Abstract
The consequences of neonatal white matter injury are devastating and represent a major societal problem as currently there is no cure. Prematurity, low weight birth and maternal pre-natal infection are the most frequent causes of acquired myelin deficiency in the human neonate leading to cerebral palsy and cognitive impairment. In the developing brain, oligodendrocyte (OL) maturation occurs perinatally, and immature OLs are particularly vulnerable. Cell replacement therapy is often considered a viable option to replace progenitors that die due to glutamate excitotoxicity. We previously reported directed specification and mobilization of endogenous committed and uncommitted neural progenitors by the combination of transferrin and insulin growth factor 1 (TSC1). Here, considering cell replacement and integration as therapeutic goals, we examined if OL progenitors (OLPs) grafted into the brain parenchyma of mice that were subjected to an excitotoxic insult could rescue white matter injury. For that purpose, we used a well-established model of glutamate excitotoxic injury. Four-day-old mice received a single intraparenchymal injection of the glutamate receptor agonist N-methyl-D-aspartate alone or in conjunction with TSC1 in the presence or absence of OLPs grafted into the brain parenchyma. Energetics and expression of stress proteins and OL developmental specific markers were examined. A comparison of the proteomic profile per treatment was also ascertained. We found that OLPs did not survive in the excitotoxic environment when grafted alone. In contrast, when combined with TSC1, survival and integration of grafted OLPs was observed. Further, energy metabolism in OLPs was significantly increased by N-methyl-D-aspartate and modulated by TSC1. The proteomic profile after the various treatments showed elevated ubiquitination and stress/heat shock protein 90 in response to N-methyl-D-aspartate. These changes were reversed in the presence of TSC1 and ubiquitination was decreased. The results obtained in this pre-clinical study indicate that the use of a combinatorial intervention including both trophic support and healthy OLPs constitutes a promising approach for long-term survival and successful graft integration. We established optimal conditioning of the host brain environment to promote long-term survival and integration of grafted OLPs into an inflamed neonate host brain. Experimental procedures were performed under the United States Public Health Service Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee at (UCLA) (ARC #1992-034-61) on July 1, 2010.
Keywords: myelin regeneration, myelination, oligodendrocytes, periventricular leukomalacia, premature birth, proteomics, trophic factors, white matter injury
Chinese Library Classification No. R456; R364; R741
Introduction
The central nervous system (CNS) communicates via transmission of electrical signals. The speed of these signals is essential for normal motor, sensory, and integrative functions. The myelin membrane is an essential component underlying this process, by insulating axons and allowing for rapid electrical transmission of these signals (Nave, 2010). Oligodendrocytes (OLs) are the cells responsible for producing and maintaining myelin in the brain. In the adult CNS, damage to or loss of neurons, axons, and OLs causes demyelination, which leads to impaired neurological function and disability (Blumenthal, 2004). The deleterious effects of myelin loss are seen in a number of inherited and acquired myelin disorders (Duncan and Radcliff, 2016). Mutations in myelin genes, known as leukodystrophies, have been implicated in several developmental human diseases, e.g., Pelizaeus-Merzbacher disease.
The two most common causes of neonatal brain injury are extreme prematurity and hypoxic ischemic encephalopathy. In the USA (du Plessis and Volpe 2002; Blumenthal, 2004), one in 8 babies is born before term (37–40 weeks), and 1.44% of babies (56,000 per year) are born with a birth weight of 1250 grams or less (Hamilton et al., 2013). These small, preterm babies are at high risk of death or neurodevelopmental impairment: approximately 20% die before hospital discharge, and 40% of survivors develop long-term intellectual or physical impairment, including cerebral palsy (O’Shea et al., 2009; Stoll et al., 2010; Juul and Ferriero, 2014). In the USA, care of preterm infants accounts for more than half of pediatric health care expenses. Premature infants represent a population vulnerable to white matter (WM) loss, and the size of this population is continuously increasing. Advances in perinatal care have led to a significant improvement in the survival of very premature (< 30 weeks gestational age) infants (Rees and Inder, 2005). From the data available in certain developed countries, the preterm birth rate has increased substantially in the past twenty years, and it continues to rise. In 2005, there were approximately 12.9 million preterm infants born globally, with about 90% of them surviving past infancy (Khwaja and Volpe, 2008; Beck et al., 2010). Of these survivors, up to a quarter develop cerebral palsy, and 25–50% have cognitive disabilities and developmental deficits (Back et al., 2007). These impairments are largely due to the developmental state of the premature infant’s cerebral WM. Using a myelin deficient rat mutant we previously showed that OLPs grafted into their brains migrate, integrate and reach naked axons to myelinate them (Espinosa-Jeffrey et al., 1992, 1993, 1996, 1997). Other researchers have also used mutant rodents extensively with the same purpose (Duncan and Radcliff, 2016). These mutant animal models have facilitated the evaluation of graft performance and host-graft interaction in a non-inflammatory microenvironment. In contrast, transplantation of healthy OLPs into a hostile microenvironment (i.e., inflammation in the host brain) still presents challenges.
Because the cerebral WM is being formed in premature infants, under low cerebral blood flow, it is susceptible to hypoxia-ischemia. This leads to periventricular leukomalacia, a common and severe form of cerebral WM injury (WMI) (Deng, 2010). Hypoxia-ischemia causes increased levels of extracellular glutamate, which in turn leads to free reactive oxygen species. Pre-OLs are vulnerable to free radical insult, leading to their death (Khwaja and Volpe, 2008). The number of glutamate N-methyl-D-aspartate (NMDA) receptors is involved in WMI together with elevated levels of extracellular glutamate that could be caused by insufficient glutamate transporters and glutamate-induced excitotoxicity (Mohammad-Gharibani et al., 2014; Tovar-y-Romero et al., 2014). Accordingly, animal models of excitotoxicity have been generated through the use of NMDA injections (Felt et al., 2002). The activation of NMDA receptors increases intracellular calcium, pro-apoptotic pathways via caspase-3 activation, free radical formation and lipid peroxidation resulting in profound and widespread injury to the developing brain (Juul and Ferreiro, 2014).
Currently, there is no cure for WMI so affected individuals will undergo life-long damaging effects of myelin loss including ventriculomegaly and impaired neurological function. Two promising therapeutic approaches include: 1) OLP transplants to provide the brain with healthy OLPs for myelination. Indeed, past studies have attempted to graft OLPs after glutamate excitotoxicity (GME) (Porambo et al., 2015). Grafted cells have been proven to survive in comparable numbers to those in control mice several weeks post-grafting. Survival and migration of grafted OLPs was extremely reduced, yet behavioral scores, myelination and neuropathological outcomes were improved, most likely due to early trophic effects of grafted cells onto host cells (Porambo et al., 2015) and 2) The introduction of additional trophic factors after GME. For example, in a mouse model of excitotoxicity, we have shown that TSC1 (a combination of transferrin and insulin growth factor 1 (IGF1) stimulates both, endogenous neural stem cells and OLP lineage progression and maturation. TSC1 promoted increased OL proliferation and migration into the corpus callosum (CC) and striatum, thereby regenerating myelin and reducing ventricular size (Espinosa-Jeffrey et al., 2013a, 2016b). In the current study, we examined OLP behavior after grafting. We hypothesized that the concurrent injection of TSC1 and OLPs would confer long-term neuroprotection to grafted OLPs.
Materials and Methods
Cells and culture system
The central glial 4 (CG4) cell line was originally derived by Varon and collaborators (Louis et al., 1992) and generously distributed around the world. Dr. T. Ogata and his group at the National Rehabilitation Center (Downey, CA, USA) undertook the task of cloning CG4 cells and characterized several lines. The newly generated line of their choice to continue studying OL properties was the clone CG4-16 OLPs (Ueno et al., 2012). For the purpose of this paper we will refer to CG4-OLPs or OLPs and it should be understood that we are referring to the CG4-16 line. These cells were maintained in our previously described (Espinosa-Jeffrey et al., 2001) GDM medium (glia defined medium containing Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12-containing 5 μg/mL insulin, 16.1 μg/mL putrescine, 50 μg/mL apo-transferrin, 4.6 μg/mL d-galactose, and 8 ng/mL sodium selenite). To promote differentiation in cell culture we usually switch to oligodendrocyte defined medium composed of DMEM and Ham’s F12-containing 0.5% FCS, 5 μg/mL insulin, 16.1 μg/mL putrescine, 4.6 μg/mL d-galactose, and 8 ng/mL sodium selenite (Espinosa-Jeffrey et al., 2002, 2016b). For cell grafting, cells were seeded onto A2B5 antibody coated dishes for soft and gentle detachment of donor OLPs, and for phenotype assessments cells were seeded onto poly-D-lysine-coated coverslips in 24-well plates.
To measure energy metabolism in OLPs after acute treatment of NMDA or NMDA with TSC1 we used human embryonic brain-derived OLPs prepared as previously described (Espinosa-Jeffrey et al., 2013b) and using our OL chemically defined medium (Espinosa-Jeffrey et al., 2016a). Fetal human tissue specimens (E17 weeks) were donated by the Department of Pathology and Laboratory of Medicine at the University of California, Los Angeles (UCLA) (http://pathology.ucla.edu/default.cfm? id=1). Samples were de-identified in accordance with the National Institutes of Health guidelines. These anonymous, pre-shelved specimens are donated for medical research purposes and are Institutional Review Board (IRB) exempt (NIH Exemption 4).
Animals and intraparenchymal injection procedures
Experimental procedures were performed under the United States Public Health Service Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee at (UCLA) (ARC #1992-034-61) on July 1, 2010. All efforts were made to minimize suffering and the number of animals used. The nestin- green fluorescent protein (GFP) transgenic mice (Yamaguchi et al., 2000) were bred at the University of California Los Angeles (UCLA, Los Angeles, CA, USA) in a restricted access, temperature-controlled vivarium under a 12:12 hour light-dark cycle, with access to food and water ad libitum. Postnatal day 4 (P4) nestin-GFP mouse pups (male and female, ~3.5 g body weight) were used. Experiments were performed to compare the neuroprotective effects of TSC1 on grafted OLPs in mice receiving NMDA as the excitotoxic agent. For effects of myelination these experiments consisted of one time point 35 days post-injection (PI) and six conditions: A total of 36 nestin-GFP transgenic mice (male and female, ~19–25 g body weight) were used for each experiment, six per group: Non-treated, saline, NMDA, NMDA + CG4, NMDA + TSC1, NMDA + TSC1 + CG4. A separate group (n = 5) was used to monitor graft survival 1 week post-grafting.
Induction of GME
We induced excitotoxicity in nestin-GFP transgenic mice through the stereotaxic injection of NMDA (Millipore, Sigma, Burlington, MA, USA) (5 μg in 1.5 μL sterile Hanks solution) into the CC. A single unilateral injection was performed on P4 mice as previously described (Espinosa-Jeffrey et al., 2006). We chose P4 mice as at this age, in contrast to adults, the WM is more susceptible to excitotoxic injury. Indeed, studies suggest that WM vulnerability in rodents at P4 better reflects WM vulnerability in pre-term infants (Choi et al., 2011). In addition, as we showed previously, simultaneous injection of NMDA with trophic factors, as opposed to sequential injection, produces optimal protection and survival of grafted cells (Espinosa-Jeffrey et al., 2013a, 2016b). The stereotaxic intraparenchymal injections of saline, NMDA, NMDA + TSC1, NMDA + CG4 or NMDA + TSC1 + CG4 into the host brain were performed as described (Espinosa-Jeffrey et al., 2013a). Briefly, P4 nestin-GFP transgenic mouse pups were injected as follows: Ophthalmology microsurgery instruments and a stereotaxic apparatus with an adjustable adapter for small (newborn) animals were used. The animals were anesthetized by inhalation of isofluorane gas (4–5 % for induction and 1–2% for maintenance) and placed on a stereotaxic frame for the intraparenchymal administration of the cells with a Hamilton syringe. The injection coordinates were 1.2 mm lateral (right) and 0.7 mm caudal to Bregma (Szulc et al., 2015). The needle was inserted 2.5 mm deep into the CC. The control group consisted of nestin-expressing mice that received saline injections, using the same procedures. We performed unilateral grafts. The total volume of the injected factors was 1.5 μL.
Preparation of trophic factors
Stock solutions were prepared in phosphate buffer (PBS) containing IGF-1 50 ng/mL, and apo-Tf 100 mg/mL and frozen in small aliquots. The final concentrations were prepared in Hanks solution: 100 ng IGF-1, and 15 μg apo-Tf per pup (Espinosa-Jeffrey et al., 2006).
Injections of CG4 cells and trophic factors
All the injections were performed as previously described (Espinosa-Jeffrey et al., 2013a; Juul and Ferreiro, 2014). The coordinates for the implant sites were the same as indicated above. The charcoal with which the tip of the needle was labeled prior to performing the injection was used to determine the implant site. The CG4 cells were pre-labeled with the fluorescent dye fast blue (FB) that has proven to be effective in showing cell migration within the host parenchyma even after several months (Espinosa-Jeffrey et al., 1992).
Collection and examination of samples
Brain collection and sectioning for the characterization of cell phenotypes was performed as previously described (Espinosa-Jeffrey et al., 2006). We examined samples at 7 days, to ascertain successful grafting, and at 35 days after treatment (40 days of age) because it allows ascertaining the myelinogenic potential of cells in an excitotoxic environment. To assess the effectiveness of the treatment, OLs and myelin were determined through the expression of cyclic nucleotide 3′-phosphohydrolase (CNPase). Cellular stress was monitored through the expression of the stress inducible heat shock protein 90 (HSP90-alpha). We used six mice per condition. Three separate experiments were performed to assess the effects of NMDA alone or with TSC1 on endogenous OLP survival, proliferation and maturation. Intraparenchymal injections were performed according to our previously described methods (Espinosa-Jeffrey et al., 2006, 2013a).
Double immunocytochemistry
Briefly, cultures were fixed with 4% paraformaldehyde. Samples were blocked for 1 h in 1% BSA (Sigma-Aldrich), 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), and 10% normal goat or donkey serum in PBS. HSP90-alpha mouse monoclonal antibody (MA5-32057, 1:200 dilution, ThermoFisher Scientific, Waltham, MA USA); CNPase rabbit polyclonal antibody (100-401-D21, 1:50 dilution, Rockland Immunochemicals Inc., Limerick, PA, USA). Primary antibodies were diluted in carrier solution (1% BSA and 0.3% Triton X-100 in PBS) and incubated overnight at 4°C. After washing with PBS, secondary antibodies (anti-rabbit Texas red, 1:800 dilution; anti-mouse IgG Alexa Fluor 488, 1:1000 dilution; and anti-mouse IgM Alexa Fluor 633 ABCAM, 1:1000 dilution) were incubated for 1 hour at room temperature, washed with TBS and mounted. Samples were imaged using the LSM 510 META confocal microscope (Zeiss, Jena, Germany) and analyzed with the Axiovision software (Zeiss).
Cellular bioenergetics
OLPs were seeded onto an XF24 Cell Culture Microplate (Seahorse Bioscience, Agilent, Santa Clara, CA, USA) at 5 × 104 cells/well in glial defined medium supplemented with transferrin and IGF-1, namely TS1, in order to obtain post-mitotic OLs. Cells were kept 24 hours in standard culture conditions (37°C, 4.5% CO2). The following day, some of the supernatant was removed from the well plates. We used an XF24 Analyzer (Agilent) to measure oxygen consumption rate and extracellular acidification rate. Oxygen consumption rate and extracellular acidification rate reflect rates of cellular respiration and glycolysis, respectively. Measurements were performed with the cells in unbuffered DMEM assay medium supplemented with 1 mM pyruvate, 2 mM glutamine, 3.1 g/L glucose, and 4.6 g/L galactose. Measures were recorded before (basal reading) and after the sequential injections of oligomycin, carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone and rotenone/myxothiazol. Mitochondrial respiration was calculated by subtracting the rotenone/myxothiazol-induced respiration. Coupled (ATP-linked) respiration indicated the oligomycin-sensitive respiration. Maximal extracellular acidification rate was the response to oligomycin. Values were normalized to total protein determined with the Protein Assay reagent (Bio-Rad, Hercules, CA, USA) in all experiments.
Proteomics
Cryostat brain sagittal sections were immersed in PBS followed by a quick wash with double distilled water. The protocol for sample preparation (protein extraction) was performed using Liquid Tissue MS protein prep kit that allows for the detection of proteins and peptides in tissues that have been fixed with paraformaldehyde. Using 27 μm frozen brain parasagittal sections containing the brain tissue without the cerebellum, the amount of total protein was determined using the micro BSA protein assay method of Markaryan et al. (2010). We obtained 50 μg/mL of protein per brain section. The protein was extracted from the tissue using the Liquid Tissue MS protein prep kit (from Expression Pathology). The proteomic analysis was performed on the extracted tryptic peptide samples fractionated on a HPLC column, and analyzed with a MALDI-TOF and TOF/TOF tandem MS/MS (AB SCIEX TOF/TOF™ 5800 System (AB SCIEX, Framingham, MA, USA). All experiments were run in duplicate.
For the categorization of proteins and pathway analysis, we used the public bioinformatics tool available from the NIH called the Database for Annotation, Visualization, and Integrated Discovery (DAVID; http://www.david.niaid.nih.gov, Huang et al., 2009a, b). The classification of proteins was not pre-set but generated by the analysis program. In detail, all the proteins entered into the analysis were first annotated into 40 annotation categories, including GO (a gene-ontology tool) terms, protein-protein interactions, protein functional domains, disease associations, bio-pathways, sequence general features, homologies, gene functional summaries, gene tissue expression, and others found in the literature. The analysis software uses a novel algorithm to measure the relationships among the annotation terms based on the degrees of their co-association genes to group the similar, redundant, and heterogeneous annotation contents from the same or different resources into annotation groups. The functional annotation chart was used to search for significantly enriched gene ontology categories. The mass spectroscopy data analysis included protein-peptide information to then identify the proteins in high confidence or low confidence. Only those proteins that were found in 95% confidence interval or higher in relation to the total ion score and strength as determined by the MASCOT software (Matrix Science Inc. Boston, MA, USA) were identified.
Statistical analysis
For statistical tests we used VassarStats, a web site for statistical computation. Quantitative data are expressed as the means ± SE. Comparison of mean values between multiple groups was evaluated using one-way ANOVA followed by a post hoc Tukey HSD multiple comparison test where data obtained with the various treatments were compared either to their respective saline control or non-treated samples within the same group. Significance was assumed when P < 0.05. For the pathway analysis we used the highest stringency P-value < 0.05 as the cutoff and Bonferroni post-hoc analysis.
Results
Trophic support is essential for graft survival
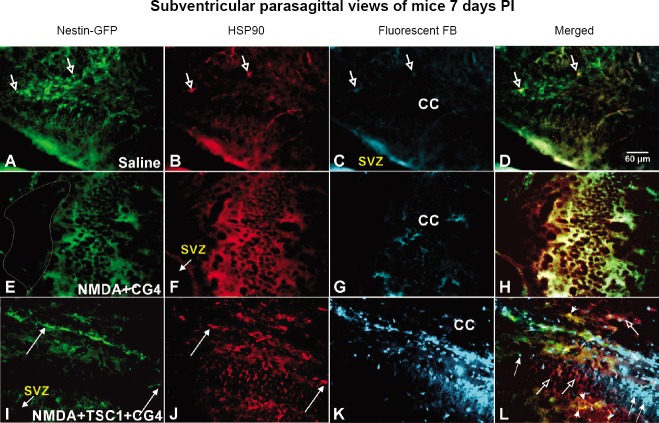
At 35 PI, a total of 36 GFP-nestin-expressing mice were used per experiment, six per group: Non-treated, Saline, NMDA, NMDA + CG4, NMDA + TSC1, NMDA + TSC1 + CG4 all mice survived. To determine graft survival, examination of the brains was performed in a separate group at 7 days PI (n = 5 mice) to assess the survival and interaction of the graft with host cells. We examined the condition of the brain parenchyma of mice after the different treatments. Saline injection did not disturb the presence and integrity of nestin-expressing cells nor the cytoarchitecture of the host parenchyma which was well preserved (Figure 1A). We also determined the expression of HSP90 in these mice and found virtually no expression of this stress marker (Figure 1B). Since there were no cells injected, the brain parenchyma did not show FB label (Figure 1C). The view of the merged frames of the same field illustrates the presence of just two host nestin-labeled neural stem cells co-expressing HSP90 (Figure 1D). The parenchyma of mice receiving NMDA + CG4 cells was very different; it looked unhealthy and deprived of small nestin-expressing cells. Instead, flat-shaped cells appeared to express nestin. Cavitation was observed in mice that did not receive TSC1 injections (Figure 1E). The same cells were intensely labeled by HSP90 (Figure 1F). To our surprise, we did not find any FB-labeled cells (Figure 1G). The merged image confirms the colocalization of HSP90 and nestin (Figure 1H). In contrast, mice that received the combined therapy of TSC1 + CG4 cells displayed small nestin-GFP positive cells (Figure 1I). Interestingly, HSP90 was extensively expressed in the host parenchyma, mainly in small cells (Figure 1J). Moreover, some regions of the brain had no immunoreactivity (Figure 1J). Successful survival of grafted cells was observed and numerous very intensely FB-labeled cells were present (Figure 1K). Careful examination of these brain sections revealed that FB-positive cells did not express HSP90 indicating that they had not suffered from the acute excitotoxic insult as can be observed in Figure 1L.
Figure 1.
Representative views of the SVZ and the floor of the CC 7 days post-injection of the various treatments.
(A) The expression of nestin by host NSCs was not affected by the saline injection. (B) Very few cells co-expressed HSP90 as shown by the arrows. (C) We know that they are host cells because they do not show the FB label. (D) The merged picture of the same field confirms the co-localization of nestin and HSP90. (E) The brain tissue of mice that received NMDA and CG4 cells did not display healthy GFP-nestin-expressing cells; some flat cells were the only ones visible and the brain tissue displayed sponge-like texture. The region delineated by the white shape shows where tissue was missing. (F) The flat layer of cells in the brain parenchyma intensely expressed the inducible form of HSP90. (G) There were not FB-labeled cells in this region. (H) The merged picture shows the presence of nestin-expressing flat cells co-labeled with HSP90 in an almost one to one basis. (I) The brains of mice that were treated with the combinatorial approach (cells and TSC1) displayed nestin-expressing cells in the vicinity of the implant site although in reduced numbers when compared with saline-treated mice. (J) Some nestin positive cells appeared to express HSP90. Nonetheless, both host nestin-negative cells of flat appearance and nestin-positive cells predominantly expressed HSP90. (K) The survival of the graft could be appreciated, as numerous intensely FB-labeled cells were present in the host parenchyma. (L) Merging the views of the same field shows some nestin/HSP90-positive flat host cells (open arrows), many FB-labeled/HSP90-negative grafted cells (thin arrows)) and very few small nestin-positive cells co-expressing HSP90 (arrowheads). CC: Corpus callosum; CG4: central glial 4 cells; FB: Fast Blue; GFP: green fluorescent protein; HSP90: heat shock protein 90; NMDA: N-methyl-D-aspartate; NSCs: neural stem cells; PI: post-injection; SVZ: subventricular zone; TSC1: the combination of transferrin and insulin growth factor 1.
CG4-16-OLPs survive when grafted with a cocktail of neurotrophic factors
At P35 PI, further characterization on the effect of the treatments showed that in the presence of NMDA alone the blood vessels had become auto-fluorescent. No nestin-expressing cells were visible in regions close to the stereotaxic NMDA injection (Figure 2A). The CC was devoid of CNPase staining (Figure 2B), these features are more visible in the merged picture (Figure 2C). TSC1 attenuated the effects of NMDA at various levels; the blood vessels were not auto-fluorescent; small cells expressing nestin-GFP at various intensities were visible in the region and in particular in the floor of the CC (Figure 2D). CNPase immunostaining was present as a diffuse label in the CC while single CNPase-expressing cells were found in the sub-ventricular zone (SVZ) (Figure 2E). The merged image allows for a better definition and visualization of nestin and CNPase expression that clearly labeled two distinct populations (nestin-expressing neural progenitors and CNPase-labeled OLs) as expected (Figure 2F). When NMDA was co-injected with CG4-16-OLPs the presence of auto-fluorescent blood vessels decreased, some were present but not as many as when NMDA was injected alone (Figure 2G). Moreover, there was no CNPase staining either from host or graft OLs (Figure 2H). The merged image (Figure 2I) illustrates the auto-fluorescence and grafted cells that were still present 35 days after grafting (arrowheads). Some FB-labeled small cells were observed and a few fibrous flat cells also appeared to be FB labeled. CG4 grafted cells were not numerous 35 days after having been co-injected with NMDA (Figure 2J). Interestingly, when CG4-16 cells were grafted in the presence of both NMDA and TSC1, two subpopulations expressing nestin were found. One comprised of flat and fibrous-like cells in the CC and also small cells expressing nestin (arrows) and small round ones (arrowheads) (Figure 2K). The same pattern was observed with the CNPase label, the two subpopulations were present (Figure 2L). The merged images delineate the cell somas of grafted cells across the CC (Figure 2M). In terms of cells bearing the FB label (thus grafted cells), the small cells were numerous in the floor of the CC distributed in rows and other cells were bipolar perpendicular to the ventricular wall as if they were migrating (Figure 2N). Figure 2O shows diagram of sagittal view or mouse brain, the rectangle indicates the area where the images were taken.
Figure 2.
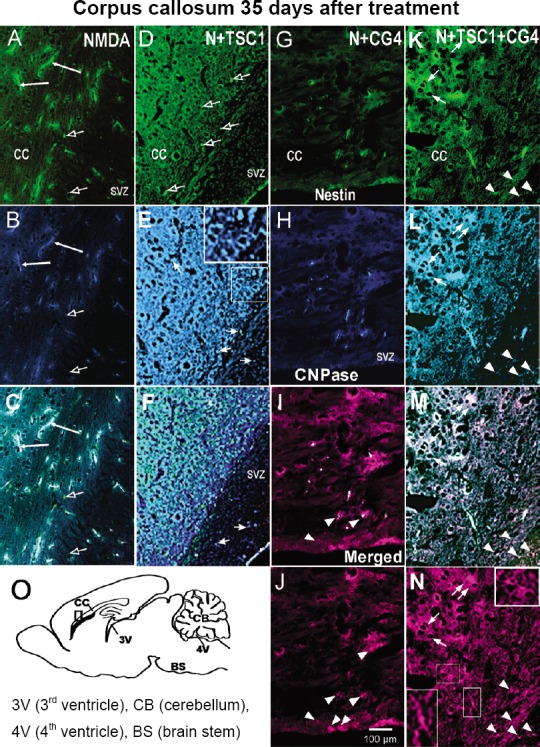
Representative views of the CC 35 days after treatment.
In the presence of NMDA (N) alone there was considerable nestin-expressing cell loss and extreme reaction of blood vessels that auto-fluoresced. Very few cells, most likely pericytes, displayed intense GFP-nestin fluorescence. (B) View of the same field where blood vessels were conspicuously auto-fluorescent, but no cells in the brain parenchyma displayed CNPase labeling. (C) The merged image allows for the visualization of auto-fluorescent blood vessels as well as very few nestin-positive/CNPase-negative cells (open arrows). (D) In animals that received N + TSC1 the CC displayed very few GFP-expressing cells besides two or three of them (open arrows). (E) Blood vessels were not auto-fluorescent and the tissue presented its cytoarchitecture undisturbed, yet only few single cells expressed CNPase in their soma (arrows). Most tissue in the CC showed a diffuse-positive CNPase label. (F). The merged image allows for the visualization of auto-fluorescent blood vessels as well as the nestin-positive/CNPase-negative cells (arrows). (G) When mice received NMDA + CG4 grafts, very few nestin expressing cells were seen in the SVZ and virtually none in the brain parenchyma. (H) The tissue displayed no CNPase immunoreactivity, yet there were significantly less blood vessels revealed by auto-fluorescence. (I) The merged image shows these cells (FB shown in magenta pseudo-color). (J) Not many FB-labeled CG4-OLPs were found in these mice but those present were either small round cells (arrowheads) or flat and more fibrous, reactive-like ones. (K) When mice received NMDA (N) + TSC1 + CG4-OLPs, the parenchyma was better preserved and two subpopulations of nestin expressing cells were present. Flat fibrous reactive-like (arrows) and single small round nestin-GFP (arrowheads). (L) The combined treatment of OLPs and TSC1 showed immunoreactivity for CNPase and the cytoarchitecture of the parenchyma was very well preserved. (M) Numerous grafted FB-labeled CG4 cells survived. From the small round cell subpopulation some expressed CNPase (an OL marker and the FB-label seen in pseudo-color magenta), these cells were nestin negative. (N) Numerous grafted OLPs, the cytoplasmic distribution of CNPase is shown by arrows. Rows of grafted cells are shown by arrowheads. Insets show higher magnification of bipolar or multipolar grafted cells and their cell processes in more detail. (O) Diagramatic representation of a sagittal view of mouse brain indicating a single site of injection and the regions from which pictures were taken. CC: Corpus callosum; CG4: central glial 4 cells; CNPase: cyclic nucleotide 3′-phosphohydrolase; FB: Fast Blue; GFP: green fluorescent protein; NMDA: N-methyl-D-aspartate; OLPs: oligodendrocyte progenitors; SVZ: subventricular zone; TSC1: the combination of transferrin and insulin growth factor 1.
Effects of the combinatorial treatment on the striatum 35 days after treatment
In the presence of NMDA, nestin-expressing progenitors were absent from the vicinity of the injection site, nonetheless pericytes along capillaries were positive for nestin. The blood vessels were auto-fluorescent both in the CC and the striatum (Figure 3A). In this region, there was no CNPase expression and in some areas the tissue was absent (Figure 3B). The merged image allows for the visualization of tissue loss, auto-fluorescence of capillaries and CNPase absence (Figure 3C). In the presence of TSC1, NMDA had a milder effect, the capillaries did not auto-fluoresce and tissue loss was reduced (Figure 3D). Nestin-expressing cells were found in small numbers in the CC and at the junction between CC and striatum. Moreover, CNPase immunostaining revealed numerous small cells in the CC. In the striatum the cells that expressed CNPase were of a fibrous, flatter aspect (Figure 3E). The merged image shows basically the CNPase cellular distribution (Figure 3F). In mice that received the grafts of CG4-OLPs in the presence of NMDA, the blood vessels were not auto-fluorescent, to some extent reminiscent of the effects of TSC1 that also prevents blood vessels auto-fluorescence. Nonetheless, there were practically no nestin-expressing small cells but rather flat cells (Figure 3G) or CNPase expression (Figure 3H). The merged image allows for a better visualization of grafted cells (Figure 3I). Some grafted CG4-OLPs were present in this region labeled with FB (magenta pseudo-color) as shown in (Figure 3J). When CG4-OLPs were grafted in the presence of NMDA and TSC1 there was not auto-fluorescence. Nestin immunoreactivity was present in the SVZ and by the wall of the ventricle (Figure 3K). Small CNPase labeled OLPs were present (Figure 3L). The merged image illustrates the distribution of some grafted cells intermingled with host nestin-expressing cells (Figure 3M). The most remarkable finding was the large number of FB-labeled CG4-OLPs found in the host brain parenchyma mostly as single cells. In the vicinity of the injection site the tissue appeared spongy but there was no visible tissue loss. In this area there were numerous small cells arranged in rows perpendicular to the ventricle (Figure 3N). Examples of areas of tissue loss are delineated in panels B and F. Figure 3O indicates where images were taken.
Figure 3.
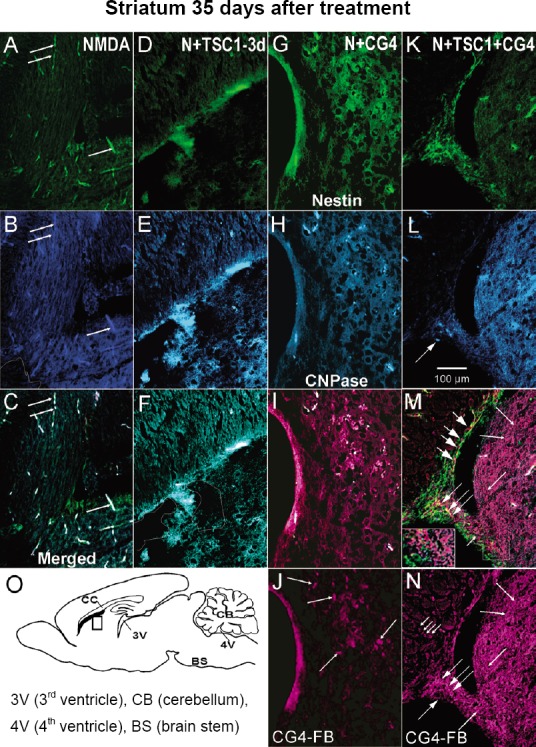
Representative views of the CC and striatum 35 days after treatment.
(A) In the presence of NMDA alone there was considerable nestin-expression loss in the brain parenchyma, just a few nestin-expressing cells were present in the SVZ. Nonetheless, pericytes were nestin-GFP labeled. The blood vessels were auto-fluorescent and the tissue presented a spongy aspect in the striatum, the white outline shows tissue loss. (B) CNPase expression was absent from the white matter regions. (C) The merged image illustrates better the presence of few nestin-expressing cells in the apical portion of the SVZ and the auto-fluorescence of capillaries. The absence of CNPase is clearly appreciated. (D) The injection of NMDA followed by TSC1 3 days after the excitotoxic insult resulted in auto-fluorescence quenching, loss of nestin-expressing cells and some tissue loss in this region. (E) Two kinds of cells expressed CNPase small cells organized as rows in the CC and large flat and fibrous-like cells. (F) The merged image confirms the presence of small nestin-positive cells and some tissue loss has been outlined. (G) When central glial 4 cells-oligodendrocyte progenitors (CG4-OLPs) were injected with NMDA nestin expression was absent in the striatum, and just a few blood vessels were auto-fluorescent. (H) The tissue appears spongy and there is not CNPase expression. (I) The merged picture shows the presence of a few grafted cells and autofluorescent blood vesels. (J) A few FB-labeled grafted cells seen in pseudo-color magenta (arrows) distributed in just one region of the tissue. (K) View of the effects of NMDA with the combinatorial treatment of TSC1 + CG4-OLP grafts where we can appreciate nestin-GFP-expressing cells in the SVZ and the wall of the ventricle. (L) Some small cells expressed CNPase (arrows). (M) The merged picture shows grafted cells intermingled with nestin-labeled host cells (short arrows) or adjacent to the nestin-expressing host cells. This image allows for the visualization of FB+ cells (seen in magenta) arranged in straight and long rows starting from the SVZ towards the striatum brain parenchyma (long arrows with short heads) point to some of these cell rows. At the level of the SVZ FB-grafted cells and nestin-GFP host cells appear to overlap showing spots of yellow fluorescence (medium size arrows). Inset shows in detail FB-labeled cells and host nestin-expressing cells. (N) The striatum was populated by CG4-OLPs FB-labeled (magenta, short headed long arrows). small arrow points to a grafted cell in the SVZ. (O) Diagramatic representation of a sagittal view of mouse brain indicating a single site of injection and the regions from which pictures were taken. CC: Corpus callosum; CG4: central glial 4 cells; CNPase: cyclic nucleotide 3′-phosphohydrolase; FB: Fast Blue; GFP: green fluorescent protein; NMDA: N-methyl-D-aspartate; OLPs: oligodendrocyte progenitors; SVZ: subventricular zone; TSC1: the combination of transferrin and insulin growth factor 1.
Protein expression profiling
The NanoLC-MS/MS approach is powerful due to its high sensitivity and specificity and it allows for the comparison of protein profiles among samples. The protein profile was obtained from the ipsilateral hemisphere and the cerebellum was not included. We considered the high confidence hits (C1 < 95%) and compared the protein profile of each group: Non-treated, NMDA alone, NMDA + CG4, NMDA + TSC1 and NMDA + CG4 + TSC1. We detected different total number of proteins/peptides depending on the treatment, ranging between 3400 and 3730, from which 249 were significant hits (high confidence).
Functional classification of proteins
From our protein list, thirty-two clusters were generated using the highest stringency. We grouped the proteins in 10 categories according to their function; structural, general metabolism, energy metabolism, DNA/RNA processing, stress proteins, proteins involved in the regulation the cell cycle, signal transduction, immune response, OL/myelin specific, and ubiquitination. The proteomic profile changed considerably across treatments. In the non-treated mice, the outstanding points were the absence of stress proteins, higher energy metabolism than mice treated with NMDA alone or NMDA + CG4-OLPs, whereas TSC1 increased energy metabolism alone or when administered in combination with the cell grafts. The percentage of ubiquitination proteins was increased by NMDA and reduced by TSC1 (Figure 4).
Figure 4.
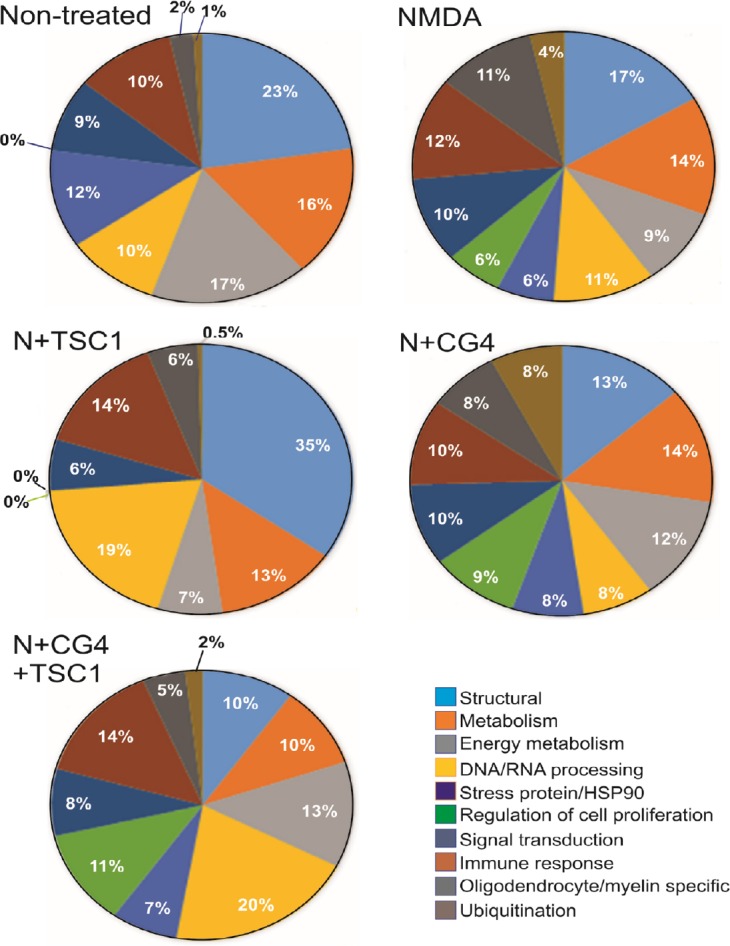
Comparison of the proteomic profile using the functional enrichment score and taking into account significant hits only.
We found that the percentage of the proteins varied considerably across treatments in the brains of non-treated mice and those exposed to the specific treatments: NMDA (N), N + TSC1, N + CG4-OLPs, N + TSC1 + CG4-OLPs, in particular structural proteins, reduction of DNA/RNA and proliferation-related proteins. There was no significant expression of HSP90 in mice treated with N + TSC1 and a reduced percentage was found in mice receiving CG4-OLPs in the presence of NMDA whereas 12% was expressed in non-treated mice, these results include both HSP90 alpha and beta. Ubiquitination fluctuated between virtually 0 and 8% where in animals receiving TSC1 with or without grafted CG4-OLPs the ubiquitination percentage ranged between 0 and 2%. In contrast, mice injected with just NMDA showed 4% ubiquitination and mice injected with NMDA + CG4-OLP grafts reached 8% indicating that CG4-OLPs, derived from rat brain, are more vulnerable to NMDA than mouse OLPs. CG4: Central glial 4 cells; HSP90: heat shock protein 90; NMDA: N-methyl-D-aspartate; OLPs: oligodendrocyte progenitors; TSC1: the combination of transferrin and insulin growth factor 1.
Bioenergetic status of OLPs
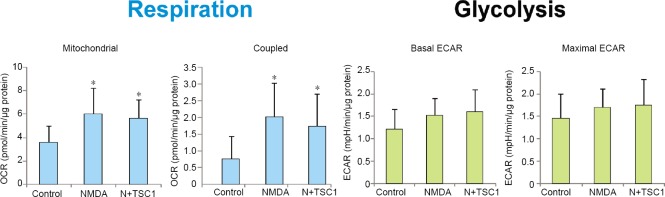
In order to ascertain the changes in energy metabolism produced by NMDA or NMDA with TSC1 on OLPs, we quantified the oxygen consumption rate and extracellular acidification rate as measures of cellular respiration and glycolysis, respectively (Figure 5). OLPs were treated overnight with NMDA or NMDA + TSC1. Total respiration was not changed by NMDA or NMDA + TSC1 treatments (data not shown) but mitochondrial and specifically coupled (ATP-linked) respirations were significantly higher in the treatment groups. It suggested a higher mitochondrial activity driven by NMDA-induced ATP demand after a short treatment, which was not prevented by TSC1 administration. In contrast, glycolysis changes were not significant across treatments with respect to control.
Figure 5.
Analysis of the bioenergetics state of human OLPs alone or treated with NMDA or NMDA + TSC1.
Representative experiment showing that NMDA increased mitochondrial respiration (oxygen consumption rate, OCR) and TSC1 did not modify this effect, indicating that cells have the need to produce more ATP in the presence of NMDA. Interestingly, glycolysis remained unchanged. Values are expressed as mean ± SE. *P < 0.05, vs. control (n = 12, one-way analysis of variance). ECAR: Extracellular acidification rate; NMDA: N-methyl-D-aspartate; OLP: oligodendrocyte progenitor; TSC1: the combination of transferrin and insulin growth factor 1.
Discussion
We demonstrated previously that survival of OLPs is increased when TSC1 and NMDA are injected simultaneously and ventricular enlargement is reduced (Espinosa-Jeffrey et al., 2013a). The present study was designed to examine if OLPs grafted into the brain parenchyma of mice subjected to an excitotoxic insult could rescue WM damage. We tested this hypothesis by implementing a combinatorial intervention of a single TSC1 + CG4 dose administered into the CC. We report survival and integration of CG4 grafts when injected in the presence of TSC1 but not when cells are grafted alone. To further explore the changes produced by the treatments, we specifically ascertained the expression of OL-specific markers in combination with stress protein HSP90. We found that energy metabolism in OLPs was significantly increased by NMDA and modulated by TSC1. We also compared the proteomic profiles produced by the different treatments as cumulative percentage of proteome ranked according to protein abundance. The proteomic profile showed elevated ubiquitination and HSP90 expression in response to NMDA. These changes were reversed in the presence of TSC1 and ubiquitination was decreased. Thus, the use of a combinatorial intervention including both trophic support and healthy OLPs constitutes a promising approach for long-term survival and successful graft integration.
Cell replacement therapies in an excitotoxic environment
We previously showed that TSC1 is neuroprotective for committed and uncommitted neural endogenous progenitors, i.e., nestin-expressing cells and OLPs (Espinosa-Jeffrey et al., 2016b). In the present study, we sought to determine if the results obtained by neuroprotection and mobilization of endogenous OLPs and nestin-expressing progenitors could be enhanced by using a combinatorial approach by grafting healthy CG4-16 OLPs in the presence of TSC1 to provide a favorable microenvironment for grafted cells. Our results demonstrate that conditioning of the microenvironment of the neonate host brain that experiences GME is a key factor for successful survival and integration of the graft in the host parenchyma. In the absence of TSC1, FB-labeled cell survival was very poor. However, in the presence of TSC1, survival, migration and integration of the graft were successful in the CC and the striatum. Other benefits were reduction of tissue loss and extensive myelination of host axons, confirming a previous report (Espinosa-Jeffrey et al., 2013a). The time of administration in a trophic factor-based therapy is critical. Trophic factors are short-lived and therefore they have a short time frame for protection of cells in distress. We have reported that the time of administration of the trophic factor cocktail is crucial and the sooner after the excitotoxic insult the more cells are neuroprotected (Espinosa-Jeffrey et al., 2013a). Trophic factors to address other conditions and diseases have been used mainly with the aim of promoting neuronal survival (Tovar et al., 2014; Wrigley et al., 2017).
Cell-based therapy continues to be a treatment option to mitigate the long-term sequelae of hypoxia-ischemia (Phillips et al., 2013). It has been previously determined that glial restricted precursor cells (GRPs) transplanted in a model of WMI weeks after the initial ischemic insult, resulted in poor graft-cell survival when compared with controls. Therefore, reduced long-term survival and migration of transplanted GRPs indicate that neonatal ischemia leads to long-lasting detrimental effects, particularly on OLs, even months after the initial insult (Porambo et al., 2015). The authors found that in spite of reduced graft survival, the differentiation capacity of transplanted GRP cells into mature OLs was comparable between healthy and WMI mice. In addition, GRP transplantation after WMI induced recovery of myelination and reduced axonal injury with improvement of behavioral cues. The authors concluded that GRP cell transplants resulted in amelioration of WMI by mechanisms not directly related to long-term survival of the grafted cells (Porambo et al., 2015). Their findings were perhaps due to the presence of a few cells that may have survived and released factors/molecules with trophic effects on host brain cells.
The success of cell replacement therapy in the brain depends not just on the capability to rewire precisely the neuronal connections but also on other concurrent events among which are the presence of healthy myelinating cells, the intrinsic properties of donor progenitors and their ability to adapt to the environmental cues provided by the host. In our animal model, the fact that HSP90 was not expressed by FB-labeled CG4-OLPs in the presence of TSC1, indicating that this approach protected grafted cells from excitotoxicity, confirming the neuroprotective effects of TSC1 on the graft. These findings are in agreement with our previous report where immunocytochemistry in acute slices stained after electrophysiology, clearly showed the severe response of endogenous OLs to GME as ascertained by the intense expression of HSP90 and its co-localization with CNPase. This response was attenuated in the presence of TSC-1 (Espinosa-Jeffrey et al., 2013a). The poor survival of grafted CG4-OLPs may be due to the inflammatory conditions elicited by NMDA creating a hostile microenvironment. Franklin et al. (1996) have shown that in the normal adult CNS there is minimal longitudinal migration of grafted “myelinating” cells, while in the X-irradiated CNS these cells migrated. Moreover, in 1997 O’Leary and Blakemore reported that OLPs (younger cells) survived poorly and did not migrate following transplantation into the normal adult spinal cord. In that case the authors used normal and X-irradiated syngeneic adult female rats, the contrast between immature OLPs grafted into the adult WM environment from the host was not supportive for OLPs survival. This is further supported by our own data where CG4 cells survived, migrated and integrated in the non-inflammatory environment of the myelin deficient rat mutant (Espinosa-Jeffrey et al., 1997). Thus, conditioning of the GME brain with TSC1 was necessary for a successful graft survival.
It was interesting to observe co-localization of nestin and HSP90 in host cells of flat appearance that resembled astrocytes. While here we did not focus on the elucidation of this point, there are reports in the literature describing transient re-expression of nestin by astrocytes (Tamagno and Schiffer, 2006). In particular, in experimental models of CNS lesions such as trauma or cerebral ischemia nestin re-expression by reactive astrocytes have been reported (Lin et al., 1995; Duggal et al., 1997; Li and Chopp, 1999).
Long-lasting changes produced by excitotoxicity in the presence of grafted CG4-OLPs and TSC1
In order to learn as much as possible from our samples, we used the “liquid tissue” mass spectrometry approach that allows for the detection of proteins and peptides in tissues that have been fixed with paraformaldehyde. Although emPAI scores provide a semi-quantitative measure of the “relative” amount of peptides/proteins in each sample, it allowed us to compare the protein profile after the various treatments. Here we discuss solely those hits that were statistically significant.
We divided the results into ten categories of peptides/proteins according to their function. The cellular protein quality control system consists of various defense mechanisms against the accumulation of misfolded/aggregated proteins. Ubiquitination is the first step of a non-lysosomal degradation pathway of proteins. The selective recognition of misfolded proteins is mainly governed by quality control E3 ubiquitin ligases mediated through ubiquitin-proteasome system. Deregulation of this pathway in neurons and the related intracellular deposition of ubiquitin-protein conjugates in pathological inclusion bodies is a common factor in all major chronic neurodegenerative disorders, such as Alzheimer’s, Parkinson’s and Huntington’s diseases as well as in amyotrophic lateral sclerosis. In multiple sclerosis, abnormal ubiquitination of axons in normally myelinated WM has been reported. Derangement of this proteolytic pathway in axons outside the plaques may be the consequence of chronic absence of myelin in the axonal segment inside the plaque. Therefore, the spectrum of axonal changes in multiple sclerosis appears to be wider than expected and involves the apparently normal WM (Giordana et al., 2002). In our research paradigm we found variations of ubiquitin-related protein content with respect to the control “non-treated” brain in which ubiquitination-related proteins accounted for 1% and when NMDA was injected it was increased to 4% suggesting that this mechanism was triggered as a neuroprotective measure. These proteins increased even more in the presence of NMDA and CG4 grafts suggesting that CG4-OLPs are more sensitive to NMDA excitotoxicity than the endogenous OLPs. The percentage of ubiquitin-related proteins was reduced by TSC1 in mice that received NMDA and CG4 to 2%. Moreover, in mice receiving NMDA and TSC1 the percentage was further reduced to levels similar to those found in non-treated mice again suggesting that CG4-OLPs may be contributing to these differences. It has been reported that besides the ubiquitin proteasome system, in SJL mice with experimental autoimmune encephalomyelitis, ubiquitin independent brain-derived proteasomes generate significant amounts (ten times more) of myelin basic protein peptides that induce cytotoxic lymphocytes to target mature OLs ex vivo (Belogurov et al., 2015).
It has been shown that binding of glutamate to its cognate NMDA receptor modulates LPS-induced innate immune reaction in a TLR4-dependent manner (Glezer et al., 2003). The authors hypothesized that this acute response may be crucial to eliminate bacterial cell wall components and minimize tissue injury. Inhibition of TLR4 by antagonists prevents cytokine production at a very early stage in an efficient manner blocking inflammation. Nonetheless, sustained deregulation of proinflammatory cytokines involving NMDA receptors leads to demyelination in such pathological state (Glezer et al., 2003). Immune modulation of neuronal excitability is crucial to consider following a brain insult because injury to neurons elicits both inflammatory and neurophysiological responses (Dileonardi et al., 2012; Ferrario et al., 2013). Moreover, it has been reported that modulation of the NMDA receptor on astrocytes leads to cytokine regulation and growth factor production in these cells (Sühs et al., 2016). In addition, glutamate excitotoxicity through the NMDA-R decreases the survival signal of IGF-1 for neurons through decreased phosphorylation. Therefore, in our experimental paradigm, inflammation appears to be intrinsically modulating the immune response at different levels and in various brain cell types.
Although interesting, we found that NMDA alone increased myelin-related peptides/proteins from 2% to 11% while in mice grafted with CG4 cells in the presence of TSC1 this percentage was reduced to 5% and the response of endogenous OLs was reduced to 6% suggesting that TSC1 may be influencing both the ubiquitin and non-ubiquitin-related proteasomes. It is known that NMDA-R is not necessary for OLPs proliferation or myelination, nonetheless, the expression of calcium permeable AMPA receptors is enhanced in NMDA-R-deficient mice. Thus, NMDA-Rs act through AMPA-R signaling (De Biase et al., 2011). More studies are necessary to analyze the mechanisms leading to these changes still visible 35 days after NMDA injection.
Energy metabolism-related peptides/proteins were more abundant in non-treated mice accounting for 17%. They were differentially reduced by NMDA depending on if it was co-injected with TSC1 or CG4 cells. In order to further elucidate these data, we examined the oxygen consumption rate and glycolysis in OLPs treated overnight with NMDA or NMDA + TSC1 and we found that differences in mitochondrial and specifically coupled respiration were significantly increased in the presence of NMDA. These data suggest that acute NMDA treatment promotes mitochondrial activity, which TSC1 cannot counteract. We acknowledge that these results were not consistent with the proteomics analysis but we cannot rule out that these early effects of NMDA on OLPs change as a function of time after treatment. In addition, it is not clear which cell type(s) are contributing to the proteomics results.
It has been shown that after different brain insults such as ischemia IGF-1 is considerably up-regulated (Schwab et al., 1997; Beilharz et al., 1998). Its neuroprotective role has been recognized in stroke where IGF-levels are increased in perilesional areas (Beilharz et al., 1998). More recently, animal models have shown that in traumatic brain injury, IGF-1 increases its expression (Mangiola et al., 2015) reaffirming its response mechanism to injury and its role in neuroprotection. Our current results show that the exogenous administration of TSC1 is essential to grafted CG4-OLPs that did not survive if grafted without this trophic support.
It is known that an array of molecular and cellular changes occurs during the acute phase of injury such as excitotoxicity. After glutamate excitotoxicity, some of these changes persist for the long-term. The present pre-clinical study has generated proof-of-concept evidence that the trophic support provided by a single dose of TSC1 is necessary and sufficient to sustain graft survival as the microenvironment becomes permissive to grafted cells that interact with and integrate into the host brain, opening avenues for OLPs-based therapeutic interventions after excitotoxicity in the neonate. We have previously performed grafts of committed and uncommitted neural stem cells (Espinosa-Jeffrey et al., 2002) and to us, the cells most suitable for transplants are those specified in culture prior to grafting in order to prevent multi-potent cells from acquiring a non-desired phenotype. To our knowledge this is the first study that establishes a comparative proteomic profile produced by NMDA with respect to non-treated animals as well as with those receiving treatments aiming at rescuing the WM by endogenous means, cell transplantation or the combinatorial approach.
Considerations for clinical translation
As the incidence of premature births rises, safe novel therapies are necessary. Preclinical work has brought substantial promise to prevent further injury and promote recovery of the developing WM. Our work and that of many scientists attest that the promise may not lie on the grafted cells, nor the endogenous or exogenous trophic factors, but rather on the unique richness of the environment that is produced by both, the host and the graft.
Acknowledgments
This article is dedicated to the memory of Dr. Jean de Vellis, exemplary mentor and friend. We would like to thank Ms. A. Espinosa de los Monteros for helping preparing the manuscript.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Financial support: The Cell Culture Core supported by grant No. PP1498: Neural Cell Culture Core and NIH grant No. 04612 Intellectual & Developmental Disabilities. The Cell, Circuits and Systems Analysis Core is supported by NICHD award No. U54HD087101-03. The content is solely the responsibility of the authors and does not represent the official views of the funding agencies.
Institutional review board statement: Experimental procedures were performed under the United States Public Health Service Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee at (UCLA) (ARC #1992-034-61) on July 1, 2010.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jo Anne Stratton, Hotchkiss Brain Institute, Comparative Biology and Experimental Medicine, Canada.
Funding: The Cell Culture Core supported by grant No. PP1498: Neural Cell Culture Core and NIH grant No. 04612 Intellectual & Developmental Disabilities. The Cell, Circuits and Systems Analysis Core is supported by NICHD award No. U54HD087101-03.
P-Reviewer: Stratton JA; C-Editors: Zhao M, Li CH; T-Editor: Jia Y
References
- 1.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beilharz EJ, Russo VC, Butler G, Baker NL, Connor B, Sirimanne ES, Dragunow M, Werther GA, Gluckman PD, Williams CE, Scheepens A. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injury. Brain Res Mol Brain Res. 1998;59:119–134. doi: 10.1016/s0169-328x(98)00122-3. [DOI] [PubMed] [Google Scholar]
- 4.Belogurov A, Jr, Kuzina E, Kudriaeva A, Kononikhin A, Kovalchuk S, Surina Y, Smirnov I, Lomakin Y, Bacheva A, Stepanov A, Karpova Y, Lyupina Y, Kharybin O, Melamed D, Ponomarenko N, Sharova N, Nikolaev E, Gabibov A. Ubiquitin-independent proteosomal degradation of myelin basic protein contributes to development of neurodegenerative autoimmunity. FASEB J. 2015;29:1901–1913. doi: 10.1096/fj.14-259333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal I. Periventricular leucomalacia: A review. Eur J Pediatr. 2004;163:435–442. doi: 10.1007/s00431-004-1477-y. [DOI] [PubMed] [Google Scholar]
- 6.Choi EK, Park D, Kim TK, Lee SH, Bae DK, Yang G, Yang YH, Kyung J, Kim D, Lee WR, Suh JG, Jeong ES, Kim SU, Kim YB. Animal models of periventricular leukomalacia. Lab Anim Res. 2011;27:77–84. doi: 10.5625/lar.2011.27.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Biase LM1, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010;6:328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- 9.Dileonardi AM, Huh JW, Raghupathi R. Differential effects of FK506 on structural and functional axonal deficits after diffuse brain injury in the immature rat. J Neuropathol Expeurol. 2012;71:959–972. doi: 10.1097/NEN.0b013e31826f5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15:151–157. doi: 10.1097/00019052-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 12.Duncan ID, Radckliff AB. Inherited and acquired disorders of myelin: the underlying myelin pathology. Exp Neurol. 2016;283:452–475. doi: 10.1016/j.expneurol.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinosa de los Monteros A, Zhang M, Gordon M, Aymie M, de Vellis J. Transplantation of cultured premyelinating oligodendrocytes into normal and myelin-deficient rat brain. Dev Neurosci. 1992;14:98–104. doi: 10.1159/000111653. [DOI] [PubMed] [Google Scholar]
- 14.Espinosa de los Monteros A, Zhang MS, de Vellis J. O2A progenitor cells transplanted into the neonatal rat brain develop into oligodendrocytes but not astrocytes. Proc Natl Acad Sci U S A. 1993;90:50–54. doi: 10.1073/pnas.90.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinosa de los Monteros A, Zhao AP, de Vellis J. Transplantation of oligodendrocyte progenitors and CG4 cells into the developing rat brain: differences and similarities. In: Jeserich G, Althaus H, Richter-Landsberg C, editors. cellular and molecular mechanisms of regeneration and functional repair in the CNS. Berlin, Heidelberg: Springer - Verlag; 1996. pp. 329–341. [Google Scholar]
- 16.Espinosa de los Monteros A, Zhao P, Huang C, Pan T, Chang R, Nazarian R, Espejo D, de Vellis J. Transplantation of CG4 oligodendrocyte progenitor cells in the myelin-deficient rat brain results in myelination of axons and enhanced oligodendroglial markers. J Neurosci Res. 1997;50:872–887. doi: 10.1002/(SICI)1097-4547(19971201)50:5<872::AID-JNR23>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa de los Monteros A, Baba H, Zhao PM, Pan T, Chang R, de Vellis J, Ikenaka K. Remyelination of the adult demyelinated mouse brain by grafted oligodendrocyte progenitors and the effect of B-104 cografts. Neurochem Res. 2001;26:673–682. doi: 10.1023/a:1010943505013. [DOI] [PubMed] [Google Scholar]
- 18.Espinosa-Jeffrey A, Becker-Catania S, Zhao PM, Cole R, de Vellis J. Phenotype specification and development of oligodendrocytes and neurons from rat stem cell cultures using two chemically defined media. J Neurosci Res. 2002;69:810–825. doi: 10.1002/jnr.10344. [DOI] [PubMed] [Google Scholar]
- 19.Espinosa-Jeffrey A, Zhao P, Awosika W, Wu N, Macias F, Cepeda C, Levine M, de Vellis J. Activation, proliferation and commitment of endogenous stem/progenitor cells to the oligodendrocyte lineage by TS1 in a rat model of dysmyelination. Dev Neurosci. 2006;28:488–498. doi: 10.1159/000095111. [DOI] [PubMed] [Google Scholar]
- 20.Espinosa-Jeffrey A, Barajas SAR, Arrazola AR, Taniguchi A, Zhao PM, Bokhoor P, Holley SM, Dejarme DP, Chu B, Cepeda C, Levine MS, Gressens P, Feria-Velasco A, de Vellis J. White matter loss in a mouse model of periventricular leukomalacia is rescued by trophic factors. Brain Sci. 2013a;3:1461–1482. doi: 10.3390/brainsci3041461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espinosa-Jeffrey A, Paez PM, Cheli VT, Spreuer V, Wanner I, de Vellis J. Impact of simulated microgravity on oligodendrocyte development: implications for central nervous system repair. PLoS One. 2013b;8:e76963. doi: 10.1371/journal.pone.0076963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinosa-Jeffrey A, Blanchi B, Biancotti JC, Kumar S, Hirose M, Mandefro B, Talavera-Adame D, Benvenisty N, de Vellis J. Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes. Curr Protoc Stem Cell Biol. 2016a;38:2D.18.1–2D.18.27. doi: 10.1002/cpsc.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinosa-Jeffrey A, Arrazola RA, Chu B, Taniguchi A, Barajas SM, Bokhoor P, please Garcia J, Feria-Velasco A, de Vellis J. Trophic factors intervention regenerate nestin progenitors in a model of perinatal brain injury. Integr Mol Med. 2016b;3:703–715. doi: 10.15761/imm.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felt BT, Schallert T, Shao J, Liu Y, Li X, Barks JD. Early appearance of functional deficits after neonatal excitotoxic and hypoxic-ischemic injury: fragile recovery after development and role of the NMDA receptor. Dev Neurosci. 2002;24:418–425. doi: 10.1159/000069053. [DOI] [PubMed] [Google Scholar]
- 25.Franklin RJ, Bayley SA, Blakemore WF. Transplanted CG4 cells (an oligodendrocyte progenitor cell line) survive, migrate, and contribute to repair of areas of demyelination in X-irradiated and damaged spinal cord but not in normal spinal cord. Exp Neurol. 1996;137:263–276. doi: 10.1006/exnr.1996.0025. [DOI] [PubMed] [Google Scholar]
- 26.Giordana MT, Richiardi P, Trevisan E, Boghi A, Palmucci L. Abnormal ubiquitination of axons in normally myelinated white matter in multiple sclerosis brain. Neuropathol Appl Neurobiol. 2002;28:35–41. doi: 10.1046/j.1365-2990.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- 27.Glezer I, Zekki H, Scavone C, Rivest S. Modulation of the innate immune response by NMDA receptors has neuropathological consequences. J Neurosci. 2003;23:11094–11103. doi: 10.1523/JNEUROSCI.23-35-11094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131:548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009a;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009b;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juul SE, Ferriero DM. Pharmacological neuroprotective strategies in neonatal brain injury. Clin Perinatol. 2014;41:119–131. doi: 10.1016/j.clp.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838:1–10. doi: 10.1016/s0006-8993(99)01502-4. [DOI] [PubMed] [Google Scholar]
- 34.Lin RC, Matesic DF, Marvin M, McKay RD, Brüstle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 35.Louis JC, Magal E, Muir D, Manthorpe M, Varon S. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J Neurosci Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 36.Mangiola A, Vigo V, Anile C, De Bonis P, Marziali G, Lofrese G. Role and importance of IGF-1 in traumatic brain injuries. Biomed Res Int. 2015;2015:736104. doi: 10.1155/2015/736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markaryan A, Nelson EG, Helseth LD, Hinojosa R. Proteomic analysis of formalin-fixed celloidin-embedded whole cochlear and laser microdissected spiral ganglion tissues. Acta Otolaryngol. 2010;130:984–989. doi: 10.3109/00016481003591749. [DOI] [PubMed] [Google Scholar]
- 38.Mohammad-Gharibani P, Modi J, Menzie J, Genova R, Ma Z, Tao R, Prentice H, Wu JY. Mode of action of S-methyl-N, N-diethylthiocarbamate sulfoxide (DETC-MeSO) as a novel therapy for stroke in a rat model. Mol Neurobiol. 2014;50:655–672. doi: 10.1007/s12035-014-8658-0. [DOI] [PubMed] [Google Scholar]
- 39.Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 40.O’Leary MT, Blakemore WF. Oligodendrocyte precursors survive poorly and do not migrate following transplantation into the normal adult central nervous system. J Neurosci Res. 1997;48:159–167. [PubMed] [Google Scholar]
- 41.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A. ELGAN Study Investigators. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips AW, Johnston MV, Fatemi A. The potential for cell-based therapy in perinatal brain injuries. Transl Stroke Res. 2013;4:137–148. doi: 10.1007/s12975-013-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porambo M, Phillips AW, Marx J, Ternes K, Arauz E, Pletnikov M, Wilson MA, Rothstein JD, Johnston MV, Fatemi A. Transplanted glial restricted precursor cells improve neurobehavioral and neuropathological outcomes in a mouse model of neonatal white matter injury despite limited cell survival. Glia. 2015;63:452–465. doi: 10.1002/glia.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–761. doi: 10.1016/j.earlhumdev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–1748. doi: 10.1161/01.str.28.9.1744. [DOI] [PubMed] [Google Scholar]
- 46.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;3:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sühs KW, Gudi V, Eckermann N, Fairless R, Pul R, Skripuletz T, Stangel M. Cytokine regulation by modulation of the NMDA receptor on astrocytes. Neurosci Lett. 2016;629:227–233. doi: 10.1016/j.neulet.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Szulc KU, Lerch JP, Nieman BJ, Bartelle BB, Friedel M, Suero-Abreu GA, Watson C, Joyner AL, Turnbull DH. 4D MEMRI atlas of neonatal FVB/N mouse brain development. Neuroimage. 2015;118:49–62. doi: 10.1016/j.neuroimage.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamagno I, Schiffer D. Nestin expression in reactive astrocytes of human pathology. J Neurooncol. 2006;80:227–233. doi: 10.1007/s11060-006-9181-6. [DOI] [PubMed] [Google Scholar]
- 50.Tovar-Y-Romo LB, Ramírez-Jarquín UN, Lazo-Gómez R, Tapia R. Trophic factors as modulators of motor neuron physiology and survival: implications for ALS therapy. Front Cell Neurosci. 2014;8:61. doi: 10.3389/fncel.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno T, Ito J, Hoshikawa S, Ohori Y, Fujiwara S, Yamamoto S, Ohtsuka T, Kageyama R, Akai M, Nakamura K, Ogata T. The identification of transcriptional targets of Ascl1 in oligodendrocyte development. Glia. 2012;60:1495–1505. doi: 10.1002/glia.22369. [DOI] [PubMed] [Google Scholar]
- 52.Wrigley S, Arafa D, Tropea D. Insulin-like growth factor 1: at the crossroads of brain development and aging. Front Cell Neurosci. 2017;11:14. doi: 10.3389/fncel.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]