Abstract
Investigating the cellular and molecular mechanisms involved in the development of topographically ordered connections in the central nervous system constitutes an important issue in neurobiology because these connections are the base of the central nervous system normal function. The dominant model to study the development of topographic maps is the projection from the retinal ganglion cells to the optic tectum/colliculus. The expression pattern of Eph/ephrin system in opposing gradients both in the retina and the tectum, labels the local addresses on the target and gives specific sensitivities to growth cones according to their topographic origin in the retina. The rigid precision of normal retinotopic mapping has prompted the chemoaffinity hypothesis, positing axonal targeting to be based on fixed biochemical affinities between fibers and targets. However, several lines of evidence have shown that the mapping can adjust to experimentally modified targets with flexibility, demonstrating the robustness of the guidance process. Here we discuss the complex ways the Ephs and ephrins interact allowing to understand how the retinotectal mapping is a precise but also a flexible process.
Keywords: axon growth, axon guidance, development, Eph and ephrin, mapping, regeneration, retinal ganglion cells, retino-tectal system
Molecular Mechanisms of Mapping: Retinotectal/Collicular System as a Model
Investigating the cellular and molecular mechanisms involved in the development of topographically ordered connections in the central nervous system (CNS) constitutes an important issue in neurobiology because these connections are the base of the CNS normal function. Axonal projections between two populations of neurons, which preserve neighborhood relationships, are called topographic maps and they are ubiquitous in the brain. The dominant model to study the development of topographic maps is the projection from the retinal ganglion cells (RGCs) to its major midbrain target namely the optic tectum of fishes, frogs and chicks or its mammalian homolog, the superior colliculus. This map is organized in two orthogonally oriented axes. Nasal RGCs project to the caudal tectum and the temporal ones project to the rostral tectum, whereas dorsal RGCs project to the ventral (lateral) tectum and ventral RGCs project to the dorsal (medial) tectum (Figure 1). This organization is the cellular base by which the visual field inverted in the retina is correctly reconstituted on the tectal surface (Vanegas and Ito, 1983; Flanagan, 2006). Therefore, the regeneration of retinofugal maps is the final objective of any regenerative strategy applied in traumatic or degenerative pathologies affecting the RGCs or the optic nerve (Scicolone et al., 2009).
Figure 1.
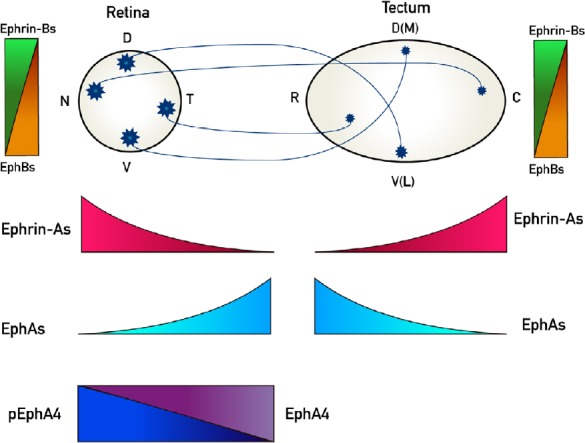
Representation of retinotectal/collicular projections and the expression patterns of EphAs and ephrin-As along its rostro-caudal axis and the EphBs and ephrin-Bs along its dorso-ventral axis.
Retinal ganglion cells (RGCs) project to contralateral tectum in chicks whereas they project bilaterally to colliculus in mice. Nasal (N) RGCs axons project to caudal (C) tectum/colliculus whereas temporal (T) RGC axons project to rostral (R) tectum/colliculus. Dorsal (D) RGCs axons project to ventral(V)-lateral (L) tectum/colliculus meanwhile ventral (V) RGC axons project to dorsal (D)-medial (M) tectum/colliculus. Ephs and ephrins are expressed in gradients both in the retina and the tectum/colliculus. EphAs (3, 5, 6) (light blue) are expressed in an increasing naso-temporal gradient in the retina, whereas EphA4 presents an even expression along the retina (purple), but it presents a decreasing nasodorsal to temporoventral gradient of phosphorylation (p) (blue). EphAs (3, 6, 7) are expressed in a decreasing rostro-caudal gradient in the tectum/colliculus. Ephrin-As (2, 5, 6) are expressed in a decreasing naso-temporal gradient in the retina and in an increasing rostro-caudal gradient in the tectum/colliculus (red). EphBs are expressed in increasing dorso-ventral gradients both in the retina and the tectum/colliculus (orange) whereas ephrin-Bs are expressed in-decreasing dorso-ventral gradients both in the retina and the tectum/colliculus (green).
Significant advances have been obtained in axonal regrowth of damaged RGCs, but no reconstitution of the retinotectal/collicular map could be obtained (Kim et al., 2018). Latest studies about retinotectal/collicular mapping have shown the existence of complex molecular mechanisms, some of which seems to present conflicting biological consequences. Analyzing these mechanisms is the purpose of this short review and their understanding could be applied to perform regeneration therapies.
We searched PubMed database. Selection criteria: activity dependent mapping; Eph ephrin binding; Eph ephrin clustering and binding; EphA4; Optic fiber regeneration and Eph ephrin; Optic nerve regeneration; Eph; Retinal projection regeneration; Retinotectal mapping; Visual system and Eph ephrin without data limits.
Eph/Ephrin System in Axon Guidance
Sperry’s classic theory predicted that RGC axons find their synaptic targets in the tectum through a process of interaction between recognition molecules, or chemoaffinity labels, differentially expressed on their growth cones and on tectal cells. Sperry suggested that each point in the tectum has a unique molecular address determined by the graded distribution of topographic guidance molecules along the two tectal axes. Each RGC has a unique profile of receptors for those molecules, resulting in a position-dependent, differential response to the guidance molecules by RGC axons (Sperry, 1963). Graded expression of Eph receptors and their ligands, the ephrins, both in the retina and the optic tectum/superior colliculus has suggested that these molecules could provide the positional information that guides the topographic movement of growth cones in the visual system (Scicolone et al., 2009). The analysis of the roles of Eph/ephrin system in mapping retinal projections onto the tectum/colliculus is the main issue of this review.
Ephs are a family of widely expressed receptor tyrosine kinases comprising ten EphAs and six EphBs. They promiscuously bind the six glycosylphosphatidylinositol (GPI)-linked ephrin-As and the three transmembrane ephrin-Bs respectively. However, this apparent class-specificity may be an oversimplification, as EphA4 binds to ephrin-Bs, and EphB2 binds to ephrin-A5 (Gale et al., 1996; Himanen et al., 2004; Truitt and Freywald, 2011; Xia et al., 2013) (Figure 2).
Figure 2.
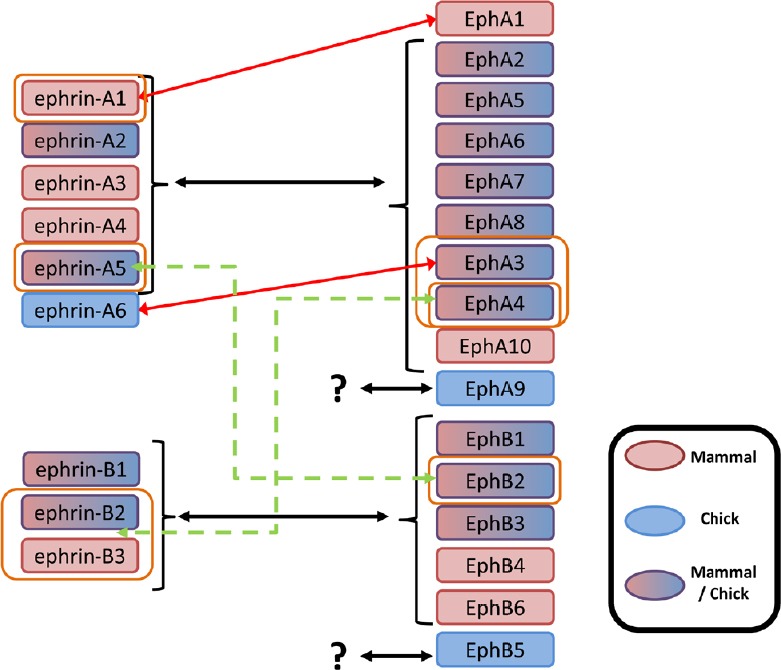
Structural classes of Eph receptors and ephrins and their binding specificities.
Despite some described high affinity ligand/receptor interactions (red arrows), binding is mostly promiscuous within each of the ephrin/Eph specificity classes (black arrows). In addition, there are two exceptions that show low affinities between members of distinct subclasses (green dashed arrows). Ligands for EphA9 and EphB5 receptors have still not been described. Ligands and receptors have been characterized in mammals (light red), chick (blue) or both (light red/blue).
The fact that the ephrins are membrane-bound proteins allows the Eph/ephrin system to signal in forward (Ephs acting as receptors) and in reverse (ephrins acting as receptors) directions (Scicolone et al., 2009). It is thought that in the absence of cell-cell interactions, these molecules exist in loosely associated clusters (microdomains) within plasma membranes, which become much more compact upon Eph/ephrin complex formation, generating clearly defined signaling centers at the cell-cell interfaces (Vearing and Lackmann, 2005).The classic model of Eph and ephrin function in neighboring cells involves ephrins acting as in trans ligands of Eph receptors, resulting in cell repulsion (ephrin:Eph, or ‘forward’ signaling). However, Eph receptors can also act as in trans ligands for ephrins (Eph:ephrin or ‘reverse’ signaling), eliciting either cell repulsion or adhesion. In addition, both Eph proteins and ephrins can simultaneously act as receptors and ligands, leading to bidirectional or parallel and antiparallel signaling, depending on the distribution of Ephs and ephrins between interacting cells, as well as the direction of signaling in single ephrin-Eph pairs. Ephrins can also induce signaling cascades independently of Eph proteins (Chin-Sang, 2002).
A number of detailed discussions of the multitude of Eph signaling mechanisms exist at present. In most cases, to elicit robust Eph receptor signaling, ephrins must be presented as multimers. This results in the formation of signaling clusters, in which Eph receptors form arrays intercalated with ephrins, the size of which correlates with the strength of the signal, and which might partly explain the diverse cellular responses that are elicited by Eph activation. Some studies argue that Ephs and ephrins can interact on the surface of the same cell (in cis), and that this attenuates Eph signal-possibly by inhibiting the formation of Ephs clusters (Carvalho et al., 2006; Kao and Kania, 2011).
One of the first forward ephrin: Eph signaling events is the activation of the kinase activity of the Eph (Kullander et al., 2001) which results in the autophosphorylation of the juxtamembrane Tyr residues, an event that is crucial (Zisch et al., 1998) for Eph-directed cellular responses. In reverse Eph:ephrin signaling, the phosphorylation of the ephrin-B intracellular domain is also an important early event (Holland et al., 1996; Brückner et al., 1997) and is mediated by Src family kinases (Palmer et al., 2002). Signals generated by ephrin binding to Eph receptors involve their interaction with specific intracellular proteins, including the non-catalytic region of Tyr kinase adaptor protein 1 and Nck2, phosphoinositide 3-kinase, Src family kinases, Vav2, Vav3 and ephexin. In turn, these effectors are coupled to Rho GTPases such as RhoA1, Cdc42 and Rac1, which can modulate the cytoskeleton (Kania and Klein, 2016).
For reverse signaling, Src family kinases seem to be crucial for signaling mediated by both ephrin classes. In addition, Ret and p75 are transmembrane effectors of class A ephrin signaling (Lim et al., 2008; Marler et al., 2008; Bonanomi et al., 2012), and the Grb4-Pak-1-Dock180 complex specifically interacts with the carboxyl terminus of B class ephrins (Xu and Henkemeyer, 2009). Many Eph-triggered cellular responses eventually lead to cytoskeletal rearrangements, such as the collapse of the cytoskeleton, by controlling the balance between small GTPase activation and inactivation (Kania and Klein, 2016). Once intracellular signaling is initiated, the repulsive cellular responses seem to rely on the dissociation of ephrins from Ephs through proteolytic cleavage of ephrins (Hattori et al., 2000; Janes et al., 2005) and/or Eph receptors (Lin et al., 2008), an event that has been proposed to terminate ephrin-Eph signaling.
Rigid and Precise Mapping versus Flexible and Robust Mapping
Rigid and precise mapping: the chemoaffinity hypothesis based on Eph/ephrin system
The rigid precision of normal retinotopic mapping has prompted the chemoaffinity hypothesis, positing axonal targeting to be based on fixed biochemical affinities between fibers and targets (Scicolone et al., 2009). However, several lines of evidence have been gathered that the mapping can adjust to experimental modified targets with flexibility demonstrating the robustness of the guidance process. The identification of ephrins and Eph-receptors as the underlying molecular cues has mostly been interpreted as supporting the fiber-target chemoaffinity hypothesis, while the evidence on mapping robustness has been neglected (Weth et al., 2014).
Eph receptors and their ephrin ligands are expressed in gradients in both the retina and the tectum, and it was shown that they represent the main molecular system controlling the mapping of retinal projections onto the tectum/colliculus (Scicolone et al., 2009). EphAs and ephrin-As define the topographic retinotectal connections along the rostro-caudal axis, whereas EphBs and ephrin-Bs have been implicated along the dorso-ventral axis. This is achieved through opposing gradients of Ephs and ephrins in both the retina and the tectum (Scicolone et al., 2009) (Figure 1). Mapping along rostro-caudal axis was focused in this review because the molecular mechanisms in this axis are better understood than in the dorso-ventral one.
Ephrin-As expressed in an increasing rostro-caudal gradient in the tectum are growth cone repellents (Drescher et al., 1995; Nakamoto et al., 1996; Monschau et al., 1997) and interstitial branching inhibitors (Yates et al., 2001; Sakurai et al., 2002) that preferentially affect temporal RGC axons by activating their EphA receptors (Brown et al., 2000; Feldheim, 2004). Thus, tectal ephrin-As prevent temporal RGCs from branching caudally to their appropriate termination zone. It was shown that axonal ephrin-As diminish the repulsive response of axonal EphA receptors to tectal ephrin-As, preventing repulsion of nasal RGC axons from the caudal tectum (Hornberger et al., 1999). However, these data do not explain why nasal RGC axons grow toward the caudal tectum without branching rostrally to their appropriate target area. Two opposing forces are required, so that each axon branches off where these forces balance (Yates et al., 2001; Flanagan, 2006; Gosse et al., 2008; Scicolone et al., 2009).
Conflicting models have been postulated about the second mapping force. One model proposes a bifunctional activity of tectal ephrin-As, showing an attractant effect at low concentrations in the rostral tectum and a repulsive effect at higher concentrations in the caudal tectum (Hansen et al., 2004; Honda, 2004). The transition from attraction to repulsion varied systematically with both ephrin concentration and retinal position, providing topographic specificity. These results support a model in which map position could be specified as a point where positive and negative forces balance out for each specific position of origin of RGCs (Hansen et al., 2004; Naoki, 2017) (Figure 3A). However, no effect on branching was evaluated. Indeed, others have posited that branch formation is induced where the balance of EphA/ephrin-A signaling in the RGC is achieved to allow for Brain-derived neurotrophic factor (BDNF)-induced branching (Triplett, 2014). Besides, these experiments employed membrane vesicles from chicken rostral tecta to provide a permissive substrate mixed with different concentrations of 293T cell membranes transfected with ephrin-As. Since an attractant effect was demonstrated for the EphA3 expressed in chicken rostral tectum (Ortalli et al., 2012), the former work cannot exclude the possibility that the attractant effect attributed to lower concentrations of ephrin-As could be partially due to parallel increasing concentrations of EphA3 (Scicolone et al., 2009) (Figure 3A). Another model proposes that this second force is produced by a decreasing rostro-caudal gradient of EphA7 which repels nasal optic fibers and prevent them from branching in the rostral tectum/colliculus throughout ephrin-As reverse signaling (Rashid et al., 2005; Lim et al., 2008) (Figure 3B). However, as optic fibers invade the tectum/colliculus throughout the highest part of this gradient, this model cannot explain how the axons invade the tectum/colliculus without being repelled from it. On the other hand, we demonstrated that the decreasing rostro-caudal tectal gradient of EphA3 promotes nasal RGC axon growth toward the caudal tectum and inhibits them from branching rostrally (Ortalli et al., 2012). Thus, its positive effect on axon growth, instead of a repellent effect, allows explaining the axonal invasion of the tectum and the axon growth of nasal RGCs toward the caudal tectum (Ortalli et al., 2012) (Figure 3C). It is possible that a combination of the bifuntional effect of tectal ephrin-As on RGC axons plus the promoting axon growth and branching inhibition of tectal EphA3 could act as partially redundant systems together with BDNF branching promoting effect (Figure 3D).
Figure 3.
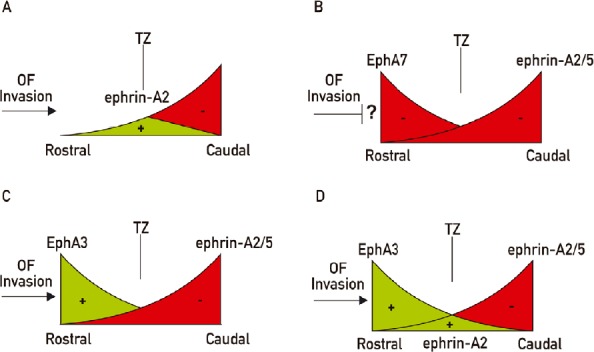
Schematic representations of different biological models that try to explain the retinotectal mapping along the rostro-caudal axis.
(A) Ephrin-A2 has an attractant role (green) at low concentrations in the rostral tectum and a repellent effect (red) at high concentrations in the caudal tectum. Termination zones (TZ) are formed where both forces are balanced according to the different sensitivity of the optic fibers. (B) EphA7 repels (red) optic fibers from the rostral colliculus meanwhile ephrin-As (red) repel them from the caudal colliculus. How are the optic fibers (OF) able to invade the colliculus throughout the repellent activity of EphA7? (C) EphA3 expressed in the rostral tectum pushes optic fibers to the caudal tectum (green) meanwhile ephrin-As repel them (red). (D) Combination of A and C. Green: Positive effect on axon growth; red: repulsive effect.
Plasticity in axon guidance and retinotectal mapping are consistent with precise distributed cues
The concept of rigid mapping was challenged by in vivo experiments. In mature goldfish, a disconnected temporal half-retina resulting from nerve transaction and nasal ablation regenerated an expanded, proper projection covering the whole tectum (expansion experiments) (Schmidt et al., 1978). Similar results were also obtained in Xenopus development. Double temporal or double nasal retinae microsurgically assembled in the larvae, instead of forming doubly occupied maps on their respective tectal halves, matured into expanded projections of each half-retina on the whole target (compound eye experiments) (Gaze et al., 1963). Conversely, tectal ablations (rostral or caudal half) in the goldfish, irrespective of concomitant nerve transaction, yielded compressed maps of the full retina on the remaining half-target (compression experiments) (Sharma, 1972). Notably, without nerve transaction, this implies mobilization of maturely connected axons of the remaining half-tectum. Thus, topographic maps can flexibly adjust to the target. As Ephs/ephrins are stable, while axons can flexibly map, chemoaffinity might not be absolute but relative (Weth et al., 2014). Neither expansion nor normal topographic mapping depends on neural activity, but they are genetically hard-wired. Pre-vision spontaneous activity, like early experience-driven activity is important for circuit refinement (Weth et al., 2014; Thompson et al., 2017).
Weth et al. (2014) concluded that expansion, compound eye and compression experiments indicate, in addition to fiber-target chemoaffinity, the existence of a second guidance influence, which they called fiber-fiber chemoaffinity. Thus, the ephrin/Eph forward and reverse signaling throughout trans and cis interactions in fiber-fiber interactions can explain the maintaining of the retinotectal map with changes in the target (Figure 4A). Thus, interaxonal and intraaxonal binding between EphAs and ephrin-As in trans and in cis, could add plasticity to the retinotectal mapping. This assumption can reconcile the seemingly conflicting findings on rigid and flexible topographic mapping. Accordingly, some works have suggested that interaxonal competition participates in the establishment of topographic ordered connections in fishes, chicks and mice (Feldheim, 2004; Honda, 2004; Yates et al., 2004; Pfeiffenberger et al., 2006; Triplett et al., 2011). Nevertheless, Gosse et al. (2008) postulated that interaxonal competition is not required for retinotopic targeting along the rostro-caudal axis in zebrafish, but serves to restrict arbor size and shape.
Figure 4.
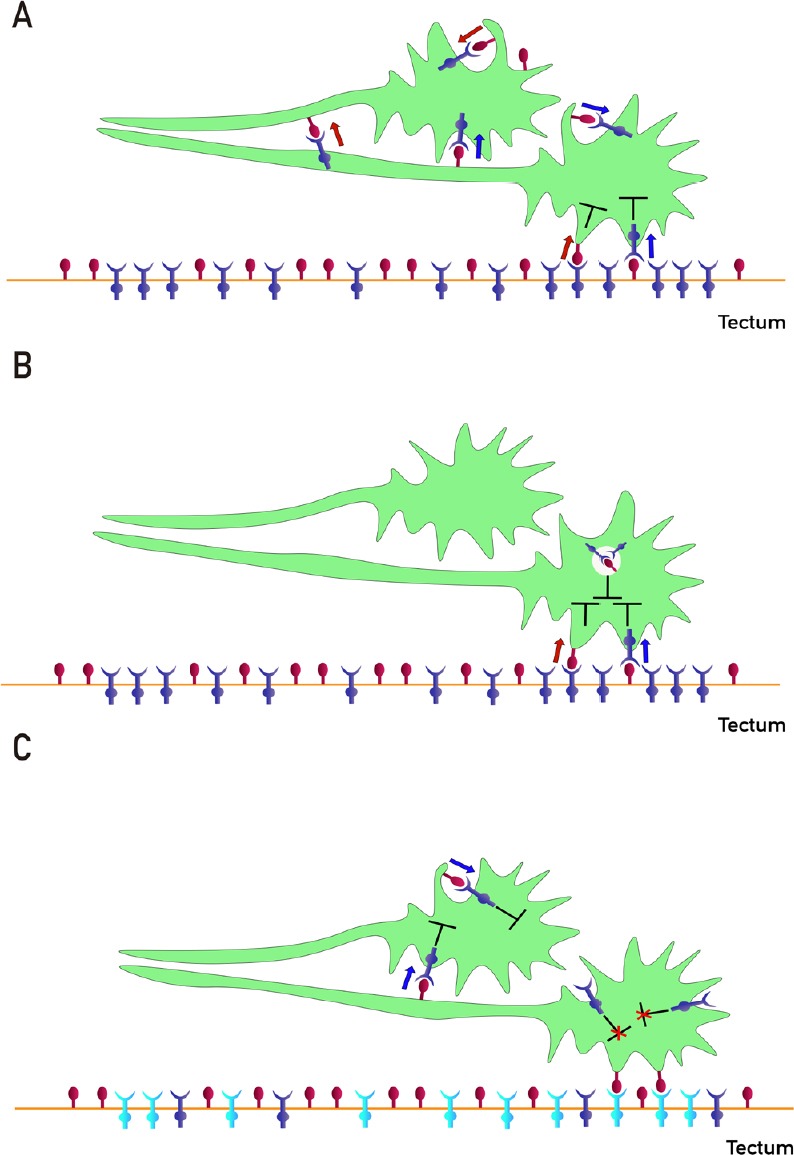
Different ways of interactions between EphAs and ephrin-As participate in retinotectal/collicular mapping.
(A) Proposed mechanisms of fiber-fiber (FF)- and fiber-target (FT)-chemoaffinity. Segregation of fibers is mediated by FF EphAs (blue)/ephrin-As (red) forward (blue arrow) and reverse (red arrow) signaling besides FT EphAs (blue)/ephrin-As (red) forward (blue arrow) and reverse (red arrow) signaling. Cis forward (blue arrow) and reverse (red arrow) signaling and inhibition (T-shaped symbol) are also shown. The guidance potential is calculated by adding all instantaneous forward and reverse signals impinging on the axons and balancing the sums. The termination zones (TZs) are formed when the guidance potential is zero. (B) Proposed mechanism of co-adaptation. EphAs (blue) and ephrin-As (red), located in membrane lipid microdomains signal in trans forward (blue arrows) and reverse (red arrows) eliciting repulsion. Dispersed, unbound sensors are constitutively endocytosed and might increase cis signaling upon internalization, as anti-parallel orientation is favored in high-curvature membrane vesicles. Enhanced cis signals desensitize growth cones (GCs) to trans signals. Sensitivity returns with the recycling of unbound sensors. (C) Regulation of ephrin-As-dependent EphA4 forward signaling by tectal EphA3 during retinotectal mapping. Ephrin-As (red)-dependent EphA4 (blue) forward signaling (blue arrows) decreases the nasal retinal ganglion cells (RGCs) axon growth before reaching the tectum. Tectal EphA3 (light blue) binds and displaces axonal ephrin-As from axonal EphA4 decreasing its forward signaling and stimulating nasal RGC axon growth toward the caudal tectum and inhibiting TZ formation in the rostral tectum.
On the other hand, novel adaptation assays demonstrated that retinal growth cones (GCs) robustly adapt towards ephrin-A/EphA forward and reverse signals (Fiederling et al., 2017). In in vitro collapse assays (in which the repulsive effect of a molecular cue is recognized by producing growth cone collapse) typically temporal GCs collapse with ephrin-As. Surprisingly, temporal GCs recover their morphology, despite the presence of the repulsive cue ephrin-As after prolonged incubation. This indicates desensitization of RGC GCs towards forward signals. The same phenomenon was observed for reverse ephrin-A/EphA signaling when EphAs were applied as ligands after prolonged incubation. This was puzzling, because topographic guidance was believed to rely on precise quantitative sensing. Computational modeling suggested that topographic accuracy and adaptability could be reconciled by a novel mechanism of coupled adaptation of forward and reverse Eph/ephrins signaling. Thus, ephrin-As ligands in the substrate are able to desensitize GCs from EphA forward and ephrin-As reverse signaling, and EphAs acting as ligands in the substrate are able to desensitize GCs from ephrin-As reverse and EphAs forward signaling (Figure 4B). Co-adaptation involves trafficking of unbound sensors between the surface membrane and recycling endosomes. Authors proposed that co-adaptative desensitization eventually relies on guidance sensor translocation into cis-signaling endosomes (Fiederling et al., 2017). Together, these findings proved the existence of a novel mechanism of signal modulation (co-adaptation), which allows for topographic mapping in the presence of GC adaptation. Co-adaptation could explain the ingrowth of optic fibers through the repulsive effect of EphA7 in the rostral tectum/colliculus (Figure 3B), but no molecule was discovered in vivo which could desensitize optic fibers from rostral tectal EphAs.
Finally, we have shown a new molecular mechanism which allows explaining how RGC axons invade the tectum and how axon growth is regulated over tectal surface (Fiore et al., 2019). Thus, we demonstrated that ephrin-As-dependent EphA4 forward signaling decreases axon growth in a target independent way. We showed that cis interactions and perhaps trans interaxonal interactions produce EphA4 forward signaling. This effect is higher in nasal RGC axons (Fiore et al., 2019). This is a long lasting effect in which EphA4 bearing axons are not retracted by axonal ephrin-As, instead, they decrease the level of axon growth. This effect has some similarities with the adaptation process described by Fiederling et al. (2017). Therefore, when nasal axons arrive the tectum, EphA3 decreases EphA4 forward signaling by competing for axonal ephrin-As (Figure 4C). Thus, target EphA3 increases axon growth by reducing ephrin-A-dependent EphA4 forward signaling. When nasal RGC axons arrive the caudal tectum, the decreasing level of EphA3 allows increasing ephrin-A-dependent EphA4 signaling which produces a decrease in the level of axon growth. Besides, the increasing levels of tectal ephrin-As stop axon growth throughout EphA forward signaling (Fiore et al., 2019).
In summary, the retinotectal mapping is a precise but also a flexible process. Several molecular ways of interactions between EphAs and ephrin-As have been described which could explain the coexistence of these two apparently opposite properties of mapping. Thus, fiber-target EphAs/ephrin-As forward and reverse signaling, competition between fiber and target EphAs for fibers ephrin-As, fiber-fiber EphAs/ephrin-As forward and reverse signaling, as a consequence of trans and cis interactions have been shown. Besides, different ways of cis interactions –in parallel with masking properties (Kania and Klein, 2016) or in anti-parallel orientation on GC surface (Fiore et al., 2019) or inside endosomes (Fiederling et al., 2017) inducing signaling have been shown (Figure 4). All of these interactions represent a part of the complex molecular network which regulates axon guidance and retinotectal mapping.
Can These Findings Collaborate for Designing Regeneration Therapies?
No clinical treatments are available to help those who suffer from loss of function due to axonal injuries associated with optic neuropathy. The optic nerve crush rodent model of traumatic optic neuropathy is a well-established system for tackling the fundamental problem of long-distance axon regeneration failure in the CNS and for determining potentially novel treatments. Few studies showed regeneration of RGC axons beyond the optic chiasm (Kim et al., 2018).
There are several hurdles to overcome in optic nerve regeneration: enhancing the intrinsic growth capacity of RGCs, overcoming the extrinsic growth-inhibitory environment of the optic nerve and optimizing the reinnervation of their targets. Some degree of optic nerve regeneration has been achieved by factors associated with inducing intraocular inflammation or by providing exogenous neurotrophic factors. Reactivating intrinsic growth capacity of mature RGCs has enabled experimental optic nerve regeneration by inhibition of cell-intrinsic suppressors of axon growth, or by activation of the intracellular signaling pathways (Chun and Cestari, 2017). Stimulation of neural activity enhanced RGC axon regeneration. However, RGC axons that recover back to their target fails during myelination and consequently undergo slower conduction of electrical potentials (Laha et al., 2017). Modifying the extrinsic growth-inhibitory environment of the optic nerve has also achieved some degree of optic nerve regeneration by suppressing receptors to cell extrinsic inhibitors, inhibiting RhoA/ROCK pathway, by chelation of mobile zinc, or by administration of calcium channel blockers (Chun and Cestari, 2017). Peripheral nerve grafts have been used to bridge tissue defects from retina to colliculus (You et al., 2016). In some experiments, axons have shown to reinnervate their targets, but they generally showed a lack of topographic order. Therefore, a major aim of visual system repair is the restoration of neural maps (You et al., 2016; Chun and Cestari, 2017). For this purpose, retinal projections must be topographically organized onto targets according to the gradients of Eph/ephrin system. It has been suggested that this system persist in the mature mammalian visual system or become upregulated after optic nerve injury, but more research is required in this area (Chun and Cestari, 2017).
Severed axonal connections in the CNS of mammals and avian generally do not regenerate. By contrast, in anamniotes (fishes and amphibians) many axonal tracts, including the optic nerve, spontaneously regrow leading to functional recovery (Stuermer et al., 1992; Bernhardt, 1999). Accordingly, these animals present a continuous retinal growth during embryonic and postnatal life. Therefore, a link between continued growth and regenerative capacity of different systems in the adult has been proposed, with the postulation that cues available to growing axons in the adult may also be available to guide regenerating axons (Holder and Clarke, 1988). Thus, anamniotes offer the opportunity to study the molecular mechanisms involved in successful axonal regrowth and, importantly, also those necessary for functional reinnervation of targets.
There are also important species-specific differences in retinotectal system about Ephs/ephrins expression and axon regeneration after injury. In goldfish (Rodger et al., 2000) and rats (Rodger et al., 2005), ephrin-A2 expression gradients persist in adult tectum and superior colliculus, respectively, although at lower levels than during development. Following optic nerve injury, ephrin-A2 expression increases in the posterior tectum/colliculus, providing the potential for enhanced retinotectal mapping information for regenerating retinal axons. These findings were described in goldfish (Rodger et al., 2000, 2004; King et al., 2004), rats and mice despite the fact that in these latter species, RGC axons do not successfully regenerate (Knöll et al., 2001; Rodger et al., 2005). In contrast to these results, it was described the persistence of ephrin-As gradients of expression in adult frog and zebrafish tectum but it was not found their up regulation following optic nerve injury although frog and zebrafish are capable of retinotectal map regeneration (Becker and Becker, 2000; Bach et al., 2003). In the goldfish retina, EphA3 and EphA5 expression increases in an ascending naso-temporal gradient following optic nerve transaction (King et al., 2003), suggesting that the recapitulation of the developmental ephrin-A guidance field in the tectum is accompanied by a complementary recapitulation of EphA-encoded axonal responsiveness in the retina. However, in rats, although the retinal EphA5 gradient persists into adulthood, its expression becomes uniform following injury, which may indicate a loss of topographic information (Rodger et al., 2005). Such differences in the way neural tissues respond to damage may therefore be involved in determining species-specific regenerative outcomes (Goldshmit et al., 2006). Rodger et al. (2004) showed that EphA/ephrin-As interactions are necessary for regenerating topographically ordered retinotectal connections in the goldfish, supporting the idea that recapitulation of developmental topographic organization of axonal guidance cues such as EphAs/ephrin-As is necessary for CNS regeneration.
Whether or not these developmental expression patterns are accurately recapitulated following CNS injuries may be an important determinant of regenerative success. However, expression of Eph/ephrin system on mature cells types, such as astrocytes and oligodendrocytes, may also have an influence that is not present during development, such as mediating astrocytic gliosis or axonal remyelination (Goldshmit et al., 2006). Thus, after spinal cord injury in adult rats, Eph/ephrin system is up-regulated on both astrocytes and oligodendrocytes and in motoneurons (Willson et al., 2002). It was shown that EphA4 regulates two important features of spinal cord injury, axonal inhibition, and astrocytic gliosis (Goldshmit et al., 2006). Accordingly, blocking Eph receptor function after spinal cord injury in mice promoted axonal regeneration and functional improvement (Fabes et al., 2007; Goldshmit et al., 2011).
This raises another question: If down-regulation of molecules that function like guidance cues during development is necessary to allow axon elongation through the injury, which are the guidance cues that allow regenerating axons to recognize the pathway and target to rebuild the topographic ordered connections?
Taking all into account, it is possible to appreciate that there are conflicting findings about roles of Eph/ephrin system after injury in the retinotectal/colliculus system and in the spinal cord. These facts, however, may not imply opposite conclusions. It is possible that the up regulation of Eph/ephrin system after spinal cord injury participate in an excessive inflammatory response that mask the appropriate axon guidance cues, so the reduction of the glial scar could reveal the axon guidance signals or could leave the pathway free to appropriate manipulation of these cues. However, in the retinotectal system, it is possible that the inflammatory response is not excessive and the recapitulation of axon guidance cues that act during development could be the main factor that define the outcome of regeneration. Thus, in this case stimulating the appropriate topographic expression of these signals could be the main point to obtain regeneration.
It is interesting to consider the possible bifunctional role of Eph/ephrin system: a) a necessary system to guide axon growth during regeneration and b) an out of control system that may mask the appropriate guidance cues with upregulated and inappropriate distributed cues that produce misrouting of axons. Thus, it is possible that better understanding about the roles of Eph/ephrin system allow us to establish more clear connections between cellular and molecular mechanisms involved in development of topographically ordered connections and their possible regeneration after injury in the adult CNS.
The complex molecular interactions of the EphA/ephrin-As system add several variables to take into account to obtain remapping and functional restitution after optic nerve crush, but these complex interactions are also the ones which make the retinotectal/collicular mapping more flexible, opening more possibilities to restitute the map. This would allow us to make a rational manipulation of different factors that could constitute the base of a therapeutic approach to obtain regeneration in the adult CNS. This goal would be the most striking consequence of the old and new studies that are increasing our knowledge about the development of topographic ordered connections in the CNS.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 00441); Universidad de Buenos Aires (M 00526BA, 00769BA, both to GS).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Bach H, Feldheim DA, Flanagan JG, Scalia F. Persistence of graded EphA/Ephrin-A expression in the adult frog visual system. J Comp Neurol. 2003;467:549–565. doi: 10.1002/cne.10941. [DOI] [PubMed] [Google Scholar]
- 2.Becker CG, Becker T. Gradients of ephrin-A2 and ephrin-A5b mRNA during retinotopic regeneration of the optic projection in adult zebrafish. J Comp Neurol. 2000;427:469–483. [PubMed] [Google Scholar]
- 3.Bernhardt RR. Cellular and molecular bases of axonal regeneration in the fish central nervous system. Exp Neurol. 1999;157:223–240. doi: 10.1006/exnr.1999.7059. [DOI] [PubMed] [Google Scholar]
- 4.Bonanomi D, Chivatakarn O, Bai G, Abdesselem H, Lettieri K, Marquardt T, Pierchala BA, Pfaff SL. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell. 2012;148:568–582. doi: 10.1016/j.cell.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A, Yates PA, Burrola P, Ortuo D, Vaidya A, Jessell TM, Pfaff SL, O’Leary DDM, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 6.Brückner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho RF, Beutler M, Marler KJM, Knöll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- 8.Chin-Sang ID. The divergent C. elegans ephrin EFN-4 functions inembryonic morphogenesis in a pathway independent of the VAB-1 Eph receptor. Development. 2002;129:5499–5510. doi: 10.1242/dev.00122. [DOI] [PubMed] [Google Scholar]
- 9.Chun BY, Cestari DM. Advances in experimental optic nerve regeneration. Curr Opin Ophthalmol. 2017;28:558–563. doi: 10.1097/ICU.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 10.Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 11.Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 12.Feldheim DA. Loss-of-Function Analysis of EphA Receptors in Retinotectal Mapping. J Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiederling F, Weschenfelder M, Fritz M, von Philipsborn A, Bastmeyer M, Weth F. Ephrin-A/EphA specific co-adaptation as a novel mechanism in topographic axon guidance. Elife. 2017 doi: 10.7554/eLife.25533. doi: doi: 10.7554/eLife.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiore L, Medori M, Spelzini G, Carreño CO, Carri NG, Sanchez V, Scicolone G. Regulation of axonal EphA4 forward signaling is involved in the effect of EphA3 on chicken retinal ganglion cell axon growth during retinotectal mapping. Exp Eye Res. 2019;178:46–60. doi: 10.1016/j.exer.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan JG. Neural map specification by gradients. Curr Opin Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 17.Gaze RM, Jacobson M, Székely G. The retino-tectal projection in Xenopus with compound eyes. J Physiol. 1963;165:484–499. doi: 10.1113/jphysiol.1963.sp007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Goldshmit Y, Spanevello MD, Tajouri S, Li L, Rogers F, Pearse M, Galea M, Bartlett PF, Boyd AW, Turnley AM. EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice. PLoS One. 2011;6:e24636. doi: 10.1371/journal.pone.0024636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosse NJ, Nevin LM, Baier H. Retinotopic order in the absence of axon competition. Nature. 2008;452:892–895. doi: 10.1038/nature06816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin-As shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 23.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: Ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 24.Holder N, Clarke JDW. Is there a correlation between continuous neurogenesis and directed axon regeneration in the vertebrate nervous system? Trends Neurosci. 1988;11:94–99. doi: 10.1016/0166-2236(88)90151-8. [DOI] [PubMed] [Google Scholar]
- 25.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 26.Honda H. Competitive interactions between retinal ganglion axons for tectal targets explain plasticity of retinotectal projection in the servomechanism model of retinotectal mapping. Dev Growth Differ. 2004;46:425–437. doi: 10.1111/j.1440-169x.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 27.Hornberger MR, Dütting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Weßel R, Logan C, Tanaka H, Drescher U. Modulation of EphA receptor function by coexpressed EphrinA ligands on retinal ganglion cell axons. Neuron. 1999;22:731–742. doi: 10.1016/s0896-6273(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 28.Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB. Adam meets Eph: An ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17:240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 30.Kao TJ, Kania A. Ephrin-mediated cis-attenuation of Eph receptor signaling is essential for spinal motor axon guidance. Neuron. 2011;71:76–91. doi: 10.1016/j.neuron.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Sajid MS, Trakhtenberg EF. The extent of extra-axonal tissue damage determines the levels of CSPG upregulation and the success of experimental axon regeneration in the CNS. Sci Rep. 2018;8:9839. doi: 10.1038/s41598-018-28209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King C, Lacey R, Rodger J, Bartlett C, Dunlop S, Beazley L. Characterisation of tectal ephrin-A2 expression during optic nerve regeneration in goldfish: Implications for restoration of topography. Exp Neurol. 2004;187:380–387. doi: 10.1016/j.expneurol.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 33.King CE, Wallace A, Rodger J, Bartlett C, Beazley LD, Dunlop SA. Transient up-regulation of retinal EphA3 and EphA5, but not ephrin-A2, coincides with re-establishment of a topographic map during optic nerve regeneration in goldfish. Exp Neurol. 2003;183:593–599. doi: 10.1016/s0014-4886(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 34.Knöll B, Isenmann S, Kilic E, Walkenhorst J, Engel S, Wehinger J, Bähr M, Drescher U. Graded expression patterns of ephrin-As in the superior colliculus after lesion of the adult mouse optic nerve. Mech Dev. 2001;106:119–127. doi: 10.1016/s0925-4773(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 35.Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 36.Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017;356:1031–1034. doi: 10.1126/science.aal5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DDM. p75NTR mediates Ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marler KJM, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger MR, Löschinger J, Pasquale EB, Siever DA, Verderame MF, Müller BK, Bonhoeffer F, Drescher U. Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. EMBO J. 1997;16:1258–1267. doi: 10.1093/emboj/16.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamoto M, Cheng HJ, Friedman GC, McLaughlin T, Hansen MJ, Yoon CH, O’Leary DDM, Flanagan JG. Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- 42.Naoki H. Revisiting chemoaffinity theory: Chemotactic implementation of topographic axonal projection. PLoS Comput Biol. 2017;13:e1005702. doi: 10.1371/journal.pcbi.1005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortalli AL, Fiore L, Di Napoli J, Rapacioli M, Salierno M, Etchenique R, Flores V, Sanchez V, Carri NG, Scicolone G. EphA3 expressed in the chicken tectum stimulates Nasal retinal ganglion cell axon growth and is required for retinotectal topographic map formation. PLoS One. 2012;7:e38566. doi: 10.1371/journal.pone.0038566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: Regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- 45.Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and Patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rashid T, Upton AL, Blentic A, Ciossek T, Knöll B, Thompson ID, Drescher U. Opposing gradients of Ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron. 2005;47:59–69. doi: 10.1016/j.neuron.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Rodger J, Bartlett CA, Beazley LD, Dunlop SA. Transient up-regulation of the rostrocaudal gradient of ephrin A2 in the tectum coincides with reestablishment of orderly projections during optic nerve regeneration in goldfish. Exp Neurol. 2000;166:196–200. doi: 10.1006/exnr.2000.7486. [DOI] [PubMed] [Google Scholar]
- 48.Rodger J, Symonds ACE, Springbett J, Shen WY, Barlett CA, Rakoczy PE, Beazley LD, Dunlop SA. Eph/ephrin expression in the adult rat visual system following localized retinal lesions: Localized and transneuronal up-regulation in the retina and superior colliculus. Eur J Neurosci. 2005;22:1840–1852. doi: 10.1111/j.1460-9568.2005.04381.x. [DOI] [PubMed] [Google Scholar]
- 49.Rodger J, Vitale PN, Tee LBG, King CE, Bartlett CA, Fall A, Brennan C, O’Shea JE, Dunlop SA, Beazley LD. EphA/ephrin-A interactions during optic nerve regeneration: Restoration of topography and regulation of ephrin-A2 expression. Mol Cell Neurosci. 2004;25:56–68. doi: 10.1016/j.mcn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Sakurai T, Wong E, Drescher U, Tanaka H, Jay DG. Ephrin-A5 restricts topographically specific arborization in the chick retinotectal projection in vivo. Proc Natl Acad Sci U S A. 2002;99:10795–10800. doi: 10.1073/pnas.162161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt JT, Cicerone CM, Easter SS. Expansion of the half retinal projection to the tectum in goldfish: An electrophysiological and Anatomical study. J Comp Neurol. 1978;177:257–277. doi: 10.1002/cne.901770206. [DOI] [PubMed] [Google Scholar]
- 52.Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res Bull. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Sharma SC. Reformation of retinotectal projections after various tectal ablations in adult goldfish. Exp Neurol. 1972;34:171–182. doi: 10.1016/0014-4886(72)90197-5. [DOI] [PubMed] [Google Scholar]
- 54.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuermer CAO, Bastmeyer M, Bähr M, Strobel G, Paschke K. Trying to understand axonal regeneration in the CNS of fish. J Neurobiol. 1992;23:537–550. doi: 10.1002/neu.480230508. [DOI] [PubMed] [Google Scholar]
- 56.Thompson A, Gribizis A, Chen C, Crair MC. Activity-dependent development of visual receptive fields. Curr Opin Neurobiol. 2017;42:136–143. doi: 10.1016/j.conb.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Triplett JW. Molecular guidance of retinotopic map development in the midbrain. Curr Opin Neurobiol. 2014;24:7–12. doi: 10.1016/j.conb.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Triplett JW, Pfeiffenberger C, Yamada J, Stafford BK, Sweeney NT, Litke AM, Sher A, Koulakov AA, Feldheim DA. Competition is a driving force in topographic mapping. Proc Natl Acad Sci U S A. 2011;108:19060–19065. doi: 10.1073/pnas.1102834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Truitt L, Freywald A. Dancing with the dead: Eph receptors and their kinase-null partners. Biochem Cell Biol. 2011;89:115–129. doi: 10.1139/o10-145. [DOI] [PubMed] [Google Scholar]
- 60.Vanegas H, Ito H. Morphological aspects of the teleostean visual system: a review. Brain Res. 1983;6:117–137. doi: 10.1016/0165-0173(83)90036-x. [DOI] [PubMed] [Google Scholar]
- 61.Vearing CJ, Lackmann M. Eph receptor signalling; dimerisation just isn’t enough. Growth Factors. 2005;23:67–76. doi: 10.1080/08977190500055869. [DOI] [PubMed] [Google Scholar]
- 62.Weth F, Fiederling F, Gebhardt C, Bastmeyer M. Chemoaffinity in topographic mapping revisited - Is it more about fiber-fiber than fiber-target interactions? Semin Cell Dev Biol. 2014;35:126–135. doi: 10.1016/j.semcdb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Willson CA, Irizarry-Ramíacrez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplantation. 2002;11:229–239. [PubMed] [Google Scholar]
- 64.Xia Y, Luo C, Dai S, Yao D. Increased EphA/ephrinA expression in hippocampus of pilocarpine treated mouse. Epilepsy Res. 2013;105:20–29. doi: 10.1016/j.eplepsyres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yates PA, Holub AD, McLaughlin T, Sejnowski TJ, O’Leary DD. Computational modeling of retinotopic map development to define contributions of EphA-EphrinA gradients, axon-axon interactions, and patterned activity. J Neurobiol. 2004;59:95–113. doi: 10.1002/neu.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yates PA, Roskies AL, McLaughlin T, O’Leary DD. Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J Neurosci. 2001;21:8548–8563. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.You SW, Hellström M, Pollett MA, LeVaillant C, Moses C, Rigby PJ, Penrose M, Rodger J, Harvey AR. Large-scale reconstitution of a retina-to-brain pathway in adult rats using gene therapy and bridging grafts: An anatomical and behavioral analysis. Exp Neurol. 2016;279:197–211. doi: 10.1016/j.expneurol.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Zisch AH, Kalo MS, Chong LD, Pasquale EB. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene. 1998;16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]


