Abstract
Stroke is one of the leading causes of death and disability in adults worldwide, resulting in huge social and financial burdens. Extracts from herbs, especially those used in Chinese medicine, have emerged as new pharmaceuticals for stroke treatment. Here we review the evidence from preclinical studies investigating neuroprotective properties of Chinese medicinal compounds through their application in acute and subacute phases of ischemic stroke, and highlight potential mechanisms underlying their therapeutic effects. It is noteworthy that many herbal compounds have been shown to target multiple mechanisms and in combinations may exert synergistic effects on signaling pathways, thereby attenuating multiple aspects of ischemic pathology. We conclude the paper with a general discussion of the prospects for novel natural compound-based regimens against stroke.
Keywords: cell death, herbal compound, immune response, ischemic stroke therapy, neuroplasticity, neuroprotection, oxidative damage, traditional Chinese medicine
Introduction
Stroke, including ischemic and hemorrhagic types, is the leading cause of death and the most common cause of disability in the adult population worldwide (Donnan et al., 2008), inflicting tremendous social and financial burdens on individuals and society. Ischemic stroke accounts for approximately 85% of all stroke cases (Donnan et al., 2008), and can be further categorized into lacunar stroke and large artery atheromatous or embolic stroke based on its etiology and clinical symptoms. Lacunar stroke (~25% of all ischemic stroke) is small in size (2–20 mm) and can be “silent” (symptom-free), while large artery atheromatous or embolic stroke generally results in a massive infarct volume and more severe symptoms (Donnan et al., 2008). Although tissue plasminogen activator (tPA) treatment and thrombectomy are the most effective methods to restore blood flow in the ischemic area in the acute phase, only a small portion of patients meet the criteria for such therapies (Yong, 2014; Powers et al., 2018; Wang and Wang, 2018). Nevertheless, in the subacute phase stroke patients can benefit from proper supportive regimen and physical rehabilitation (Powers et al., 2018).
To achieve improved stroke outcome, vast resources have been-and are being-devoted to develop safe, effective therapeutic interventions (Junhua et al., 2009; Behravan et al., 2014). These efforts have led to the discovery of various compounds that achieve neuroprotection when delivered in preclinical models. However, because of the complex nature of stroke, treatment-related efficacy has not been validated in clinical trials. Meanwhile, the last decades have witnessed the resurgence of interest in herbal usage for medicinal and/or health promoting applications (Feng et al., 2006; Gurib-Fakim, 2006; Feigin, 2007; Bousser, 2013). Traditional Chinese medicine (TCM) has a long history of empirical usage of herbal and mineral products for medical purposes, representing an extensive, practice-based and information-rich foundation of regimens and medical knowledge. A variety of herbs have been utilized in TCM and in complementary therapies from other world regions for the treatment of stroke and stroke-associated symptoms (Junhua et al., 2009) for thousands of years (Craig, 1999; Dufresne and Farnworth, 2001; Balunas and Kinghorn, 2005; Ganesan, 2008; Wu et al., 2010; Bousser, 2013). From these herbs, bioactive herbal compounds have been extracted and extensively tested (Kumar and Khanum, 2012; Shen et al., 2015; Ting et al., 2018). Even so, the majority of this treasure of herbal compounds is still waiting to be explored (Gong and Sucher, 1999; Bauer and Bronstrup, 2013).
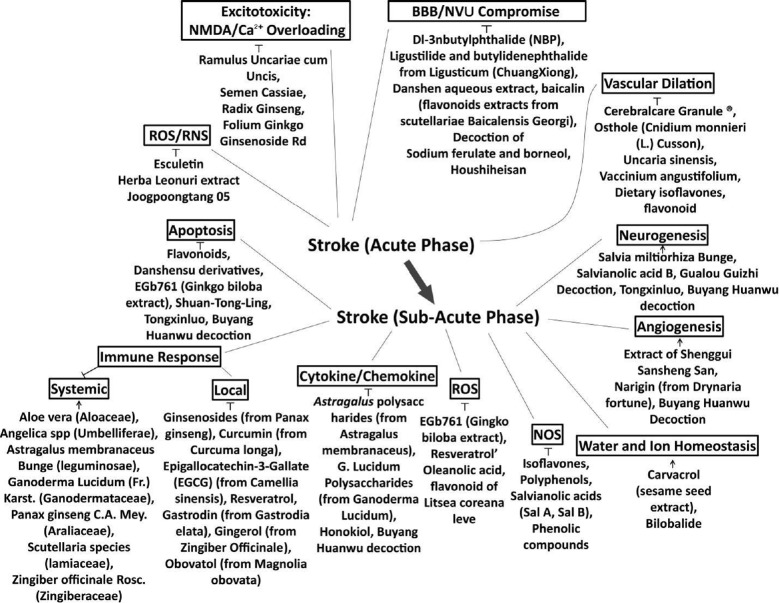
This review focuses on natural compounds that have been extracted from TCM herbs and tested for their efficacy in the treatment of ischemic stroke at acute and subacute phases (Figure 1).
Figure 1.
Natural compounds for stroke therapy involved in the current review.
At acute and sub-acute phases of stroke, natural compounds can perform pluripotent functions to ameliorate stroke injury (┬) and promote reparative mechanisms (↑) through various mechanisms. BBB: Blood-brain barrier; NMDA: N-methyl-D-aspartate; NOS: nitric oxide synthase; NVU: neurovascular unit; RNS: reactive nitrogen species; ROS: reactive oxygen species.
Search Strategy and Selection Criteria
We searched original studies and reviews in regard to TCM therapy for stroke on PubMed, Google Scholar, and ScienceDirect in the range of 1980–2018, with a focus on the peer-reviewed publications in the last two decades. Key words utilized in search were “Traditional Chinese medicine (TCM)”, “stroke”, “herbal medicine”, and those involved in each section subtitles. Initially over 500 reports were identified, of which 154 were selected for the current review. Exclusion criteria included: not TCM herb-based study or therapy, therapy window beyond the subacute phase, opinion or commentary without peer review, and outside the focus of current review (such as side effects, toxicity, formulation development and fabrication, and patient/hospital management).
Natural Compounds in the Treatment of Ischemic Stroke: Acute Phase
In the acute phase (from minutes to hours) of an ischemic stroke, interruption of blood supply leads to events including rapid cell necrosis, cellular membrane and subcellular organelle injury, DNA damage, excitotoxicity, acidotoxicity, oxidative stress, mitochondria swelling and dysfunction, ionic homeostasis disturbance and cell lysis-related cell oedema (Dirnagl et al., 1999; Donnan et al., 2008; Doyle et al., 2008; Lo, 2008). Prevention or attenuation of any of these pathological factors can be beneficial and may ameliorate the damage to the brain.
Attenuation of cellular oxidative stress
Occlusion of the cerebral artery deprives the brain of metabolic substrates and oxygen to mitochondrial enzymes, disrupts the respiratory chain and results in leakage of superoxide, along with activated NADPH oxidase and inducible nitric oxide synthase (iNOS). Together these lead to the overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS), inducing further damage to organelle membranes, lipids, proteins and DNA (Soobrattee et al., 2008; Loh et al., 2009; Wang et al., 2009; Jiang et al., 2010). These injuries can be alleviated by treatment with antioxidant molecules, including a large array of natural compounds, such as members of the phenol and/or flavonoid families (Perron et al., 2009). For example, neural protection against stroke by flavonoid of Litsea coreana leve, esculetin (a coumarin derivative) (Wang et al., 2012), Herba Leonuri extract (Loh et al., 2009), and Joongpoongtang 05 (Lagouge et al., 2006) can be attributed, in part, to their oxidant-scavenging effects when applied in the acute phase of stroke (Lagouge et al., 2006).
Alleviation of excitotoxicity
Ischemia drives the neuronal release of excitatory neurotransmitters, such as glutamate, and disrupts their absorbance or clearance by neurons and astrocytes. Excessive extracellular glutamate results in overactivation of N-methyl-D-aspartic acid receptors (NMDA-Receptor, NMDA-R), cellular Ca2+ overload, and eventually neuronal death. This phenomenon, called excitotoxicity, has been identified as a crucial therapeutic target in stroke treatment (Brouns and De Deyn, 2009; Debet al., 2010; Moskowitz et al., 2010).
Several herbal compounds have been identified as having NMDA-R antagonist properties or the capability of inhibiting Ca2+ responses, including those from Ramulus Uncariae cum Uncis, Semen Cassiae, Radix Ginseng, and Folium Ginkgo (Sun et al., 2005; Kaneko et al., 2010; Wu et al., 2010; Zhao et al., 2011; Liang et al., 2013). Similarly, entry of Ca2+ through NMDA linked receptor-operated calcium channels (ROCC), which is the main reason for cytosolic Ca2+ elevation after stroke, can be blocked by Ginsenoside Rd, a dammarane-type steroid glycoside extracted from ginseng plants (Ye et al., 2013). Interestingly, TCM herbal reagents may exert their protective function through direct blockage of Ca2+ channels or through secondary mechanisms, such as modulation of the PKC cell signaling pathway (Li et al., 2007; Wang et al., 2008; Chen, 2012). Although their effects against excitotoxicity need confirmation in vivo, these reagents’ therapeutic potential is worthy of further exploration.
Maintenance of blood-brain barrier and neurovascular unit functions
The cerebral microvasculature is crucial for two physiological processes which maintain brain homeostasis: 1) prevention of blood-borne pathogen intrusion into the parenchyma via the blood-brain barrier (BBB), which relies on the unique tight junction proteins that seal along the endothelial cells in the brain microvasculature; and 2) maintenance of cerebral blood supply and the active exchange of oxygen/metabolites and CO2/metabolic waste between the blood and brain. Both processes rely on the normal functioning of the neurovascular unit (NVU), which is composed of cerebral endothelial cells, pericytes, astrocytes and neurons (Peknyand and Nilsson, 2005; Giaume et al., 2010; Sofroniew and Vinters, 2010).
Ischemia induces necrosis in the infarct core and disrupts the normal function of the NVU and BBB in the penumbra. This results in vasculature leakage and disturbance of astrocyte-neuron communication (Peknyand and Nilsson, 2005; Giaume et al., 2010).
Recovery of the disrupted endothelium is critical for the functional restoration of the BBB and the NVU after stroke. Natural compounds such as dl-3n-butylphthalide (NBP) have demonstrated the capacity for improving endothelium function of cerebral microvessels through increasing numbers of perfused microvessels in infarct area in a stroke model using stroke-prone renovascular hypertensive rats (Liu et al., 2007). Extracts from Ligusticum (Chuanxiong), namely ligustilide and butylidenephthalide, can relax the vascular constriction of rat arteries induced by norepinephrine bitartrate and calcium chloride (CaCl2) (Liang et al., 2005). Moreover, in a cerebral ischemia-reperfusion model in rats, treatment with Danshen aqueous extract decreased serum levels of hs-C-reactive protein, interleukin-8, interleukin-10, and tumor necrosis factor-α (TNF-α), and attenuated secondary endothelium damage (Liang et al., 2013). Experimental evidence suggests that BBB function can be preserved following stroke by modifying the levels and/or localization of tight junction proteins with baicalin (flavonoid extracts from Scutellariae Baicalensis Georgi) (Lin, 2011) treatment or decoction that is composed of sodium ferulate and borneol (Chen et al., 2010).
Salvage of the penumbra
The ischemic penumbra is the tissue in the vicinity of the stroke core which is not yet necrotic/lost, but is damaged and receives lower than normal perfusion (Dirnagl et al., 1999; Weinberger, 2006; Lo, 2008). Penumbra salvage is a therapeutic target in both acute and sub-acute phases of stroke. Natural compounds contribute to such salvage through multiple mechanisms, such as anti-apoptotic, anti-inflammatory responses and regulation of local blood flow.
Improvement of collateral blood flow from surrounding areas supports the penumbra’s survival (Wei et al., 2001). It has been reported that natural compounds with vascular dilation effects, including Cerebralcare Granule® (Sun et al., 2010), osthole (Cnidium monnieri (L.) Cusson) (Fusi et al., 2012), Uncaria sinensis (Park et al., 2011), Vaccinium angustifolium (Kristo et al., 2010), dietary isoflavones (Mann et al., 2009; Siow and Mann, 2010) and industrialized flavonoid (Lapchak, 2012), increase collateral blood flow through 1) vascular dilation in the acute phase or 2) reconstruction of the vascular network through angiogenesis in the sub-acute phase (Vauzour et al., 2008; Bu et al., 2013). Their biological effects include increased nitric oxide (NO) availability and induction of angiogenic growth factors.
Natural Compounds in the Treatment of Ischemic Stroke: Sub-Acute Phase
More complicated mechanisms and regulatory factors are involved in the sub-acute phase than in the acute phase. The mechanisms include shifting of cell death mechanisms from necrosis to apoptosis, activation of local and systemic immune responses, infiltration of neutrophils and monocytes, increased cytokine and ROS production, oedema from water and ion imbalances, and activation of protective mechanisms and repair processes, including neurogenesis and angiogenesis (Figure 1).
Prevention of apoptotic cell death
In the sub-acute phase, damaged cells, especially neurons, can be rescued by preventing them from entering the apoptotic cascade (Akpan and Troy, 2013). Thus, attenuation of mitochondrial damage, maintenance of ion homeostasis, inhibition of caspase activities and other mediators of the apoptotic cascade will contribute to the survival of neurons after stroke.
In many cases natural compounds can at least partially achieve these effects, and the mechanisms involved have been investigated. Flavonoids are known to affect the phosphatidylinositol 3 kinase (PI3K)-Akt/PKB pathway, and subsequently inhibit the activation of central proteins in the cell death machinery, such as the proapoptotic Bcl-2 family member BAD and members of the caspase family (Spencer, 2008; Zhou et al., 2010; Zhang et al., 2010, 2013; Su and Hsieh, 2011; Wang et al., 2012; Pan et al., 2013). Flavonoids also show effects in modifying mitogen-activated protein kinase signaling pathway through changes in extracellular signal-regulated kinase 1/2 and c-jun amino-terminal kinase. Similarly, Danshensu derivatives can dose-dependently attenuate cell damage and death, lower ROS production, preserve mitochondrial potential, and modulate Bcl-2, Bax, caspase-3 and p53 protein expression in cells that are challenged with oxidants in vitro (Pan et al., 2013). Such observations have been further confirmed in vivo. One of the Danshensu derivatives has demonstrated antioxidant capacity through increasing the activity of superoxide dismutase (SOD) and glutathione peroxidase while decreasing the level of malondialdehyde and ROS production (Seetapun et al., 2013).
Ginkgo biloba extract EGb761 has been shown to ameliorate apoptosis through attenuation of caspase activation, thus blocking the extrinsic apoptotic pathway in a permanent middle cerebral artery occlusion model using female ovariectomized mice (Tulsulkar et al., 2016). The extract is also known to increase heme oxygenase-1 (a potent antioxidant enzyme) expression and activate the Wnt signaling pathway (Saleem et al., 2008; Nada and Shah, 2012, 2015; Nada et al., 2014). Oleanolic acid, a well-characterized triterpenoid from medicinal plants and herbs, can also increase heme oxygenase-1 expression and improve stroke outcome in preclinical studies (Caltana et al., 2015). Alternatively, gavage feeding a mixture of various herbal compounds following a traditional Chinese prescription attenuated neuronal loss and mitigated stroke volume in a transient cerebral ischemia/reperfusion model in rats (Mu et al., 2014).
Regulation of immune responses: microglia and infiltrating macrophages
Stroke induces both systemic and local immune responses (Iadecola and Anrather, 2011). Systemic immune depression after stroke can worsen patients’ prognosis due to higher occurrence of infectious complications such as pneumonia (Kamel and Iadecola, 2012). Chinese herbal medicines contain compounds that selectively modulate immune cells such as lymphocytes and natural killer cells and thus can be used to support the immune system through regulation of immune cells’ population and activation, and manipulation of circulatory cytokines to fight infectious complications (Tan and Vanitha, 2004). Among the most commonly used are Aloe vera. (Aloaceae), Angelica spp. (Umbelliferae), Astragalus membranaceus Bunge. (Leguminosae), Ganoderma lucidum (Fr.) Karst. (Ganodermataceae), Panax ginseng C.A. Mey. (Araliaceae), Scutellaria species (Lamiaceae) and Zingiber officinale Rosc. (Zingiberaceae) (Tan and Vanitha, 2004).
In contrast, local immune responses at the site of stroke damage are highly activated, and these responses have been reported to be determinant factors of stroke outcome. Microglia are the immune surveillance cells in the brain that can be directly activated upon stroke and, along with monocyte-derived macrophages that infiltrate through the disrupted BBB, are key contributors to the inflammatory responses in the ischemic brain (Yenari et al., 2010).
Activated microglia and newly migrated peripheral immune cells produce an array of factors (e.g., interleukins, TNF-α, NO, prostaglandins; refer to next section) that are toxic to neurons. Natural compounds regulate the local immune response by altering the production of cytokines and chemokines or acting as antagonists of cytokine receptors (Shao et al., 2004a, b; Zhang et al., 2010; Choi et al., 2011; Liang et al., 2011; Sun and Hsieh, 2011; Spencer et al., 2012; Fischer et al., 2013; Gu et al., 2014; Fu et al., 2018) to inhibit microglia-mediated neurotoxicity (see below).
Modulation of cytokine/chemokine production
At the injury site, damaged cells and infiltrated neutrophils produce a variety of inflammatory cytokines and chemokines, including TNF-α, interleukin-1β, interleukin-6, intercellular adhesion molecule 1, and sphingosine-1-phosphate, which closely correlate with damage severity and extent. Restricting peripheral inflammatory cell recruitment through the preservation of endothelium function is therefore of primary importance in stroke therapy. Various natural compounds are reported to attenuate stroke-induced elevations of cytokines and lessen tissue damage (Shao et al., 2004a, b; Sun and Hsieh, 2011; Shichita et al., 2012; Spencer et al., 2012; Fischer et al., 2013), effects that can be observed with injection of combined herbal components as well (Chen et al., 2015). The most commonly studied components are Ginsenosides (from Panax ginseng), Curcumin (from Curcuma longa), Epigallocatechin-3-Gallate (from Camellia sinensis), Resveratrol, Gastrodin (from Gastrodia elata), Gingerol (from Zingiber officinale) and Obovatol (from Magnolia obovata) (Shao et al., 2004a, b; Zhang et al., 2010; Choi et al., 2011; Liang et al., 2011; Sun and Hsieh, 2011; Spencer et al., 2012; Fischer et al., 2013; Gu et al., 2014; Fu et al., 2018). For example, Ginsenosides suppresses nuclear factor-κB and mitogen-activated protein kinase activities, upstream signaling molecules in the inflammatory response. It also inhibits expression of iNOS and elevation of TNF-α under pathological stresses (Zhang et al., 2010; Su and Hsieh, 2011).
Interestingly, independent reports have shown that in a model of cerebral ischemia-reperfusion honokiol exhibits both anti-inflammatory effects through inhibition of nuclear factor-κB and cytokine production in glial cells (Zhang et al., 2013) and neuroprotective effects through inhibition of NMDA current and disruption of PSD95-nNOS interaction to alleviate excessive, toxic NO production (Hu et al., 2013). These reports support TCM’s “one reagent, multiple targets” and “integral therapy through mutual effects” principles.
Attenuation of oxidative stress/nitric oxide stress
Ischemia induces oxidative stress and NO stress, both of which are deleterious in causing secondary damage. Antioxidant effects are among the earliest identified properties in phytochemicals. Initially they were considered as oxidant scavengers; later, extensive studies revealed their potential to reduce ROS and NO production through modulation of gene expression and signaling pathways (Soobrattee et al., 2005; Stevenson and Hurst, 2007; Lin, 2011; Rubio et al., 2013). Recent studies have shown long-term intake of resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction, suggesting a regulatory role in cellular metabolism (Pearson et al., 2008) and potential for maintaining long-term cardiac and cerebrovascular health. Of note, natural compounds execute their multi-functional effects through different mechanisms, including attenuation of ROS and NO production (see below), some of which are cell-specific (Conforti and Menichini, 2011; Lin, 2011; Rubio et al., 2013).
Reduction of reactive oxygen species production
Reduction of ROS production by natural compounds can be achieved through multiple mechanisms: (a) direct oxidant scavenging, which attenuates mitochondrial damage and reduces superoxide leakage; (b) upregulation of antioxidant genes; or (c) suppression of oxidant genes (Esposito et al., 2002; Soobrattee et al., 2005; Stevenson and Hurst, 2007; Perron and Brumaghim, 2009; Campos-Esparza Mdel and Torres-Ramos, 2010; Wu et al., 2010).
For example, resveratrol, a naturally occurring stilbene-class of polyphenol, is well recognized for its anti-oxidant benefits. The chemical structure of resveratrol allows it to behave directly as a free radical scavenger, which can decrease oxidative damage in a dose-dependent manner (Shang et al., 2009). The neuroprotective effect of resveratrol was first identified in a model of systemic injection of kainic acid in rats, as it ameliorated kainate-induced excitotoxicity in the hippocampus and olfactory bulb (Virgili and Contestabile, 2000). Since then, resveratrol has been shown to reduce pathology and improve behavioral outcomes in numerous animal models of CNS injuries, including stroke. Although the exact mechanism of resveratrol-related neuroprotection is not fully understood, the beneficial effects are thought to be related to activation of silent mating type information regulation 2 homolog 1 (Borra et al., 2005), AMP-activated kinase (Dasgupta and Milbrandt, 2007) and nuclear factor (erythroid derived 2)-like 2 (Chen et al., 2005; Ungvari et al., 2010). Activation of silent mating type information regulation 2 homolog 1 and AMP-activated kinase can both improve metabolism and lifespan, which promote a pro-survival environment in the injured CNS, while activation of nuclear factor (erythroid derived 2)-like 2 enhances the transcription of genes involved in anti-oxidative activity, such as SOD, heme oxygenase-1 (HO-1), catalase and many other phase II defense enzymes (Kansanen et al., 2013; Zhang et al., 2013). In a mouse cerebral ischemia-reperfusion model, resveratrol has been demonstrated to have neural protective effects through upregulation of HO-1 (Sakata et al., 2010). In addition, it has also been shown that resveratrol can activate PPARγ coactivator 1α (Lorenz et al., 2003; Lagouge et al., 2006), which then mitigates oxidative stress by modulating mitochondrial function and the expression of anti-oxidant enzymes, such as SOD2 and glutathione peroxidase 1 (St-Pierre et al., 2006; Lu et al., 2010). Similarly, treatment with EGb 761, an extract from Gingko biloba leaves, has been shown to produce anti-oxidant effects through increases in HO-1 expression in both transient and permanent ischemic stroke models (Saleem et al., 2008; Shah et al., 2011; Tulsulkar and Shah, 2013).
Regulation of nitric oxide production
NO, a critical molecule in cell signaling and regulation of local blood flow, is generated by neuronal nitric oxide synthase and endothelial nitric oxide synthase under physiological conditions. At the onset of stroke, however, NO reaches toxic levels due to the activation of iNOS.
Isoflavones and polyphenols regulate endothelial nitric oxide synthase production and transcriptional activation of antioxidant defense genes in the vasculature via the transcription factors NFκB and nuclear factor (erythroid derived 2)-like 2 (Siow et al., 2007). Salvianolic acids, especially salvianolic acid A and salvianolic acid B, have been identified to have similar transcriptional regulatory effects (Ho and Hong, 2011). Interestingly, some phenolic compounds from plants can also activate endothelial nitric oxide synthase and neuronal nitric oxide synthase, improving NO availability and blood flow in the damaged area. By inhibiting iNOS activation or expression, they prohibit NO overproduction and subsequent neural toxicity (Siow et al., 2007; Siow and Mann, 2010; Conforti and Menichini, 2011). Such observations partially explain these herbal compounds’ antioxidant, antithrombic and vascular relaxing properties, suggesting their inclusion in the prevention and treatment of cardiovascular disease and reducing risk factors (Layne and Ferro, 2017; Peng et al., 2017).
Maintenance of water and ion homeostasis
Ischemia disturbs water and ion homeostasis in the brain (Brouns and De Deyn, 2009; Aronowski and Zhao, 2011; Mann, 2011; Kim et al., 2018). Dysfunction of astrocytes and the microvasculature leads to cytotoxic vasogenic edema, which further compromises transportation of oxygen and metabolic substrates from the vessels to the parenchyma.
Aquaporin-4, a water channel on the cell membrane of astrocytes, is critical for the elevation of ion and water levels in the damaged area (Brouns and De Deyn, 2009; Aronowski and Zhao, 2011; Mann, 2011; Kim et al., 2018). Carvacrol (a sesame seed extract) and bilobalide have been shown to have neuroprotective effects by attenuating cerebral edema through inhibition of aquaporin-4 expression in both intracranial hemorrhagic and ischemic animal models (Lee et al., 2012; Zhong et al., 2013).
Activation of endogenous reparative function
In addition to initiating cell damage and death, ischemic injury also activates the endogenous reparative functions of the brain. Those mechanisms include angiogenesis, which restores the cerebral vasculature and cerebral blood flow, and neurogenesis, which rebuilds the neural network to enhance neuronal function (Lin et al., 2015; Ma et al., 2015; Ruan et al., 2015; Cassidy and Cramer, 2017). Activation and proper regulation of these processes improve neurological function and stroke outcome. Accumulating evidence shows that active ingredients from natural compounds can manipulate these processes through various mechanisms (Liu et al., 2018; Chen et al., 2015; Ren et al., 2015; Udalamaththa et al., 2016; Zhao et al., 2018).
At the periphery of the injury site, endothelial progenitor cells can bud from the existing vasculature and develop into new microvessels, which elongate and penetrate through the glial barrier, and eventually form mature, functional microvessels and improve blood flow in the infarct area (Ma et al., 2015). For example, it has been shown that extract from Shengui Sansheng San increases VEGF signaling pathways, and facilitates vasculature formation in the infarct area in vivo and tube formation of cultured brain microvascular endothelial cells in vitro (Liu et al., 2018). In addition, narigin, a major active ingredient in the Chinese herb Drynaria fortune, has been shown to promote tube formation of endothelial progenitor cells in vitro through the activation of the CXCL12/CXCR4/PI3K/Akt signaling pathway (Zhao et al., 2018). In a rat model of focal cerebral ischemia, post-stroke gavage feeding of Houshiheisan preserved NVU integrity and salvaged neurons in the penumbra (Wang et al., 2014).
The majority of neurogenesis from activated neural stem cells takes place in the subventricular zone and hippocampal subgranular zone and contributes to neural replacement and restoration of neural circuits (Lin et al., 2015; Ruan et al., 2015; Cassidy and Cramer, 2017). Several studies have demonstrated that natural compounds or decoction can regulate the activation and proliferation of neural stem cells and mesenchymal stem cells, as well as guide their neural differentiation by manipulating multiple signaling pathways (Lin et al., 2013; Si et al., 2014; Chen et al., 2015; Ren et al., 2015; Gao and Shen, 2017; Qin et al., 2017). For example, Salvia miltiorrhiza Bunge attenuated apoptosis and improved cell viability of injected mesenchymal stem cells in a rat stroke model (Kim et al., 2018). Moreover, Salvianolic acid B promoted neural differentiation of induced pluripotent stem cells via the PI3K/Akt pathway (Shu et al., 2018) and active ingredients of radix astragali promoted neural stem cell proliferation and guided their differentiation into dopaminergic neurons in vitro (Gao et al., 2018).
Summary and Future Prospects for the Clinical Use of Natural Compounds
In summary, natural compounds have been demonstrated to have neuroprotective actions including anti-oxidative, anti-apoptotic, anti-neuroinflammatory, and neuromodulatory effects, as well as promoting brain tissue repair and functional recovery (Figure 1) (Soobrattee et al., 2005; Sucher, 2006). Importantly, the efficacy of natural compounds, such as those derived from Danshen, may be derived from either a reduction of cardiovascular risk factors for stroke and/or through multiple bioactivities, including gene modulation and interactions with cell signaling cascades, rather than merely as classic oxidant scavengers. Most studies reviewed here reported beneficial treatment effects of natural compounds using in vitro and/or in vivo experimental stroke models, suggesting a promising therapeutic potential of natural compounds in treating stroke (Wu et al., 2010; Han et al., 2017; Gaire, 2018).
Moreover, studies using combined ingredients support the therapeutic principles of TCM, namely, “one reagent, multiple targets” and “integral therapy through mutual effects” (Chen et al., 2018). The benefits derived from prescriptions of multiple components can be from mutually enhancing or synergetic effects of the ingredients targeting multiple events in stroke pathology. Thus a combination of multiple natural compounds may be a better remedy achieving synergic effects (Liu et al., 2018). Indeed, several small-scale clinical trials for stroke have already been undertaken to examine the efficacy of herbal regimens consisting of multiple ingredients (Butler, 2008; Huang et al., 2015). Although current evidence is still incomplete, preliminary results from those trials show that administration of a single compound or a combination of different compounds, as an addition to the conventional western style therapeutic strategies, benefits patients in recovery after stroke (Gao et al., 2009; Junhua et al., 2009; Butler et al., 2014; Chen et al., 2015; Hung et al., 2015; Peng et al., 2017).
In conclusion, natural compounds and their derivatives are emerging as effective novel therapeutic reagents against stroke. While the mechanisms of their protective properties require further clarification as do issues with regard to quality control, mechanisms of drug-drug interactions and toxicity, we expect to see the increasing validation of these gifts from nature and acceleration of their translation into clinical regimens.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was supported by AHA Award 14SDG20480186 (to LC), Kentucky Spinal Cord & Head Injury Research Trust Grant 14-12A (to KES), Startup Funds from Shaanxi University of Chinese Medicine to Young Investigators (1410170078) (to BZ).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by AHA Award 14SDG20480186 (to LC), Kentucky Spinal Cord & Head Injury Research Trust Grant 14-12A (to KES), Startup Funds from Shaanxi University of Chinese Medicine to Young Investigators (1410170078) (to BZ).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Akpan N, Troy CM. Caspase inhibitors: prospective therapies for stroke. Neuroscientist. 2013;19:129–136. doi: 10.1177/1073858412447875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Bauer A, Bronstrup M. Industrial natural product chemistry for drug discovery and development. Nat Prod Rep. 2013;31:35–60. doi: 10.1039/c3np70058e. [DOI] [PubMed] [Google Scholar]
- 5.Behravan E, Razavi BM, Hosseinzadeh H. Review of plants and their constituents in the therapy of cerebral ischemia. Phytother Res. 2014;28:1265–1274. doi: 10.1002/ptr.5187. [DOI] [PubMed] [Google Scholar]
- 6.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 7.Bousser MG. Drugs from natural substances: why study them in cerebral infarction. Cerebrovasc Dis. 2013 doi: 10.1159/000346226. doi:10.1159/000346226. [DOI] [PubMed] [Google Scholar]
- 8.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111:483–495. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Bu Y, Lee K, Jung HS, Moon SK. Therapeutic effects of traditional herbal medicine on cerebral ischemia: a perspective of vascular protection. Chin J Integr Med. 2013;19:804–814. doi: 10.1007/s11655-013-1341-2. [DOI] [PubMed] [Google Scholar]
- 10.Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 11.Butler MS, Robertson AA, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612–1661. doi: 10.1039/c4np00064a. [DOI] [PubMed] [Google Scholar]
- 12.Caltana L, Nieto ML, Brusco A. Oleanolic acid: a promising neuroprotective agent for cerebral ischemia. Neural Regen Res. 2015;10:540–541. doi: 10.4103/1673-5374.155414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos-Esparza Mdel R, Torres-Ramos MA. Neuroprotection by natural polyphenols: molecular mechanisms. Cent Nerv Syst Agents Med Chem. 2010;10:269–277. doi: 10.2174/187152410793429728. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2017;8:33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Song J, Luo Y, Li M, Gao L. Individualized traditional Chinese medicine treatment of acute stroke. Science (Special Issue, Translational Medicine at Capital Medical University: Investigating Major Chronic Disease) 2015;2015:68–71. [Google Scholar]
- 17.Chen MM, Zhao GW, He P, Jiang ZL, Xi X, Xu SH, Ma DM, Wang Y, Li YC, Wang GH. Improvement in the neural stem cell proliferation in rats treated with modified “Shengyu” decoction may contribute to the neurorestoration. J Ethnopharmacol. 2015;165:9–19. doi: 10.1016/j.jep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 18.Chen XH, Lin ZZ, Liu AM, Ye JT, Luo Y, Luo YY, Mao XX, Liu PQ, Pi RB. The orally combined neuroprotective effects of sodium ferulate and borneol against transient global ischaemia in C57 BL/6J mice. J Pharm Pharmacol. 2010;62:915–923. doi: 10.1211/jpp.62.07.0013. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Sun Y, Li W, Wei H, Long T, Li H, Xu Q, Liu W. Systems pharmacology dissection of the anti-stroke mechanism for the Chinese traditional medicine Xing-Nao-Jing. J Pharmacol Sci. 2018;136:16–25. doi: 10.1016/j.jphs.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Chen YF. Traditional Chinese herbal medicine and cerebral ischemia. Front Biosci (Elite Ed) 2012;4:809–817. doi: 10.2741/E420. [DOI] [PubMed] [Google Scholar]
- 21.Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16:1021–1043. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conforti F, Menichini F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr Med Chem. 2011;18:1137–1145. doi: 10.2174/092986711795029690. [DOI] [PubMed] [Google Scholar]
- 23.Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70:491S–499S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 27.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 28.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–421. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 30.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 31.Feigin VL. Herbal medicine in stroke: does it have a future? Stroke. 2007;38:17341–17346. doi: 10.1161/STROKEAHA.107.487132. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Wu Z, Zhou X, Zhou Z, Fan W. Knowledge discovery in traditional Chinese medicine: state of the art and perspectives. Artif Intell Med. 2006;38:219–236. doi: 10.1016/j.artmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Fischer W, Urban N, Immig K, Franke H, Schaefer M. Natural compounds with P2X7 receptor-modulating properties. Purinergic Signal. 2013;10:313–326. doi: 10.1007/s11302-013-9392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Yang J, Wang X, Yang P, Zhao Y, Li K, Chen Y, Xu Y. Herbal compounds play a role in neuroprotection through the inhibition of microglial activation. J Immunol Res. 2018;2018:9348046. doi: 10.1155/2018/9348046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusi F, Sgaragli G, Ha le M, Cuong NM, Saponara S. Mechanism of osthole inhibition of vascular Ca(v)1. 2 current. Eur J Pharmacol. 2012;680:22–27. doi: 10.1016/j.ejphar.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 36.Gaire BP. Herbal medicine in ischemic stroke: challenges and prospective. Chin J Integr Med. 2018;24:243–246. doi: 10.1007/s11655-018-2828-2. [DOI] [PubMed] [Google Scholar]
- 37.Ganesan A. The impact of natural products upon modern drug discovery. Curr Opin Chem Biol. 2008;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Gao C, Shen J. Metabolic factors and adult neurogenesis: impacts of Chinese herbal medicine on brain repair in neurological diseases. Int Rev Neurobiol. 2017;135:117–147. doi: 10.1016/bs.irn.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Gao H, Dou L, Shan L, Sun Y, Li W. Proliferation and committed differentiation into dopamine neurons of neural stem cells induced by the active ingredients of radix astragali. Neuroreport. 2018;29:577–582. doi: 10.1097/WNR.0000000000000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L, Ji X, Song J, Liu P, Yan F, Gong W, Dang S, Luo Y. Puerarin protects against ischemic brain injury in a rat model of transient focal ischemia. Neurol Res. 2009;31:402–406. doi: 10.1179/174313209X444017. [DOI] [PubMed] [Google Scholar]
- 41.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 42.Gong X, Sucher NJ. Stroke therapy in traditional Chinese medicine (TCM): prospects for drug discovery and development. Trends Pharmacol Sci. 1999;20:191–196. doi: 10.1016/s0165-6147(98)01276-0. [DOI] [PubMed] [Google Scholar]
- 43.Gu Y, Chen J, Shen J. Herbal medicines for ischemic stroke: combating inflammation as therapeutic targets. J Neuroimmune Pharmacol. 2014;9:313–339. doi: 10.1007/s11481-014-9525-5. [DOI] [PubMed] [Google Scholar]
- 44.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Han SY, Hong ZY, Xie YH, Zhao Y, Xu X. Therapeutic effect of Chinese herbal medicines for post stroke recovery: A traditional and network meta-analysis. Medicine (Baltimore) 2017;96:e8830. doi: 10.1097/MD.0000000000008830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho JH, Hong CY. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci. 2011;18:30. doi: 10.1186/1423-0127-18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Z, Bian X, Liu X, Zhu Y, Zhang X, Chen S, Wang K, Wang Y. Honokiol protects brain against ischemia-reperfusion injury in rats through disrupting PSD95-nNOS interaction. Brain Res. 2013;1491:204–212. doi: 10.1016/j.brainres.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Hung IL, Hung YC, Wang LY, Hsu SF, Chen HJ, Tseng YJ, Kuo CE, Hu WL, Li TC. Chinese herbal products for ischemic stroke. Am J Chin Med. 2015;43:1365–1379. doi: 10.1142/S0192415X15500779. [DOI] [PubMed] [Google Scholar]
- 49.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W, Zhang S, Fu F, Zhu H, Hou J. Inhibition of nuclear factor-kappaB by 6-O-acetyl shanzhiside methyl ester protects brain against injury in a rat model of ischemia and reperfusion. J Neuroinflammation. 2010;7:55. doi: 10.1186/1742-2094-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung HW, Mahesh R, Bae HS, Kim YH, Kang JS, Park YK. The antioxidant effects of Joongpoongtang 05 on brain injury after transient focal cerebral ischemia in rats. J Nat Med. 2011;65:322–329. doi: 10.1007/s11418-010-0497-3. [DOI] [PubMed] [Google Scholar]
- 52.Junhua Z, Menniti-Ippolito F, Xiumei G, Firenzuoli F, Boli Z, Massari M, Hongcai S, Yuhong H, Ferrelli R, Limin H, Fauci A, Guerra R, Raschetti R. Complex traditional Chinese medicine for poststroke motor dysfunction: a systematic review. Stroke. 2009;40:2797–2804. doi: 10.1161/STROKEAHA.109.555227. [DOI] [PubMed] [Google Scholar]
- 53.Kamel H, Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol. 2012;69:576–581. doi: 10.1001/archneurol.2011.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaneko Y, Eve DJ, Yu S, Shojo H, Bae EC, Park DH, Roschek B, Jr, Alberte RS, Sanberg PR, Sanberg CD, Bickford PC, Borlongan CV. Acute treatment with herbal extracts provides neuroprotective benefits in in vitro and in vivo stroke models, characterized by reduced ischemic cell death and maintenance of motor and neurological functions. Cell Med. 2010;1:137–142. doi: 10.3727/215517910X552818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim R, Lee S, Lee CY, Yun H, Lee H, Lee MY, Kim J, Jeong JY, Baek K, Chang W. Salvia miltiorrhiza enhances the survival of mesenchymal stem cells under ischemic conditions. J Pharm Pharmacol. 2018;70:1228–1241. doi: 10.1111/jphp.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kristo AS, Kalea AZ, Schuschke DA, Klimis-Zacas DJ. A wild blueberry-enriched diet (Vaccinium angustifolium) improves vascular tone in the adult spontaneously hypertensive rat. J Agric Food Chem. 2010;58:11600–11605. doi: 10.1021/jf101839u. [DOI] [PubMed] [Google Scholar]
- 58.Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6:81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Lapchak PA. A series of novel neuroprotective blood brain barrier penetrating flavonoid drugs to treat acute ischemic stroke. Curr Pharm Des. 2012;18:3694–3703. doi: 10.2174/138161212802002652. [DOI] [PubMed] [Google Scholar]
- 61.Layne K, Ferro A. Traditional Chinese medicines in the management of cardiovascular diseases: a comprehensive systematic review. Br J Clin Pharmacol. 2017;83:20–32. doi: 10.1111/bcp.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee K, Jo IY, Park SH, Kim KS, Bae J, Park JW, Lee BJ, Choi HY, Bu Y. Defatted sesame seed extract reduces brain oedema by regulating aquaporin 4 expression in acute phase of transient focal cerebral ischaemia in rat. Phytother Res. 2012;26:1521–1527. doi: 10.1002/ptr.4599. [DOI] [PubMed] [Google Scholar]
- 63.Li N, Liu B, Dluzen DE, Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2007;111:458–463. doi: 10.1016/j.jep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 64.Liang MJ, He LC, Yang GD. Screening, analysis and in vitro vasodilatation of effective components from Ligusticum Chuanxiong. Life Sci. 2005;78:128–133. doi: 10.1016/j.lfs.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 65.Liang Q, Wu Q, Jiang J, Duan J, Wang C, Smith MD, Lu H, Wang Q, Nagarkatti P, Fan D. Characterization of sparstolonin B, a Chinese herb-derived compound, as a selective Toll-like receptor antagonist with potent anti-inflammatory properties. J Biol Chem. 2011;286:26470–26479. doi: 10.1074/jbc.M111.227934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang W, Lam WP, Tang HC, Leung PC, Yew DT. Current Evidence of Chinese Herbal Constituents with Effects on NMDA Receptor Blockade. Pharmaceuticals (Basel) 2013;6:1039–1054. doi: 10.3390/ph6081039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang XY, Li HN, Yang XY, Zhou WY, Niu JG, Chen BD. Effect of Danshen aqueous extract on serum hs-CRP, IL-8, IL-10 TNF-alpha levels, and IL-10 mRNA, TNF-alpha mRNA expression levels, cerebral TGF-beta1 positive expression level and its neuroprotective mechanisms in CIR rats. Mol Biol Rep. 2013;40:3419–3427. doi: 10.1007/s11033-012-2419-9. [DOI] [PubMed] [Google Scholar]
- 68.Lin B. Polyphenols and neuroprotection against ischemia and neurodegeneration. Mini Rev Med Chem. 2011;11:1222–1238. doi: 10.2174/13895575111091222. [DOI] [PubMed] [Google Scholar]
- 69.Lin R, Cai J, Nathan C, Wei X, Schleidt S, Rosenwasser R, Iacovitti L. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol Dis. 2015;74:229–239. doi: 10.1016/j.nbd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Liu B, Luo C, Zheng Z, Xia Z, Zhang Q, Ke C, Liu R, Zhao Y. Shengui Sansheng San extraction is an angiogenic switch via regulations of AKT/mTOR, ERK1/2 and Notch1 signal pathways after ischemic stroke. Phytomedicine. 2018;44:20–31. doi: 10.1016/j.phymed.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 71.Liu CL, Liao SJ, Zeng JS, Lin JW, Li CX, Xie LC, Shi XG, Huang RX. dl-3n-butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP. J Neurol Sci. 2007;260:106–113. doi: 10.1016/j.jns.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Liu J, Shen F, Qin Z, Jiang M, Zhu J, Wang Z, Zhou J, Fu Y, Chen X, Huang C, Xiao W, Zheng C, Wang Y. Systems pharmacology analysis of synergy of TCM: an example using saffron formula. Sci Rep. 2018;8:380. doi: 10.1038/s41598-017-18764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 74.Loh KP, Huang SH, Tan BK, Zhu YZ. Cerebral protection of purified Herba Leonuri extract on middle cerebral artery occluded rats. J Ethnopharmacol. 2009;125:337–343. doi: 10.1016/j.jep.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 75.Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9:64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–1022. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma F, Morancho A, Montaner J, Rosell A. Endothelial progenitor cells and revascularization following stroke. Brain Res. 2015;1623:150–159. doi: 10.1016/j.brainres.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Mann GE. Molecular mechanisms underlying neurovascular protection in stroke. J Physiol. 2011;589:4103–4104. doi: 10.1113/jphysiol.2011.217455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9:139–145. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 80.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mu Q, Liu P, Hu X, Gao H, Zheng X, Huang H. Neuroprotective effects of Buyang Huanwu decoction on cerebral ischemia-induced neuronal damage. Neural Regen Res. 2014;9:1621–1627. doi: 10.4103/1673-5374.141791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nada SE, Shah ZA. Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis. 2012;46:180–189. doi: 10.1016/j.nbd.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nada SE, Tulsulkar J, Shah ZA. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761(R)) after permanent ischemic stroke in mice. Mol Neurobiol. 2014;49:945–956. doi: 10.1007/s12035-013-8572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nash KM, Shah ZA. Current perspectives on the beneficial role of ginkgo biloba in neurological and cerebrovascular disorders. Integr Med Insights. 2015;10:1–9. doi: 10.4137/IMI.S25054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan LL, Liu XH, Jia YL, Wu D, Xiong QH, Gong QH, Wang Y, Zhu YZ. A novel compound derived from danshensu inhibits apoptosis via upregulation of heme oxygenase-1 expression in SH-SY5Y cells. Biochim Biophys Acta. 2013;1830:2861–2871. doi: 10.1016/j.bbagen.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 86.Park SH, Kim JH, Park SJ, Bae SS, Choi YW, Hong JW, Choi BT, Shin HK. Protective effect of hexane extracts of Uncaria sinensis against photothrombotic ischemic injury in mice. J Ethnopharmacol. 2011;138:774–779. doi: 10.1016/j.jep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 87.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 89.Peng W, Lauche R, Ferguson C, Frawley J, Adams J, Sibbritt D. Efficacy of Chinese herbal medicine for stroke modifiable risk factors: a systematic review. Chin Med. 2017;12:25. doi: 10.1186/s13020-017-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 91.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL American Heart Association Stroke Council. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 92.Qin W, Chen S, Yang S, Xu Q, Xu C, Cai J. The effect of traditional Chinese medicine on neural stem cell proliferation and differentiation. Aging Dis. 2017;8:792–811. doi: 10.14336/AD.2017.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren C, Wang B, Li N, Jin K, Ji X. Herbal formula Danggui-Shaoyao-San promotes neurogenesis and angiogenesis in rat following middle cerebral artery occlusion. Aging Dis. 2015;6:245–253. doi: 10.14336/AD.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruan L, Wang B, ZhuGe Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015;1623:166–173. doi: 10.1016/j.brainres.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubio L, Motilva MJ, Romero MP. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles. Crit Rev Food Sci Nutr. 2013;53:943–953. doi: 10.1080/10408398.2011.574802. [DOI] [PubMed] [Google Scholar]
- 96.Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol. 2010;224:325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saleem S, Zhuang H, Biswal S, Christen Y, Dore S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seetapun S, Yaoling J, Wang Y, Zhu YZ. Neuroprotective effect of Danshensu derivatives as anti-ischaemia agents on SH-SY5Y cells and rat brain. Biosci Rep. 2013 doi: 10.1042/BSR20130032. doi: 10.1042/BSR20130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shang YJ, Qian YP, Liu XD, Dai F, Shang XL, Jia WQ, Liu Q, Fang JG, Zhou B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: influence of the solvent, radical, and substitution. J Org Chem. 2009;74:5025–5031. doi: 10.1021/jo9007095. [DOI] [PubMed] [Google Scholar]
- 101.Shao BM, Dai H, Xu W, Lin ZB, Gao XM. Immune receptors for polysaccharides from Ganoderma lucidum. Biochem Biophys Res Commun. 2004a;323:133–141. doi: 10.1016/j.bbrc.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 102.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004b;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 103.Shen YC, Lu CK, Liou KT, Hou YC, Lin YL, Wang YH, Sun HJ, Liao KH, Wang HW. Common and unique mechanisms of Chinese herbal remedies on ischemic stroke mice revealed by transcriptome analyses. J Ethnopharmacol. 2015;173:370–382. doi: 10.1016/j.jep.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 104.Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem. 2012;123:29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- 105.Shu T, Liu C, Pang M, He L, Yang B, Fan L, Zhang S, Wang X, Liu B, Rong L. Salvianolic acid B promotes neural differentiation of induced pluripotent stem cells via PI3K/AKT/GSK3beta/beta-catenin pathway. Neurosci Lett. 2018;671:154–160. doi: 10.1016/j.neulet.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 106.Si YC, Li Q, Xie CE, Niu X, Xia XH, Yu CY. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin Med. 2014;9:13. doi: 10.1186/1749-8546-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siow RC, Li FY, Rowlands DJ, de Winter P, Mann GE. Cardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic Biol Med. 2007;42:909–925. doi: 10.1016/j.freeradbiomed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 108.Siow RC, Mann GE. Dietary isoflavones and vascular protection: activation of cellular antioxidant defenses by SERMs or hormesis? Mol Aspects Med. 2010;31:468–477. doi: 10.1016/j.mam.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soobrattee MA, Bahorun T, Neergheen VS, Googoolye K, Aruoma OI. Assessment of the content of phenolics and antioxidant actions of the Rubiaceae, Ebenaceae, Celastraceae, Erythroxylaceae and Sterculaceae families of Mauritian endemic plants. Toxicol In Vitro. 2008;22:45–56. doi: 10.1016/j.tiv.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 111.Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 112.Spencer JP. Flavonoids: modulators of brain function? Br J Nutr. 2008;99:ES60–77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- 113.Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Stevenson DE, Hurst RD. Polyphenolic phytochemicals--just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 116.Su SY, Hsieh CL. Anti-inflammatory effects of Chinese medicinal herbs on cerebral ischemia. Chin Med. 2011;6:26. doi: 10.1186/1749-8546-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sucher NJ. Insights from molecular investigations of traditional Chinese herbal stroke medicines: implications for neuroprotective epilepsy therapy. Epilepsy Behav. 2006;8:350–362. doi: 10.1016/j.yebeh.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 118.Sun K, Hu Q, Zhou CM, Xu XS, Wang F, Hu BH, Zhao XY, Chang X, Chen CH, Huang P, An LH, Liu YY, Fan JY, Wang CS, Yang L, Han JY. Cerebralcare Granule, a Chinese herb compound preparation, improves cerebral microcirculatory disorder and hippocampal CA1 neuron injury in gerbils after ischemia-reperfusion. J Ethnopharmacol. 2010;130:398–406. doi: 10.1016/j.jep.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 119.Sun X, Chan LN, Sucher NJ. Magnesium as NMDA receptor blocker in the traditional Chinese medicine Danshen. Phytomedicine. 2005;12:173–177. doi: 10.1016/j.phymed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 120.Tan BK, Vanitha J. Immunomodulatory and antimicrobial effects of some traditional chinese medicinal herbs: a review. Curr Med Chem. 2004;11:1423–1430. doi: 10.2174/0929867043365161. [DOI] [PubMed] [Google Scholar]
- 121.Ting HC, Chang CY, Lu KY, Chuang HM, Tsai SF, Huang MH, Liu CA, Lin SZ, Harn HJ. Targeting cellular stress mechanisms and metabolic homeostasis by Chinese herbal drugs for neuroprotection. Molecules. 2018 doi: 10.3390/molecules23020259. doi: 10.3390/molecules23020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tulsulkar J, Glueck B, Hinds TD, Shah ZA. Ginkgo biloba extract prevents female mice from ischemic brain damage and the mechanism is independent of the HO1/Wnt pathway. Transl Stroke Res. 2016;7:120–131. doi: 10.1007/s12975-015-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tulsulkar J, Shah ZA. Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int. 2013;62:189–197. doi: 10.1016/j.neuint.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Udalamaththa VL, Jayasinghe CD, Udagama PV. Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:110. doi: 10.1186/s13287-016-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett. 2000;281:123–126. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- 128.Wang C, Pei A, Chen J, Yu H, Sun ML, Liu CF, Xu X. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J Neurochem. 2012;121:1007–1013. doi: 10.1111/j.1471-4159.2012.07744.x. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Wang L, Zhang N, Zhang Q, Zhao H. Houshiheisan compound prescription protects neurovascular units after cerebral ischemia. Neural Regen Res. 2014;9:741–748. doi: 10.4103/1673-5374.131580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang SJ, Lin TY, Lu CW, Huang WJ. Osthole and imperatorin, the active constituents of Cnidium monnieri (L. ) Cusson, facilitate glutamate release from rat hippocampal nerve terminals. Neurochem Int. 2008;53:416–423. doi: 10.1016/j.neuint.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 131.Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, Chen JG. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Wang Y. Stroke research in 2017: surgical progress and stem-cell advances. Lancet Neurol. 2018;17:2–3. doi: 10.1016/S1474-4422(17)30421-0. [DOI] [PubMed] [Google Scholar]
- 133.Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- 134.Weinberger JM. Evolving therapeutic approaches to treating acute ischemic stroke. J Neurol Sci. 2006;249:101–109. doi: 10.1016/j.jns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 135.Wu PF, Zhang Z, Wang F, Chen JG. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin. 2010;31:1523–1531. doi: 10.1038/aps.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ye R, Zhao G, Liu X. Ginsenoside Rd for acute ischemic stroke: translating from bench to bedside. Expert Rev Neurother. 2013;13:603–613. doi: 10.1586/ern.13.51. [DOI] [PubMed] [Google Scholar]
- 137.Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yong E. First response: race against time. Nature. 2014;510:S5. doi: 10.1038/510S5a. [DOI] [PubMed] [Google Scholar]
- 139.Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: role of resveratrol in microglial activation. Eur J Pharmacol. 2010;636:1–7. doi: 10.1016/j.ejphar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 140.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang P, Liu X, Zhu Y, Chen S, Zhou D, Wang Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-kappaB activation and cytokine production of glial cells. Neurosci Lett. 2013;534:123–127. doi: 10.1016/j.neulet.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 142.Zhang S, Qi Y, Xu Y, Han X, Peng J, Liu K, Sun CK. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem Int. 2013;63:522–532. doi: 10.1016/j.neuint.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 143.Zhao Y, Dou J, Luo J, Li W, Chan HH, Cui W, Zhang H, Han R, Carlier PR, Zhang X, Han Y. Neuroprotection against excitotoxic and ischemic insults by bis(12)-hupyridone, a novel anti-acetylcholinesterase dimer, possibly via acting on multiple targets. Brain Res. 2011;1421:100–109. doi: 10.1016/j.brainres.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 144.Zhao Z, Ma X, Ma J, Sun X, Li F, Lv J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem Biol Interact. 2018;286:45–51. doi: 10.1016/j.cbi.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Zhong Z, Wang B, Dai M, Sun Y, Sun Q, Yang G, Bian L. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci Lett. 2013;555:24–29. doi: 10.1016/j.neulet.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 146.Zhou ZY, Tang YP, Xiang J, Wua P, Jin HM, Wang Z, Mori M, Cai DF. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J Ethnopharmacol. 2010;131:154–164. doi: 10.1016/j.jep.2010.06.023. [DOI] [PubMed] [Google Scholar]