Abstract
In the peripheral nervous system, the vast majority of axons are accommodated within the fibre bundles that constitute the peripheral nerves. Axons within the nerves are in close contact with myelinating glia, the Schwann cells that are ideally placed to respond to, and possibly shape, axonal activity. The mechanisms of intercellular communication in the peripheral nerves may involve direct contact between the cells, as well as signalling via diffusible substances. Neurotransmitter glutamate has been proposed as a candidate extracellular molecule mediating the cross-talk between cells in the peripheral nerves. Two types of experimental findings support this idea: first, glutamate has been detected in the nerves and can be released upon electrical or chemical stimulation of the nerves; second, axons and Schwann cells in the peripheral nerves express glutamate receptors. Yet, the studies providing direct experimental evidence that intercellular glutamatergic signalling takes place in the peripheral nerves during physiological or pathological conditions are largely missing. Remarkably, in the central nervous system, axons and myelinating glia are involved in glutamatergic signalling. This signalling occurs via different mechanisms, the most intriguing of which is fast synaptic communication between axons and oligodendrocyte precursor cells. Glutamate receptors and/or synaptic axon-glia signalling are involved in regulation of proliferation, migration, and differentiation of oligodendrocyte precursor cells, survival of oligodendrocytes, and re-myelination of axons after damage. Does synaptic signalling exist between axons and Schwann cells in the peripheral nerves? What is the functional role of glutamate receptors in the peripheral nerves? Is activation of glutamate receptors in the nerves beneficial or harmful during diseases? In this review, we summarise the limited information regarding glutamate release and glutamate receptors in the peripheral nerves and speculate about possible mechanisms of glutamatergic signalling in the nerves. We highlight the necessity of further research on this topic because it should help to understand the mechanisms of peripheral nervous system development and nerve regeneration during diseases.
Keywords: AMPA receptors, axons, glutamate, metabotropic glutamate receptors, myelination, nerve injury, NMDA receptors, peripheral nervous system, PNS, Schwann cells, synaptic signalling
Introduction
The peripheral nervous system (PNS) is a part of the nervous system that encompasses nerves and ganglia outside the brain and spinal cord. The PNS connects the central nervous system (CNS) to every other organ of the body. The peripheral nerves accommodate the axons of many different classes of motor, sensory, and autonomic neurons located in the brain, the spinal cord, and the peripheral ganglia. Axons within the peripheral nerves are in close contact with glial cells, the Schwann cells (SCs). In the embryonic nerves of rodents, all axons are unmyelinated, and the SC lineage is represented by SC precursors and/or immature SCs (Jessen and Mirsky, 2005). In the adult nerves, each thick axon (diameter above ~1 μm, usually motor and some sensory axons) is tightly enwrapped with multiple layers of myelin, while the bundles of small calibre axons (diameter below ~1 μm, usually C-fibres) are loosely engulfed with one or few layers of myelin (Jessen and Mirsky, 2005). The compact myelin sheaths are produced by the myelinating SCs, while the engulfment is accomplished by the Remak SCs. The third type of SCs, the terminal SCs located at the neuromuscular junction, is not involved in the generation of myelin but is indispensable for the maintenance of synapses and modulation of synaptic activity at the neuromuscular junction (Hyung et al., 2018). Terminal SCs are outside the scope of this review.
Contiguous spatial arrangement between neuronal axons and SCs suggests that the two cell types communicate extensively with each other, and that SCs may respond to, and possibly shape axonal activity in the peripheral nerves. Of great interest are the mechanisms by which axons may influence the development of SCs and trigger them to myelinate, as well as the idea that SC function and myelination process may be coupled to axonal activity (Stevens and Fields, 2000). SCs, in turn, may modulate development and function of axons in the peripheral nerves. A number of key molecules and cellular mechanisms that mediate bidirectional communication between axons and SCs have been identified. For instance, release of neuregulin-1 (NRG1) from axons and binding to epidermal growth factor receptor 2 (ErbB2) in SCs is important for determining the thickness of the myelin sheath in vivo (Quintes et al., 2010), release of adenosine triphosphate (ATP)/adenosine and their binding to corresponding purinergic receptors in SCs inhibits proliferation of SCs in vitro (Lecca et al., 2012), transfer of vesicles from SCs to axons is important for axonal maintenance and regeneration after nerve damage (Lopez-Verrilli and Court, 2012), while serine/threonine kinase liver kinase B1 (LKB1) plays a key role in metabolic regulation in SCs in vivo (Beirowski et al., 2014). However, many other mechanisms mediating axon-SC communication in the peripheral nerves remain poorly characterised. In addition to axon-SC signalling, communication between SCs or between neighbouring axons may take place in the peripheral nerves. But very little, if any, knowledge is currently available regarding this type of communication.
Several earlier studies suggested that classical neurotransmitters, particularly glutamate and acetylcholine, may mediate communication between axons and/or SCs in the peripheral nerve (Lissak, 1939; Dettbarn and Rosenberg, 1966; Wheeler et al., 1966; DeFeudis, 1971; Weinreich and Hammerschlag, 1975; Vizi et al., 1983; Wieraszko and Ahmed, 2009). However, little research has been performed on this topic up to date, and the information regarding neurotransmitter-mediated signalling in the peripheral nerves and its functional role during physiological or pathological conditions remains very limited. Remarkably, in the fibre tracts of the CNS, axons are involved in glutamatergic signalling with myelinating glia. Glutamate can be released via several mechanisms and can bind to different types of ionotropic (iGluRs) and metabotropic (mGluRs) glutamate receptors expressed by the oligodendrocyte lineage cells (Kula et al., 2019). One of the most intriguing types of glutamatergic axon-glia communication in the CNS fibre tracts is fast (on the scale of few milliseconds) synaptic signalling between axons and oligodendrocyte precursor cells (OPCs) that has been first discovered in the corpus callosum and the optic nerve of rodents (Kukley et al., 2007; Ziskin et al., 2007), and subsequently described in the human fimbria (Gallo et al., 2008). Other types of glutamatergic signaling in the CNS fibre tracts may involve non-synaptic vesicular or non-vesicular release of glutamate from axons onto OPCs and oligodendrocytes (Kula et al., 2019). Glutamate-mediated communication plays an important role in regulation of proliferation, migration and differentiation of OPCs in vitro and/or in vivo, is crucial for survival of oligodendrocytes, may modulate different events during myelination process, and is important for re-myelination of axons after damage (Kula et al., 2019). On the other hand, if glutamate is released in excess it may be damaging to myelinating glia, and antagonists of different types of glutamate receptors have been reported to protect CNS fibre tracts during pathological conditions (Karadottir and Attwell, 2007; Matute, 2011).
The myelinating glia, the properties of neuronal axons, and the myelination process in the CNS and in the PNS have similarities and differences. Do SCs in the peripheral nerves communicate with axons, or between themselves, via glutamate release? Are the mechanisms of glutamate-mediated signalling in the peripheral nerves similar to the mechanisms in the CNS? What is the functional role of glutamatergic signalling in the peripheral nerves during health and disease? The goal of the present article is to bring into the light the limited knowledge regarding glutamate receptors and glutamatergic signalling in the peripheral nerves, and to inspire thinking about new research aiming at investigation of the neurotransmitter-mediated signalling in the PNS. This research is of great importance because it should foster discoveries of new mechanisms of the PNS development and open new avenues for treatment of diseases involving the peripheral nerves.
Search Strategy
The articles used in this review were retrieved using an electronic search of the MEDLINE database for literature describing glutamate receptors and glutamatergic signalling in the peripheral nerves. The search was performed for all years until 2019 using the following conditions: peripheral nerves OR peripheral nervous (MeSH Terms) AND glutamate (MeSH Terms); peripheral nerves OR peripheral nervous (MeSH Terms) AND AMPA (MeSH Terms); peripheral nerves OR peripheral nervous (MeSH Terms) AND NMDA (MeSH Terms); peripheral nerves OR peripheral nervous (MeSH Terms) AND metabotropic glutamate (MeSH Terms). The results were pooled and further screened by the text. The searches also retrieved articles describing glutamatergic signalling only in the spinal cord but those were excluded because they are out of scope of the present review.
Glutamate Release in the Peripheral Nerves
Axons as a possible source of glutamate in the nerves
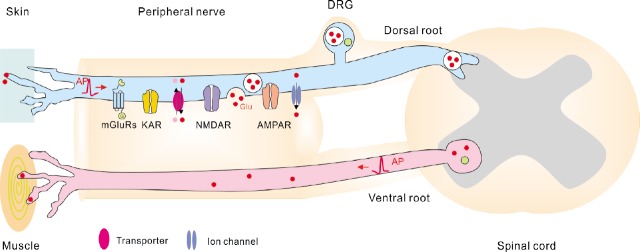
In the PNS, glutamate is synthesized by pseudo-unipolar sensory neurons located in the sensory ganglia (Malet and Brumovsky, 2015), e.g., in the dorsal root ganglia (DRG) and in the sensory cranial ganglia. In addition, immunohistochemical data show that glutamate-like immunoreactivity is present in the large cell bodies in the ventral horn of rat spinal cord (Meister et al., 1993), suggesting that motor neurons also contain glutamate. Neurons located in sensory ganglia project one branch of their axon through the peripheral nerves to the peripheral organs, and another branch through the dorsal roots, or the roots of the cranial nerves, to the CNS (Figure 1). The motor neurons located in the spinal cord project their axons to the muscles (Figure 1). Remarkably, glutamate is present not only in the cell soma of sensory and motor neurons, but also in their axons and terminals (Figure 1). Particularly, glutamate has been detected in the dorsal roots and/or the peripheral nerves of hen, cat, frog, rat and monkey (Porcellati and Thompson, 1957; Graham et al., 1967; Wheeler and Boyarsky, 1968; Duggan and Johnston, 1970; Johnson and Aprison, 1970; Roberts et al., 1973; Osborne et al., 1974; Roberts and Keen, 1974; Battaglia and Rustioni, 1988; Westlund et al., 1989), as well as in the motor nerve terminals (Meister et al., 1993; Waerhaug and Ottersen, 1993). Furthermore, the activity of the enzyme glutaminase which generated glutamate from glutamine has been detected in the sensory ganglia, in the dorsal roots and in the peripheral nerves (Graham and Aprison, 1969; McDougal et al., 1981; Hassel et al., 2003; Miller et al., 2011). Taken together, these findings indicate that glutamate is located in the axons within the peripheral nerves and/or may also be synthesized locally in these axons.
Figure 1.
Scheme showing Glu receptors and Glu release from the peripheral axons.
Note that information regarding Glu receptors is based on morphological findings that report presence of receptors on the peripheral axons but do not verify whether these receptors are functional. Information regarding the mechanisms of axonal Glu release is based on the findings in the central nervous system because the mechanisms of Glu release along the axonal shafts in the peripheral nervous system remain unknown. AMPAR: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AP: action potential; DRG: dorsal root ganglion; Glu: glutamate; KAR: kainate receptor; mGluR: metabotropic Glu receptor; NMDAR: N-methyl-D-aspartate receptor.
If glutamate is present in the axoplasm, can it be released along the axons shafts? Earlier experiments suggested that axon-SC signalling mediated by extracellular glutamate takes place in the giant axon of the squid. When individual SCs in the isolated giant axon were impaled with microelectrodes and axons were stimulated electrically at high frequency (100 Hz), changes of the SC membrane potential were recorded, and these changes were abolished by application of glutamate receptor antagonists (Villegas et al., 1987; Lieberman et al., 1989; Lieberman and Sanzenbacher, 1992). Subsequently it has been demonstrated that if peripheral nerves or nerve segments dissected from frog or mouse are pre-loaded with labelled glutamate (14C-glutamate) or aspartate (d-2,3-(3)H-aspartic acid) and stimulated electrically or magnetically, the loaded substances can be detected in the bath solution (Wheeler et al., 1966; DeFeudis, 1971; Weinreich and Hammerschlag, 1975; Wieraszko and Ahmed, 2009). Glutamate release from the peripheral nerve segments has also been demonstrated upon chemical stimulation, i.e., application of high concentration (53.5 mM) of K+ (Drescher et al., 1987). Taken together, these experiments suggest that glutamate can be secreted from axonal shafts in the peripheral nerves (Figure 1), although they do not completely exclude that SCs located along the axons may also release glutamate.
SCs as a possible source of glutamate in the nerves
Do SCs contain glutamate and can they be a source of glutamate in the peripheral nerves, in addition to or instead of axons? The reports regarding presence of glutamate in SCs are very limited and not fully consistent. Immunohistochemical experiments in rat nodose ganglion and branches of vagus nerve showed that only sensory axons, but not SCs, label positively for glutamate (Schaffar et al., 1997) indicating that source of glutamate in the PNS is only neuronal. However, later studies using rat and mouse sciatic nerve demonstrated that SCs in vitro and in the nerve slices contain enzymes related to the metabolism of glutamate: glutamate dehydrogenase, glutamic acid decarboxylase (GAD67), and glutamine synthetase (Miller et al., 2002; Magnaghi et al., 2010; Saitoh and Araki, 2010). Importantly, these enzymes are functional proteins in SCs because they have been detected using not only immunohistochemistry and Western blotting, but also functional assays which showed that cultured SCs use GAD67 to synthesize γ-aminobutyric acid (Magnaghi et al., 2010) and that glutamine synthetase promotes myelination by decreasing glutamate concentration in SCs (Saitoh and Araki, 2010). Furthermore, in vitro studies showed that SCs isolated from rat sciatic nerve express functional excitatory amino acid transporter-1 (Perego et al., 2011), indicating that SCs take up glutamate from the extracellular space. Taken together, these studies suggest that SCs contain glutamate in the cytoplasm and are also able to metabolise glutamate.
If SCs contain glutamate, can they release it and what the mechanisms of release are? SCs from rat DRG or sciatic nerve are able to release glutamate in vitro (Parpura et al., 1995; Jeftinija and Jeftinija, 1998; Wu et al., 2005). Glutamate release from cultured SCs can be induced by ATP and bradykinin and is mediated by mobilization of Ca2+ from the internal stores (Parpura et al., 1995; Jeftinija and Jeftinija, 1998). ATP-stimulated glutamate release involves activation of P2-purinoceptors on SCs (Jeftinija and Jeftinija, 1998). The mechanism of bradykinin-induced release remains unknown but does not involve reversal of glutamate transporters or cell swelling (Parpura et al., 1995).
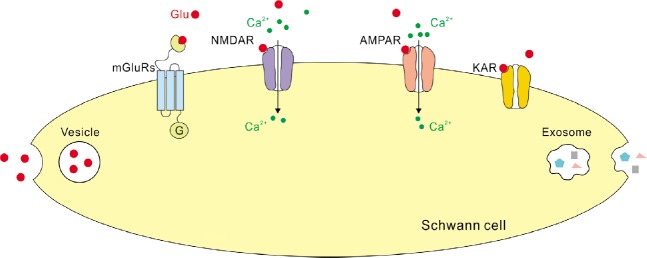
A major mechanism of glutamate release from neurons is vesicular exocytosis that requires a specialized complex set of proteins, and glial cells in the CNS may be capable of vesicular exocytosis as well (Savtchouk and Volterra, 2018). Hence it is interesting to ask whether this mechanism plays a role during glutamate release from SCs. Some studies indeed reported that cultured SCs express proteins required for regulated secretion of vesicles, including synaptosome associated protein 23, syntaxin I, synaptobrevin/vesicle-associated membrane protein 2 and cellubrevin (Verderio et al., 2006). Other studies demonstrate that synaptotagmin and synaptophysin, that are also crucial for vesicle fusion in neurons, are absent from SCs (Parpura et al., 1995; Jeftinija and Jeftinija, 1998). Evidence regarding presence of synaptosome associated protein 25 in SCs remains contradictory (Verderio et al., 2006; Marinelli et al., 2012). Hence, SCs in culture may be capable of vesicular glutamate release (Figure 2) but this release does not necessarily involve exactly the same set of proteins which are used for exocytosis of glutamatergic vesicles in neurons.
Figure 2.
Scheme showing Glu receptors and glutamate release from Schwann cells located in the peripheral nerves.
Note that several studies report that AMPARs, NMDARs and mGluRs are functional proteins in SCs, but only morphological evidence is available regarding KARs in SCs. Also note that mechanisms of Glu release from SCs are hypothetical as no functional studies are available on this topic. AMPAR: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; Glu: glutamate; KAR: kainate receptor; mGluR: metabotropic Glu receptor; NMDAR: N-methyl-D-aspartate receptor; SC: Schwann cell.
Taking together, several pieces of evidence suggest that axonal shafts and SCs in the peripheral nerves contain glutamate and can release it (Figure 2). However, the research on this topic has been mainly performed in cell cultures or using artificial conditions (e.g., loading the radioactively-labelled glutamate). And it currently remains unclear which cells in the peripheral nerves release glutamate in vivo, and which conditions foster this release.
Glutamate Receptors in the Peripheral Nerves
The amino acid glutamate acts on two types of receptors: the iGluRs and the mGluRs. The iGluRs are ligand-gated ionotropic receptors for glutamate that comprise α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), N-methyl-D-aspartate receptors (NMDARs), and kainate receptors (KARs). A major function of iGluRs in the CNS is to mediate fast synaptic signalling between neurons, between neurons and OPCs, and between neurons and Bergmann glia of the cerebellum. The mGluRs are G-coupled metabotropic receptors for glutamate that encompass group I (mGluR1 and mGluR5), group II (mGluR2 and mGluR3), and group III (mGluR4, mGluR6, mGluR7, mGluR8) receptors (Niswender and Conn, 2010). The mGluRs induce activation of intracellular signalling cascades upon glutamate binding, may trigger Ca2+ release from the internal stores, and the opening of G-protein-coupled inwardly-rectifying K+ channels (Sherman, 2014). Which types of glutamate receptors are expressed in the peripheral nerves?
Glutamate receptors in SCs
Mammalian SCs express various types of iGluRs and mGluRs that may detect released glutamate and mediate glutamatergic signalling in the nerves (Figure 2). Particularly, immunohistochemical and ultrastructural analysis showed that SCs surrounding neurons and fibres in the vestibular ganglia of rat and guinea pig are positively labelled for GluA2/A3 and GluA4 subunits of AMPARs (Dememes et al., 1995), while processes of SCs in skin nerve biopsies obtained from humans are positively labelled for either NMDARs or KARs (Kinkelin et al., 2000). Reverse transcription-polymerase chain reaction experiments demonstrated that SCs isolated from rat sciatic nerve express mRNAs for NR1, NR2B, NR2C, NR2D, and NR3B subunits of NMDARs, mRNAs for all AMPAR subunits (although GluA4 subunit seems to be expressed at lower level than other subunits), as well as mRNAs for GluK2, GluK3, GluK4, and GluK5 subunits of KARs (Campana et al., 2017). Importantly, the iGluRs and the mGluRs are functional proteins in SCs because application of glutamate, NMDA, AMPA, or kainate triggers transient increase in intracellular Ca2+-concentration and/or release of ATP or brain-derived neurotrophic factor from SCs, activation of ERK1/2 signalling pathways, or phosphorylation of Akt in SC in vitro (Fink et al., 1999; Liu and Bennett, 2003; Verderio et al., 2006; Campana et al., 2017). The application of mGluR agonists or antagonists affects proliferation and differentiation of SCs, and myelination in vitro and in vivo (Saitoh et al., 2016). Recent findings using single-cell electrophysiology corroborated and extended the in vitro studies of iGluRs in SCs by demonstrating that functional Ca2+-permeable AMPARs are expressed by the developing mammalian SCs ex vivo, and that the single-channel conductance of AMPARs in SCs (8–11 pS) is comparable to that in neurons (Chen et al., 2017). However, functional expression of AMPARs likely decreases when SCs become mature and myelinate because only NMDARs, but not AMPARs, trigger morphological changes in the adult PNS myelin ex vivo (Christensen et al., 2016).
Glutamate receptors in the peripheral axons
Glutamate receptors have been detected on axonal cylinders within various peripheral nerves using immunohistochemistry and electron microscopy (Figure 1) (Fernandez-Montoya et al., 2017). Rat postganglionic sympathetic axons in grey rami (grey rami are the branches from the paravertebral ganglion of the sympathetic trunk which contact each spinal nerve) express NR1 subunit of NMDARs and GluA1 subunit of AMPARs (Carlton et al., 1998). These subunits, as well as GluK1, 2, 3 are also present in the subpopulations of myelinated and unmyelinated axons in the sural and medial plantar nerves and of unmyelinated cutaneous axons at the dermal-epidermal junction in the glabrous rat skin (Carlton et al., 1995; Coggeshall and Carlton, 1998; Carlton and Coggeshall, 1999; Kinkelin et al., 2000). Interestingly, the proportion of axons labelled for these iGluRs increased during experimentally-induced inflammation (Carlton and Coggeshall, 1999), indicating that iGluRs may play an important role during inflammatory damage of peripheral organs. Labelling for mGluR2/3 has been detected in axons within the rat digital nerve (Carlton et al., 2001), while labelling for mGluR5 has been found on the axons of the peripheral nerve trunks within the dermis of the rat skin (Walker et al., 2001). The immunoreactivity for iGluRs and mGluR2/3 was diffusely distributed within the axoplasm and/or localized to membrane (Carlton et al., 1995, 2001) suggesting that receptors can be transported along the axons or targeted to the membrane. Axons of the mouse dorsal root also express functional AMPARs and NMDARs because application of glutamate or specific receptor agonists to these axons ex vivo induce Ca2+ elevations in the axons (Christensen et al., 2016). Furthermore, activation of NMDARs, but not AMPARs, induced morphological damage to axon cylinders in the dorsal roots (Christensen et al., 2016).
Notably, nerve bundles within the rat heart also show labelling for GluA1 and GluA2/3 subunits of AMPARs, GluK5 subunit of KARs, NR1 subunit of NMDARs, as well as for mGluR2/3 (Gill et al., 1998, 1999). However, it has not been investigated whether axons or SCs express glutamate receptors in the heart nerves.
Does Glutamatergic Signalling Occur in the Peripheral Nerves?
If axons and SCs in the peripheral nerves are capable of releasing glutamate, and also express functional glutamate receptors, does glutamatergic signalling take place between the cells in the peripheral nerves?
Glutamatergic signalling between axons and SCs in the peripheral nerves
In the squid, SCs located along the giant axon detect axonal glutamate release because they show changes in the membrane potential in response to electrical stimulation of axons, and these changes are abolished by glutamate receptor antagonists (Lieberman et al., 1989). More recent studies in mammals showed that functional glutamate uptake system exists in the SCs (Perego et al., 2011), and that application of glutamate to the sciatic nerve in vivo triggers immature phenotype and proliferation of SCs (Saitoh et al., 2016). Based on these findings and on the fact that SCs express glutamate receptors, the authors suggested that glutamate is a signalling molecule in the peripheral nerves (Figure 3) (Perego et al., 2011; Saitoh et al., 2016), however a direct evidence for this has not been yet provided.
Figure 3.
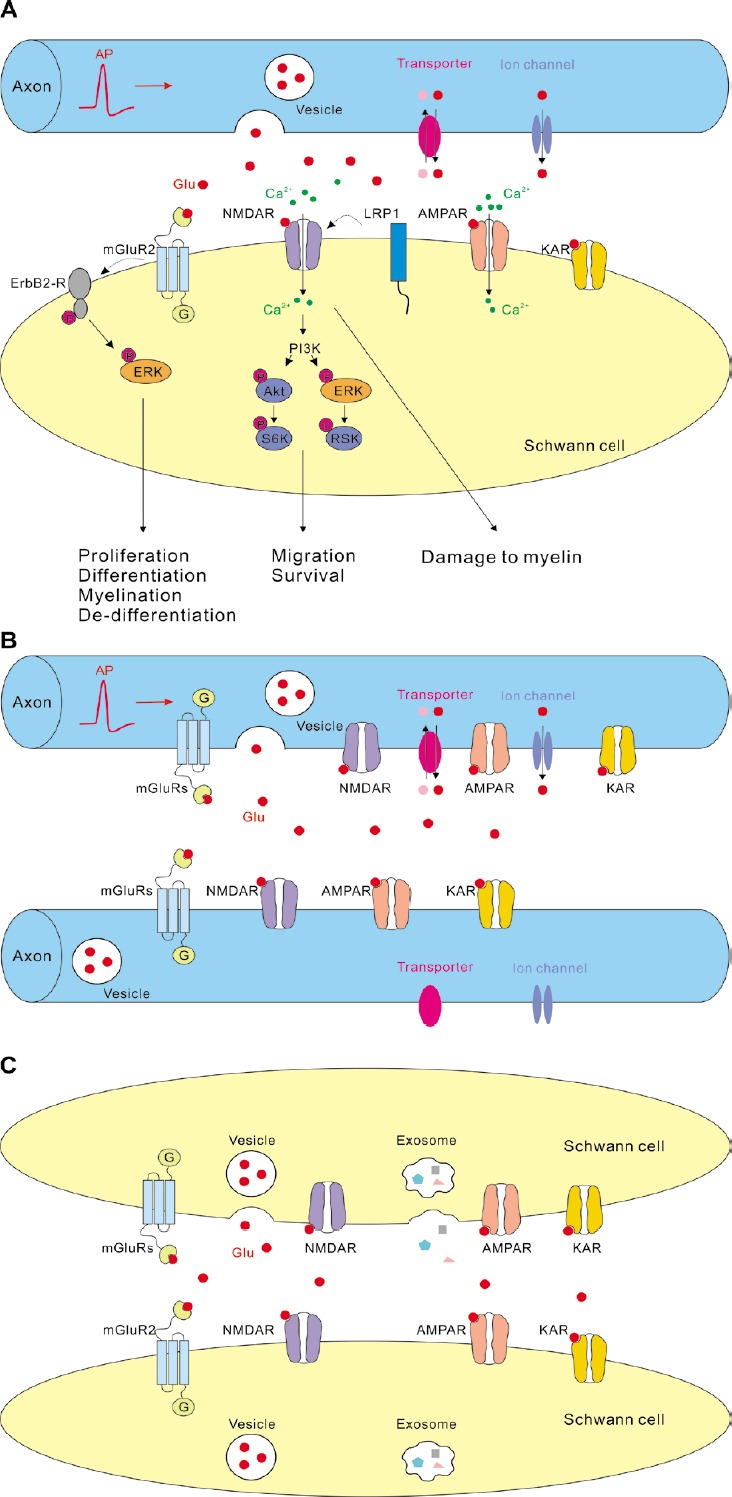
Hypothetical scheme showing glutamatergic signalling in the peripheral nerves (A) between axons and SCs, (B) between axons, and (C) between SCs.
Information regarding the glutamate receptors in SCs is based on various findings in cell culture and ex vivo. Information regarding the mechanisms of axonal glutamate release is based on the findings in the central nervous system because the mechanisms of glutamate release along the axonal shafts in the peripheral nervous system remain unknown. Note, that “Axon” represents both myelinated and non-myelinated axons, while “Schwann cell” represents different developmental stages of SC lineage. Akt: Serine/threonine-specific protein kinase; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AP: action potential; ErbB2-R: tyrosine kinase epidermal growth factor receptor 2; ERK: extracellular signal-regulated kinase; KAR: kainate receptor; LRP1: low density lipoprotein receptor-related protein 1; mGluR: metabotropic glutamate receptor; NMDAR: N-methyl-D-aspartate receptor; P: phosphorylation; PI3K: phosphoinositide 3-kinase; pink dots: ions or molecules which are co-transported with glutamate; red dots (Glu): glutamate; RSK: ribosomal S6 kinase; S6K: p70 S6 kinase; SCs: Schwann cells. Ion channel represents P2X7 receptor, TWIK1-related K+ channel 1 (TREK-1), bestrophin 1 (BEST1), or any other channel through which glutamate may be released. Transporter represents reversed excitatory amino acid transporter, cystine-glutamate exchanger, or any other transporter through which glutamate may be released.
The forms of axon-SC communication in the peripheral nerves are unknown, but some studies suggest that glutamate release in the nerve resembles synaptic release because it depends on extracellular Ca2+ and is stimulated by elevated extracellular K+ (DeFeudis, 1971; Wieraszko and Ahmed, 2009). As axons in the CNS fibre tracts establish synaptic junctions with OPCs and fast vesicular glutamate release occurs at these junctions (Kukley et al., 2007; Ziskin et al., 2007; Gallo et al., 2008), it is possible that glutamatergic signalling between axons and SCs in the peripheral nerves takes place at specialized synaptic junctions as well. Recently, different approaches have been used to test for synaptic axon-SC communication in a new preparation of peripheral nerve slices where the tissue architecture is preserved. However, synaptic AMPARs-mediated currents in SCs were detected neither upon electrical stimulation of axons nor using pharmacological paradigms which induce massive glutamate release at neuronal and neuron-OPCs synapses in the CNS (Chen et al., 2017). Thus, electrophysiological evidence in favour of synaptic junctions with functional presynaptic release machinery along the peripheral axons and clustered postsynaptic AMPARs in the developing SCs could not be provided. However, synaptic axon-SC signalling may be restricted to certain stages of animal development, e.g., takes place only before the embryonic day 16 which was the earliest time-point investigated in the recent study (Chen et al., 2017). With this regard, it is interesting to note that developing SCs located along the intramuscular motor axons in the phrenic nerve respond to vesicular release of ATP from axons with Ca2+-transients only during early development but gradually lose the response as animal mature, although SCs continue to express ATP receptors and respond to the application of agonists (Heredia et al., 2018).
If functional synapses are not established between axons and SCs, which alternative forms of glutamatergic axon-SC signalling may exist in the peripheral nerves and how is glutamate released? Hypothetically, axon-SC signalling may involve non-synaptic vesicular exocytosis of glutamate and/or non-vesicular glutamate release (Figures 1 and 3A). The fact that vesicles are found in some types of peripheral axons (Carlton et al., 1995), and that glutamate as well as glutaminase activity is detected in axonal shafts of the peripheral nerves (Miller et al., 2011) suggest that release of glutamate in the nerves may occur from vesicles. Interestingly, the axons of DRG neurons, that are a source of glutamate in the spinal nerves, are capable of vesicular glutamate release in vitro although they do not build synaptic junctions with glial cells (Wake et al., 2015). These findings may be in favour of vesicular non-synaptic mechanism of glutamate release in the peripheral nerves, although such conclusion should be made with care as it is not known whether axons of cultured DRG neurons represent the peripheral or the central branches.
In addition to vesicular exocytosis, several mechanisms of non-vesicular glutamate release from different cells are known in the CNS and may also occur in the PNS (Figures 1 and 3A). For instance, glutamate can be released via cystine-glutamate exchangers, P2X7 ion channels, glutamate-permeable two-pore domain potassium channels TWIK1-related K+ channel 1 (TREK-1), glutamate-permeable Ca2+-activated anion channel bestrophin 1, or reversed electrogenic glutamate transporters (Szatkowski et al., 1990; Duan et al., 2003; Woo et al., 2012; Massie et al., 2015). However, neither of these mechanisms has been investigated in the peripheral nerves.
Remarkably, a recent study proposed an interesting mechanism of bi-directional signalling between axons and SCs involving glutamate (Hu et al., 2019). The authors showed that glutamate can induce Ca2+-elevations and secretion of exosomes from SCs in vitro and that glutamate is released from DRG cells during electrical stimulation. Based on these findings, the authors put forward an idea that in vivo, glutamate released from DRG neurons during neuronal activity may foster SCs to secrete exosomes which contain substances modulating neuronal activity, for instance growth factors and/or cyclic adenosine monophosphate. These substances can influence behaviour and function of the DRG neurons/axons (Hu et al., 2019).
Do SCs in the peripheral nerves use neurotransmitter glutamate to signal back to axons? Several studies suggest that SCs are able to transfer ribosomes and various molecules (e.g., receptors, sodium channels) to axons, and that this transfer is crucial during peripheral nerve regeneration (Lopez-Verrilli and Court, 2012). Yet, it remains un-known whether SCs release glutamate onto axons and/or whether glutamate, glutamate receptors, or molecules required for metabolism of glutamate are packed into the exosomes which SCs transfer to axons.
Glutamatergic signalling between axons in the peripheral nerves
Supposedly, glutamate released from axonal shafts in the peripheral nerves may activate not only glutamate receptors on the neighbouring SCs, but also glutamate receptors on the axons themselves (Figure 3B). In the CNS, NMDARs and mGluRs are found on the presynaptic axonal boutons, and are involved in modulation of synaptic transmission and plasticity (Upreti et al., 2013; Banerjee et al., 2016). Analogous autocrine regulation of neurotransmitter release may occur in the peripheral nerves. In line with this idea, ultrastructural evidence demonstrates that clusters of small vesicles in some types of peripheral axons are frequently associated with iGluRs (Carlton et al., 1995). These vesicles may be glutamatergic and, upon exocytosis, may activate glutamate auto-receptors or receptors on the neighbouring axons. Yet, direct demonstration of axo-axonic glutamatergic signalling in the peripheral nerves is currently missing.
Glutamatergic signalling between SCs in the peripheral nerves
As SCs express glutamate receptors and may release glutamate, it is possible that they are capable of autocrine glutamatergic signalling and/or release glutamate onto the neighbouring SCs (Figure 3C). It has been suggested that SCs may communicate between themselves, for instance during development or during injury when they get denervated from axons (Glenn and Talbot, 2013). This communication may involve an autocrine signal from the SC extracellular matrix with subsequent activation of G-protein-coupled receptor 126 (Gpr126) and signalling mediated by neuregulin. However it remains unknown whether glutamate and glutamate receptors are involved in communication between SCs.
Functional Role of Glutamate Receptors and Glutamatergic Signalling in the Peripheral Nerves
How glutamatergic signalling may influence physiological function of the peripheral nerves? Functional role of AMPARs in SCs in vivo is unknown. Two studies investigating role of AMPARs in SCs in vitro found that AMPARs are not involved in glutamine-induced proliferation of SCs (Saitoh and Araki, 2010), and do not mediated activation of ERK1/2 in response to application of 80 μM glutamate (Campana et al., 2017).
Functional role of NMDARs in SCs in vivo is also un-known. In vitro, NMDARs influence migration of SCs via PI3K and ERK1/2 pathway (Campana et al., 2017), but are not involved in regulation of glutamine-induced proliferation of SCs (Saitoh and Araki, 2010) (Figure 3A).
The mGluR2 control the balance between proliferation of SCs, their differentiation, and myelination in vitro and in vivo (Figure 3A): activation of mGluR2 promotes immature phenotype of SCs via phosphorylation of ERK, while downregulation of mGluR2 stimulates expression of myelin genes Krox-20 and MBP, and reduces expression of c-Jun, a negative regulator of myelination (Saitoh et al., 2016).
The newest evidence shows that glutamate triggers Ca2+ increase and promotes secretion of exosomes from SCs in vitro (Hu et al., 2019) suggesting that glutamate receptors may be involved in this process, although the mechanisms have not been investigated in further details.
Glutamate receptors may also regulate SC development and function in concert with other molecules. Although little information is currently available regarding this issue, it deserves attention. For instance, NRG1-ErbB signalling is a well-defined factor influencing development of SCs and myelination in the PNS. Activation of mGluR2 enhances NRG1-induced ERK phosphorylation that induces proliferation of SCs in vitro (Saitoh et al., 2016). It remains unknown whether iGluRs in SCs also interact with NRG1-ErbB but it is plausible, in analogy to other cells. In neurons, some types of ErbBs co-localize with postsynaptic AMPARs and NMDARs (Garcia et al., 2000; Ma et al., 2003), presynaptic NRG1 regulates trafficking of postsynaptic AMPARs (Zhong et al., 2017), and NRG1-ErbB signalling can regulate glutamatergic synaptic plasticity (Fenster et al., 2012). In the CNS, NRG-ErbB signalling also regulates the switch from the activity-independent to the activity-dependent mode of myelination involving NMDARs (Lundgaard et al., 2013). Hence, NRG1-ErbB signalling may also regulate development and/or function of SCs in the peripheral nerves in concert with glutamate receptors.
Does Glutamatergic Signalling in the Peripheral Nerves Play a Role during Pathological Conditions?
In the fibre tracts of the CNS, glutamate is a key player during pathological conditions including ischemia, inflammation, traumatic injuries, and mental disorders (Spitzer et al., 2016). The sources of glutamate may include axons, glial cells, or inflammatory cells; and, if released in excess, glutamate mediates excitotoxic cell damage. However, glutamatergic signalling may also be beneficial during pathological conditions: for instance, NMDARs in oligodendrocytes are essential during re-myelination of axons in a toxin-induced demyelinated lesion in the cerebellum (Lundgaard et al., 2013). Although the role of glutamatergic signalling in pathophysiology of the PNS received much less attention, evidence is emerging that excessive glutamate level may trigger or potentiate damage of axons and glia in the peripheral nerves. For instance, genetic deletion of glutamate carboxypeptidase II, an enzyme which generates glutamate from N-acetyl-aspartyl-glutamate, results in attenuation of injury after sciatic nerve crush (Bacich et al., 2005). Activation of NMDARs triggers disordering of the parallel profiles of the PNS myelin ex vivo (Christensen et al., 2016), while NMDAR antagonist MK-801 is protective during ischemia/reperfusion injury of the rat sciatic nerve (Ke et al., 2016). However, glutamate is likely not merely a negative player during PNS pathologies. For example, NMDARs are up-regulated after sciatic nerve injury in vivo, and this may be beneficial for repair because activation of NMDARs promotes migration and survival of SCs in vitro through ERK1/2 and Akt pathway, and application of NMDA on the injured sciatic nerve in vivo activates ERK1/2 (Campana et al., 2017). Interestingly, if applied on normal sciatic nerve in vivo, glutamate induces de-differentiation of myelinating SCs, i.e., a process of substantial phenotypic transformation of SCs to the immature state, with subsequent increase in proliferation of SCs. These effects are mediated by mGluR2 (Saitoh et al., 2016). Similar events may be triggered by glutamate in the nerve during pathological conditions. Thus, it is possible that glutamatergic signalling is transiently required at certain stages of nerve regeneration to promote migration and proliferation of de-differentiated SCs and generation of new immature SCs which can differentiate into re-myelinating cells.
Besides its role during PNS injury, glutamatergic signalling may also be essential during tumorigenesis in the peripheral nerves. For example, gria4 gene which encodes the GluA4 subunit of AMPARs is among the ten top genes up-regulated in human schwannomas (Sarver et al., 2015). Yet, the sources of glutamate, the mechanisms of glutamate release, and the forms of glutamatergic signalling in schwannomas are currently unknown.
Glutamatergic signalling also plays an important role in generation and modulation of pain. Nociceptive Aδ-fibres and C-fibres, that are the axons of the DRG neurons, are glutamatergic. Various types of iGluRs and mGluRs are localized in the pain pathway not only in the CNS but also in the PNS, i.e., in the DRG neurons and on the peripheral terminals of their axons located in the skin (Bleakman et al., 2006). Multiple studies suggest that glutamate is a peripheral mediator of pain because (a) subcutaneous injection of glutamate or glutamate receptor agonists evokes hyperalgesic responses, (b) concentration of glutamate in the skin rises and expression of glutamate receptors is up-regulated in animal models of pain and in the inflamed skin of human patients, (c) peripherally applied antagonists of glutamate receptors attenuate nociceptive behaviour (Westlund et al., 1992; Carlton et al., 1995; Jackson et al., 1995; Zhou et al., 1996; Davidson et al., 1997; Omote et al., 1998; Carlton and Coggeshall, 1999; Dogrul et al., 2000; Bhave et al., 2001; Carlton et al., 2001; Du et al., 2001; Walker et al., 2001; Du et al., 2003; Jang et al., 2004; Jung et al., 2006; Lee et al., 2006; Tan et al., 2008; Jin et al., 2012). Functional role of glutamate and its receptors during pain has also been under extensive investigation in the CNS (Bleakman et al., 2006). In contrast, role of glutamatergic signalling in the peripheral nerve trunk during pain remains largely unexplored. It is known that in response to painful stimuli, trains of action potentials propagate along the nociceptive axons, and the frequency of firing correlates with pain intensity (Momin and McNaughton, 2009). Therefore, one can speculate that if nociceptive axons are capable of releasing glutamate along their shafts in the peripheral nerves, this release may increase during pain. Excessive level of glutamate may then trigger de-differentiation of SCs and/or damage to myelin because these processes have been observed upon application of glutamate to normal nerves (Christensen et al., 2016; Saitoh et al., 2016). Demyelination in the peripheral nerves is known to elicit spontaneous action potential firing in the afferents and to induce pain, even without direct damage to axons (Wallace et al., 2003). Therefore, if release of glutamate occurs within the nerves it may promote pain via feed-forward mechanism, and altered crosstalk between axons and SCs in the nerves may be an important pathophysiological mechanism of pain.
Conclusion
In contrast to the recent boost of interest regarding glutamatergic axon-glia signalling in the CNS, intercellular communication mediated by glutamate in the peripheral nerves remains in a shadow. Several studies demonstrated that SCs and axons in the PNS express glutamate receptors, and that glutamate may be released from the peripheral nerves. However, the majority of available information on this topic stems from cell culture studies and/or from the experiments using artificial conditions. Some authors proposed that glutamate is a signalling molecule in the peripheral nerves but no study has so far directly demonstrated this in mammals in vitro or in vivo. The newest findings also suggest that glutamate may play a dual role during peripheral nerve injury or other PNS pathologies, being harmful during some circumstances and beneficial during the others. In the future, it would be important to address the open questions regarding glutamatergic signalling in the PNS, putting particular focus on studying this signalling in preparations with preserved cellular architecture and in vivo because this would help understanding the mechanism of PNS development and repair. Targeting glutamatergic signalling in the peripheral nerves may appear to be an important approach for promoting regeneration and repair, as well as for pain management.
Additional file: Open peer review report 1 (98.4KB, pdf) .
Acknowledgments
We thank Bartosz Kula (Tübingen) for comments on the manuscript.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: The work in the laboratory of Maria Kukley was supported by the Excellence Strategy Program of the University of Tübingen (Deutsche Forschungsgemeinschaft, ZUK63).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Susana R. Cerqueira, University of Miami School of Medicine, USA.
Funding: The work in the laboratory of Maria Kukley was supported by the Excellence Strategy Program of the University of Tübingen (Deutsche Forschungsgemeinschaft, ZUK63).
P-Reviewer: Cerqueira SR; C-Editors: Zhao M, Yu J; T-Editor: Jia Y
References
- 1.Bacich DJ, Wozniak KM, Lu XC, O’Keefe DS, Callizot N, Heston WD, Slusher BS. Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury. J Neurochem. 2005;95:314–323. doi: 10.1111/j.1471-4159.2005.03361.x. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee A, Larsen RS, Philpot BD, Paulsen O. Roles of presynaptic NMDA receptors in neurotransmission and plasticity. Trends Neurosci. 2016;39:26–39. doi: 10.1016/j.tins.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988;277:302–312. doi: 10.1002/cne.902770210. [DOI] [PubMed] [Google Scholar]
- 4.Beirowski B, Babetto E, Golden JP, Chen YJ, Yang K, Gross RW, Patti GJ, Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhave G, Karim F, Carlton SM, Gereau RWt Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- 6.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Campana WM, Mantuano E, Azmoon P, Henry K, Banki M, Kim JH, Pizzo DP, Gonias SL. Ionotropic glutamate receptors activate cell signaling in response to glutamate in Schwann cells. FASEB J. 2017;31:1744–1755. doi: 10.1096/fj.201601121R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlton SM, Coggeshall RE. Inflammation-induced changes in peripheral glutamate receptor populations. Brain Res. 1999;820:63–70. doi: 10.1016/s0006-8993(98)01328-6. [DOI] [PubMed] [Google Scholar]
- 9.Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- 10.Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105:957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 11.Carlton SM, Chung K, Ding Z, Coggeshall RE. Glutamate receptors on postganglionic sympathetic axons. Neuroscience. 1998;83:601–605. doi: 10.1016/s0306-4522(97)00406-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen TJ, Frohlich N, Kula B, Barzan R, Kukley M. Glutamate activates AMPA receptor conductance in the developing Schwann cells of the mammalian peripheral nerves. J Neurosci. 2017;37:11818–11834. doi: 10.1523/JNEUROSCI.1168-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen PC, Welch NC, Brideau C, Stys PK. Functional ionotropic glutamate receptors on peripheral axons and myelin. Muscle Nerve. 2016;54:451–459. doi: 10.1002/mus.25078. [DOI] [PubMed] [Google Scholar]
- 14.Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Davidson EM, Coggeshall RE, Carlton SM. Peripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin test. Neuroreport. 1997;8:941–946. doi: 10.1097/00001756-199703030-00025. [DOI] [PubMed] [Google Scholar]
- 16.DeFeudis FV. Effects of electrical stimulation on the efflux of L-glutamate from peripheral nerve in vitro. Exp Neurol. 1971;30:291–296. doi: 10.1016/s0014-4886(71)80008-0. [DOI] [PubMed] [Google Scholar]
- 17.Dememes D, Lleixa A, Dechesne CJ. Cellular and subcellular localization of AMPA-selective glutamate receptors in the mammalian peripheral vestibular system. Brain Res. 1995;671:83–94. doi: 10.1016/0006-8993(94)01322-9. [DOI] [PubMed] [Google Scholar]
- 18.Dettbarn WD, Rosenberg P. Effect of ions on the efflux of acetylcholine from peripheral nerve. J Gen Physiol. 1966;50:447–460. doi: 10.1085/jgp.50.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dogrul A, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000;292:115–118. doi: 10.1016/s0304-3940(00)01458-0. [DOI] [PubMed] [Google Scholar]
- 20.Drescher MJ, Drescher DG, Hatfield JS. Potassium-evoked release of endogenous primary amine-containing compounds from the trout saccular macula and saccular nerve in vitro. Brain Res. 1987;417:39–50. doi: 10.1016/0006-8993(87)90177-6. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 23.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggan AW, Johnston GA. Glutamate and related amino acids in cat spinal roots, dorsal root ganglia and peripheral nerves. J Neurochem. 1970;17:1205–1208. doi: 10.1111/j.1471-4159.1970.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 25.Fenster C, Vullhorst D, Buonanno A. Acute neuregulin-1 signaling influences AMPA receptor mediated responses in cultured cerebellar granule neurons. Brain Res Bull. 2012;87:21–29. doi: 10.1016/j.brainresbull.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Montoya J, Avendano C, Negredo P. The glutamatergic system in primary somatosensory neurons and its involvement in sensory input-dependent plasticity. Int J Mol Sci. 2017;19:E69. doi: 10.3390/ijms19010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink T, Davey DF, Ansselin AD. Glutaminergic and adrenergic receptors expressed on adult guinea pig Schwann cells in vitro. Can J Physiol Pharmacol. 1999;77:204–210. [PubMed] [Google Scholar]
- 28.Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586:3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill SS, Pulido OM, Mueller RW, McGuire PF. Molecular and immunochemical characterization of the ionotropic glutamate receptors in the rat heart. Brain Res Bull. 1998;46:429–434. doi: 10.1016/s0361-9230(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 31.Gill SS, Pulido OM, Mueller RW, McGuire PF. Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res Bull. 1999;48:143–146. doi: 10.1016/s0361-9230(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 32.Glenn TD, Talbot WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol. 2013;23:1041–1048. doi: 10.1016/j.conb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham LT, Jr, Aprison MH. Distribution of some enzymes associated with the metabolism of glutamate, aspartate, gamma-aminobutyrate and glutamine in cat spinal cord. J Neurochem. 1969;16:559–566. doi: 10.1111/j.1471-4159.1969.tb06855.x. [DOI] [PubMed] [Google Scholar]
- 34.Graham LT, Jr, Shank RP, Werman R, Aprison MH. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967;14:465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- 35.Hassel B, Boldingh KA, Narvesen C, Iversen EG, Skrede KK. Glutamate transport, glutamine synthetase and phosphate-activated glutaminase in rat CNS white matter. A quantitative study. J Neurochem. 2003;87:230–237. doi: 10.1046/j.1471-4159.2003.01984.x. [DOI] [PubMed] [Google Scholar]
- 36.Heredia DJ, Feng CY, Agarwal A, Nennecker K, Hennig GW, Gould TW. Postnatal restriction of activity-induced Ca(2+) responses to schwann cells at the neuromuscular junction are caused by the proximo-distal loss of axonal synaptic vesicles during development. J Neurosci. 2018;38:8650–8665. doi: 10.1523/JNEUROSCI.0956-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu M, Hong L, Liu C, Hong S, He S, Zhou M, Huang G, Chen Q. Electrical stimulation enhances neuronal cell activity mediated by Schwann cell derived exosomes. Sci Rep. 2019;9:4206. doi: 10.1038/s41598-019-41007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyung S, Jung K, Cho SR, Jeon NL. The Schwann cell as an active synaptic partner. Chemphyschem. 2018;19:1123–1127. doi: 10.1002/cphc.201701299. [DOI] [PubMed] [Google Scholar]
- 39.Jackson DL, Graff CB, Richardson JD, Hargreaves KM. Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur J Pharmacol. 1995;284:321–325. doi: 10.1016/0014-2999(95)00449-u. [DOI] [PubMed] [Google Scholar]
- 40.Jang JH, Kim DW, Sang Nam T, Se Paik K, Leem JW. Peripheral glutamate receptors contribute to mechanical hyperalgesia in a neuropathic pain model of the rat. Neuroscience. 2004;128:169–176. doi: 10.1016/j.neuroscience.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 41.Jeftinija SD, Jeftinija KV. ATP stimulates release of excitatory amino acids from cultured Schwann cells. Neuroscience. 1998;82:927–934. doi: 10.1016/s0306-4522(97)00310-2. [DOI] [PubMed] [Google Scholar]
- 42.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 43.Jin YH, Takemura M, Furuyama A, Yonehara N. Peripheral glutamate receptors are required for hyperalgesia induced by capsaicin. Pain Res Treat. 2012;2012:915706. doi: 10.1155/2012/915706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson JL, Aprison MH. The distribution of glutamic acid, a transmitter candidate, and other amino acids in the dorsal sensory neuron of the cat. Brain Res. 1970;24:285–292. doi: 10.1016/0006-8993(70)90107-1. [DOI] [PubMed] [Google Scholar]
- 45.Jung CY, Lee SY, Choi HS, Lim EJ, Lee MK, Yang GY, Han SR, Youn DH, Ahn DK. Participation of peripheral group I and II metabotropic glutamate receptors in the development or maintenance of IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Neurosci Lett. 2006;409:173–178. doi: 10.1016/j.neulet.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 46.Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke T, Li R, Chen W. Inhibition of the NMDA receptor protects the rat sciatic nerve against ischemia/reperfusion injury. Exp Ther Med. 2016;11:1563–1572. doi: 10.3892/etm.2016.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinkelin I, Brocker EB, Koltzenburg M, Carlton SM. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci Lett. 2000;283:149–152. doi: 10.1016/s0304-3940(00)00944-7. [DOI] [PubMed] [Google Scholar]
- 49.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 50.Kula B, Chen TJ, Kukley M. Glutamatergic signaling between neurons and oligodendrocyte lineage cells: Is it synaptic or non-synaptic? Glia. 2019 doi: 10.1002/glia.23617. doi: doi: 10.1002/glia.23617. [DOI] [PubMed] [Google Scholar]
- 51.Lecca D, Ceruti S, Fumagalli M, Abbracchio MP. Purinergic trophic signalling in glial cells: functional effects and modulation of cell proliferation, differentiation, and death. Purinergic Signal. 2012;8:539–557. doi: 10.1007/s11302-012-9310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HJ, Choi HS, Ju JS, Bae YC, Kim SK, Yoon YW, Ahn DK. Peripheral mGluR5 antagonist attenuated craniofacial muscle pain and inflammation but not mGluR1 antagonist in lightly anesthetized rats. Brain Res Bull. 2006;70:378–385. doi: 10.1016/j.brainresbull.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Lieberman EM, Sanzenbacher E. Mechanisms of glutamate activation of axon-to-Schwann cell signaling in the squid. Neuroscience. 1992;47:931–939. doi: 10.1016/0306-4522(92)90041-y. [DOI] [PubMed] [Google Scholar]
- 54.Lieberman EM, Abbott NJ, Hassan S. Evidence that glutamate mediates axon-to-Schwann cell signaling in the squid. Glia. 1989;2:94–102. doi: 10.1002/glia.440020205. [DOI] [PubMed] [Google Scholar]
- 55.Lissak K. Liberation of acetylcholine and adrenaline by stimulating isolated nerves. Am J Physiol. 1939;127:263–271. [Google Scholar]
- 56.Liu GJ, Bennett MR. ATP secretion from nerve trunks and Schwann cells mediated by glutamate. Neuroreport. 2003;14:2079–2083. doi: 10.1097/00001756-200311140-00014. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Verrilli MA, Court FA. Transfer of vesicles from schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front Physiol. 2012;3:205. doi: 10.3389/fphys.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Charles F-C, Attwell D, Karadottir RT. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013;11:e1001743. doi: 10.1371/journal.pbio.1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma L, Huang YZ, Pitcher GM, Valtschanoff JG, Ma YH, Feng LY, Lu B, Xiong WC, Salter MW, Weinberg RJ, Mei L. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magnaghi V, Parducz A, Frasca A, Ballabio M, Procacci P, Racagni G, Bonanno G, Fumagalli F. GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J Neurochem. 2010;112:980–990. doi: 10.1111/j.1471-4159.2009.06512.x. [DOI] [PubMed] [Google Scholar]
- 61.Malet M, Brumovsky PR. VGLUTs and glutamate synthesis-focus on DRG neurons and pain. Biomolecules. 2015;5:3416–3437. doi: 10.3390/biom5043416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marinelli S, Vacca V, Ricordy R, Uggenti C, Tata AM, Luvisetto S, Pavone F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One. 2012;7:e47977. doi: 10.1371/journal.pone.0047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massie A, Boillee S, Hewett S, Knackstedt L, Lewerenz J. Main path and byways: non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission. J Neurochem. 2015;135:1062–1079. doi: 10.1111/jnc.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011;219:53–64. doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDougal DB, Jr, Yu MJ, Gorin PD, Johnson EM., Jr Transported enzymes in sciatic nerve and sensory ganglia of rats exposed to maternal antibodies against nerve growth factor. J Neurochem. 1981;36:1847–1852. doi: 10.1111/j.1471-4159.1981.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 66.Meister B, Arvidsson U, Zhang X, Jacobsson G, Villar MJ, Hokfelt T. Glutamate transporter mRNA and glutamate-like immunoreactivity in spinal motoneurones. Neuroreport. 1993;5:337–340. doi: 10.1097/00001756-199312000-00040. [DOI] [PubMed] [Google Scholar]
- 67.Miller KE, Richards BA, Kriebel RM. Glutamine-glutamine synthetase-glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002;945:202–211. doi: 10.1016/s0006-8993(02)02802-0. [DOI] [PubMed] [Google Scholar]
- 68.Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 2011;130:283–309. doi: 10.1016/j.pharmthera.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Momin A, McNaughton PA. Regulation of firing frequency in nociceptive neurons by pro-inflammatory mediators. Exp Brain Res. 2009;196:45–52. doi: 10.1007/s00221-009-1744-2. [DOI] [PubMed] [Google Scholar]
- 70.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787:161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- 72.Osborne NN, Wu PH, Neuhoff V. Free amino acids and related compounds in the dorsal root ganglia and spinal cord of the rat as determined by the micro dansylation procedure. Brain Res. 1974;74:175–181. doi: 10.1016/0006-8993(74)90122-x. [DOI] [PubMed] [Google Scholar]
- 73.Parpura V, Liu F, Jeftinija KV, Haydon PG, Jeftinija SD. Neuroligand-evoked calcium-dependent release of excitatory amino acids from Schwann cells. J Neurosci. 1995;15:5831–5839. doi: 10.1523/JNEUROSCI.15-08-05831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perego C, Cairano ES, Ballabio M, Magnaghi V. Neurosteroid allopregnanolone regulates EAAC1-mediated glutamate uptake and triggers actin changes in Schwann cells. J Cell Physiol. 2011;227:1740–1751. doi: 10.1002/jcp.22898. [DOI] [PubMed] [Google Scholar]
- 75.Porcellati G, Thompson RH. The effect of nerve section on the free amino acids of nervous tissue. J Neurochem. 1957;1:340–347. doi: 10.1111/j.1471-4159.1957.tb12091.x. [DOI] [PubMed] [Google Scholar]
- 76.Quintes S, Goebbels S, Saher G, Schwab MH, Nave KA. Neuron-glia signaling and the protection of axon function by Schwann cells. J Peripher Nerv Syst. 2010;15:10–16. doi: 10.1111/j.1529-8027.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- 77.Roberts PJ, Keen P. (14C)glutamate uptake and compartmentation in glia of rat dorsal sensory ganglion. J Neurochem. 1974;23:201–209. doi: 10.1111/j.1471-4159.1974.tb06935.x. [DOI] [PubMed] [Google Scholar]
- 78.Roberts PJ, Keen P, Mitchell JF. The distribution and axonal transport of free amino acids and related compounds in the dorsal sensory neuron of the rat, as determined by the dansyl reaction. J Neurochem. 1973;21:199–209. doi: 10.1111/j.1471-4159.1973.tb04239.x. [DOI] [PubMed] [Google Scholar]
- 79.Saitoh F, Araki T. Proteasomal degradation of glutamine synthetase regulates schwann cell differentiation. J Neurosci. 2010;30:1204–1212. doi: 10.1523/JNEUROSCI.3591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saitoh F, Wakatsuki S, Tokunaga S, Fujieda H, Araki T. Glutamate signals through mGluR2 to control Schwann cell differentiation and proliferation. Sci Rep. 2016;6:29856. doi: 10.1038/srep29856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarver AE, Sarver AL, Thayanithy V, Subramanian S. Identification, by systematic RNA, sequencing, of novel candidate biomarkers and therapeutic targets in human soft tissue tumors. Lab Invest. 2015;95:1077–1088. doi: 10.1038/labinvest.2015.80. [DOI] [PubMed] [Google Scholar]
- 82.Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci. 2018;38:14–25. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res. 1997;778:302–308. doi: 10.1016/s0006-8993(97)01058-5. [DOI] [PubMed] [Google Scholar]
- 84.Sherman SM. The function of metabotropic glutamate receptors in thalamus and cortex. Neuroscientist. 2014;20:136–149. doi: 10.1177/1073858413478490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spitzer S, Volbracht K, Lundgaard I, Karadottir RT. Glutamate signalling: A multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology. 2016;110:574–585. doi: 10.1016/j.neuropharm.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 86.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 87.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 88.Tan PH, Yang LC, Chiang PT, Jang JS, Chung HC, Kuo CH. Inflammation-induced up-regulation of ionotropic glutamate receptor expression in human skin. Br J Anaesth. 2008;100:380–384. doi: 10.1093/bja/aem398. [DOI] [PubMed] [Google Scholar]
- 89.Upreti C, Zhang XL, Alford S, Stanton PK. Role of presynaptic metabotropic glutamate receptors in the induction of long-term synaptic plasticity of vesicular release. Neuropharmacology. 2013;66:31–39. doi: 10.1016/j.neuropharm.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verderio C, Bianco F, Blanchard MP, Bergami M, Canossa M, Scarfone E, Matteoli M. Cross talk between vestibular neurons and Schwann cells mediates BDNF release and neuronal regeneration. Brain Cell Biol. 2006;35:187–201. doi: 10.1007/s11068-007-9011-6. [DOI] [PubMed] [Google Scholar]
- 91.Villegas J, Evans PD, Reale V. Modulation of the membrane potential of the Schwann cell of the squid giant nerve fiber. J Physiol (Paris) 1987;82:322–326. [PubMed] [Google Scholar]
- 92.Vizi ES, Gyires K, Somogyi GT, Ungvary G. Evidence that transmitter can be released from regions of the nerve cell other than presynaptic axon terminal: axonal release of acetylcholine without modulation. Neuroscience. 1983;10:967–972. doi: 10.1016/0306-4522(83)90234-8. [DOI] [PubMed] [Google Scholar]
- 93.Waerhaug O, Ottersen OP. Demonstration of glutamate-like immunoreactivity at rat neuromuscular junctions by quantitative electron microscopic immunocytochemistry. Anat Embryol (Berl) 1993;188:501–513. doi: 10.1007/BF00190144. [DOI] [PubMed] [Google Scholar]
- 94.Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun. 2015;6:7844. doi: 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini F, Kuhn R, Urban L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 96.Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weinreich D, Hammerschlag R. Nerve impulse-enhanced release of amino acids from non-synaptic regions of peripheral and central nerve trunks of bullfrog. Brain Res. 1975;84:137–142. doi: 10.1016/0006-8993(75)90807-0. [DOI] [PubMed] [Google Scholar]
- 98.Westlund KN, McNeill DL, Coggeshall RE. Glutamate immunoreactivity in rat dorsal root axons. Neurosci Lett. 1989;96:13–17. doi: 10.1016/0304-3940(89)90235-8. [DOI] [PubMed] [Google Scholar]
- 99.Westlund KN, Sun YC, Sluka KA, Dougherty PM, Sorkin LS, Willis WD. Neural changes in acute arthritis in monkeys. II. Increased glutamate immunoreactivity in the medial articular nerve. Brain Res Brain Res Rev. 1992;17:15–27. doi: 10.1016/0165-0173(92)90003-5. [DOI] [PubMed] [Google Scholar]
- 100.Wheeler DD, Boyarsky LL. Influx of glutamic acid in peripheral nerve--characteristics in influx. J Neurochem. 1968;15:1019–1031. doi: 10.1111/j.1471-4159.1968.tb11645.x. [DOI] [PubMed] [Google Scholar]
- 101.Wheeler DD, Boyarsky LL, Brooks WH. The release of amino acids from nerve during stimulation. J Cell Physiol. 1966;67:141–147. doi: 10.1002/jcp.1040670116. [DOI] [PubMed] [Google Scholar]
- 102.Wieraszko A, Ahmed Z. Axonal release of glutamate analog, d-2, 3-3H-Aspartic acid and l-14C-proline from segments of sciatic nerve following electrical and magnetic stimulation. Neurosci Lett. 2009;458:19–22. doi: 10.1016/j.neulet.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 103.Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, Park JY, Lee CJ. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151:25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Wu SZ, Jiang S, Sims TJ, Barger SW. Schwann cells exhibit excitotoxicity consistent with release of NMDA receptor agonists. J Neurosci Res. 2005;79:638–643. doi: 10.1002/jnr.20401. [DOI] [PubMed] [Google Scholar]
- 105.Zhong C, Akmentin W, Du C, Role LW, Talmage DA. Axonal type III Nrg1 controls glutamate synapse formation and GluA2 trafficking in hippocampal-accumbens connections. eNeuro 4:ENEURO.0232-16.2017. 2017 doi: 10.1523/ENEURO.0232-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7:895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]
- 107.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.