Focus of perspective: Numbers of visually-impaired adults are rising rapidly around the globe. Neuronal transplantation therapies show great promise to restore vision, but have been severely impeded by poor synaptic positioning within retinal host. This perspectives article highlights our group’s abilities to remarkably direct the migration of transplantable cells by applying electro-chemical fields within retina-customized microtechnology. Integration of our in vitro results with current ex vivo and in vivo studies has the potential to transformatively impact outcomes of cell replacement therapy.
Age-related vision loss: Progressive vision loss from age-related retinal disorders will impair an unprecedented additional 150 million adults, worldwide, by the year 2050 (Flaxman et al., 2017). The Human retina is a layered, neurosensory tissue that lines the eye posterior to transmit signals to the visual cortex via the optic nerve (Thoreson and Dacey, 2019) (Figure 1A). Retinal dysfunction is a leading cause of irreversible blindness from diseases like age-related macular degeneration, proliferative diabetic retinopathy and primary open angle glaucoma, which disproportionately affect mature and elderly adults (Figure 1B).
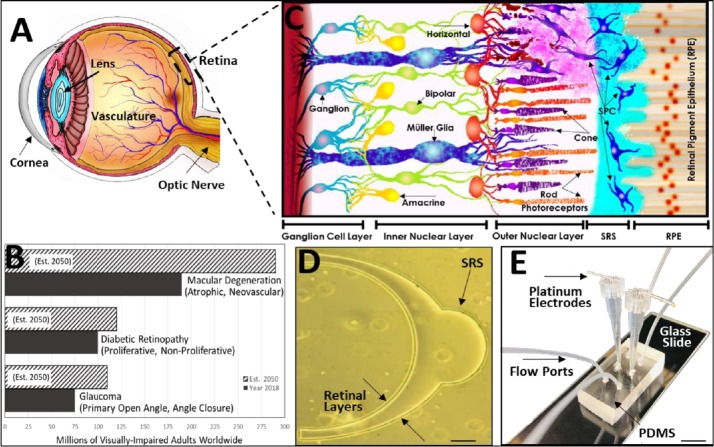
Figure 1.
Cell replacement therapy to treat age-related retinal degeneration requires the honed, directional migration of transplanted stem-like and progenitor cell donors (SPC).
(A) Rendering of the Human eye from corneal membrane at the eye anterior to retinal lining of the eye posterior. The eye lens, vasculature and optic nerve are also shown. (B) Current 2018 and projected 2050 numbers of mature adults visually-impaired by the three leading causes of retinal degeneration, worldwide: Macular degeneration, diabetic retinopathy and glaucoma (Flaxman et al., 2017). (C) Schematic of retinal cross-section from the retinal pigment epithelium (RPE) of the blood retinal barrier to the axons of the ganglion cell layer comprising the optic nerve (Mishra et al., 2019). SPC are transplanted into sub-retinal spaces (SRS) surgically created between the RPE and outer nuclear layer (ONL), where they must migrate to achieve positioning among host rod and cone photoreceptors. SPC able to functionally replace degenerated photoreceptors synapse with endogenous horizontal and/or bipolar neurons, which in turn synapse with amacrine and ganglion cells to transmit phototransductive signals for vision. (D) Retina-customized, microfluidic models developed by our group (Mishra et al., 2015) mimic the anatomical scale and spherical geometry of human retinal laminae, i.e., layers, alongside SRS microenvironments focal to surgical transplantation (scale bar: 100 μm). (E) Microfluidic systems readily-fabricated via poly-dimethylsiloxane (PDMS) elastomeric molding incorporate electrodes and flow ports within its system reservoirs to facilitate study of SPC migratory behaviors in response to external electric and biochemical fields (Unachukwu et al., 2016; Mishra and Vazquez, 2017), individually and in combination (scale bar: 1 mm).
Interfaces of technology and treatment: Modern diagnoses of retinal disorders and their commensurate treatments have been technologically-enriched by translational imaging modalities, biomimetic materials and tissue engineered, cellular constructs (Hunt et al., 2018). Advances in biotechnology have further pioneered the growth of regenerative medicine, in which the transplantation of specialized genes and stem cells is used to treat genetic disorders and diseases, respectively (Bobba et al., 2018). Surprisingly, precision microtechnologies have been sparingly applied in the development or advancement of contemporary cell replacement therapies, despite the dramatic rise of microfluidics applications in developmental biology (Sonnen and Merten, 2019). However, microfluidics enable singular opportunities for quantitative scrutiny of stem cell responses to a range of therapeutic and clinically-applied stimuli, including electromagnetic fields, pharmacological agents and customized extracellular matrix (ECM).
Cell replacement therapy: Vision loss via retinal degeneration is due in large part to the limited regeneration capacity of retinal photoreceptor cells, which absorb and convert light into electrochemical signals for vision via phototransduction (Thoreson and Dacey, 2019) (Figure 1C). Cell replacement therapies have transformative potential to restore vision by transplanting specialized donor cells to replace degenerated rod and cone photoreceptors. A wide variety of stem-like and progenitor cell donors (SPC) have been surgically inserted into sub-retinal spaces (SRS) created between photoreceptors of the outer nuclear layer (ONL) and the retinal pigment epithelium to re-activate damaged retinal circuitry. Here, signaling cues are believed to stimulate SPC into acquiring the phototransduction capabilities of photoreceptors, which enable them to serve as rod or cone replacements (Bobba et al., 2018). Unfortunately, the synaptic integration of SPC needed to re-establish vision in degenerated retina has yet to be realized, in large part because the process of SPC integration is highly complex and has been further compounded by recent evidence of SPC material transfer in a number of genetic models (Bobba et al., 2018). Donor SPC cells in an idealized integration model of age-degenerated retina must: 1) Migrate out of SRS transplantation sites; 2) Navigate into damaged retinal laminae; 3) Achieve functional positioning within host ONL; and 4) Synapse with endogenous secondary neurons (e.g., bipolar, horizontal cells) to restore vision (Figure 1C). While advancements in stem cell biology have cultivated sophisticated molecular and genetic techniques to derive photoreceptor replacements, low cell viability and unrealized differentiation have diminished success (Bobba et al., 2018). In response, a complement of cellular biomaterials and organoid models have been developed to foster and deliver viable donor cells as well as evaluate desired SPC differentiation using defined ECM structures, precise rheological properties and targeted biochemical factors that mimic the host retinal environment (Hunt et al., 2018). Despite these exciting innovations, however, the directional migration of surgically-inserted SPC into damaged laminae lies paramount to success in many transplantation models but remains largely underexplored. Modulation of these migratory processes requires a deeper understanding of SPC behaviors in response to the multivariate signaling cues produced by damaged, adult retina (Thoreson and Dacey, 2019).
Endogenous signals from neurosensory retina: The adult retinal microenvironment produces spatial and temporal gradients of multiple signals that include concentrations of biochemical factors, electrical potentials, substrate rigidity and adhesion kinetics of ECM (Thoreson and Dacey, 2019). Cell movement in response to these signaling factors is essential to the development and function of the visual system as well as to its plasticity and repair. A wealth of data has elucidated biochemical pathways able to regulate cellular positioning within complex retinal architectures across species (Herrera et al., 2019; Thoreson and Dacey, 2019). Key cytokines, such as stromal cell-derived factor 1, glial cell-derived neurotrophic factor and others expressed during late-stage development, stimulate SPC motility to configure the intricate neural networks needed for phototransduction and vision. Many of these proteins have also been implicated in SPC movement during retinal damage and repair via chemotactic mechanisms induced by their binding to cognate receptors. In parallel, electric fields (EF) play critical roles in the synaptic, physiological processes of both healthy and damaged neural tissues (Cortese et al., 2014). EF orient the extension of growth cones during development and support SPC survival, as well as increase neurite extension to promote synaptogenesis during repair. EF can additionally stimulate electrotaxis, i.e. the aligned migration of cells along electric potentials, in response to both developmental signals and cues of neural damage. Mechanisms of electrotactic movement have been proposed to occur via polarizing of membrane signaling molecules, attraction of membrane lipids via electro-osmosis, purinergic signaling from extracellular ATP as well as gated ion channels (Cortese et al., 2014).
External stimuli central to contemporary therapies: The use of external biochemical and electric fields to guide the migration of SPC during cell replacement therapy has been surprisingly underexplored despite inclusion of these stimuli in contemporary retinal treatments. Pharmacological inhibitors of vascular endothelial growth factor (e.g., Avastin, Eylea) are widely-used to limit cell migration during angiogenesis in diabetic retinopathy (proliferative and non-proliferative DR), while anti-transforming growth factor compounds (e.g., galunisertib, fresolimumab) are currently utilized to minimize Muller cell movement and glial scarring in age-related macular degeneration (atrophic and neovascular age-related macular degeneration). EF have been similarly applied to increase synaptic cell communication and improve visual acuity in patients with retinal disorders. Modern implants and prostheses (e.g., Argus II, Retina Implant Alpha AMS) have electrically stimulated damaged retinal ganglion cells to restore partial vision in patients with inherited and age-related retinal dysfunctions (Lorach et al., 2015). Such ongoing clinical progress has motivated our group to examine the use of external biochemical fields and EF to direct the migration and synaptic communication of transplantable SPC, respectively. We have developed retina-customized testing platforms using microfluidics to investigate the impacts of external chemotactic and EF stimuli in guiding the migration of photoreceptor replacement cells.
Cell migratory responses to microfluidically-enhanced stimuli: The complex nature of concurrent signals, and the range of SPC responses to them, requires experimental systems able to parse the impact of each field, individually, for comparison against combinatory effects. Microfluidics provide singular opportunities to create controlled microenvironments within which to expose SPC to tunable stimuli that are, both, analytically well-defined and experimentally-validated (Sonnen and Merten, 2019). Synergies between microfluidics and cell replacement therapy include miniaturized features that can be tailor-fabricated to mimic the hemispherical anatomy and scale of laminated retinal architecture, as well as annexation of sub-retinal microenvironments focal to transplantation. While a host of projects have used microdevices to measure cell motility, the focus of our microtechnology has been to recreate signals of endogenous retinal ligands and EF to modulate SPC migratory behaviors. Our testing platforms have incorporated different ECM molecules to represent the substrate rigidity and composition of the inter-photoreceptor matrix in which donor cells must migrate and reside, as well as evaluated the cell density, lineage composition and differentiation state of SPC groups needed to promote in vitro viability of primary donor cells (Unachukwu et al., 2016) (Figure 1D).
Using microfluidic systems comprised of poly-dimethyl siloxane elastomers and glass, we have reported that SPC were able to acquire motility in response to injury ligands expressed in degenerated retina. Further, experiments measured preferential SPC chemotaxis toward increasing concentration gradients of select ligands (Mishra et al., 2015; Unachukwu et al., 2016). Results illustrated significant increases in the phosphorylated cognate receptors of motile cells and further validated activation of canonical signaling pathways, such as MAPK/ERK. Our in vitro measurements also reported that approximately one quarter of SPC exhibited migratory responses to signaling from exogenous ligand fields (Mishra et al., 2015). Notably, our modest motile fractions have been overwhelmingly corroborated by in vivo studies reporting equally low numbers of motile SPC in damaged host retina (Bobba et al., 2018). These consistent SPC migration results present severe challenges, however, as large numbers of motile donor cells are desired to increase the probability of restored vision via synaptic SPC integration (Bobba et al., 2018). As a result, we developed a new strategy to increase, both, numbers of motile SPC and their directionality of movement by applying concurrent ligand and EF fields that better simulate multivariate retinal cues (Thoreson and Dacey, 2019).
Combinatory electro-chemotactic signals hone SPC migration in vitro: Our galvano-microfluidic systems apply ligand gradient fields in combination with EF without altering the chemical distribution applied onto cells (Mishra and Vazquez, 2017). This technology enabled individual study of electrotaxis and chemotaxis directly alongside combinatory electro-chemotactic migration (Figure 1E). The first stage of experimentation used a low EF stimulus similar to fields reported for corneal wound healing, without chemotactic supplement. Tests measured a tremendous increase in average motile fraction, with over 95% of SPC exhibiting migration toward the negatively-charged cathode (Mishra and Vazquez, 2017). However, while EF produced large numbers of motile SPC, net migration distances were smaller than those measured via ligand-induced chemotaxis. The next stage of experimentation then combined EF with chemotactic stimulus to report that electro-chemotactic (EC) stimuli yielded over three times the SPC migration distances recorded to either field, individually, with significant increases in directionality (Mishra et al., 2019). Further, immunofluorescence assays revealed no significant differences in the distribution or expression of cognate receptors, such that ligand binding alone did not regulate the EC-enhanced migration observed. This EC data implied mechanistic interplay between electro- and chemotactic pathways that was further investigated using bioinformatics computing. Statistical analyses (P < 10–4) indicated that EC stimuli activated pathways, such as focal adhesion kinase and Ral signaling, able to regulate cytoskeletal movement via, both cell adhesion to ECM for displacement and distribution of membrane signaling molecules for directionality (Mishra et al., 2019). Our findings suggest interfaces between electro- and chemotactic mechanisms that corroborate independent studies able to electrically-induce migration by redistributing acetyl choline and ionotropic glutamate (N-methyl-D-aspartate) receptors to trigger PI3K signaling pathways and activate ligand-gated ion channels (Cortese et al., 2014), respectively.
Future directions and clinical strategy: The exciting findings from our retinal microfluidic platforms highlight EC stimuli as a new strategy to increase the directed migration of SPC in cell replacement therapy. Future directions will examine the extent to which EC-enriched migratory behaviors can be recapitulated in retinal explants before progressing to in vivo models. Clinical applications can further leverage currently-used types of EF stimuli, e.g., direct trans-corneal electro stimulation, repetitive trans-orbital alternating current stimulation, as well as biochemically-conjugated biomaterials, e.g., hyaluronic acid, methylcellulose, to promote directional SPC displacement along tissue engineered constructs. EC stimuli, thereby, represent an exciting and translational avenue with which to enrich outcomes of cell replacement therapy via guided SPC migration.
This work was supported by the US National Institutes of Health (NIH R21 EY026752).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Steven Levy, The Healing Institute, USA; Evgeniya Vladislavovna Pushchina, Russian Academy of Sciences, Russia.
P-Reviewers: Levy S, Pushchina EV; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Bobba S, Di Girolamo N, Munsie M, Chen F, Pébay A, Harkin D, Hewitt AW, O’Connor M, McLenachan S, Shadforth AMA, Watson SL. The current state of stem cell therapy for ocular disease. Exp Eye Res. 2018;177:65–75. doi: 10.1016/j.exer.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Cortese B, Palamà IE, D’Amone S, Gigli G. Influence of electrotaxis on cell behaviour. Integr Biol (Camb) 2014;6:817–830. doi: 10.1039/c4ib00142g. [DOI] [PubMed] [Google Scholar]
- 3.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 4.Herrera E, Erskine L, Morenilla-Palao C. Guidance of retinal axons in mammals. Semin Cell Dev Biol. 2019;85:48–59. doi: 10.1016/j.semcdb.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Hunt NC, Hallam D, Chichagova V, Steel DH, Lako M. The application of biomaterials to tissue engineering neural retina and retinal pigment epithelium. Adv Healthc Mater. 2018;7:e1800226. doi: 10.1002/adhm.201800226. [DOI] [PubMed] [Google Scholar]
- 6.Lorach H, Goetz G, Smith R, Lei X, Mandel Y, Kamins T, Mathieson K, Huie P, Harris J, Sher A, Palanker D. Photovoltaic restoration of sight with high visual acuity. Nat Med. 2015;21:476–482. doi: 10.1038/nm.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra S, Peña JS, Redenti S, Vazquez M. A novel electro-chemotactic approach to impact the directional migration of transplantable retinal progenitor cells. Exp Eye Res. 2019;185:107688. doi: 10.1016/j.exer.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra S, Thakur A, Redenti S, Vazquez M. A model microfluidics-based system for the human and mouse retina. Biomed Microdevices. 2015;17:107. doi: 10.1007/s10544-015-0002-6. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Vazquez M. A gal-mmicroS device to evaluate cell migratory response to combined galvano-chemotactic fields. Biosensors (Basel) 2017 doi: 10.3390/bios7040054. doi: 10.3390/bios7040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonnen KF, Merten CA. Microfluidics as an emerging precision tool in developmental biology. Dev Cell. 2019;48:293–311. doi: 10.1016/j.devcel.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Thoreson WB, Dacey DM. Diverse cell types, circuits, and mechanisms for color vision in the vertebrate retina. Physiol Rev. 2019;99:1527–1573. doi: 10.1152/physrev.00027.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unachukwu UJ, Warren A, Li Z, Mishra S, Zhou J, Sauane M, Lim H, Vazquez M, Redenti S. Predicted molecular signaling guiding photoreceptor cell migration following transplantation into damaged retina. Sci Rep. 2016;6:22392. doi: 10.1038/srep22392. [DOI] [PMC free article] [PubMed] [Google Scholar]