Phl p 2, Phl p 5 and Phl p 6 were measured by ELISA and ImmunoCAP as previously described [21]. For IgG, IgG1 and IgG4 measurements ELISA plates (Thermo Scientific Nunc, Roskilde, Denmark) were coated with 2 μg/mL of each of the grass pollen allergens rPhl p 1, 2, 5, or 6. Allergen-specific human IgG responses (total IgG, and IgG1 and IgG4 subclasses) were measured by diluting sera 1:100 for determination of the total IgG antibodies, and 1:20 for determination of IgG1 and IgG4 subclasses. Total IgG antibodies were detected with rabbit anti-Fc fragment of human IgG (1:10,000) (Jackson ImmunoResearch, ME, USA), followed by donkey anti-rabbit horseradish peroxidase (HRP-coupled IgG antibodies (1:2000), (NA 934; GE Healthcare UK Limited, Chalfont St Giles, United Kingdom). Subclasses IgG1 and IgG4 were detected using mouse monoclonal anti-human IgG1 (clone JDC-1) and IgG4 (clone JDC-14) antibodies (1:1000, BD Bioscience, San Jose, CA, USA) followed by HRP-coupled sheep anti-mouse IgG (1:2000) (GE Healthcare, Waukesha, WI, USA). Colorimetric detection for all ELISAs was done with 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulphonic acid (Sigma) and optical density (OD405nm-OD490nm) was measured on a Spectra-Max-spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Plate-to-plate normalization was done by a testing control serum pool with established antibody levels for the antigens on each plate. Unspecific binding of detection antibodies was excluded by performing buffer controls (i.e., omission of sera). All determinations were carried out in duplicates and results are shown as means for each sample.
Allergen-specific IgE antibody levels specific for Phl p 1, Phl p 2, Phl p 5 and Phl p 6 were quantified by ImmunoCAP technology (Thermofisher, Uppsala, Sweden).
2.4. Measurement of allergen- and peptide-specific PBMC proliferation and secretion of cytokines in cultured PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated from patients’ heparinized blood samples using Ficoll gradient (GE Healthcare, Chicago, IL, USA) and cultured as described [21]. Briefly, PBMCs were washed twice in phosphate buffered saline (PBS) and re-suspended in serum-free UltraCULTURE medium (BioWhittaker, Rockland, ME, USA) supplemented with 2 mmol/ L-glutamine (Sigma, St Louis, MO, USA), 50 μmol/L β-mercaptoethanol (Sigma), and 0.02 g/L of gentamicin (Sigma) before culture in 96 well plates (200,000 cells/well; Nunclone; Nalgene Nunc International, Rosklide, Denmark). Cells were stimulated for one week in the absence or presence of the following antigenic stimuli: (i) mix of 0.25 µg/well of each of the four recombinant grass pollen allergens: rPhl p 1, rPhl p 2, rPhl p 5, rPhl p 6 (Biomay AG, Vienna, Austria), (ii) a mix of synthetic allergen-derived peptides (Phl p 1–5: 0.03298 µg/well; Phl p 2–4: 0.08528 µg/well; Phl p 5–1, Phl p 5–2, Phl p 5–5, Phl p 5–6: each 0.03002 µg/well; Phl p 6–1:0.07589 µg/well); Fig. S1, Table S2), (iii) a mix of 0.25 μg per well of each of the 4 BM32 fusion proteins (BM321, BM322, BM325 and BM326)(Biomay AG), (iv) each of the recombinant grass pollen allergens alone (Phl p 1, Phl p 2, Phl p 5, Phl p 6, 0.25 μg per well), (v) recombinant PreS alone (0.15 µg/well) [21] (vi) each of the synthetic allergen-derived peptides alone (Phl p 1–5: 0.03298 µg/well; Phl p 2–4: 0.08528 µg/well; Phl p 5–1, Phl p 5–2, Phl p 5–5, Phl p 5–6: each 0.03002 µg/well; Phl p 6–1:0.07589 µg/well), (vii) IL-2 (4 U/well) (BioLegend, San Diego, CA, USA) as a positive control or (viii) medium alone as negative control.
For measurement of PBMC proliferation, 0.5 µCi of 3H thymidine (PerkinElmer, Waltham, MA, USA) were added to cultures 16 h before harvesting and counts per minutes (cpm) were measured upon harvesting. The stimulation index (SI) was calculated as follows: cpm (stimulated cells)/cpm (unstimulated cells). Experiments were performed in triplicates. For measurement of cytokines, supernatants were collected from PBMC cultures, which had been prepared identically to the proliferations after one week of culture and frozen at −80 °C until measurements were performed. Supernatants of triplicates were pooled and measured with a human Bio-Plex ProTM Th1/Th2 17-plex immunoassay (Bio-Rad Inc., Hercules, CA, USA) according to the manufacturer's instructions. The levels for the following cytokines, i.e., IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, GMCSF, GCSF, MIP, MCP, IFN-γ, TNF-α, were measured using a Luminex 100 System (Luminex Corp., Austin, TX, USA) [29].
2.5. Flow cytometry for levels of CD23 and FcεRI
PBMCs were isolated using Ficoll gradient and washed twice in PBS. Thereafter, cells were blocked using PBS 1% v/v bovine serum albumin (BSA, Sigma) and 10% v/v mouse serum (Thermo Fisher Scientific) for 20 min on ice. After washing, cells were incubated with antibodies or isotype controls for 20 min on ice in PBS 1% v/v BSA. The following antibodies (all Thermo Fisher Scientific) were used: anti-CD23PE (clone EBVCS2), anti-CD19 APC (clone HIB 19), anti-CD27 PECy7 (clone O323), anti-CD123 FITC (clone 6H6), anti-FcεRI PE (clone AER-37), anti-CCR3 APC (clone 5E8-49-B4), viability dye e780 or respective isotype controls. After washing, cells were re-suspended in PBS 1% v/v BSA and samples were acquired using a BD FACS Canto II (BD Bioscience, San Jose, CA, USA). Quantibrite beads (BD Bioscience) were used for quantification of the numbers of molecules of CD23 and FcεRI according to manufacturer's instructions and as described [30]. A minimum of 20,000 events per sample (samples in triplicates) were recorded. Data was analysed using Flowjo Software (Treestar, Ashland, OR, USA).
2.6. Statistical analyses
The observed time trajectories of antibody levels and other parameters were visualized in terms of scatter plots with a small amount of jittering added to allow for distinction of single values. The mean trajectory was estimated for each group and is shown in the plots. To test whether the trajectories for a given antibody level or parameter are different between the treatment and control group, the null hypothesis of no mean difference was first separately tested for each time-point using an unpaired t-test. Subsequently, the obtained p-values were adjusted for multiple testing across time-points by the Holm–Sidak method. Adjusted p-values below 0.05 were considered significant. In all calculations and figures measurements below the detection limit entered the analysis with a value of zero. Detection limits of the applied assays were between 0.09 and 3.63 pg/ml. Given the small value of the detection limits compared to the overall range of observed values and given the small number of measurements below the limit, this approximation does not affect the results of the statistical analysis. The statistical analysis was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com).
3. Results
3.1. Kinetics of allergen-specific IgG, IgG1 and IgG4 antibody responses during two years of AIT with BM32
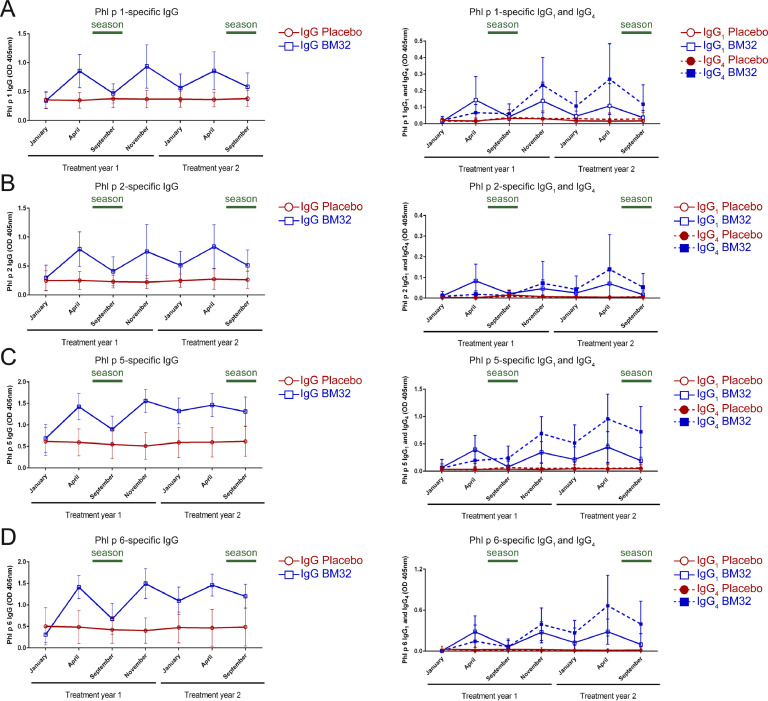
Fig. 3 shows the development of IgG, IgG1 and IgG4 responses to the four major timothy grass pollen allergens, Phl p 1, Phl p 2, Phl p 5 and Phl p 6 during the two years of treatment with BM32 and placebo. The three pre-seasonal injections of BM32 given in year 1 induced an increase of IgG antibodies specific for each of the four major grass pollen allergens which was not observed for the placebo-treated subjects (Figs. 3(A)–(D) and S2, left panels). This IgG response declined 5 months later (September year 1) but could be boosted with the booster injection in October and with the next pre-seasonal three injections in year 2 (Fig. 3(A)–(D), and S2 left panels). We noted interesting differences regarding the kinetics of allergen-specific IgG1 and IgG4 responses (Figs. 3(A)–(D) and S2, right panels). Allergen-specific IgG1 levels induced already after the first treatment course, were higher than allergen-specific IgG4 levels but dropped quicker than allergen-specific IgG4 responses after the first course of treatment, after the booster injection in October and even after the second course of pre-seasonal treatment (Figs. 3(A)–(D) and S2, right panels). No changes of allergen-specific IgG1 or IgG4 responses were noted for the placebo group during the two years of treatment.
Fig 3.
Allergen-specific IgG, IgG1, IgG4 levels during the two treatment years. (A) Phl p 1-, (B) Phl p 2-, (C) Phl p 5- and (D) Phl p 6-specific IgG (left graphs), IgG1 and IgG4 (right graphs) levels were measured by ELISA (Optical density levels OD405nm, y-axes) at indicated time points (x-axes). Lines (Placebo: red circles, BM32: blue squares) represent means with standard deviation shown. Symbols for IgG subclasses are indicated in the legends.
3.2. Vaccination with BM32 induces a continuously increasing grass pollen allergen-specific IgG4 response
Allergen-specific IgG4 responses induced by BM32 showed a different kinetic behaviour as compared to allergen-specific IgG1 responses. While IgG1 responses rose quickly after each vaccination, IgG4 responses developed slower and were lower than IgG1 responses in year 1 (Fig. 3(A)–(D) and S2, right panels). However, in contrast to IgG1, allergen-specific IgG4 responses did not drop quickly and continued to increase during treatment, especially in year 2. In fact, allergen-specific IgG4 levels were much higher in year 2 than in year 1 and were maintained at high levels four months after the last treatment in September of year 2 whereas IgG1 levels had declined (Figs. 3(A)–(D) and S2, right panels).
3.3. A single booster injection of BM32 is sufficient to restore allergen-specific IgG1 responses and to further increase allergen-specific IgG4 levels
After the first course of three pre-seasonal injections given until March of year 1, the levels of allergen-specific IgG1 dropped markedly until September (Figs. 3(A)–(D), and S2, right panels). Interestingly, the single booster injection administered in October brought allergen-specific IgG1 levels back to the same level which had been reached with the first course of three injections (Figs. 3(A)–(D), and S2, right panels). Even more interesting, the next course of three pre-seasonal injections did not increase allergen-specific IgG1 much more than the single booster injection in October (Figs. 3(A)–(D) and S2, right panels).
The effect of the single booster injection on allergen-specific IgG4 was different than that on IgG1. Instead of only restoring allergen-specific IgG4 levels, the single booster injection further increased allergen-specific IgG4 levels (Figs. 3(A)–(D) and S2, right panels).
3.4. Vaccination with BM32 does not boost allergen-specific IgE levels and blunts rises of allergen-specific IgE induced by natural allergen exposure
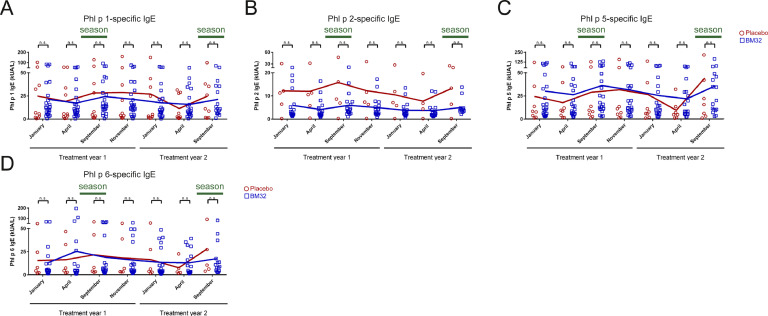
Fig. 4 shows the IgE levels specific for the four major timothy grass pollen allergens as measured in the BM32- and placebo treated patients during the two years course of the study. In contrast to other forms of AIT (e.g., SLIT, AIT with native allergen extracts, hydrolysed allergens, allergoids or other recombinant approaches) [31–37], vaccination with BM32 did not induce any increases of allergen-specific IgE levels after the first course of treatment (January–April, year 1), after the booster injection (October, year 1) or after the second course of pre-seasonal injections (January–April, year 2) (Fig. 4).
Fig 4.
Allergen-specific IgE levels during two treatment years with BM32 or placebo in patients. (A) Phl p 1-, (B) Phl p 2-, (C) Phl p 5- and (D) Phl p 6-specific IgE levels (y-axes: kUA/l) are shown for the patients sensitized to the corresponding allergens at indicated time points (x-axes) Dots (Placebo: red circles, BM32: blue squares) represent individual patients and lines represent the means. Differences between BM32-treated and placebo group are indicated. (* = p < .05 and n.s. = not significant).
Natural respiratory allergen exposure induces rises of systemic allergen-specific IgE antibodies [38,39]. Accordingly we found increases of allergen-specific IgE levels after the first and much more pronounced after the second pollen season (i.e., comparing allergen-specific IgE levels in April versus September) in the placebo group whereas these were partially blunted in the BM32 group (Fig. 4).
3.5. Vaccination with BM32 does not induce relevant boosts of T cell responses against native grass pollen allergens
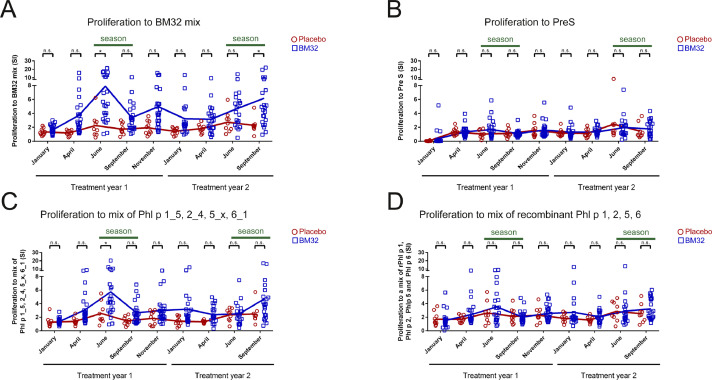
Lymphoproliferative responses to different antigens in BM32- and placebo-treated patients were determined. First, we studied responses to a mix of the BM32 fusion proteins used for treatment which consisted of hepatitis B-derived PreS and attached grass pollen allergen-derived peptides. BM32- but not placebo-treated patients showed increases of lymphoproliferative responses after vaccination (Fig. 5(A)). Basically no relevant lymphoproliferative responses or increases of responses to hepatitis B-derived PreS alone were observed in the BM32 and placebo-treated patients (Fig. 5(B)). This is in agreement with data from earlier studies indicating that in vitroT cell responses to certain HBV antigens in HBV-vaccinated persons are low [40]. Furthermore it has been found that PreS fusion proteins induce the production of the tolerogenic cytokine IL-10 in PBMC cultures from allergic patients [41]. We also tested a mix comprising the isolated unfolded allergen-derived synthetic peptides which had been part of the BM32 fusion proteins and found markedly increased lymphoproliferative responses against the peptide mix in the BM32 but not in the placebo-treated patients (Fig. 5(C)). Interestingly, when we stimulated patients’ PBMCs with a mix of folded, native and wildtype-like allergen, we did not find significant differences regarding allergen-specific lymphoproliferative responses after vaccination between BM32- and placebo-treated patients. A detailed analysis of the lymphoproliferative responses to the individual allergen-derived synthetic peptides and allergens revealed that mainly Phl p 5-derived peptides and the Phl p 6-derived peptide (Phl p 6_1), which is structurally related to one of the Phl p 5-derived peptides (i.e., Phl p 5_x: P2) (Fig. S1, Table S2) contribute to allergen-specific lymphoproliferative responses (Fig. S3).
Fig. 5.
Development of peptide- and allergen-specific T cell proliferation in patients during two years of treatment with BM32 or placebo. (A–D) Proliferation of patients PBMCs (x-axes: stimulation indices, SIs) in response to (A) a mix of the four BM32 fusion proteins (BM32 mix), (B) PreS alone, (C) a mix of Phl p 1_5, 2_4, 5_x, 6_1 peptides or (D) a mix of recombinant Phl p 1, 2, 5, 6 allergens. Dots (Placebo: red circles, BM32: blue squares) represent results from individual patients and lines represent the means. Differences between BM32-treated and placebo group are indicated. (* = p < .05 and n.s. = not significant).
3.6. Vaccination with BM32 does not induce relevant boosts of allergen-specific cytokine responses
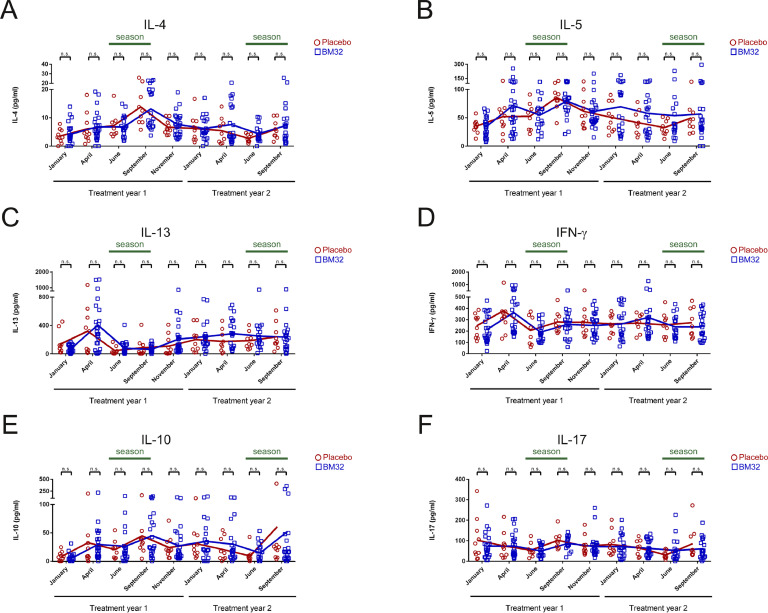
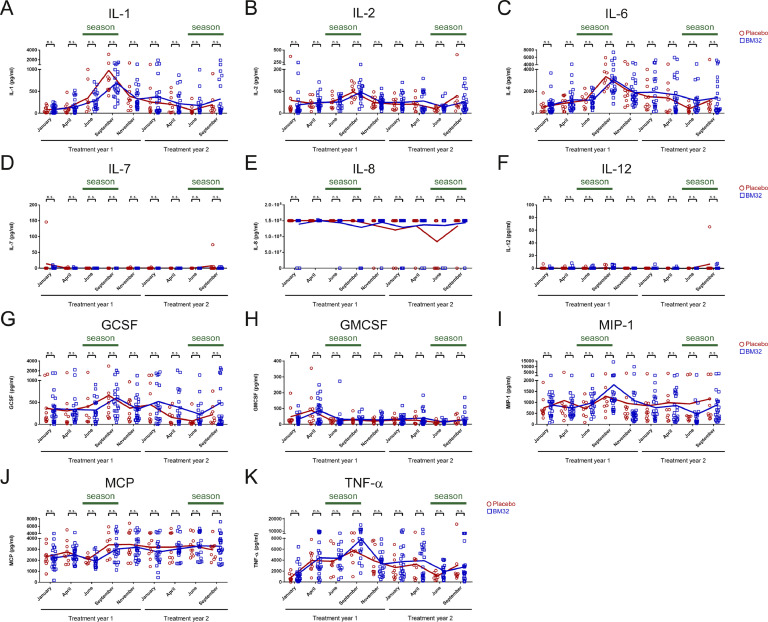
To further elucidate the effect of BM32 on allergen-specific T cell responses, we analysed the supernatants of PBMC cultures stimulated with a mix of the four major timothy grass pollen allergens, Phl p 1, 2, 5 and 6 for secretion of cytokines. Importantly, vaccination with BM32 did not induce relevant boosts of Th2, Th1 or other pro-inflammatory allergen-specific cytokine responses (Figs. 6 and 7). However, also no boost of an allergen-specific IL-10 response was noted (Fig. 6(E)).
Fig 6.
Allergen-specific cytokine responses of cultured PBMCs from patients treated with BM32 (n = 25) or placebo (n = 10). (A–F) Shown are the levels (y-axes, pg/ml) of (A) IL-4, (B) IL-5 (C) IL-13, (D) IFN-γ, (E) IL-10 and (F) IL-17 produced by cultured PBMCs at different time points of the study (x-axes) in response to a mix of recombinant Phl p 1, 2, 5 and 6. Dots (Placebo: red circles, BM32: blue squares) represent individual patients and lines represent the means. Differences between BM32-treated and placebo group are indicated. (* = p < .05 and n.s. = not significant).
Fig 7.
Allergen-specific cytokine responses of cultured PBMCs from patients treated with BM32 (n = 25) or placebo (n = 10). (A–F) Shown are the levels (y-axes, pg/ml) of (A) IL-1, (B) IL-2, (C) IL-6, (D) IL-7, (E) IL-8, (F) IL-12, (G) GCSF, (H) GMCSF, (I) MIP-1, (J) MCP and (K) TNF-α produced by cultured PBMCs at different time points of the study (x-axes) in response to a mix of recombinant Phl p 1, 2, 5 and 6. Dots (Placebo: red circles, BM32: blue squares) represent individual patients and lines represent the means. Differences between BM32-treated and placebo group are indicated. (* = p < .05 and n.s. = not significant).
In all patients, BM32- as well as placebo-treated patients, Th2 cytokines IL-4 (Fig. 6(A)) and IL-5 (Fig. 6(B)) showed a trend of increase towards September of the first treatment year, but there was no significant difference in IL-4 or IL-5 secretion between placebo and BM32 treated groups at any of the time points analysed. There was also no difference observed regarding levels of IL-13 (Fig. 6(C)) or the Th1 cytokine IFN-γ (Fig. 6(D)) between both treatment groups during the two years of treatment. Furthermore, when we investigated the levels of the regulatory cytokine IL-10 (Fig. 6 (E)) and of IL-17 (Fig. 6(F)) again no differences were observed between BM32-treated and placebo groups. There was also no relevant difference between the treatment groups noted for the other cytokines assessed, namely IL-1, IL-2, IL-6, IL-7, IL-8, IL-12, GCSF, GMCSF, MIP-1, MCP, TNF-α in PBMC cultures stimulated with a mix of major allergens (Figs. 7(A)–(K)).
3.7. Vaccination with BM32 has no strong effects on B cell subsets and expression of the high (FcεRI) and low (CD23) affinity receptor for IgE in peripheral blood
Finally we assessed the effect of BM32 vaccination on changes in B cell subsets as well as in expression levels of high (FcεRI) and low (CD23) affinity receptor for IgE by flow cytometry. Absolute levels of receptor expression were quantified using Quantibrite beads [30]. The percentage of B cell subsets and basophils showed no difference between BM32 and placebo treated groups (Figs. S4 A-C and S5 A). Furthermore the absolute levels of expression of low (Fig. S4 D–F) and high affinity receptor (Fig. S5 B and C) for IgE did not differ in the BM32 treated as compared to the placebo group at any of the time points assessed.
4. Discussion
We have previously analysed allergen-specific immune responses in grass pollen allergic subjects who had been treated with three doses of three monthly injections with the recombinant grass pollen allergy vaccine BM32 outside the grass pollen season [21]. In this previous AIT trial effects of treatment were studied by exposing subjects to grass pollen in an exposure chamber but the immunological effects of treatment with BM32 in a field study have not yet been investigated. Here we report effects of vaccination with BM32 on immune responses in grass pollen allergic subjects in a double-blind, placebo-controlled field study over two years of treatment and natural grass pollen exposure. The study was designed to treat patients with a course of only three monthly pre-seasonal injections of BM32 given before the pollen season of the first and second treatment year [28]. A single booster injection was given in the autumn of the first treatment year, after the pollen season, thus allowing us to determine the effects of a single injection on immune responses. We found that BM32 induced an allergen-specific IgG response consisting of an early onset but a rather short-lived allergen-specific IgG1 response, which was followed by a continuously increasing allergen-specific IgG4 response. Notably, in another sub-study allergen-specific IgG levels induced by BM32 were shown to be comparable to those induced by conventional grass pollen allergen extract-based AIT but required five times less injections during the observation period of 2 years [33]. The allergen-specific IgG1 response appeared quickly and returned to almost baseline levels whereas the allergen-specific IgG4 response continued to increase during the treatment and was higher in treatment year 2 than in year 1. The different kinetics of IgG1 and IgG4 cannot be explained by differences in half-life [42], which are actually comparable, but may be attributed to different binding to Fcγ receptor subtypes. In this respect IgG1 shows stronger association constants for most Fcγ receptors as compared to IgG4 [43]. This together with the continuing affinity maturation of IgG4 may explain for the continuous rise of this IgG subclass in our study.
The development of allergen-specific IgG antibodies is considered as an important surrogate marker associated with the clinical efficacy of AIT [13]. In this context it is important to note that it has been found that AIT with BM32 improved symptoms of grass pollen allergy as measured by three different parameters, i.e., first, reduction of a combined symptom medication score, second, improvement of well-being determined by visual analogue score and third, rhinoconjunctivitis-related quality of life. These effects were observed to be more pronounced in year 2 compared to year 1, in parallel with higher allergen-specific IgG4 levels [28]. As there were no big differences regarding cumulative pollen exposures in the evaluated peak pollen seasons in both years, the better treatment effect in year 2 as compared to year one cannot be attributed to varying pollen exposure but seems to be due to the continuously rising IgG4 response [28]. Based on these observations we speculate that treatment for more than two years would further increase clinical efficacy and eventually result in long-term effects. However, it is a limitation of our work that this could not be investigated in our study which was designed only for two years.
Thus this finding indicates that clinical efficacy of BM32 could be improved in the first treatment year by giving additional pre-seasonal injections in order to achieve higher IgG4 levels. According to results of a recent AIT trial (NCT02643641) this indeed seems to be the case (Valenta et al., unpublished). Another important notion was that a single booster injection given in October of treatment year 1 after the pollen season was sufficient to boost the allergen-specific IgG1 response to levels comparable to three monthly injections and to further increase allergen-specific IgG4 responses. This finding indicates that during prolonged treatment with BM32 eventually fewer than three injections may be sufficient to boost allergen-specific IgG production after the first treatment year. Thus there seems to be further potential for increasing efficacy and convenience of treatment with BM32 employing a more vigorous building up of IgG responses by four to five pre-seasonal injections in the first year followed eventually by only one pre-seasonal injection in further treatment years.
BM32 is also unique among other AIT forms because it did not induce any boosts of allergen-specific IgE production, which occurs with SLIT and SCIT using allergen extracts, allergoids, hydrolized allergen preparations, recombinant hypoallergens and allergen-derived long synthetic peptides 31, 32, 33, 34, 35, 36, 37. By contrast, treatment with BM32 rather seemed to reduce boosts of allergen-specific IgE production induced by seasonal allergen exposure. While there is no evidence that boosts of IgE production by conventional allergen extract-based vaccines are a problem for therapeutic vaccination, it may represent a bottle neck for prophylactic allergy vaccination. In this context it should be noted that secondary preventive AIT has been shown to prevent the progression of mild forms of allergy (e.g., rhinoconjunctivitis) to severe forms such as asthma [10] and trials are being conducted with the goal of preventing the progression of clinically silent IgE sensitization towards symptomatic allergy [44,45]. Moreover, one may consider using AIT also in a primary preventive setting which either may be done very early in childhood before IgE sensitization has taken place or by inducing protective allergen-specific IgG in mothers to be transmitted to their children with the goal to prevent allergic sensitization [46, 47, 48, 49]. A B cell epitope-based vaccine such as BM32 which only induces allergen-specific IgG responses without boosting or inducing IgE responses would be useful for each of these preventive AIT strategies [24].
The analysis of allergen-specific lymphoproliferative responses and cytokine responses in our study further indicates that BM32 is an AIT vaccine with a very low inflammatory potential because we observed only minor increases of allergen-induced T cell proliferation and no induction of pro-inflammatory cytokine responses as compared to placebo treatment. Increases of T cell proliferation were mainly noted for the fusion proteins containing PreS as immunological carrier and to synthetic, non-IgE-reactive allergen-derived peptides whereas the mix of the native-like allergens to which the patient is exposed in the course of natural allergen exposure was not different from that observed for placebo and only a minor induction of specific responses to the structurally related allergens Phl p 5 and Phl p 6 (Fig. S1, Table S2) was noted in treatment year 1. These findings are in agreement with the good safety profile of BM32 with regards to the occurrence of late-phase reactions. In fact, treatment with BM32 did not induce any IgE-mediated anaphylactic reactions and late-phase, T cell-mediated side effects were rare and occurred at much lower frequency than those observed for other hypoallergenic AIT vaccines [37,50].
The results of our immunological analysis thus explain the low inflammatory potential of BM32 and provide information for refining the treatment with the goal to optimize the induction of protective allergen-specific IgG antibodies to make treatment with BM32 more effective and convenient. Furthermore, the immunological analysis indicates that BM32 may be very useful for preventive AIT.
Data sharing
All original data, the study protocol and methods for analysis are available upon reasonable request after approval of a proposal with a signed data access agreement.
Declaration of Competing Interest
RV has received research grants from Biomay AG and Viravaxx, Vienna, Austria and serves as a consultant for these companies. He is also recipient of a Megagrant of the Government of the Russian Federation, grant No 14.W03.31.0024 (Project title: “From the immune recognition of the major birch pollen allergen Bet v 1 towards specific diagnostic, therapeutic and preventive strategies for birch pollen-associated allergy"). The other authors declare that they have no conflicts of interest.
Funding
This study was supported by grants F4605, F4613 and DK 1248-B13 of the Austrian Science Fund (FWF). Funders did not have any role in role in study design, data collection and data analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2019.11.006.
Appendix. Supplementary materials
References
- 1.Valenta R., Karaulov A., Niederberger V., Gattinger P., van Hage M., Flicker S. Molecular aspects of allergens and allergy. Adv Immunol. 2018;138:195–256. doi: 10.1016/bs.ai.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Pawankar R, Sánchez-Borges M, Bonini S, Kaliner MA. Chapter 2. The burden of allergic diseases. In: World Allergy Organization (WAO) White Book on Allergy: Update 2013. Milwaukee, WI: World Allergy Organization (2013) 27-32
- 3.Anto J.M., Bousquet J., Akdis M., Auffray C., Keil T., Momas I. Mechanisms of the development of allergy (MeDALL): introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139(2):388–399. doi: 10.1016/j.jaci.2016.12.940. [DOI] [PubMed] [Google Scholar]
- 4.Siroux V., Boudier A., Nadif R., Lupinek C., Valenta R., Bousquet J. Association between asthma, rhinitis, and conjunctivitis multimorbidities with molecular IgE sensitization in adults. Allergy. 2019;74(4):824–827. doi: 10.1111/all.13676. [DOI] [PubMed] [Google Scholar]
- 5.Elisyutina O., Fedenko E., Campana R., Litovkina A., Ilina N., Kudlay D. Bet v 1-specific IgE levels and PR-10 reactivity discriminate silent sensitization from phenotypes of birch allergy. Allergy. 2019 doi: 10.1111/all.13931. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickman M., Lupinek C., Andersson N., Belgrave D., Asarnoj A., Benet M. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. 2017;26:91–99. doi: 10.1016/j.ebiom.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posa D., Perna S., Resch Y., Lupinek C., Panetta V., Hofmaier S. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139(2) doi: 10.1016/j.jaci.2016.08.014. 541-9 e8. [DOI] [PubMed] [Google Scholar]
- 8.Larche M., Akdis C.A., Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6(10):761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 9.Durham S.R., Walker S.M., Varga E.M., Jacobson M.R., O'Brien F., Noble W. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen L., Niggemann B., Dreborg S., Ferdousi H.A., Halken S., Host A. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the Pat study. Allergy. 2007;62(8):943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 11.Incorvaia C., Gritti B.L., Ridolo E. The economic advantage of allergen immunotherapy over drug treatment in respiratory allergy. J Med Econ. 2018;21(6):553–555. doi: 10.1080/13696998.2018.1423567. [DOI] [PubMed] [Google Scholar]
- 12.Shamji M.H., Durham S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140(6):1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Shamji M.H., Kappen J.H., Akdis M., Jensen-Jarolim E., Knol E.F., Kleine-Tebbe J. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI position paper. Allergy. 2017;72(8):1156–1173. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 14.Incorvaia C. One more step towards a deeper understanding of the mechanisms of allergen immunotherapy. EBioMedicine. 2018;37:34–35. doi: 10.1016/j.ebiom.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckl-Dorna J., Villazala-Merino S., Linhart B., Karaulov A.V., Zhernov Y., Khaitov M. Allergen-Specific antibodies regulate secondary allergen-specific immune responses. Front Immunol. 2018;9:3131. doi: 10.3389/fimmu.2018.03131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenta R., Karaulov A., Niederberger V., Zhernov Y., Elisyutina O., Campana R. Allergen extracts for in vivo diagnosis and treatment of allergy: is there a future? J Allergy Clin Immunol Pract. 2018;6(6) doi: 10.1016/j.jaip.2018.08.032. 1845-1855.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K.W., Zieglmayer P., Zieglmayer R., Lemell P., Horak F., Bunu C.P. Selection of house dust mite-allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2019;143(3) doi: 10.1016/j.jaci.2018.10.048. 1248-52 e12. [DOI] [PubMed] [Google Scholar]
- 18.Curin M., Khaitov M., Karaulov A., Namazova-Baranova L., Campana R., Garib V. Next-Generation of allergen-specific immunotherapies: molecular approaches. Curr Allergy Asthma Rep. 2018;18(7):39. doi: 10.1007/s11882-018-0790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhernov Y., Curin M., Khaitov M., Karaulov A., Valenta R. Recombinant allergens for immunotherapy: state of the art. Curr Opin Allergy Clin Immunol. 2019;19(4):402–414. doi: 10.1097/ACI.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Focke-Tejkl M., Weber M., Niespodziana K., Neubauer A., Huber H., Henning R. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135(5):1207. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zieglmayer P., Focke-Tejkl M., Schmutz R., Lemell P., Zieglmayer R., Weber M. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBio Medicine. 2016;11:43–57. doi: 10.1016/j.ebiom.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelius C., Schoneweis K., Georgi F., Weber M., Niederberger V., Zieglmayer P. The recombinant B cell epitope-based grass pollen vaccine BM32 induces antibodies protecting against hepatitis B virus (HBV) infection. Allergy. 2017;72:370–371. [Google Scholar]
- 23.Gangl K., Niederberger V., Valenta R. Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin Exp Allergy. 2013;43(11):1202–1216. doi: 10.1111/cea.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valenta R., Campana R., Niederberger V. Recombinant allergy vaccines based on allergen-derived B cell epitopes. Immunol Lett. 2017;189:19–26. doi: 10.1016/j.imlet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenta R., Campana R., Focke-Tejkl M., Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137(2):351–357. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber M., Niespodziana K., Linhart B., Neubauer A., Huber H., Henning R. Comparison of the immunogenicity of BM32, a recombinant hypoallergenic B cell epitope-based grass pollen allergy vaccine with allergen extract-based vaccines. J Allergy Clin Immunol. 2017;140(5):1433–1436. doi: 10.1016/j.jaci.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niederberger V., Marth K., Eckl-Dorna J., Focke-Tejkl M., Weber M., Hemmer W. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136(4):1101–1103. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niederberger V., Neubauer A., Gevaert P., Zidarn M., Worm M., Aberer W. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J Allergy Clin Immunol. 2018;142(2):497–509. doi: 10.1016/j.jaci.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campana R., Moritz K., Marth K., Neubauer A., Huber H., Henning R. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J Allergy Clin Immunol. 2016;137(2):601–609. doi: 10.1016/j.jaci.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selb R., Eckl-Dorna J., Neunkirchner A., Schmetterer K., Marth K., Gamper J. CD23 surface density on B cells is associated with IgE levels and determines IgE-facilitated allergen uptake, as well as activation of allergen-specific t cells. J Allergy Clin Immunol. 2017;139(1) doi: 10.1016/j.jaci.2016.03.042. 290-9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durham S.R., Yang W.H., Pedersen M.R., Johansen N., Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(4):802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 32.Francis J.N., James L.K., Paraskevopoulos G., Wong C., Calderon M.A., Durham S.R., Till S.J. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121(5) doi: 10.1016/j.jaci.2008.01.072. 1120-1125.e2. [DOI] [PubMed] [Google Scholar]
- 33.Rauber M.M., Möbs C., Campana R., Henning R., Schulze-Dasbeck M., Greene B. Allergen immunotherapy with the hypoallergenic B cell epitope-based vaccine BM32 modifies IL-10- and IL-5-secreting T cells. Allergy. 2019 doi: 10.1111/all.13996. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Mösges R., Koch A.F., Raskopf E., Singh J., Shah-Hosseini K., Astvatsatourov A. Lolium perenne peptide immunotherapy is well tolerated and elicits a protective B-cell response in seasonal allergic rhinitis patients. Allergy. 2018;73(6):1254–1262. doi: 10.1111/all.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleich G.J., Zimmermann E.M., Henderson L.L., Yunginger J.W. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70(4):261–271. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 36.Niederberger V., Horak F., Vrtala S., Spitzauer S., Krauth M.T., Valent P. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spertini F., DellaCorte G., Kettner A., de Blay F., Jacobsen L., Jutel M., Worm M., Charlon V., Reymond C. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase IIb study. J Allergy Clin Immunol. 2016;138(1):162–168. doi: 10.1016/j.jaci.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 38.Henderson L.L., Larson J.B., Gleich G.J. Maximal rise in IgE antibody following ragweed pollination season. J Allergy Clin Immunol. 1975;55(1):10–15. doi: 10.1016/s0091-6749(75)80003-0. [DOI] [PubMed] [Google Scholar]
- 39.Niederberger V., Ring J., Rakoski J., Jager S., Spitzauer S., Valent P. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int Arch Allergy Immunol. 2007;142(2):133–144. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- 40.Cupps T.R., Gerin J.L., Purcell R.H., Goldsmith P.K., Fauci A.S. In vitro antigen-induced antibody responses to hepatitis B surface antigen in man. J Clin Invest. 1984;74(4):1204–1213. doi: 10.1172/JCI111529. Kinetic and cellular requirements. Cupps TR, Gerin JL, Purcell RH, Goldsmith PK, Fauci AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marth K., Breyer I., Focke-Tejkl M., Blatt K., Shamji M.H., Layhadi J. A nonallergenic birch pollen allergy vaccine consisting of hepatitis Pres-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013;190(7):3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morell A., Terry W.D. Waldmann TA. Metabolic properties of IgE subclasses in man. J Clin Invest. 1970;49(4):673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidarsson G., Dekkers G., Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matricardi P.M. Allergen-specific immunoprophylaxis: toward secondary prevention of allergic rhinitis? Pediatr Allergy Immunol. 2014;25(1):15–18. doi: 10.1111/pai.12200. [DOI] [PubMed] [Google Scholar]
- 45.Szépfalusi Z., Bannert C., Ronceray L., Mayer E., Hassler M., Wissmann E. Preventive sublingual immunotherapy in preschool children: first evidence for safety and pro-tolerogenic effects. Pediatr Allergy Immunol. 2014;25(8):788–795. doi: 10.1111/pai.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kündig T.M., Senti G., Schnetzler G., Wolf C., Prinz Vavricka B.M., Fulurija A. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006;117(6):1470–1476. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Campana R., Marth K., Zieglmayer P., Weber M., Lupinek C., Zhernov Y. Vaccination of nonallergic individuals with recombinant hypoallergenic fragments of birch pollen allergen Bet v 1: safety, effects, and mechanisms. J Allergy Clin Immunol. 2019;143(3):1258–1261. doi: 10.1016/j.jaci.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valenta R., Campana R., Marth K., van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272(2):144–157. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupinek C., Hochwallner H., Johansson C., Mie A., Rigler E., Scheynius A. Maternal allergen-specific IgG might protect the child against allergic sensitization. J Allergy Clin Immunol. 2019;144(2):536–548. doi: 10.1016/j.jaci.2018.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mösges R., Kasche E.M., Raskopf E., Singh J., Sohlich L., Astvatsatourov A. A randomized, double-blind, placebo-controlled, dose-finding trial with Lolium perenne peptide immunotherapy. Allergy. 2018;73(4):896–904. doi: 10.1111/all.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.