Abstract
Objective:
Studies have shown that docosahexaenoic acid (DHA) has a beneficial effect in the treatment of spinal cord injury. A meta-analysis was used to study the effect of DHA on the neurological recovery in the rat spinal cord injury model, and the relationship between the recovery of motor function after spinal cord injury and the time and method of administration and the dose of DHA.
Data source:
Published studies on the effect of DHA on spinal cord injury animal models from seven databases were searched from their inception to January 2019, including PubMed, MEDLINE, EMBASE, the China National Knowledge Infrastructure, Wanfang, VIP, and SinoMed databases. The search terms included “spinal cord injury” “docosahexaenoic acid”, and “rats”.
Data selection:
Studies that evaluated the influence of DHA in rat models of spinal cord injury for locomotor functional recovery were included. The intervention group included any form of DHA treatment and the control group included treatment with normal saline, vehicle solution or no treatment. The Systematic Review Centre for Laboratory animal Experimentation’s risk of bias assessment tool was used for the quality assessment of the included studies. Literature inclusion, quality evaluation and data extraction were performed by two researchers. Meta-analysis was then conducted on all studies that met the inclusion criteria. Statistical analysis was performed on the data using RevMan 5.1.2. software.
Outcome measures:
The primary outcome measure was the score on the Basso, Beattie, and Bresnahan scale. Secondary outcome measures were the sloping plate test, balance beam test, stair test and grid exploration test.
Results:
A total of 12 related studies were included, 3 of which were of higher quality and the remaining 9 were of lower quality. The highest mean Basso, Beattie, and Bresnahan scale score occurred at 42 days after DHA treatment in spinal cord injury rats. At 21 days after treatment, the mean difference in Basso, Beattie, Bresnahan scores between the DHA group and the control group was the most significant (pooled MD = 4.14; 95% CI = 3.58–4.70; P < 0.00001). In the subgroup analysis, improvement in the Basso, Beattie, and Bresnahan scale score was more significant in rats administered DHA intravenously (pooled MD = 2.74; 95% CI = 1.41–4.07; P < 0.0001) and subcutaneously (pooled MD = 2.99; 95% CI = 2.29–3.69; P < 0.00001) than in the groups administered DHA orally (pooled MD = 3.04; 95% CI = –1.01 to 7.09; P = 0.14). Intravenous injection of DHA at 250 nmol/kg (pooled MD = 2.94; 95% CI = 2.47–3.41; P < 0.00001] and 1000 nmol/kg [pooled MD = 3.60; 95% CI = 2.66–4.54; P < 0.00001) significantly improved the Basso, Beattie, and Bresnahan scale score in rats and promoted the recovery of motor function.
Conclusion:
DHA can promote motor functional recovery after spinal cord injury in rats. The administration of DHA by intravenous or subcutaneous injection is more effective than oral administration of DHA. Intravenous injection of DHA at doses of 250 nmol/kg or 1000 nmol/kg is beneficial. Because of the small number and the low quality of the included studies, more high-quality research is needed in future to substantiate the results.
Keywords: DHA, docosahexaenoic acid, fatty acid, meta-analysis, motor function, motor function recover, polyunsaturated fatty acid, PUFA, spinal cord injury, systematic review
Chinese Library Classification No. R453; R364; R363
Introduction
Spinal cord injury (SCI) is usually caused by concussive force or direct trauma to the spinal cord, and can lead to loss of both sensory and voluntary motor activity (Efeoglu et al., 2014; Pannek et al., 2019; Sharma et al., 2019). A systematic review on the epidemiology of SCI shows that its incidence rate ranges from 12.06 to 61.6 per million, with most patients ranging from 26.8 to 56.6 years old (Ning et al., 2013). In the USA, the total cost of SCI-related hospitalizations was approximately 1.69 billion dollars in 2009 (Mahabaleshwarkar and Khanna, 2014). SCI not only encompasses immediate mechanical primary damage, but also subsequent acute secondary damage, including inflammation, electrolytic shift, edema, vascular insult, calcium-mediated injury, excitotoxicity, neurogenic shock, ischemic cell death, and other cellular and molecular disturbances (Dumont et al., 2002; Hulsebosch, 2002).
At present, the treatment strategy for SCI is mainly neuroprotective and targets nerve regeneration (Mu et al., 2014; Beckers and Moons, 2019). Current treatments are mainly achieved through drug therapy and cell transplantation (Rouanet et al., 2017; Vismara et al., 2017; Ma et al., 2019). However, because the secondary processes resulting from SCI are complex, it is difficult to treat all complications by any current conventional single drug treatment (Estrada and Müller, 2019). Many of these drugs, especially steroid hormones, also have serious side effects (Rouanet, 2017). Therefore, drug treatment of SCI is an active area of current research, especially regarding natural substances such as curcumin, melatonin, and polyunsaturated fatty acids (Yao et al., 2015; Yang et al., 2016).
In vivo, polyunsaturated fatty acids are oxidized by CYP2J2 via NADPH-dependent epoxidation, and the process plays an important role in inflammation (Kamel et al., 2018). Docosahexaenoic acid (DHA) is an omega-3 polyunsaturated fatty acid. DHA is widely used clinically in the therapy of nerve-related diseases such as cognitive dysfunction, traumatic brain injury, Alzheimer’s disease and stroke. (Wu et al., 2011; Schober et al., 2016), DHA can alter neurotrophic factor, synapsin I, cAMP-responsive element-binding protein, and calcium/calmodulin-dependent kinase II, which may also affect recovery after SCI (Wu et al., 2011). Thus, it is necessary to systematically evaluate the efficacy of DHA for the treatment of SCI. Here, we performed systematic reviews and meta-analyses on motor function studies using rat SCI models to value the efficiency of DHA treatment after SCI.
Data and Methods
Study selection
We searched the research literature for this review, which was limited to animal study results published before January 2019. Studies were identified by searching PubMed, MEDLINE, EMBASE, Wanfang, SinoMed databases, the VIP, and the China National Knowledge Infrastructure (including China Doctor/Master Dissertation Full Text Database and China Proceedings Conference Full Text Database). We also searched the reference lists of included studies, to identify additional studies. The following key terms were used: “spinal cord injury”, “spinal cord injuries”, “spinal cord disease”, “spinal cord diseases”, “spinal lesion”, “spinal lesions”, “docosahexaenoic acid”, “DHA”, “n-3 polyunsaturated”, “omega-3”, “long chain polyunsaturated fatty acid”, “polyunsaturated fatty acid”, “n-3 PUFA”, “n-3 fatty acid”, “omega-3 fatty acid”, “alpha-linolenic acid”, “eicosapentaenoic acid”, “fatty acid”, “rat” and “rats”.
Identification of studies
The titles and abstracts of the identified studies were screened individually according to the selection criteria by two experienced investigators (MY and ZRT) independently. Thereafter, the two experienced investigators (MY and ZRT) individually retrieved and assessed the full text of all eligible studies according to the inclusion or exclusion criteria, and a third investigator (XJC) decided the outcome of any disagreement of a study’s eligibility.
Inclusion criteria
Types of studies
Studies were included that evaluated the influence of DHA on locomotor functional recovery in rat SCI models. There was no language restriction.
Types of participants
Laboratory rats of any age, gender, or strain were included in research models. The methods of SCI in rats included compression, hemisection, transection, or contusion.
Types of interventions
We included the studies of any type of DHA intervention that was compared with a placebo control. Time, dose, and the method of DHA administration were unrestricted.
Types of comparisons
Comparisons included physiological saline, vehicle (saline), normal diet, or no treatment.
Exclusion criteria
Types of studies
The studies of solely in vitro and clinical case reports were excluded.
Types of participants
We excluded the studies which used genetically-modified animals and radiation or electrical stimulation from systematic analysis.
Types of interventions
We excluded the study of DHA combined with other intervention treatments, which was compared with a placebo control.
Types of comparisons
We excluded the study of any comparisons, which may have therapeutic effects.
Outcome measures
Behavioral evaluation
Behavioral changes were assessed and recorded by the beam walk test, inclined plane test, horizontal ladder, Montoya staircase test, grid exploratory test and the Basso, Beattie, and Bresnahan (BBB) scale. The BBB locomotor rating scale is the preferred and main outcome, and other behavioral evaluations were considered secondary outcomes, including the beam walk test, inclined plane test, horizontal ladder, Montoya staircase test, grid exploratory test.
Primary outcome measure
The BBB scale (BBBs) was set as the primary outcome measure (Basso et al., 1995). The BBBs records scores are designed to analyze the functional behavior of rats. The BBBs ranges from 0 (i.e., no detectable hind limb movement) to 21 (normal locomotion). BBB allots scores according to definite behavioral categories. Operationally defined terms that reflect a series of motion (e.g., joint movement, stepping, coordination, hind limb movements, trunk position and stability, paw placement and tail position) yield a stable scoring system, within a quiet and stable assessment environment (Basso et al., 1995; Ferguson et al., 2004).
Secondary outcome measures
The secondary outcome measures include the beam walk test, inclined plane test, horizontal ladder, Montoya staircase test and grid exploratory test, to name a few. In the inclined plane test, a researcher placed the rat on an inclined plane and the researcher recorded the highest angle the rat could maintain without slipping (Rivlin and Tator, 1977; Jiang et al., 2016). The beam walk test is performed by assessing the distance the rat could walk along the wooden beam to evaluate its ability to traverse a wooden beam (King et al., 2006). In the horizontal ladder test, the number of foot slips made when the rat crosses a horizontal ladder is assessed (King et al., 2006). The Montoya staircase test is designed to assess the forelimb motor function of rats and has been demonstrated to provide a repeatable and sensitive assessment of motor impairment (Liu et al., 2015, 2017). The grid exploratory test is examined by assessing the ability of rats to precisely control and place their forelimbs and hindlimbs onto grid wires as they roam around (Liu et al., 2017).
Data extraction
Data were extracted from the included studies, including the first author, publication year, animal strain, age, weight and gender, number of animals in each group, method used to induce SCI, SCI level, DHA administration (method, dose and duration of treatment), and measured outcome of BBBs in the form of mean ± SD. In studies with multiple intervention groups, we only considered the data on BBBs from DHA treatment groups and negative control groups. The authors were e-mailed for these data if they were not reported. If the authors were not contactable, data were extracted from the published figures by GetData Graph Digitizer 2.24 (Yao et al., 2015). In addition, to avoid the risk of bias, two investigators (ZRT and LYZ) individually extracted the details from the studies. Mean data were used if errors were in the acceptable scope (error ≤ 1% mean data). Disagreements were resolved by a third investigator (MY), who extracted the data again and made the final decision.
Methodological quality of individual studies
In this study, the Systematic Review Centre for Laboratory animal Experimentation’s Risk of Bias tool (SYRCLE’s RoB tool; http://www.biomedcentral.com/1471-2288/14/43) was used to appraise the methodological quality of the animal experimental studies (Hooijmans et al., 2014). This tool is founded on the Cochrane Collaboration RoB Tool, and aims to evaluate methodological quality (Higgins et al., 2011). It is designed for risk bias assessment in animal experiments, and is related to six types of bias: selection, detection, performance, reporting, attrition and other biases. Two investigators individually evaluated the quality of the methodology of the included studies. A “yes” judgment signals a low risk of bias; a “no” judgment signals high risk of bias; and an “unclear” judgment signals that there were insufficient details to adequately evaluate the risk of bias in the studies (Hooijmans et al., 2014).
Statistical analyses
Meta-analyses
The data of all included studies were summarized and analyzed by the Cochrane Collaboration’s RevMan 5.1.2 software (https://community.cochrane.org/help/tools-and-software/revman-5) (Walker et al., 2018). GraphPad Prism (version 5.01) software was used to draw the graphs (https://www.graphpad.com/scientific-software/prism/) (Fernandez-Ruiz et al., 2018). If the outcomes had been measured using the same unit, the results were described as mean differences (MD), otherwise, described as standardized MD (SMD). The 95% confidence intervals (CIs) were calculated for both types of outcomes. A value of P ≤ 0.05 was considered statistically significant.
Heterogeneity analysis and sensitivity analysis
The heterogeneity of included studies was assessed by I2. If the heterogeneity was insignificant (i.e., P > 0.1; I2 ≤ 50%), the fixed effect models were used to analyze pooled effects; when P ≤ 0.1; I2 > 50%, we tried to find out the source of heterogeneous by sensitivity analysis. The included studies were excluded one by one to determine which study was the source of heterogeneous by the change of I2. If there were no explicit studies of heterogeneity, random effect models were used for the analysis (Higgins et al., 2011).
Results
Description of studies
In total 750 possible related studies were retrieved. The duplicate studies were eliminated, and the title and abstracts of the studies were further screened. Of these, 731 studies were excluded and the full-texts of 19 studies were assessed. Finally, after full-texts screening, we excluded 7 studies (Ward et al., 2010; Hall et al., 2012; Holly et al., 2012; Figueroa and De Leon, 2014; Maixent et al., 2014; Manzhulo et al., 2018b; Nie et al., 2018; Figure 1). The reasons for exclusion were for not using any behavioral analysis outcome or using a mixed intervention of DHA with other drugs. Of the 12 full publications that met the predetermined inclusion criteria, ten (King et al., 2006; Huang et al., 2007; S. Tural Emon et al., 2011; Figueroa et al., 2012, 2013, 2016; Liu et al., 2015, 2017; Tremoleda et al., 2016; Manzhulo et al., 2018a) were published in English and two (Jiang et al., 2016; Yu and Guo, 2017) were published in Chinese (Table 1).
Figure 1.
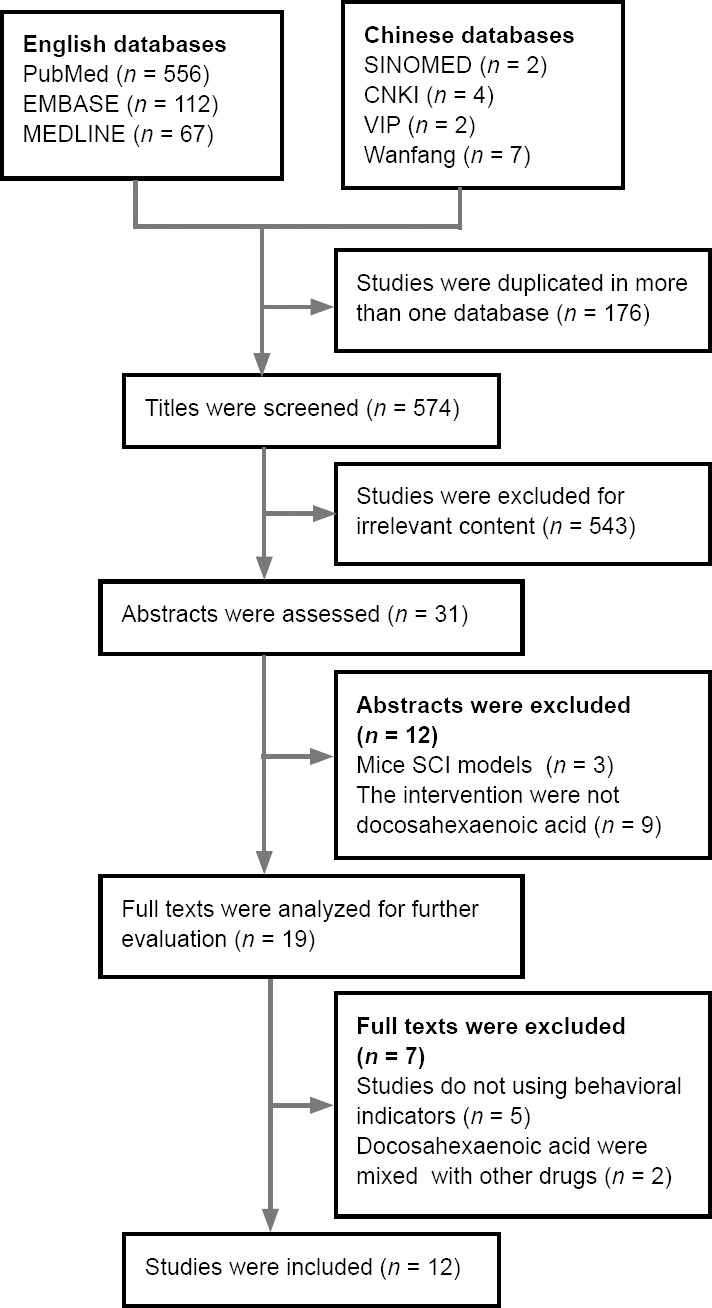
Flow diagram of studies examined in this systematic review.
A total of 750 potentially relevant studies were identified. After removing duplicates and further screening of the title and abstracts, 731 studies were excluded and 19 studies were used for full-text assessment. From this, seven studies were excluded after the full texts were analyzed and screened. CNKI: China National Knowledge Infrastructure; SCI: spinal cord injury.
Table 1.
Description of the characteristics of studies included in the meta-analysis
| Study | Animals (body weight) | Model | Groups (n) | Behavioral outcome | Motor function assessment time |
|---|---|---|---|---|---|
| King et al. (2006) | Male Wistar rats (200–250 g) | 1. T8, hemisection | SCI + vehicle (n = 8) | Beam walk | 1, 3, 5, 7 d |
| SCI + DHA, 250 nmol/kg, iv (n = 10) | Horizontal ladder | ||||
| SCI + OA, 250 nmol/kg, iv (n = 10) | BBB | ||||
| SCI + ALA, 250 nmol/kg, iv (n = 5) | |||||
| SCI + AA, 250 nmol/kg, iv (n = 5) | |||||
| 2. T8, contusion | SCI + vehicle (n = 5) SCI + DHA, 250 nmol/kg, iv (n = 5) |
BBB | 1, 4, 6 wk | ||
| Huang et al. (2007) | Female SD rats (230–255 g) | T12, contusion | SCI + vehicle (n = 5) SCI + DHA, 250 nmol/kg, iv (n = 5) SCI + DHA, iv + DHA, 400 mg/kg per day, po (n = 5) |
BBB | 1–14 d three times a week, for 3–6 wk |
| Emon et al. (2011) | Male SD rats (250–300 g) | Contusion | SCI + vehicle (n = 9) SCI + Fish-oil , 5 mL/kg, ip (DHA 72–154.5 mg/kg) (n = 9) |
BBB | 0, 7, 14, 21, 28, 35 d |
| Figueroa et al. (2012) | Female SD rats (200–250 g) | T10, contusion | Sham + vehicle (n = 6) Sham + DHA, 250 nmol/kg, iv (n = 4) SCI + vehicle (n = 9) SCI + DHA, 250 nmol/kg, iv (n = 10) |
BBB | 1, 3, 5, 7 d |
| Figueroa et al. (2013) | Female SD rats (182–212 g ) | T10, contusion | Sham + control diet (n = 8) Sham + DHA diet (DHA 500 mg/kg per day) (n = 19) SCI + control diet (n = 19) SCI + DHA diet (n = 8) |
BBB | 1–8 wk |
| Liu et al. (2015) | Female SD rats (250–300 g) | C5, hemisection | Sham (n = 5) | BBB | 1–14 d |
| SCI + vehicle (n = 5) | Staircase test | ||||
| C. SCI + DHA, 250 nmol/kg, iv (n = 5) | Grid exploration test | ||||
| Figueroa et al. (2016) | Female SD rats | T10, contusion | SCI + control diet (n = 24) SCI + DHA diet (DHA 500 mg/kg per day) (n = 24) |
BBB | 7 d |
| Jiang et al. (2016) | Male SD rats (200–250 g) | T10, contusion | Sham (n = 20) SCI (n = 20) SCI + DHA, 1000 nmol/kg, iv (n = 20) |
BBB Inclined plane test | 1, 3, 7, 21 d |
| Tremoleda et al. (2016) | Male Wistar rats (265 ± 35.4 g) | T10, contusion | Non-injured (n = 6) Sham (n = 8) SCI (n = 10) SCI + vehicle (n = 6) SCI + DHA, 250 nmol/kg, iv (n = 6) |
BBB | 6–7 d |
| Liu et al. (2017) | Female SD rats (250–300 g) | C4–5, hemisection | SCI + vehicle (n = 14) SCI + DHA, 250 nmol/kg, iv (n = 14) SCI + vehicle + rehabilitation training (n = 14) SCI + DHA + rehabilitation training (n = 14) |
Staircase test Grid exploratory test | Staircase test for 1–20 d; grid exploratory test for 1, 2, 3 wk |
| Yu and Guo (2017) | Male SD rats (300 ± 20 g) | T9–10, contusion | Sham (n = 36) SCI (n = 36) SCI + DHA, 500 mg/kg, ip (n = 36) |
BBB | 10, 24, 48, 72 h |
| Manzhulo et al. (2018a) | Female Wistar rats (240 ± 20 g) | T9, compression | Sham + vehicle (n = 14) SCI + vehicle (n = 14) SCI + DHA, 45 mg/kg, ih (n = 14) |
BBB | 1–5 wk |
AA: Arachidonic acid; ALA: α-linolenic acid; BBB: Basso, Beattie, and Bresnahan scale; C: Cervical vertebrae; DHA: docosahexaenoic acid; ih: hypodermic injection; ip: intraperitoneal injection; iv: intravenous injection; OA: oleic acid; po: peros; SCI: spinal cord injury; SD: Sprague-Dawley; T: thoracic vertebrae.
Characteristics of the studies included in the meta-analysis
The sample size of the included studies ranged from 15 to 108. Nine studies reported the use of a spinal cord contusion or compression rat model (Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2012, 2013, 2016; Tremoleda et al., 2016; Yu and Guo, 2017; Manzhulo et al., 2018a). Two used a rat model of spinal cord hemisection (Liu et al., 2015, 2017). One reported both a spinal cord contusion rat model and spinal cord hemisection rat model (King et al., 2006). In two reports, DHA was administered using a fish-oil enriched diet (DHA, 500 mg/kg per day; Figueroa et al., 2013, 2016). In six reports, DHA was administered by tail vein injection: five (King et al., 2006; Figueroa et al., 2012; Liu et al., 2015, 2017; Tremoleda et al., 2016) used a dose of 250 nmol/kg, and one (Jiang et al., 2016) used a dose of 1000 nmol/kg. In one report, DHA was administered by intraperitoneal injection of fish-oil at a dose of 5 mL/kg (DHA 72.0–154.5 mg/kg) (Emon et al., 2011), while in another, DHA was administered by intraperitoneal injection at a dose of 500 mg/kg (Yu and Guo, 2017). In one study, DHA was administered by hypodermic injection at a dose of 45 mg/kg (Manzhulo et al., 2018a). In another report, one group of rats received 250 nmol/kg DHA by tail vein injection and control diet, another group receiving 250 nmol/kg DHA by tail vein injection and DHA-enriched diet (containing 400 mg/kg per day DHA), while the control group received saline injection and control diet (Huang et al., 2007). BBBs was used to assess the motor function of rats in eleven experiments (King et al., 2006; Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2012, 2013, 2016; Liu et al., 2015; Jiang et al., 2016; Tremoleda et al., 2016; Yu and Guo, 2017; Manzhulo et al., 2018a), while the beam walk horizontal ladder (King et al., 2006), inclined plane test (Jiang et al., 2016), grid and exploratory test (Liu et al., 2017) were each used once, and the Montoya staircase test (Liu et al., 2015, 2017) was used twice.
Quality of the included studies
The risk of bias in all the included studies was assessed with SYRCLE’s RoB tool (Figure 2 and Table 2). Three were considered to be of relatively high quality (Emon et al., 2011; Jiang et al., 2016; Tremoleda et al., 2016). Three studies described allocation concealment, which was achieved using sealed envelopes and random number tables (Emon et al., 2011; Jiang et al., 2016; Yu and Guo, 2017). Eight of the studies described blinding of assessors (Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2012, 2016; Liu et al., 2015, 2017; Jiang et al., 2016; Yu and Guo, 2017). No study described whether or not sequence generation, random housing, blinding of caregivers, random outcome assessment or contamination-free environment had been performed or if any new animals were added to the sample set. All the studies were of high quality regarding baseline characteristics and all were free of units of analysis errors, with no design-specific risk of bias. All studies described similar outcomes. Two studies were unable to show complete data because of death in a group, yet neither provided a reason in the data (Huang et al., 2007; Figueroa et al., 2012). Four studies reported potential conflicts of interest and sources of study funding (Figueroa et al., 2012, 2013, 2016; Tremoleda et al., 2016; Manzhulo et al., 2018a). Assessment of overall study quality indicated that in the majority the quality was not high.
Figure 2.
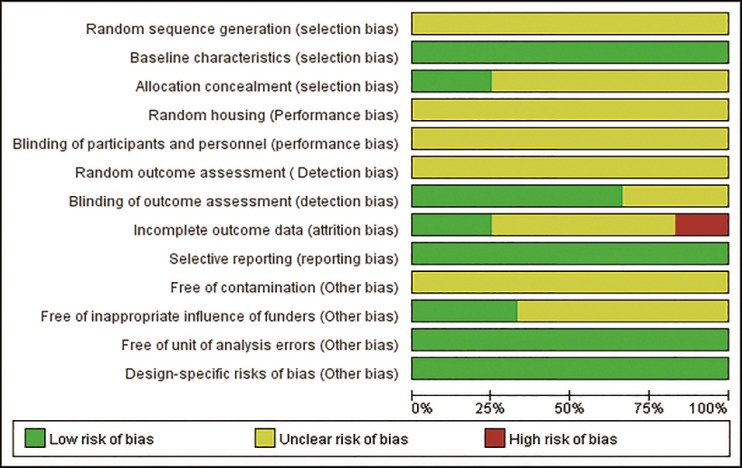
Risk of bias evaluated by SYRCLE’s RoB tool in the literature.
The figure shows the percentage of research with different levels of bias. Three were considered to be of relatively high quality. These studies described allocation concealment, which was achieved using sealed envelopes and random number tables. Eight of the studies described blinding of assessors. No study described sequence generation, random housing, blinding of caregivers, random outcome assessment, contamination-free environment, and whether new animals were added to the sample set, although these may have been performed as well. All the studies were of high quality regarding baseline characteristics and were free of units of analysis errors, with no design-specific risk of bias. All studies described the similar results. Two studies were unable to show complete data because of death in the sample, yet neither provided a solution on the data. It may cause attriton bias. Four studies reported potential conflicts of interest and sources of study funding.
Table 2.
Risk of bias evaluated by SYRCLE’s RoB tool
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation | Baseline characteristics | Allocation concealment | Random housing | Blinding | Random outcome assessment | Blinding | Incomplete outcome data | Selective outcome reporting | Free of contamination | Free of inappropriate influence of funders | Free of unit of analysis errors | Design-specific risks of bias | New animals added to replace drop-outs | |
| King et al. (2006) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Huang et al. (2007) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | No | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Emon et al. (2011) | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Figueroa et al. (2012) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | No | Yes | Unclear | Yes | Yes | Yes | Unclear |
| Figueroa et al. (2013) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes | Unclear |
| Liu et al. (2015) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Figueroa et al. (2016) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Unclear |
| Jiang et al. (2016) | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Tremoled et al. (2016) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear |
| Liu et al. (2017) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Yu and Guo (2017) | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Manzhulo et al. (2018a) | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear |
Neurological recovery effect of DHA
BBB score at different time points after SCI
Our analysis indicated that rats receiving DHA therapy obtained a higher BBB score, the mean BBB score of the DHA group reaching a peak at 42 days after SCI (BBBs = 15.54 ± 1.07) (Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2013; Jiang et al., 2016; Manzhulo et al., 2018a; Figure 3). The therapeutic effect of DHA, measured as the differences in BBBs between the DHA and control groups, increased rapidly during the first 7 days after SCI (King et al., 2006; Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2012, 2013; Liu et al., 2015; Tremoleda et al., 2016; Figure 4). This increase slowed down from the 7th (King et al., 2006; Emon et al., 2011; Figueroa et al., 2012, 2013; Huang et al., 2007; Liu et al., 2015; Tremoleda et al., 2016) (pooled MD = 3.16; 95% CI: 2.77–3.56; P < 0.00001) to the 21st day after SCI (Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2013; Jiang et al., 2016; Manzhulo et al., 2018a), and the mean difference of BBB score between the two group reaching a peak at 21 days after SCI (pooled MD = 4.14; 95% CI = 3.58–4.70; P < 0.00001). There was little change in inter-group difference between the 7th day to the 42nd day (pooled MD = 2.72; 95% CI = 2.04–3.40; P < 0.00001) after SCI.
Figure 3.
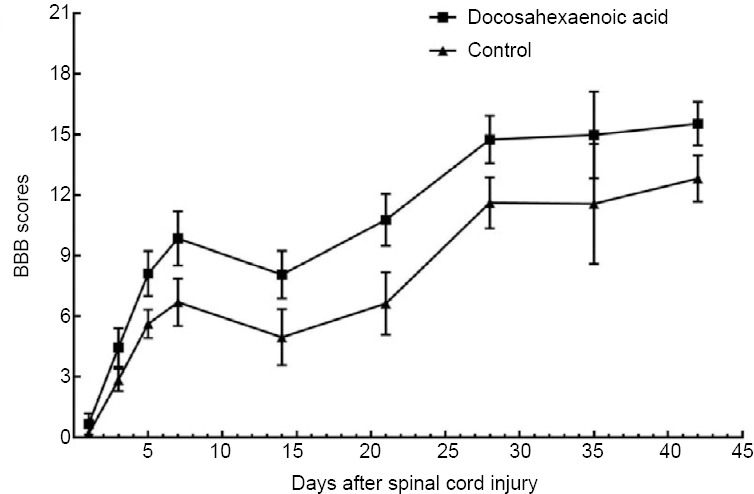
BBB scores of the docosahexaenoic acid and control groups at different time points.
By summarizing BBB scores in spinal cord injury (SCI) rats at different time points, we found that the rats received docosahexaenoic acid therapy obtained a higher BBB score, reaching a peak at 42 days after SCI (BBB scores = 15.54 ± 1.07). During the first 7 days after SCI, BBB scores of the docosahexaenoic acid group increased rapidly. The BBB scores of the control group have a similar but slower trend. BBB: Basso, Beattie, and Bresnahan scale.
Figure 4.
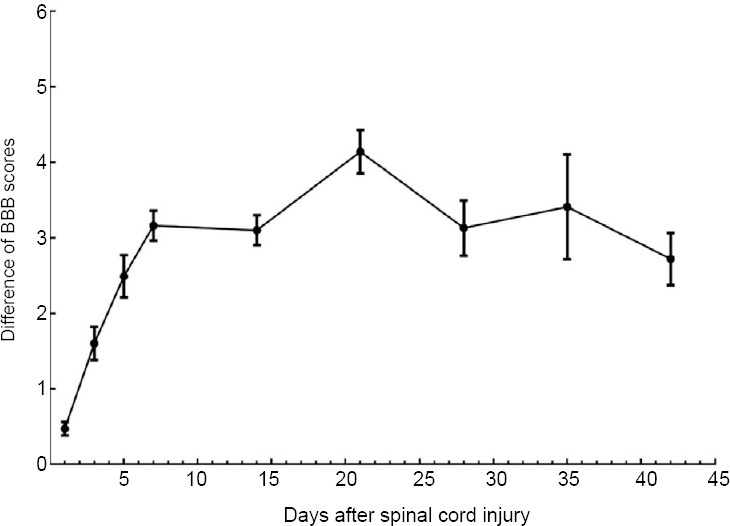
Difference of BBB scores between the docosahexaenoic acid and control groups.
The therapeutic effect of docosahexaenoic acid was expressed as the difference of BBB scores in the docosahexaenoic acid group minus BBB scores in the control group. During the first 7 days after spinal cord injury, the differences in BBB scores between the docosahexaenoic acid and control groups increased rapidly, then plateaued. BBB: Basso, Beattie, and Bresnahan scale.
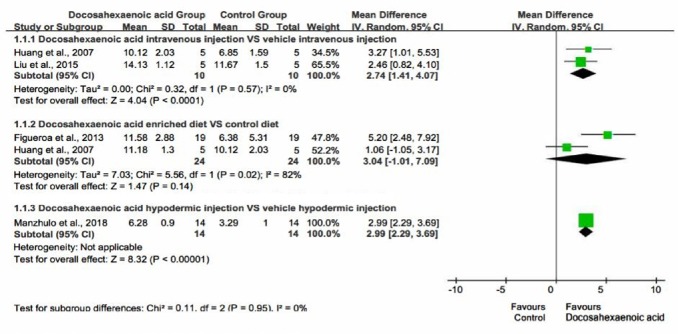
Effect of oral, intravenous injection and hypodermic injection of DHA on BBBs
Subgroup analysis was performed based on the method of DHA administration (Huang et al., 2007; Figueroa et al., 2013; Liu et al., 2015; Manzhulo et al., 2018a). Because the largest number of rats received the DHA enriched diet and those injected with DHA hypodermically had their BBBs assessed 14 days after SCI, we chose the BBBs at 14th day after SCI as the outcome indicator. Intravenous injection (Huang et al., 2007; Liu et al., 2015) (pooled MD = 2.74; 95% CI = 1.41–4.07; P < 0.0001) and hypodermic injection (Manzhulo et al., 2018a) (pooled MD = 2.99; 95% CI = 2.29–3.69; P < 0.00001) of DHA significantly promoted the recovery of motor function in SCI rats. However, this was not as marked in rats that received oral administration of DHA (pooled MD = 3.04; 95% CI = –1.01 to 7.09; P = 0.14) compared with control group (Huang et al., 2007; Figueroa et al., 2013; Figure 5). In one study, DHA was administrated by intraperitoneal injection, but the data are not clear enough for subgroup meta-analysis (Emon et al., 2011).
Figure 5.
Effect of oral, intravenous, and hypodermic injection of docosahexaenoic acid on Basso, Beattie, and Bresnahan scale scores.
Intravenous and hypodermic injection of docosahexaenoic acid significantly promoted the recovery of motor function in spinal cord injury rats. However, the recovery of motor function was not as marked in rats that received oral administration of docosahexaenoic acid compared with intravenous injection docosahexaenoic acid.
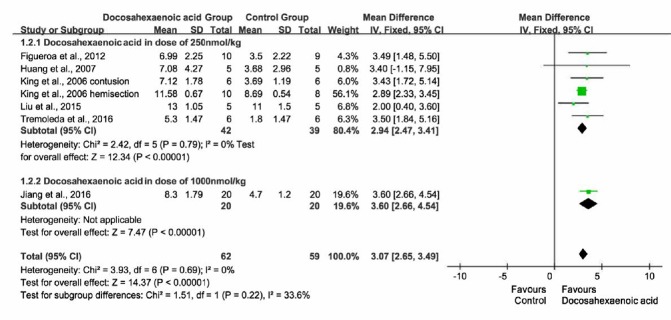
Effect of intravenous injection of different doses of DHA on BBBs
Subgroup analysis was performed based on DHA injection doses. Rats which received DHA injection in doses of 1000 nmol/kg, had BBBs measured at 7 days after SCI, therefore BBBs at 7 days after SCI were chosen as the outcome indicator. Eight studies were included (King et al., 2006; Huang et al., 2007; Figueroa et al., 2012, 2013, 2016; Liu et al., 2015; Jiang et al., 2016; Tremoleda et al., 2016). Intravenous injection of DHA at a dose of 250 nmol/kg (King et al., 2006; Huang et al., 2007; Figueroa et al., 2012; Liu et al., 2015; Tremoleda et al., 2016) (pooled MD = 2.94; 95% CI = 2.47–3.41; P < 0.00001) resulted in a similar effect on recovery of motor function as a dose of 1000 nmol/kg (pooled MD = 3.60; 95% CI = 2.66 to 4.54; P < 0.00001; Jiang et al., 2016; Figure 6).
Figure 6.
Effect of intravenous injection of different doses of docosahexaenoic acid on Basso, Beattie, and Bresnahan scale scores.
Intravenous injection of docosahexaenoic acid at a dose of 250 nmol/kg resulted in a similar effect on the recovery of motor function to a dose of 1000 nmol/kg.
Further evaluation of motor function after DHA treatment
In one study, locomotor performance after spinal cord hemisection was performed in animals that received a single intravenous injection of either saline or various polyunsaturated fatty acids (King et al., 2006). DHA-treated animals performed significantly improved on the beam walk (King et al., 2006). Furthermore, the DHA group performance, measured by the number of hindlimb slips in the horizontal ladder test, improved more when compared with the other PUFA groups (including an α-linolenic acid group) and the SCI only group (King et al., 2006). In another study, at four different time points (10, 24, 48, and 72 hours after SCI), the DHA group tolerated a greater angle than the control SCI group in the inclined plane test. In two studies by the same researcher, the performance in the DHA group was better than that in the control group on the staircase test and grid exploratory test (Liu et al., 2015, 2017).
Publishing bias
A funnel plot has been made to analyze the publishing bias. The BBBs at 7 days after SCI was chosen for analysis, as most of the studies measured the BBBs at this time point. There were 10 studies involving 11 experiments (King et al., 2006; Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2012, 2013, 2016; Liu et al., 2015; Jiang et al., 2016; Tremoleda et al., 2016; Manzhulo et al., 2018a). Most of the studies chose DHA administrated by intravenous injection (King et al., 2006; Huang et al., 2007; Figueroa et al., 2012; Liu et al., 2015; Jiang et al., 2016; Tremoleda et al., 2016) and we analyzed the publishing bias of these 6 studies (7 experiments). We found that there was no obvious publishing bias in the research (Figure 7).
Figure 7.
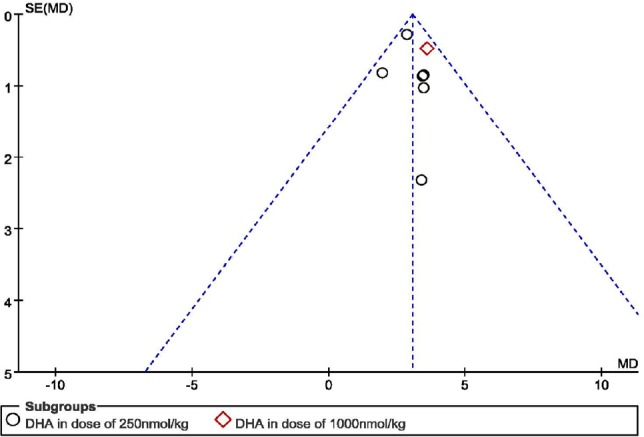
Publication bias in the studies regarding Basso, Beattie, and Bresnahan scale scores in rats at 7 days after spinal cord injury.
Discussion
Summary of the evidence
To our knowledge this is the first systematic review to assess the effect of DHA in rats of SCI. This review summarizes 12 recent studies on the laboratory administration of DHA in rat models of SCI to give a preclinical indication of its effectiveness. Meta-analysis confirmed individual studies that DHA administration significantly improved functional recuperation in rat models of SCI.
In general, the methodological quality of the included studies was low. There are 13 items in SYRCLE’s RoB tool, yet the three highest quality studies contained only seven items that conformed to SYRCLE’s RoB tool.
We found that, contrasted with the SCI control group, the ability of DHA to promote recovery of motor function significantly and positively correlated with time over a one-week period. After one week, although motor function continued to improve more gradually, the ability of DHA to promote the recovery of motor function, relative to SCI only, did not improve further. From 28 days after SCI, this ability did not change and remained at the same level. Different dosages and manner of administration of DHA gave slight changes in the degree of recovery. At 14 days after SCI, the motor function of rats fed a DHA-enriched diet did not significantly improve compared with rats fed a control diet. DHA was injected intravenously at doses of 250 nmol/kg in many studies and 1000 nmol/kg in one, with both doses significantly promoting motor function recovery. These variations make it difficult for us to reach a quantitative conclusion.
Strengths and weaknesses
This is the first systematic review on DHA treatment in SCI rat models and that searched Chinese databases to include Chinese studies. In this review, the relationship between time and ability of DHA has been analyzed to promote the recovery of motor function. We also analyzed the effect of different doses and dosage forms of DHA on motor function recovery of SCI rats.
As in other systemic reviews, our study suffers from the shortage of original studies. Although we retrieved English and Chinese databases, we still cannot be sure that all relevant studies were identified, especially regarding the “gray” unpublished literature and conference proceedings. Indeed, the bias caused by selective publishing and reporting must be considered. Studies of large sample size and positive results are often easy to publish, which is most likely to cause publication bias, which affects the authenticity of our result. Considering publication bias, these estimations may be unjustifiable given the evidence. There are little resources for publishing negative results, especially for animal studies. Financial aid is an important source of heterogeneity. None of the included studies show that there was inappropriate influence from funders. None of the studies mentioned sequence generation, random housing, blinding of caregivers, random outcome, contamination, and inclusion of new animals. Only three studies described allocation concealment. Although we tried to analyze the efficacy of the different doses and dosage forms of DHA, there were too few related studies. Therefore, our conclusions are not unequivocal.
Possible mechanism for the effect of DHA in SCI
Polyunsaturated fatty acids are oxidized by CYP2J2 via NADPH-dependent epoxidation, and the process plays an important role in inflammation (Kamel et al., 2018). Arachidonic acid, an omega-6 polyunsaturated fatty acid, can respond to stimuli and promote the release of inflammatory factors which may increase the damage of SCI (Tettamanti et al., 2018). Whereas, DHA is an omega-3 polyunsaturated fatty acid that promotes the anti-inflammatory action of immune cells, thus can protect nerve tissue from secondary damage in acute models of SCI (Manzhulo et al., 2018a). In all the studies contributing to our analysis, DHA showed neuroprotective effects in SCI rats (King et al., 2006; Huang et al., 2007; Emon et al., 2011; Figueroa et al., 2012, 2013, 2016; Liu et al., 2015, 2017; Jiang et al., 2016; Tremoleda et al., 2016; Yu and Guo, 2017; Manzhulo et al., 2018a). Specifically, DHA has been shown to reduce cavities in the white matter and neuronal edema, and it protects neurons and oligodendrocytes from damage against apoptosis and inflammation (King et al., 2006; Huang et al., 2007; Emon et al., 2011). DHA can promote pAKT expression, which can protect neurons and oligodendrocytes from apoptosis (King et al., 2006; Figueroa et al., 2012). DHA has a significant antioxidant function, decreases RNA/DNA oxidation and protein oxidation, which have disruptive effects on neurons, oligodendrocytes, and other normal cells (King et al., 2006; Huang et al., 2007; Manzhulo et al., 2018a).
We found that in contrast to the control group, the ability of DHA to promote the recovery of motor function in rats was temporal and rapidly progressive, increasing in the first week after SCI. From one-week to three-weeks after SCI, this increase slowed down. At 21 days after SCI, the ability of DHA to promote the recovery of motor function reached a peak, and then plateaued. Nonetheless, BBBs in both DHA and control groups was sustained after initially increasing. We believe the reason for this is that the self-healing ability of rats increases BBBs in control groups, whereas the motor function regained never decreases in DHA groups. After SCI, the spinal canal circulation loop may partially rearrange to compensate for loss of function, which may reflect a long-term change (Bareyre et al., 2004). After SCI, glial cells proliferate and secrete neurotrophic factors (brain-derived neurotrophic factor, nerve growth factor, and glial cell-line derived neurotrophic factor) which are potent neuroprotective agents (Shibuya et al., 2003; Kimura et al., 2016). The induced endogenous neural stem cell proliferation and differentiation promote the recovery of spinal cord function (Yamamoto et al., 2004). Other studies showed that increases in stem cell number are found but are not marked at 3 days after SCI. After 14 days, most stem cells differentiate primarily into astrocytes and are concentrated around the injury site (Mothe and Tator, 2005). These findings are consistent with our conclusions.
Comparison of different doses and dosage forms of DHA
DHA is usually administrated by vein injection or DHA-enriched daily diet. Intravenous injections of DHA clearly improve BBB and promote motor function recovery using SCI rat models. However, compared with vein injection, a DHA-enriched daily diet may have a smaller short-term effect on motion function recovery after SCI. This conclusion is similar to the previously held view that DHA treatment is ineffective if administered for 1 week solely by diet (Huang et al., 2007). We suggest that oral DHA may suffer a change in structure due to action by the liver, thus affecting its efficacy. However, there are few studies on this aspect, and we cannot make a definitive conclusion on this point. For this reason, pharmacokinetic studies of DHA in rats are necessary for future research. In a few cases, the investigator had administered DHA by intraperitoneal or subcutaneous injection and these also promoted the functional recovery of SCI rats (Emon et al., 2011; Manzhulo et al., 2018a). We compared the treatment effect of intravenous injection of different doses of DHA in SCI rat models. We found no significant differences among the different doses. However, all the included studies, bar one at 1000 nmol/kg, used a dose of 250 nmol/kg. Thus, it is difficult for us to demonstrate a dose-effect curve. In the future, more research is needed to test a comprehensive dose-effect relationship between DHA and motor recovery.
In summary, DHA administration significantly improves the functional recuperation in rat models of SCI. Intravenous injection of DHA can improve motor function recovery in SCI rats, while a DHA-enriched daily diet has a small short-term effect. Doses of DHA, intravenously injected, at 250 nmol/kg and 1000 nmol/kg have similar effects. The effects of different forms of administration and the dose-effect relationship of DHA need further investigation before clinical trials are instigated.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81704096, 81603635, 81873317 (to MY, JY, XJC); Shanghai Science and Technology Commission - Key Project of Traditional Chinese Medicine, No. 16401970100 (to YJW); the Shanghai Traditional Chinese Medicine Medical Center of Chronic Disease of China, No. 2017ZZ01010 (to YJW); the National Thirteenth Five-Year Science and Technology Major Special Project for New Drug Innovation and Development of China, No. 2017ZX09304001 (to YJW); the Program for Innovative Research Team of Ministry of Science and Technology of China, No. 2015RA4002 (to YJW); the “Innovation Team” Development Projects of China, No. IRT1270 (to YJW); the Three Years Action to Accelerate the Development of Traditional Chinese Medicine Plan of China, No. ZY(2018-2020)-CCCX-3003 (to YJW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Reporting statement: This study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the bio-statistician of Institute of Spine Disease, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81704096, 81603635, 81873317 (to MY, JY, XJC); Shanghai Science and Technology Commission - Key Project of Traditional Chinese Medicine, No. 16401970100 (to YJW); the Shanghai Traditional Chinese Medicine Medical Center of Chronic Disease of China, No. 2017ZZ01010 (to YJW); the National Thirteenth Five-Year Science and Technology Major Special Project for New Drug Innovation and Development of China, No. 2017ZX09304001 (to YJW); the Program for Innovative Research Team of Ministry of Science and Technology of China, No. 2015RA4002 (to YJW); the “Innovation Team” Development Projects of China, No. IRT1270 (to YJW); the Three Years Action to Accelerate the Development of Traditional Chinese Medicine Plan of China, No. ZY(2018-2020)-CCCX-3003 (to YJW).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Dawes EA, Frenchman B, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 2.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 3.Beckers A, Moons L. Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration? Neural Regen Res. 2019;14:1313–1316. doi: 10.4103/1673-5374.253505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumont AS, Dumont RJ, Oskouian RJ. Will improved understanding of the pathophysiological mechanisms involved in acute spinal cord injury improve the potential for therapeutic intervention? Curr Opin Neurol. 2002;15:713–720. doi: 10.1097/01.wco.0000044768.39452.5b. [DOI] [PubMed] [Google Scholar]
- 5.Efeoglu M, Akoglu H, Akoglu T, Eroglu SE, Onur OE, Denizbasi A. Spinal trauma is never without sin: a tetraplegia patient presented without any symptoms. Turk J Emerg Med. 2014;14:188–192. doi: 10.5505/1304.7361.2014.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emon ST, Irban AG, Bozkurt SU, Akakin D, Konya D, Ozgen S. Effects of parenteral nutritional support with fish-oil emulsion on spinal cord recovery in rats with traumatic spinal cord injury. Turk Neurosurg. 2011;21:197–202. doi: 10.5137/1019-5149.JTN.3523-10.2. [DOI] [PubMed] [Google Scholar]
- 7.Estrada V, Müller HW. Micromechanical adaptation as a treatment for spinal cord injury. Neural Regen Res. 2019;14:1909–1911. doi: 10.4103/1673-5374.259605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson AR, Hook MA, Garcia G, Bresnahan JC, Beattie MS, Grau JW. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Ruiz JC, Ramos-Remus C, Sanchez-Corona J, Castillo-Ortiz JD, Castaneda-Sanchez JJ, Bastian Y, Romo-Garcia MF. Analysis of miRNA expression in patients with rheumatoid arthritis during remission and relapse after a 5-year trial of tofacitinib treatment. Int Immunopharmacol. 2018;63:35–42. doi: 10.1016/j.intimp.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa JD, Cordero K, Baldeosingh K, Torrado AI, Walker RL, Miranda JD, Leon MD. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J Neurotrauma. 2012;29:551–566. doi: 10.1089/neu.2011.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueroa JD, Cordero K, Llan MS, De Leon M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. J Neurotrauma. 2013;30:853–868. doi: 10.1089/neu.2012.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa JD, De Leon M. Neurorestorative targets of dietary long-chain omega-3 fatty acids in neurological injury. Mol Neurobiol. 2014;50:197–213. doi: 10.1007/s12035-014-8701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa JD, Serrano-Illan M, Licero J, Cordero K, Miranda JD, De Leon M. Fatty Acid binding protein 5 modulates docosahexaenoic acid-induced recovery in rats undergoing spinal cord injury. J Neurotrauma. 2016;33:1436–1449. doi: 10.1089/neu.2015.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall JCE, Priestley JV, Perry VH, Michael-Titus AT. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J Neurochem. 2012;121:738–750. doi: 10.1111/j.1471-4159.2012.07726.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holly LT, Blaskiewicz D, Wu A, Feng C, Ying Z, Gomez-Pinilla F. Dietary therapy to promote neuroprotection in chronic spinal cord injury. J Neurosurg Spine. 2012;17:134–140. doi: 10.3171/2012.5.SPINE1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Method. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, Priestley JV. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 19.Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- 20.Jiang CS, Lv Y, Chen JC, Meng XS, Zhang J, Liu Y. Effect of docosahexaenoic acid on neurological recovery in rats with spinal cord injury and its mechanism. Shan Dong Yi Yao. 2016;56:32–34. [Google Scholar]
- 21.Kamel S, Ibrahim M, Awad ET, El-Hindi H, Abdel-Aziz SA. Differential expression of CYP2j2 gene and protein in Camelus dromedarius. J Biol Regul Homeost Agents. 2018;32:1473–1477. [PubMed] [Google Scholar]
- 22.Kimura A, Namekata K, Guo X, Harada C, Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int J Mol Sci. 2016;17:E1584. doi: 10.3390/ijms17091584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26:4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu ZH, Yip PK, Adams L, Davies M, Lee JW, Michael GJ, Priestley JV. A Single bolus of docosahexaenoic acid promotes neuroplastic changes in the innervation of spinal cord interneurons and motor neurons and improves functional recovery after spinal cord injury. J Neurosci. 2015;35:12733–12752. doi: 10.1523/JNEUROSCI.0605-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ZH, Yip PK, Priestley JV, Michael-Titus AT. A single dose of docosahexaenoic acid increases the functional recovery promoted by rehabilitation after cervical spinal cord injury in the rat. J Neurotrauma. 2017;34:1766–1777. doi: 10.1089/neu.2016.4556. [DOI] [PubMed] [Google Scholar]
- 26.Ma JH, Zhang D, Huo YL, Wei YY, Sun L, Zhao Y. Lentiviral-mediated transplantation of olfactory ensheathing cells modified by ciliary neurotrophic factor for treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:2709–2715. [Google Scholar]
- 27.Mahabaleshwarkar R, Khanna R. National hospitalization burden associated with spinal cord injuries in the United States. Spinal Cord. 2014;52:139–144. doi: 10.1038/sc.2013.144. [DOI] [PubMed] [Google Scholar]
- 28.Maixent JM, Fares M, Francois C, Delmotte A, Rigoard P. Docosahexaenoic acid (DHA) injection in spinal cord transection stimulates Na(+), K(+)-ATPase in skeletal muscle via beta 1 subunit. Cell Mol Biol (Noisy-le-grand) 2014;60:22–29. [PubMed] [Google Scholar]
- 29.Manzhulo I, Tyrtyshnaia A, Kipryushina Y, Dyuizen I, Ermolenko E, Manzhulo O. Docosahexaenoic acid improves motor function in the model of spinal cord injury. Neurosci Lett. 2018a;672:6–14. doi: 10.1016/j.neulet.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Manzhulo O, Tyrtyshnaia A, Kipryushina Y, Dyuizen I, Manzhulo I. Docosahexaenoic acid induces changes in microglia/macrophage polarization after spinal cord injury in rats. Acta Histochem. 2018b;120:741–747. doi: 10.1016/j.acthis.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Mu Q, Liu P, Hu X, Gao H, Zheng X, Huang H. Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage. Neural Regen Res. 2014;9:1621–1627. doi: 10.4103/1673-5374.141791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie J, Chen J, Yang J, Pei Q, Li J, Liu J, Xu L. Inhibition of mammalian target of rapamycin complex 1 signaling by n-3 polyunsaturated fatty acids promotes locomotor recovery after spinal cord injury. Mol Med Rep. 2018;17:5894–5902. doi: 10.3892/mmr.2018.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning G, Wu Q, Li Y, Feng S. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2013;35:229–239. doi: 10.1179/2045772312Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannek J, Krebs J, Wöllner J. Influence of bladder management on long-term quality of life in patients with neurogenic lower urinary tract dysfunction. Asia Pac J Clin Trials Nerv Syst Dis. 2019;4:29–32. [Google Scholar]
- 36.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 37.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuropsiquiatr. 2017;75:387–393. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 38.Schober ME, Requena DF, Abdullah OM, Casper TC, Beachy J, Malleske D, Pauly JR. Dietary docosahexaenoic acid improves cognitive function, tissue sparing, and magnetic resonance imaging indices of edema and white matter injury in the immature rat after traumatic brain injury. J Neurotrauma. 2016;33:390–402. doi: 10.1089/neu.2015.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Goel SA, Sharma S, Chhabra HS. Polyetheretherketone cages used in anterior cervical discectomy and fusion surgery: a meta-analysis. Clin Trials Orthop Disord. 2019;4:29–33. [Google Scholar]
- 40.Shibuya S, Miyamoto O, Itano T, Mori S, Norimatsu H. Temporal progressive antigen expression in radial glia after contusive spinal cord injury in adult rats. Glia. 2003;42:172–183. doi: 10.1002/glia.10203. [DOI] [PubMed] [Google Scholar]
- 41.Tettamanti L, Kritas SK, Gallenga CE, D’Ovidio C, Mastrangelo F, Ronconi G, Caraffa A. IL-33 mediates allergy through mast cell activation: potential inhibitory effect of certain cytokines. J Biol Regul Homeost Agents. 2018;32:1061–1065. [PubMed] [Google Scholar]
- 42.Tremoleda JL, Thau-Zuchman O, Davies M, Foster J, Khan I, Vadivelu KC, Yip PK. In vivo PET imaging of the neuroinflammatory response in rat spinal cord injury using the TSPO tracer [(18)F]GE-180 and effect of docosahexaenoic acid. Eur J Nucl Med Mol Imaging. 2016;43:1710–1722. doi: 10.1007/s00259-016-3391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current options for cell therapy in spinal cord injury. Trends Mol Med. 2017;23:831–849. doi: 10.1016/j.molmed.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Walker RM, Gillespie BM, Thalib L, Higgins NS, Whitty JA. Foam dressings for treating pressure injuries in patients of any age in any care setting: an abridged Cochrane systematic review. Int J Nurs Stud. 2018;87:140–147. doi: 10.1016/j.ijnurstu.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage after spinal cord injury. J Neurotrauma. 2010;27:1769–1780. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- 46.Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28:2113–2122. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto S, Uchimura K, Wakayama M, Tachiki T. Purification and characterization of glutamine synthetase of Pseudomonas taetrolens Y-30: an enzyme usable for production of theanine by coupling with the alcoholic fermentation system of baker’s yeast. Biosci Biotechnol Biochem. 2004;68:1888–1897. doi: 10.1271/bbb.68.1888. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Yao M, Lan Y, Mo W, Sun YL, Wang J, Wang YJ, Cui XJ. Melatonin for spinal cord injury in animal models: a systematic review and network meta-analysis. J Neurotrauma. 2016;33:290–300. doi: 10.1089/neu.2015.4038. [DOI] [PubMed] [Google Scholar]
- 49.Yao M, Yang L, Wang J, Sun YL, Dun RL, Wang YJ, Cui XJ. Neurological recovery and antioxidant effects of curcumin for spinal cord injury in the rat: a network meta-analysis and systematic review. J Neurotrauma. 2015;32:381–391. doi: 10.1089/neu.2014.3520. [DOI] [PubMed] [Google Scholar]
- 50.Yu SM, Guo WC. Discuss the function of docosahexaenoic acid in the early inflammatory response after acute spinal cord injury. Linchuang Waike Zazhi. 2017;25:301–303. [Google Scholar]