Abstract
Purpose
Ponatinib is the only approved tyrosine kinase inhibitor (TKI) suppressing BCR-ABL1T315I-mutated cells in chronic myeloid leukemia (CML). However, due to side effects and resistance, BCR-ABL1T315I-mutated CML remains a clinical challenge. Hydroxyurea (HU) has been used for cytoreduction in CML for decades. We found that HU suppresses or even eliminates BCR-ABL1T315I+ sub-clones in heavily pretreated CML patients. Based on this observation, we investigated the effects of HU on TKI-resistant CML cells in vitro.
Methods
Viability, apoptosis and proliferation of drug-exposed primary CML cells and BCR-ABL1+ cell lines were examined by flow cytometry and 3H-thymidine-uptake. Expression of drug targets was analyzed by qPCR and Western blotting.
Findings
HU was more effective in inhibiting the proliferation of leukemic cells harboring BCR-ABL1T315I or T315I-including compound-mutations compared to cells expressing wildtype BCR-ABL1. Moreover, HU synergized with ponatinib and ABL001 in inducing growth inhibition in CML cells. Furthermore, HU blocked cell cycle progression in leukemic cells, which was accompanied by decreased expression of CDK4 and CDK6. Palbociclib, a more specific CDK4/CDK6-inhibitor, was also found to suppress proliferation in primary CML cells and to synergize with ponatinib in producing growth inhibition in BCR-ABL1T315I+ cells, suggesting that suppression of CDK4/CDK6 may be a promising concept to overcome BCR-ABL1T315I-associated TKI resistance.
Interpretation
HU and the CDK4/CDK6-blocker palbociclib inhibit growth of CML clones expressing BCR-ABL1T315I or complex T315I-including compound-mutations. Clinical studies are required to confirm single drug effects and the efficacy of `ponatinib+HU´ and ´ponatinib+palbociclib´ combinations in advanced CML.
Funding
This project was supported by the Austrian Science Funds (FWF) projects F4701-B20, F4704-B20 and P30625.
Keywords: CML, BCR-ABL1 mutations, TKI resistance, Hydroxyurea, CDK4/CDK6 – Palbociclib
Research in context.
Evidence before this study
Tyrosine kinase inhibitor (TKI)-resistant chronic myeloid leukemia (CML) represents a clinical challenge, especially when clonal cells display T315I mutant forms of BCR-ABL1. Ponatinib is a multi-targeted TKI that blocks BCR-ABL1T315I. However, resistance or intolerance against ponatinib may occur.
Added value of this study
We here demonstrate that drugs regulating CDK4/CDK6 expression or function in CML cells, such as hydroxyurea (HU) or palbociclib, exert strong anti-neoplastic effects on CML cells expressing BCR-ABL1T315I or T315I-including compound mutations. The growth-inhibitory effects of HU on CML cells exhibiting BCR-ABL1T315I was demonstrable in vivo in heavily pretreated and highly resistant CML patients as well as in vitro in various cell line models. Furthermore, synergistic growth-inhibitory effects of the drug-combinations HU+ponatinib, palbociclib+ponatinib, and HU+ABL-001, were demonstrable.
Implications of all the available evidence
Patients with CML who develop TKI-resistance in BCR-ABL1T315I-mutated sub-clones have a grave prognosis. Ponatinib may overcome this form of resistance in a subset of patients whereas others have or develop resistance against ponatinib. We are the first to provide evidence that simultaneous targeting of BCR-ABL1 and CDK4/CDK6 is a reasonable strategy to overcome drug resistance in CML, an observation that may help develop therapeutic concepts for advanced, multi-resistant CML. Our findings are relevant clinically, since, apart from stem cell transplantation, effective therapies for these patients are lacking and the drug combination HU+ponatinib can easily be applied as both drugs are FDA-approved and available. Furthermore, our data suggest that the application of novel drug combinations, such as ABL001+HU or ponatinib+palbociclib in multi-resistant CML expressing BCR-ABL1T315I or T315I-including compound mutations, may represent effective new therapies, which should be tested in clinical studies.
Alt-text: Unlabelled box
1. Introduction
In most patients with chronic myeloid leukemia (CML) in chronic phase (CP), imatinib treatment results in long-lasting deep molecular responses [1], [2], [3]. However, resistance against imatinib may develop, often in the context of BCR-ABL1 point mutations or because of activation of BCR-ABL1-independent signaling pathways [4], [5], [6], [7]. In these patients, second-generation tyrosine kinase inhibitors (TKI), such as nilotinib, dasatinib or bosutinib, are usually prescribed [8,9]. One major problem in these patients is the outgrowth of sub-clones harboring the T315I-mutated variant of BCR-ABL1, which confers resistance against all second-generation TKI [9], [10], [11].
As of now, ponatinib is the only approved TKI that exerts major growth-inhibitory effects against sub-clones bearing BCR-ABL1T315I [12,13]. Indeed, ponatinib was found to induce clinically meaningful responses in a high proportion of patients with BCR-ABL1T315I-positive CML [13]. However, occurrence of BCR-ABL1T315I still represents a clinical challenge. First, ponatinib has been reported to cause severe cardiovascular side effects and may therefore not be an optimal drug for long-term treatment in all patients, especially in elderly patients or in patients exhibiting cardiovascular risk factors [13], [14], [15], [16]. Second, sub-clones bearing T315I-positive compound mutations of BCR-ABL1 or the E255V mutation are usually resistant against ponatinib therapy [17], [18], [19], [20]. In these cases, therapeutic options are very limited. One treatment option for such advanced CML patients is allogeneic hematopoietic stem cell transplantation (HSCT) [21], [22], [23]. However, HSCT can only be offered to a smaller number of patients who are fit and can tolerate such intensive therapy. In addition, prior to HSCT, sufficient debulking is often required. Overall most of the TKI-resistant patients have to be managed using continuous drug therapy which is associated with side effects. One strategy is to apply lower doses of ponatinib or to test new targeted drugs directed against mutant forms of BCR-ABL1, such as asciminib (ABL001) or PF-114 [24], [25], [26], [27], [28], [29]. However, not all patients may respond and little is known about long-term side effects and toxicity profiles of these novel BCR-ABL1-targeting drugs [27], [28], [29]. Therefore, current research is seeking additional therapeutic strategies to control BCR-ABL1T315I+ CML.
Hydroxyurea (HU) is a ribonucleotide reductase-inhibitor that is used for the treatment of sickle cell disease and palliative cytoreduction of end-stage (resistant) myeloid leukemias, including CML as well as palliative therapy of BCR-ABL1-negative myeloproliferative neoplasms [30], [31], [32], [33], [34]. In CML, HU has been described to exert major effects on cell cycle progression and proliferation of leukemic cells [[30], [31], [32], [33],35,36]. However, the mechanisms of action of HU on CML cells are not well understood. In addition, the effects of HU on growth and survival of TKI-resistant CML sub-clones harboring various BCR-ABL1 mutations have not yet been investigated.
We here describe that HU exerts major anti-leukemic effects on leukemic sub-clones expressing BCR-ABL1T315I in patients with TKI-resistant CML. In addition, we questioned whether HU would produce cooperative effects with other drugs as single drug effects may not be sufficient to overcome resistance in multi-mutated TKI-resistant CML cells. Indeed, we were able to show that HU and ponatinib synergize in inhibiting growth of leukemic cells in TKI-resistant CML. We also examined the mechanisms and potential targets involved in HU-induced effects on CML cells. In these studies, we found that CDK4 and CDK6 may serve as potential targets of therapy in BCR-ABL1T315I+ CML. Finally, we provide evidence that the CDK4/CDK6 inhibitor palbociclib blocks the growth and survival of BCR-ABL1T315I+ CML cells in the same way as HU. These data have clinical implications and may lead to the development of new therapeutic concepts in advanced multi-resistant BCR-ABL1T315I+ CML.
2. Subjects and methods
2.1. Patients
Four CML patients receiving HU for cytoreduction were analyzed retrospectively. CML CP was diagnosed between 1999 and 2004. Three patients received imatinib as initial therapy and one patient received imatinib after HSCT. After 2–4 years of imatinib therapy, all 4 patients had lost their hematologic and molecular response. In one of these patients, a BCR-ABL1T315I+ sub-clone developed during imatinib therapy. In the other 3 patients, therapy had been switched to a second-generation TKI (nilotinib and/or dasatinib) and BCR-ABL1T315I was detected in their second (#1 and #2) or third (#3 and #4) relapse. Of note, BCR-ABL1T315I was detected in all patients between 2006 and 2011. Ponatinib treatment was not available at that time. In 2 patients (#1 and #4), additional BCR-ABL1-mutations were detected. One patient (#3) had progressed to blast phase (BP), whereas 3 patients were still in CP. The patients´ characteristics are summarized in Table 1. Treatment response was evaluated following European LeukemiaNet guidelines [9,37,38]. After detection of BCR-ABL1T315I, HU (1000–3000 mg/day) was prescribed to suppress growth of leukemic cells.
Table 1.
Patients´ characteristics (HU-treated patients).
| Patient no. | Gender | Age at diagnosis (years) | Disease duration before HU- treatment (months) | Therapies received before HU-treatment | CML-phase at start of HU-treatment | BCR-ABL1 mutations detected | WBC at HU-start (G/l) | HU dose (mg/day) | Duration of HU treatment (months) | Follow up - outcome and duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | m | 41 | 22 | imatinib, cytarabin, 6-mercaptopurine, dasatinib | CP | T315I G250E E255K | 11.6 | 1500–2000 | 18 | HSCT MMR (20) |
| #2 | m | 65 | 108 | interferon-α, cytarabine, imatinib | CP | T315I | 9.6 | 1000–3000 | 11 | PD, † (13) |
| #3 | m | 53 | 104 | HSCT, DLI interferon-α imatinib, dasatinib, nilotinib | BP | T315I | 29.9 | 1500 | 2 | PD, † (2) |
| #4 | m | 51 | 92 | imatinib, dasatinib, nilotinib, interferon-α | CP | T315I E255K | 80.0 | 500–3000 | 4 | HSCT MMR |
HU, hydroxyurea; m, male; HSCT, allogenic hematopoietic stem cell transplantation; DLI, donor lymphocyte infusion; CP, chronic phase; BP, blast phase; MMR, major molecular response; PD, progressive disease.
Deceased.
For in vitro studies, a total of 23 primary leukemic cell samples were obtained from the peripheral blood (PB) or bone marrow (BM) of additional patients with CML as summarized in Table 2. Furthermore, control BM cells were obtained from 6 lymphoma patients without BM involvement. All investigations were approved by the local ethics committee of the Medical University of Vienna (ethic vote number: 224/206). Informed consent was obtained from all patients.
Table 2.
Patients´ characteristics: CML samples used for in vitro studies.
| Patient no. | Age (years) | Gender | Source | CML phase | BCR-ABL1 mutations | Therapy before cell sampling | HU IC50 (µM) | Palbociclib IC50 (nM) | `HU + ponatinib´ |
|---|---|---|---|---|---|---|---|---|---|
| #5 | 64 | m | PB | CP | n.t. | HU | 175 | n.t. | n.t. |
| #6.1 | 48 | m | PB | CP | n.t. | None | 74 | n.t. | Cooperative |
| #6.2 | 48 | m | PB | BP | T315I | imatinib, dasatinib (dis), nilotinib (dis) | 116 | 31 | n.t. |
| #7 | 34 | m | BM | CP | n.t. | None | 50 | n.t. | n.t. |
| #8 | 50 | m | PB | CP | n.t. | None | 46 | n.t. | Cooperative |
| #9 | 59 | m | PB | CP | n.t. | None | 73 | n.t. | n.t. |
| #10 | 63 | m | BM | CP | n.t. | None | 75 | n.t. | Cooperative |
| #11 | 54 | f | PB | CP | n.t. | None | 96 | n.t. | Cooperative |
| #12.1 | 48 | m | BM | BP | G250E | imatinib (res), dasatinib (res), bosutinib (res), ponatinib (res), HU | 236 | n.t. | n.t. |
| #12.2 | 48 | m | BM | BP | G250E E255V | imatinib (res), dasatinib (res), bosutinib (res), ponatinib (res), HU | 30 | n.t. | n.t. |
| #13 | 78 | f | PB | BP | F317L, L248V, K274del | interferon-alpha, imatinib (res), dasatinib (res), bosutinib (res), ponatinib (res), HU, rapamycin | 285 | n.t. | n.t. |
| #14 | 55 | m | BM | CP | n.t. | None | 50 | n.t. | Cooperative |
| #15 | 48 | f | PB | CP | n.t. | None | 56 | n.t. | Cooperative |
| #16 | 81 | f | BM | CP | n.t. | imatinib (dis) | 36 | 5 | n.t. |
| #17 | 29 | f | BM | CP | n.t. | None | 33 | 11 | n.t. |
| #18 | 69 | f | PB | CP | n.t. | None | 41 | 2 | n.t. |
| #19 | 71 | m | BM | BP | n.t. | None | 148 | 338 | n.t. |
| #20 | 67 | m | BM | CP | n.t. | None | 43 | 5 | n.t. |
| #21 | 34 | f | PB | CP | n.t. | None | 72 | 16 | Cooperative |
| #22 | 78 | m | BM | CP | n.t. | None | 31 | 19 | n.t. |
| #23 | 18 | m | BM | CP | n.t. | None | 14 | 1 | Cooperative |
| #24 | 59 | f | BM | CP | n.t. | None | 30 | 48 | n.t. |
| #25 | 34 | f | PB | CP | n.t. | None | n.t. | n.t. | Cooperative |
Abbreviations: CML, chronic myeloid leukemia; HU, hydroxyurea; m, male; f, female; PB: peripheral blood; BM: bone marrow; CP, chronic phase; BP, blast phase; n.t., not tested; none, no therapy (diagnostic sample); res, resistant; dis, discontinued due to intolerance; µM, micromolar. Responses of cells to HU, palbociclib and `HU + ponatinib´ were assessed by 3H-thymidine uptake.
2.2. Laboratory investigations
During follow-up, routine blood investigations, including serial determinations of blood counts and differential counts, were performed in certain time intervals (1–12 weeks). In addition, BCR-ABL1 mRNA levels were quantified in the peripheral blood (PB) in 1–6 month intervals. The BCR-ABL1 transcript burden was quantified by real-time PCR according to the International Scale (IS) [39]. Screening for mutations in the BCR-ABL1 tyrosine kinase domain (TKD) was performed essentially as described [40]. To quantify the mutant allele burden of BCR-ABL1T315I, ligation-dependent PCR (LD-PCR) was employed using the thermocycler AB-9600 (Applied Biosystems, Foster City, USA) as reported [41]. The percentage of BCR-ABL1T315I was expressed as percent of total BCR-ABL1 mRNA [41].
2.3. Reagents
Reagents used in this study are described in the Supplemental file and Supplemental Table S1.
2.4. Cell lines and culture conditions
The human CML cell lines KU812, KCL22 and K562 were used in this study. KU812 cells were kindly provided by Kenji Kishi (Niigata University, Niigata, Japan). KCL22 and K562 cells were purchased from the German Collection of Microorganism and Cell Culture (DSMZ, Braunschweig, Germany). In case of KCL22, a BCR-ABL1T315I+ sub-clone was generated by culturing cells in medium containing imatinib and dasatinib essentially as described (KCL22T315I) [42]. In addition, we employed untransfected (BCR-ABL1-negative) Ba/F3 cells, Ba/F3 cells harboring wild type (WT) BCR-ABL1 (Ba/F3p210WT) or BCR-ABL1T315I (Ba/F3p210T315I) [43]. Ba/F3 cells expressing T315I-based compound mutations (BCR-ABL1T315I/E255K, BCR-ABL1T315I/F311L, BCR-ABL1T315I/F359V, BCR-ABL1T315I/G250E) were generated as described recently [44]. Primary PB and BM mononuclear cells (MNC) were kept in culture as reported [45]. All cell lines and primary cells were cultured in RPMI 1640 medium with 10% fetal calf serum (FCS) and antibiotics. KCL22T315I cells were kept in the presence of 5 µM imatinib. Untransfected Ba/F3 cells were kept in the presence of 0.1 ng/ml IL-3. For analysis of CDK4 and CDK6 expression, cell lines were kept in RPMI 1640 medium supplemented with 1% FCS and antibiotics for up to 72 h.
2.5. Measurement of proliferation and competitive outgrowth, apoptosis and cell cycle arrest of BCR-ABL1+ cells
To examine proliferation, cell lines and primary cells were incubated with control medium (Co) or in various concentrations of drugs (HU, ponatinib, ABL001, palbociclib, cytarabine, homoharringtonine and interferon alpha (IFNα) alone or in (two- or three) drug combinations at 37 °C for 48 h. Then, 3H-thymidine-uptake was measured as described [45]. To determine the effects of HU, ponatinib, and the combination ´HU+ponatinib´ on clonal outgrowth of mutant-bearing sub-clones, experiments were performed using mixtures of BCR-ABL1+ Ba/F3 cells. In these experiments, Ba/F3p210WT cells (labeled with Venus fluorescent protein), Ba/F3p210T315I cells (labeled by green fluorescence protein, GFP) and Ba/F3p210T315I/E255V cells (labeled by tdTomato fluorescent protein) were mixed in a 1:1:1 ratio and incubated together in control medium or in the presence of HU (100 µM), ponatinib (10 nM) or a combination of both drugs for 72 h. Thereafter, cell viability was measured by trypan blue exclusion, and the relative percentage of ´sub-clone cells´ in each condition was measured on a FACScan (Becton Dickinson, San Diego, CA). Three independent experiments were performed.
2.6. Evaluation of apoptosis and cell cycle arrest in drug-exposed cells
For analysis of apoptosis, CML cell lines were kept in control medium or in the presence of HU or/and ponatinib for 48 h. Then, apoptosis was determined by staining for AnnexinV-FITC and propidium iodide (PI) or AnnexinV-FITC and 4′,6-diamidino-2-phenylindole (DAPI). For analysis of cell cycle progression, cells were kept in the presence or absence of various concentrations of HU or palbociclib for 24 h. Thereafter, PI was added and cell cycle distribution was analyzed as described previously [45]. In all cases, 3 independent experiments were performed.
2.7. Western blotting and qPCR
To study the effects of HU on expression of CDK4, CDK6, retinoblastoma gene product (Rb) and phosphorylated Rb (pRb), Western blot experiments were performed on CML cell lines. Cells were kept in RPMI 1640 medium supplemented with 1% FCS and antibiotics in the presence or absence of HU (500 µM) or palbociclib (0.5 µM) for 24–72 h. After incubation, cells were recovered and lysed in lysing buffer and then examined by Western blotting essentially as described [45] using antibodies against CDK4, CDK6, pRb, Rb, as well as β-tubulin and β-actin (loading controls). To determine drug-induced apoptosis, antibodies directed against cleaved poly-ADP-ribose polymerase (PARP) and total PARP were employed. A list of antibodies is provided in the supplemental file (Supplemental Table S2). To determine mRNA expression levels in CML cell lines, cells (as indicated) were kept in control medium or in the presence of HU (at the same condition as for Western blot analyses). RNA was isolated from CML cell lines using the RNeasy MinEluteCleanupKit (Qiagen, Hilden, Germany). cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA), random primers, first strand buffer, dNTPs (100 mM), and RNasin (all from Invitrogen) according to the manufacturer's instructions. PCR was performed as reported [46] using primers specific for CDK4 and CDK6. A list of PCR primers used in qPCR experiments is provided in the supplemental file (Supplemental Table S3). mRNA levels were quantified on a QuantStudio 3 PCR System (Applied Biosystem, Foster City, CA, USA) using iTAq SYBR Green Supermix with ROX (Bio-Rad, Hercules, CA, USA). mRNA expression levels were normalized to ABL1 mRNA levels and expressed as percentage of ABL1. Calculations were based on standard curves established for CDK4, CDK6 and ABL1 mRNA expression. All Western blotting and qPCR-experiments were repeated three times.
2.8. shRNA induced knockdown of CDK4 and CDK6
To learn more about the role of CDK4 and CDK6 on proliferation and survival of CML cells, K562 and KCL22 cells were transfected simultaneously with shRNA constructs directed against CDK4 and CDK6 or with control shRNAs as described in the supplemental file (Supplemental methods and Supplemental Table S4).
2.9. Statistical analysis
The Student´s t-test (two-sided) for dependent samples was applied. Results were considered statistically significant when p was <0.05. All data analysed for statistically significance using the t-test met the assumptions of the test. In drug combintion experiments, drug interaction-types were determined by calculating combination index (CI) values using Calcusyn software as reported [47].
3. Results
3.1. Response to HU treatment in 4 patients with BCR-ABL1T315I+ CML
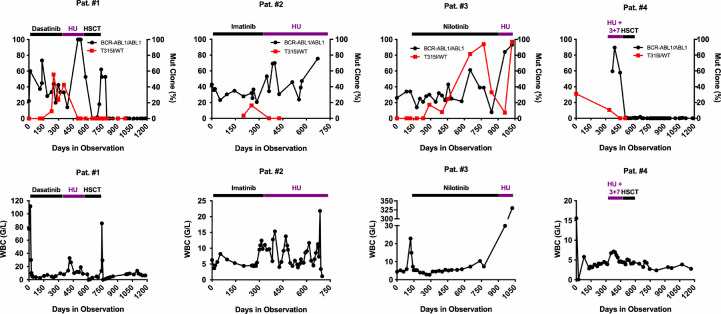
We examined 4 patients with advanced BCR-ABL1T315I+ CML who were treated with HU for bridging to HSCT (n = 2) or for palliative cytoreduction (n = 2). HU treatment resulted in stabilization of the leukocyte counts in 3 of 4 patients, but failed to induce a molecular response (Fig. 1). However, surprisingly, the percentage of BCR-ABL1T315I compared to total BCR-ABL1, assessed by LD-PCR, decreased significantly in all 4 patients during HU treatment, and in 3 of the 4 patients, the T315I mutant was no longer detectable after therapy (Fig. 1). These data suggest that HU is able to eliminate BCR-ABL1T315I+ leukemic cells. After 2 months of HU therapy, HSCT could be performed in 2 patients (#1 and #4). These patients remained in complete hematologic and molecular remission during the observation period (20 and 40 months) (Fig. 1). In patient #2 (palliative HU) a clinically and hematologically stable disease (leukocytes: 3400–15,000/µL) was observed over 18 months. Thereafter, the patient developed a BCR-ABL1T315I-negative BP of CML and died. In patient #3 in whom HU was started at the time of BP, no substantial decrease in blood leukocyte, blasts or total BCR-ABL1 could be observed during HU treatment, despite a temporary suppression of BCR-ABL1T315I from 94% to 7.3% of total BCR-ABL1 (Fig. 1). This patient died 2 months after the start of HU treatment. Together, these observations suggest that HU is able to suppress or even eliminate mutant CML sub-clones harboring BCR-ABL1T315I+ in vivo in patients with CML CP.
Fig. 1.
Hydroxyurea (HU) induces molecular response and suppresses the T315I-positive sub-clone(s) in advanced CML. Four heavily pretreated CML patients (#1–#4) were treated with HU, BCR-ABL1 tyrosine kinase inhibitors (imatinib, 400 mg/day; dasatinib, 100 mg/day; nilotinib, 2 × 400 mg/day per os), polychemotherapy, or hematopoietic stem cell transplantation (HSCT) as indicated. The percentage of BCR-ABL1 mRNA relative to ABL1 mRNA (according to the international scale) as well as the percentage of BCR-ABL1T315I mRNA relative to BCR-ABL1 mRNA (BCR-ABL1T315I/BCR-ABL1, determined by ligase-dependent PCR) are shown in the upper panels. The white blood count (WBC) of the same patients are shown in the lower panels. 3 + 7: combined chemotherapy following the 3 + 7-protocol consisting of daunorubicine (60 mg/m2 per day, days 1–3) and cytosine arabinoside (200 mg/m2 per day, days 1–7).
3.2. HU suppresses proliferation and survival of TKI-sensitive and TKI-resistant BCR-ABL1+ cells in vitro
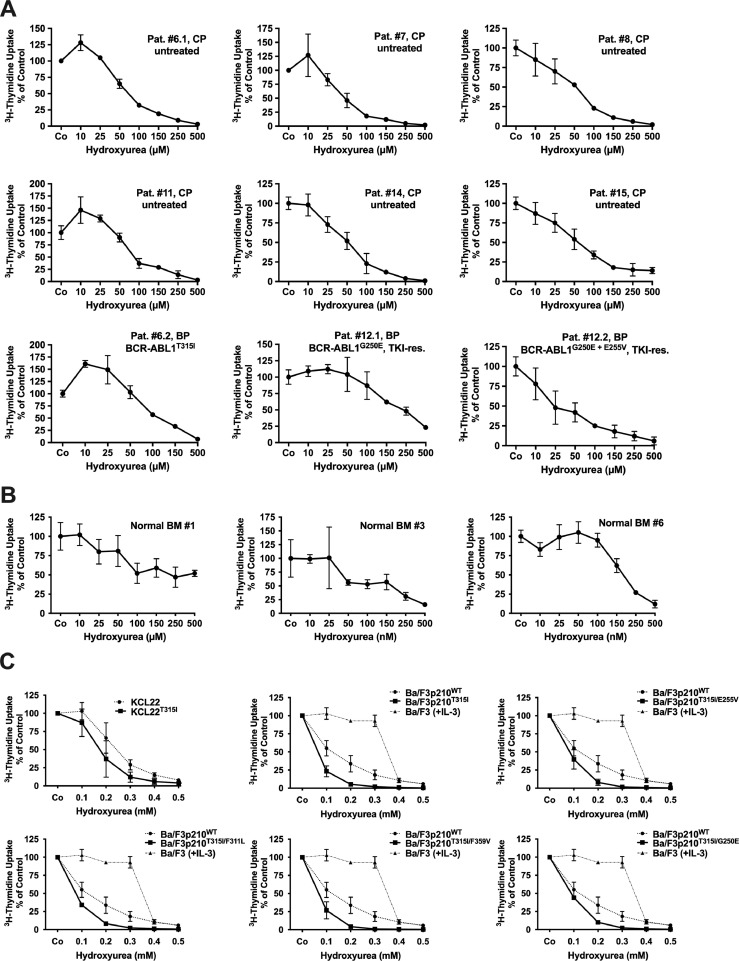
To explore the effects of HU on drug-resistant CML cells in more detail, we performed a series of in vitro experiments. As visible in Fig. 2(A) and Table 2, HU suppressed the proliferation of primary cells isolated from the PB or BM of 20 patients with CML, including 4 TKI-resistant cases (one exhibiting BCR-ABL1T315I) with IC50-values ranging between 25 and 300 µM (mean IC50: 85.81 µM). In normal BM MNC isolated in 6 donors, HU was found to inhibit proliferation at higher concentrations (mean IC50: 176.7 µM; Fig. 2(B)). These data are in line with the clinical observation that HU (compared to other cytoreductive drugs) exerts only moderate myelo-suppressive effects and is therefore considered to be a relatively save drug. HU effects were also seen in KCL22T315I cells, Ba/F3 cells expressing BCR-ABL1WT, and Ba/F3 cells harboring BCR-ABL1T315I (Fig. 2(C)). Moreover, HU was found to inhibit the proliferation of Ba/F3 cells expressing T315I-including BCR-ABL1 compound mutants (Fig. 2(C)). An intriguing observation was that HU was more effective in sub-clones harboring mutant BCR-ABL1 than BCR-ABL1WT (IC50 in Ba/F3 cells with mutant BCR-ABL1, including T315I-involving compound mutants: <100 µM; IC50 in Ba/F3p210WT: 100–200 µM; IC50 in KCL22T315I cells: <200 µM; IC50 in un-transduced KCL22 cells: 200–300 µM) (Fig. 2(C)). In untransfected Ba/F3 cells, IC50 values for HU were significantly higher than in Ba/F3 cells exhibiting various mutant forms of BCR-ABL1 (Fig. 2(C)) which may point at a therapeutic window.
Fig. 2.
Hydroxyurea (HU) inhibits the proliferation of primary CML cells and TKI-resistant cell lines harboring T315I-mutated BCR-ABL1. Primary leukemic cells obtained from CML patients (A), normal BM cells (B) and KCL22 cells expressing BCR-ABL1WT (C, left upper image, dotted line), KCL22 cells expressing BCR-ABL1T315I (C, left upper image, black line), untransfected (BCR-ABL1-negative) Ba/F3 cells (kept in 0.1 ng/ml IL-3) (C, upper right, middle and lower panels, stippled lines) and Ba/F3 cells expressing BCR-ABL1WT (C, dotted lines) or various mutant forms of BCR-ABL1 (B, black lines) were incubated in control medium (Co) or medium containing various concentrations of HU at 37 °C for 48 h. Thereafter, 3H-thymidine-uptake was measured. (A and B) Results are expressed as percent of control and represent the mean±S.D. from triplicates. Patients´ numbers refer to Table 2. TKI-res., TKI-resistant. (C) Results are expressed as percent of control and represent the mean±S.D. from 3 independent experiments.
For comparison, three additional cytoreductive agents that have been used for the treatment of CML, namely cytarabine, homoharringtonine, and IFNα [48], [49], [50], were also tested in our Ba/F3 cells and in our human CML cell line models. In these experiments, cytarabine and homoharringtonine produced relatively strong anti-proliferative effects in all cell lines examined (Supplemental Fig. 1A and B), whereas IFNα did not produce significant effects at concentrations up to 5 × 104 U/mL (not shown). More importantly, contrasting HU, neither cytarabine nor homoharringtonine exerted more potent effects in cells harboring BCR-ABL1T315I than in cells lacking BCR-ABL1T315I (Supplemental Fig. 1A and B).
Finally, we examined the effects of HU on survival of CML cells. In these experiments, HU was found to induce apoptosis in all human CML cell lines tested (Supplemental Fig. S1C). Interestingly, the apoptosis-inducing effect of HU was stronger in KCL22T315I cells than in KCL22 cells.
Collectively, these data show that HU inhibits growth and survival of CML cells, and that HU effects are stronger in CML cells expressing BCR-ABL1T315I or T315I-including compound mutants of BCR-ABL1 compared to CML cells expressing BCR-ABL1WT.
3.3. HU cooperates with ponatinib in inducing growth arrest in primary CML cells and in BCR-ABL1+ cell lines
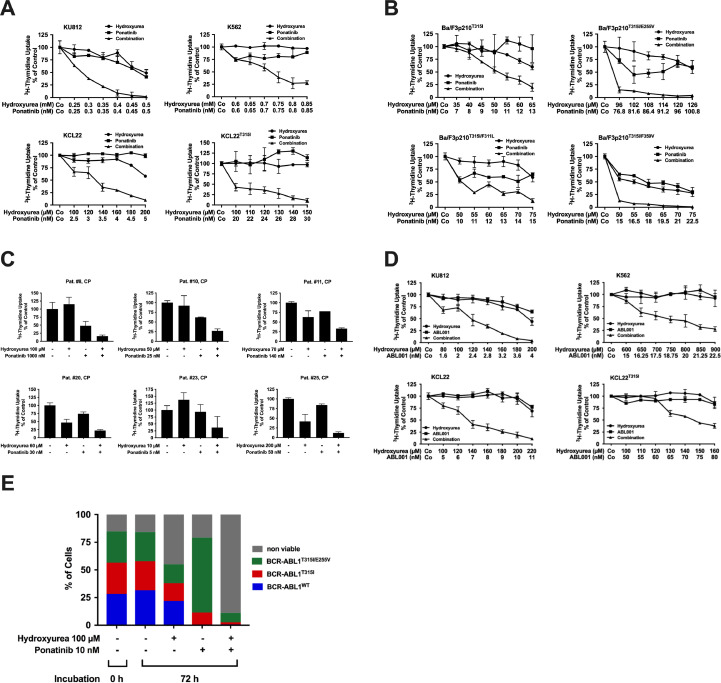
Drug combinations may be a suitable strategy to overcome drug resistance in patients with advanced CML who failed TKI therapy. We examined cooperative (potentially synergistic) effects of the drug combination ´HU+ponatinib´. As shown in Fig. 3(A), ponatinib and HU produced strong synergistic effects on proliferation in all TKI-sensitive and TKI-resistant CML cell lines, including KCL22T315I. Synergism was confirmed using Calcusyn software (Supplemental Fig. S2A). For comparison, cytarabine, homoharringtonine and IFNα were also combined with ponatinib. Cooperative effects were observed when combining cytarabine or homoharringtonine with ponatinib (Supplemental Fig. 3A), whereas IFNα failed (even in combination) to produce anti-neoplastic effects (not shown). Furthermore, HU and ponatinib were found to cooperate in inducing apoptosis in all CML cell lines examined (Supplemental Fig. S3B). Next, we evaluated the effects of the drug combination in Ba/F3 cells harboring BCR-ABL1T315I or T315I+ compound mutants. In these experiments, HU and ponatinib were found to produce synergistic growth-inhibitory effects in all sub-clones examined (Fig. 3(B) and Supplemental Fig. S2B). We also tested the drug combination in primary leukemic cells obtained from 9 patients with newly diagnosed CML CP. As visible in Fig. 3(C) and Table 2, the drug combination ´HU+ponatinib´ produced additive or even synergistic growth-inhibitory effects in these cells. Collectively, these data suggest that HU and ponatinib exert synergistic anti-leukemic effects in CML cells and may overcome TKI resistance. Currently, ponatinib is the only approved TKI that blocks BCR-ABL1T315I. However, other, novel BCR-ABL1 blocker, such as ABL001 (asciminib) [51], are currently tested in clinical trials and may be useful for third-line treatment of CML in the future. Therefore, we extended our experiments to ABL001. As a single drug, ABL001 (0.1–1 µM) was found to inhibit proliferation of Ba/F3 cells harboring BCR-ABL1WT, BCR-ABL1T315I or BCR-ABL1T315I/G250E. However, cells harboring other T315I-including compound mutants showed relative resistance against ABL001 (Supplemental Fig. S3C). Next we combined ABL001 with HU. As visible in Fig. 3(D) and Supplemental Fig. S2C, synergistic growth-inhibitory effects were also obtained with the combination ‘HU+ABL001’ in K562, KU812, KCL22 and KCL22T315I cells. Moreover, synergistic effects between ABL001 and HU were also observed in all Ba/F3 cell clones tested, including Ba/F3 cells harboring BCR-ABL1T315I or T315I-including compound mutants of BCR-ABL1 (Supplemental Fig. S3D). These data show that HU may be a suitable combination partner for various TKI used to treat CML.
Fig. 3.
Hydroxyurea (HU) synergizes with ponatinib in inducing growth inhibition in BCR-ABL1 positive cell lines and suppresses out-growth of cells harboring T315I-inculding BCR-ABL1 mutations. (A-C) Human CML cell lines (A), Ba/F3 cells expressing various mutant forms of BCR/ABL1 (B), and primary leukemic cells obtained from CML patients (C), were incubated in control medium (Co) or in various concentrations of HU, ponatinib or the combination of both drugs as indicated at 37 °C for 48 h. Thereafter, 3H-thymidine-uptake was measured. Results are expressed as percent of control and represent the mean±S.D. from triplicates. Patients' numbers in (C) refer to Table 2. (D) Human CML cell lines were incubated with control medium (Co) or medium containing various concentrations of HU, ABL001 or the combination of both drugs as indicated at 37 °C for 48 h. Thereafter, 3H-thymidine-uptake was measured Results are expressed as percent of control and represent the mean±S.D. of triplicates. (E) Ba/F3p210WT (labeled by Venus), Ba/F3p210T315I (labeled by GFP) and Ba/F3p210T315I/E255V (labeled by tdTomato) were mixed in a 1:1:1 ratio and incubated together in control medium or in the presence of HU (100 µM), ponatinib (10 nM) or the combination of both drugs for 72 h (h). Thereafter, the ratio between clones in each condition was measured by flow cytometry. The percentage of non-viable cells was determined by trypan blue staining. Results show one typical experiment. Almost identical data were obtained in 2 other independent experiments.
3.4. The drug combination ´HU+ponatinib´ suppresses the competitive outgrowth of sub-clones harboring T315I-inclusive compound mutants of BCR-ABL1
To explore whether the combination ´HU+ponatinib´ can suppress the outgrowth of sub-clones expressing BCR-ABL1 compound mutations, Ba/F3 cells expressing BCR-ABL1WT, BCR-ABL1T315I or BCR-ABL1T315I/E255 were mixed at a ratio of 1:1:1 and then exposed to HU, ponatinib or a combination of both drugs. As expected, ponatinib was found to be more effective in suppressing cells expressing BCR-ABL1WT compared to cells expressing BCR-ABL1 mutants (Fig. 3(E)). By contrast, HU was found to be more effective against the outgrowth of cells harboring BCR-ABL1T315I or BCR-ABL1T315I/E255V compared to BCR-ABL1WT. Only the combination ´HU+ponatinib´ was found to suppress the survival of all three co-cultured sub-clones (BaF3p210WT, BaF3p210T315I, BaF3p210T315I/E255V) and to induce cell death in all these sub-clones (Fig. 3(E)). These data suggest that the combination ´HU+ponatinib´ is effective in suppressing the outgrowth of TKI-resistant sub-clones in multi-resistant CML.
3.5. Effects of HU on cell cycle progression and on expression of the cell cycle regulators CDK4 and CDK6
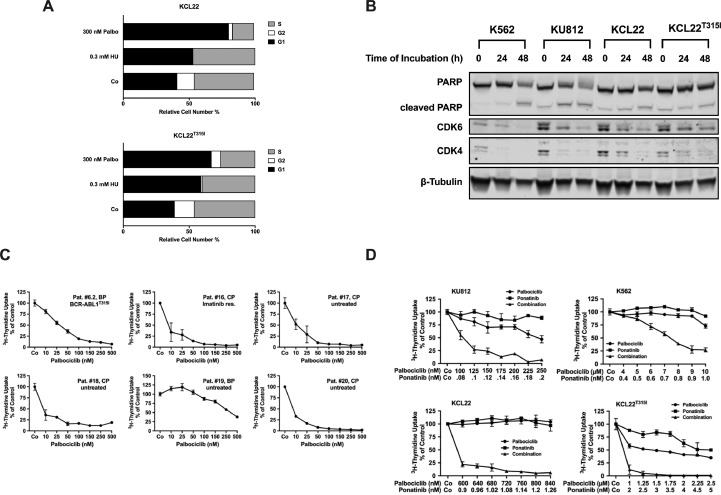
Previous data suggest that HU inhibits cell survival by interfering with cell cycle progression. In addition, it has been described that HU suppresses the expression of the cell cycle regulator CDK6 [36,52]. To learn more about the mechanism of action of HU in TKI-resistant CML cells, cell cycle progression was analyzed. As visible in Fig. 4(A), HU induced cell cycle arrest in KCL22 cells. The same effect was seen with palbociclib, a more specific inhibitor of CDK4/CDK6 [53,54]. HU effects on cell cycle progression were more pronounced in KCL22 cells harboring BCR-ABL1T315I than in cells expressing native BCR-ABL1 (Fig. 4(A)). Next we examined the effects of HU on expression of CDK4 and CDK6 in CML cells. As shown in Fig. 4(B) and Supplemental Fig. S4A, HU decreased the expression of CDK4 and CDK6 at the mRNA and protein level in all CML cell lines tested, confirming previous data [36,52]. By contrast, cytarabine, homoharringtonine and IFNα failed to reduce CDK4/CDK6 expression in all CML cell lines tested (Supplemental Fig. S4B).
Fig. 4.
Synergistic effects between HU and ponatinib are mediated by suppression of CDK4/CDK6. (A) KCL22 and KCL22T315I cells were kept in control medium (Co) or incubated in various concentrations of hydroxyurea (HU) or palbociclib as indicated for 24 h. Thereafter, PI was added and cell cycle distribution was determined by flow cytometry. The percentage of cells in G1-phase, G2/M-phase and S-phase in each condition are shown. Results represent the mean of 3 independent experiments. (B) K562, KU812, KCL22 and KCL22T315I cells were kept in RPMI medium supplemented with 1% fetal calf serum (FCS) in the absence (“0”) or presence of 500 µM HU for 24 or 48 h as indicated. Thereafter, cells were subjected to Western blot analysis using antibodies against, PARP, CDK4, CDK6 or β-tubulin as indicated. (C) Primary CML cells were kept in control medium (Co) or in various concentrations of palbociclib (as indicated) for 48 h before 3H-thymidine-uptake was measured. Results are expressed as percent of control and represent the mean±S.D. from triplicates. Patients' numbers refer to Table 2. res., resistant. (D) KU812, K562, KCL22 and KCL22T315I were incubated in control medium (Co) or in various concentrations of palbociclib, ponatinib or the combination of both drugs (as indicated) at 37 °C for 48 h. Then, 3H-thymidine-uptake was measured. Results are expressed as percent of control and represent the mean±S.D. from triplicates.
3.6. Effects of CDK4/CDK6 knock-down and palbociclib on growth and survival of CML cells
To learn more about the role of CDK4 and CDK6 as potential therapeutic targets in CML cells, we performed shRNA-mediated simultaneous knockdowns of CDK4 and CDK6 in K562 and KCL22 cells. In both cell lines, transfection with the 2 shRNA constructs resulted in decreased expression of CDK4 and CDK6 protein as assessed by Western Blotting (Supplemental Fig. S5A). Interestingly, knock-down of CDK4 and CDK6 did not lead to induction of apoptosis but resulted in a growth-disadvantage in both cell lines when compared to cells treated with control shRNA (Supplemental Fig. S5B). These data suggest, that inhibition of CDK4/CDK6 interferes with proliferation in CML cells. Knock-down of either CDK4 or CDK6 did not produce any effect on proliferation of K562 and KCL22 cells (not shown). Next, we tested the effects of palbociclib – a pharmacologic inhibitor of CDK4/CDK6 - on growth and survival of TKI-resistant CML cells. Palbociclib profoundly suppressed the proliferation of primary leukemic cells obtained from 10 CML patients, including 8 with CP CML (IC50: 1–48 nM) and 2 in BP (IC50: 31 and 338 nM, respectively) (Fig. 4(C), Table 2). In addition, palbociclib inhibited the proliferation of all CML cell lines tested, although the concentrations required to block proliferation in cell lines were higher than in primary CML cells (Supplemental Table S5). Moreover, palbociclib was found to synergize with ponatinib in inducing growth inhibition in KU812, K562, KCL22 and KCL22T315I cells in the same way as HU (Fig. 4(D), Supplemental Fig. S2D). These data suggest, that CDK4/CDK6-inhibitors sensitize CML cells against ponatinib. However, specific knock-down of CDK4/CDK6 failed to exert the same antineoplastic effects, suggesting, that additional targets of HU and palbociclib may play an certain role for synergistic drug interactions. As expected, palbociclib suppressed the phosphorylation of Rb in all CML cell lines tested (Supplemental Fig. S6A and B). By contrast, HU, albeit suppressing the expression of total CDK4 and CDK6, did not downregulate Rb phosphorylation (Supplemental Fig. S6A and B). Finally, we examined the effect of the drug combination ´HU+palbociclib´. As shown in Supplemental Fig. S7A and B, palbociclib was found to synergize with HU in inhibiting the proliferation in all CML cell lines examined, including KCL22T315I. Furthermore, the 3-drug combination `HU+ponatinib+palbociclib´ was found to produce stronger anti-proliferative effects than the single compounds (applied at very low concentrations) or the 2-drug combinations (Supplemental Fig. S7C and D).
4. Discussion
BCR-ABL1T315I occurs in 20–30% of all TKI-resistant CML patients exhibiting BCR-ABL1 mutations and thus represents a major clinical challenge [10], [11], [12]. Ponatinib suppresses BCR-ABL1T315I but is not an optimal drug for all patients due to its side effects [12], [13], [14], [15]. Therefore, other strategies to control CML sub-clones expressing BCR-ABL1T315I or more complex BCR-ABL1-mutations are currently being developed. HU is a well-tolerated drug that has been used for palliative treatment of CML over decades [31,32]. We here report that HU inhibits the growth of CML cells and Ba/F3 cells expressing BCR-ABL1T315I or T315I-involving compound mutations. In addition, we show that HU cooperates with ponatinib in suppressing the growth of leukemic cells expressing BCR-ABL1T315I or T315I-containing compound mutants. We also show that the growth-inhibitory effect of HU on BCR-ABL1T315I-positive cells is stronger than HU effects on CML cells displaying native BCR-ABL1 and that HU treatment results in a decrease in expression of CDK4 and CDK6. Finally, we show that treatment of patients with TKI-resistant CML with HU results in selective suppression or even depletion of BCR-ABL1T315I-positive sub-clones. These observations have clinical implications and may lead to the development of new treatment concepts.
Our initial observation was that during therapy with HU, the BCR-ABL1T315I+ sub-clone decreased in size in 4 heavily pretreated, TKI-resistant CML patients. Remarkably, in 3 of 4 patients, the BCR-ABL1T315I+ sub-clone was no longer detectable after therapy with HU although total BCR-ABL1 mRNA levels did not decrease. In one patient, HU showed no major response and the leukocyte counts even increased during HU therapy. In this case, the disease rapidly progressed to BP and the patient died within short time. However, even in this patient, the percentage of BCR-ABL1T315I (relative to total BCR-ABL1) decreased from 94% to 7.3%. Three out of these four patients received only HU without any other cytoreductive or targeted drugs at the time when BCR-ABL1T315I decreased. However, one patient received HU and 3 + 7 chemotherapy before HSCT. In this patient, it remains unclear whether the decrease in BCR-ABL1T315I was only caused by HU or the combination of HU and 3 + 7. Our in vivo observations suggest, that the BCR-ABL1T315I-bearing sub-clone is particularly sensitive against HU. Similar observations were made by Hanfstein et al. [55]. In their study, 4/5 patients showed a decrease in BCR-ABL1T315I under HU, but only one patient became BCR-ABL1T315I-negative [55]. It has also been hypothesized that the decrease in mutated BCR-ABL1 is due to a simple deselection of clonal cells. However, in most patients who did not receive HU in their study a decrease in BCR-ABL1 was not observed, and in the few other patients where BCR-ABL1T315I also decreased, other cytoreductive drugs were applied [55], arguing against a simple deselection scenario. In this regard it is also worth noting that the growth disadvantage of BCR-ABL1T315I+ cells over wt BCR-ABL1+ cells is only seen in vitro when no additional pro-oncogenic pathways are activated [56]. However, in the clinical setting, BCR-ABL1T315I-bearing sub-clones, once detected, did already undergo clonal selection by further acquisition of such additional pro-oncogenic pathways. Therefore, one would not expect that a fully established BCR-ABL1T315I-bearing sub-clone can be deselected by just discontinuing the TKI.
Our in vitro data confirmed the assumption that HU is particularly effective in BCR-ABL1 T315I-mutated cells. In fact, HU was found to inhibit proliferation and viability of human and Ba/F3 cells expressing BCR-ABL1T315I or T315I-inclusive compound mutatios. Most significantly, leukemic cells harboring BCR-ABL1-mutations involving T315I were more sensitive against HU than cells expressing BCR-ABL1WT suggesting, that HU may be particularly effective patients in whom TKI-resistant sub-clones express BCR-ABL1T315I or BCR-ABL1T315I-involving compound mutations.
The biochemical mechanisms underlying the particular, strong effect of HU on CML sub-clones exhibiting BCR-ABL1T315I remain unknown. A direct effect of HU on BCR-ABL1 mutants seems unlikely. Rather, other molecular targets may explain responses to HU. Indeed, HU is well known to suppress proliferation in neoplastic cells by interfering with cell-cycle progression [31,35,57,58]. Bruchova et al. described that HU, when applied in vivo to patients with CML, inhibits the expression of CDK6 in leukemic cells [36]. In the present study, we were able to confirm this effect of HU in vitro using various CML cell lines. In addition, HU was found to counteract cell cycle progression in CML cells. Since CDK6 has recently been identified as a major drug target in applied oncology [53,59,60] we were also interested to learn whether specific blockage of CDK6 would lead to cell cycle arrest and growth inhibition in CML cells. Indeed, we found that simultaneous knockdown of CDK4 and CDK6 with shRNA or application of palbociclib, a potent CDK4/CDK6 inhibitor, counteract cell cycle progression and proliferation in CML. These data point at the possible role of CDK4 and CDK6 as potential drug targets in CML which confirms previous observations [61,62]. Concerning the effects of palbociclib, our results are also in line with recent data demonstrating high anti-leukemic activity of this drug against cell lines reflecting CML in lymphatic BP or BCR-ABL1+ acute lymphoblastic leukemia [60]. Whether this concept can be translated to application in TKI-resistant CML remains at present unknown. The advantage of palbociclib over HU would be that palbociclib is a stronger CDK inhibitor compared to HU. In addition, palbociclib is a selective inhibitor that may have a more favorable side effect profile than HU, at least in long-term treated patients.
A particular problem in the treatment of TKI-resistant CML is the occurrence of sub-clones expressing T315I-involving compound mutants of BCR-ABL1 [17], [18], [19], [20]. Our data show, that sub-clones expressing highly-resistant BCR-ABL1 compound mutants respond to HU and palbociclib, suggesting, that application of these drugs in the context of complex BCR-ABL1 mutations may be a reasonable approach.
Despite the remarkable effects HU exerts on BCR-ABL1T315I-mutated CML cells, no hematologic or molecular response was observed in our patients, suggesting that other sub-clones are less sensitive to HU. In addition, HU was not able to eliminate the BCR-ABL1T315I-mutated sub-clone in all advanced CML patients which is in line with previous observations [55]. Therefore, drug-combinations including HU or palbociclib and BCR-ABL1 TKI may be a preferable approach. We found that HU synergizes with ponatinib in producing growth inhibition in all primary CML cells and all CML cell lines tested, including cells harboring BCR-ABL1WT, BCR-ABL1T315I or BCR-ABL1 compound mutations. In addition, palbociclib also induced synergistic growth-inhibitory effects on CML cells when combined with BCR-ABL1 TKI. These results may have clinical implications and point at a new concept in which drug combinations are applied to increase anti-CML effects and to reduce ponatinib doses to avoid side effects, at the same time. Finally, synergistic anti-neoplastic effects on CML cells were seen when HU was combined with ABL001, a new BCR-ABL1T315I-targeting drug that binds the myristate-binding pocket domain of BCR-ABL1 [51]. This combination may be applied in patients failing or not tolerating ponatinib in future studies.
It has previously been described, that some drugs applied in advanced CML, including cytarabine, homoharringtonine, and IFNα, are able to suppress BCR-ABL1T315I+ cells in vivo [48], [49], [50]. However, these drugs have not been investigated in detail in the context of BCR-ABL1 mutant-expressing sub-clones so far. We examined whether these drugs would produce similar effects in CML clones harboring BCR-ABL1T315I compared to HU. We here show that despite strong anti-proliferative effects of cytarabine and homoharringtonine alone or in combination with ponatinib in all BCR-ABL1+ cell lines tested, none of the 3 compounds exerted more potent anti-neoplastic effects in cells harboring BCR-ABL1T315I than in cells lacking BCR-ABL1T315I. Furthermore, none of these drugs led to a decrease in CDK4/CDK6 expression in CML cells. These data point at a unique effect of HU in TKI-resistant CML cells expressing BCR-ABL1T315I and related mutations.
The observation that HU exerts particularly strong effects on CML sub-clones exhibiting BCR-ABL1T315I and cooperates with ponatinib and other BCR-ABL1 TKI in suppressing growth of CML cells, even in the context of compound mutations, may have clinical implications. First, these results are in favor of using HU alone or in combination with other drugs in patients with multi-mutated CML exhibiting BCR-ABL1T315I as palliative therapy or as preparation (bridging) for HSCT, as exemplified in this study. Second, HU may be useful to suppress the outgrowth of new sub-clones exhibiting BCR-ABL1T315I or BCR-ABL1T315I-containing compound mutations. Especially for TKI-resistant, non-transplantable patients who are at high risk of developing BCR-ABL1T315I-expressing sub-clones (which is often fatal), addition of HU to TKI therapy as ´T315-prophylaxis´ might be considered.
Together, we show that targeting of CDK4/CDK6 may be a potent approach to overcome TKI resistance in CML sub-clones exhibiting BCR-ABL1T315I. We also show that HU, a ´palliative´ antineoplastic drug, suppresses CDK4/CDK6 expression and exerts potent effects on Ph+ cells harboring BCR-ABL1T315I or T315I-inclusive compound mutations. HU effects were seen in vitro as well as in vivo in patients with TKI-resistant CML. We also show that HU synergizes with ponatinib, ABL001 and palbociclib in inhibiting the growth of TKI-resistant and TKI-sensitive CML cells in vitro. Whether these drug combinations are effective in all patients with TKI-resistant (BCR-ABL1T315I+) CML, remains to be determined in forthcoming studies.
Declaration of Competing Interest
G.H.: research funding from Novartis and honoraria from Novartis, BMS and Pfizer. W.R.S.: honoraria from Novartis, Celgene, Jazz, Pfizer, Abbvie, Daiichi Sankyo and Teva. T.L.: honoraria from Incyte, Pfizer, Angelini, Novartis, Amgen, and a research grant from Novartis. M.D.: Paid consultant for Novartis, Pfizer, Blueprint, Takeda; Research funding: SPARC, Gilead, BMS. K.G.: honoraria from Novartis, Ariad, Roche, BMS and Pfizer. P.V.: research funding and honoraria from Novartis and Incyte, and honoraria from BMS, Pfizer and Ariad. The other authors (M.S., K.B., G.S., D.B., C.B.L., S.H., S.P., N.W., G.G., K.O., P.P. and G.E.) have no conflicts of interest to disclose.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.11.004.
Contributor Information
Peter Valent, Email: peter.valent@meduniwien.ac.at.
Karoline V. Gleixner, Email: karoline.gleixner@meduniwien.ac.at.
Appendix. Supplementary materials
References
- 1.Druker B.J., Guilhot F., O'Brien S.G., Gathmann I., Kantarjian H., Gattermann N. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Hehlmann R., Müller M.C., Lauseker M., Hanfstein B., Fabarius A., Schreiber A. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–423. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 3.Castagnetti F., Gugliotta G., Breccia M., Stagno F., Iurlo A., Albano F. GIMEMA CML Working Party. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29:1823–1831. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 4.Shah N.P., Nicoll J.M., Nagar B., Gorre M.E., Paquette R.L., Kuriyan J. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 5.Hochhaus A., Erben P., Ernst T., Mueller M.C. Resistance to targeted therapy in chronic myelogenous leukemia. Semin Hematol. 2007;44(S1):S15–S24. doi: 10.1053/j.seminhematol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Balabanov S., Braig M., Brümmendorf T.H. Current aspects in resistance against tyrosine kinase inhibitors in chronic myelogenous leukemia. Drug Discov Today Technol. 2014;11:89–99. doi: 10.1016/j.ddtec.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Jabbour E.J., Cortes J.E., Kantarjian H.M. Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk. 2013;13(5):515–529. doi: 10.1016/j.clml.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branford S., Melo J.V., Hughes T.P. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter. Blood. 2009;114(27):5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 9.Baccarani M., Cortes J., Pane F., Niederwieser D., Saglio G., Apperley J. European Leukemianet. Chronic myeloid leukemia: an update of concepts and management recommendations of European Leukemianet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintás-Cardama A., Cortes J. Therapeutic options against BCR-ABL1 T315I-positive chronic myelogenous leukemia. Clin Cancer Res. 2008;14(14):4392–4399. doi: 10.1158/1078-0432.CCR-08-0117. [DOI] [PubMed] [Google Scholar]
- 11.Patel A.B., O'Hare T., Deininger M.W. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin N Am. 2017;31:589–612. doi: 10.1016/j.hoc.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hare T., Shakespeare W.C., Zhu X., Eide C.A., Rivera V.M., Wang F. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes J.E., Kim D.W., Pinilla-Ibarz J., le Coutre P., Paquette R., Chuah C. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valent P., Hadzijusufovic E., Schernthaner G.H., Wolf D., Rea D., le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125(6):901–906. doi: 10.1182/blood-2014-09-594432. [DOI] [PubMed] [Google Scholar]
- 15.Mayer K., Gielen G.H., Willinek W., Müller M.C., Wolf D. Fatal progressive cerebral ischemia in CML under third-line treatment with ponatinib. Leukemia. 2014;28(4):976–977. doi: 10.1038/leu.2013.320. [DOI] [PubMed] [Google Scholar]
- 16.Jain P., Kantarjian H., Jabbour E., Gonzalez G.N., Borthakur G., Pemmaraju N. Ponatinib as first-line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol. 2015;2(9):e376–e383. doi: 10.1016/S2352-3026(15)00127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khorashad J.S., Kelley T.W., Szankasi P., Mason C.C., Soverini S., Adrian L.T. BCR-ABL1 compound mutations in tyrosine kinase inhibitor-resistant CML: frequency and clonal relationships. Blood. 2013;121(3):489–498. doi: 10.1182/blood-2012-05-431379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbons D.L., Pricl S., Posocco P., Laurini E., Fermeglia M., Sun H. Molecular dynamics reveal BCR-ABL1 polymutants as a unique mechanism of resistance to PAN-BCR-ABL1 kinase inhibitor therapy. Proc Natl Acad Sci USA. 2014;111(9):3550–3555. doi: 10.1073/pnas.1321173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zabriskie M.S., Eide C.A., Tantravahi S.K., Vellore N.A., Estrada J., Nicolini F.E. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26(3):428–442. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrgazov K., Lucini C.B., Valent P., Hantschel O., Lion T. BCR-ABL1 compound mutants display differential and dose-dependent responses to ponatinib. Haematologica. 2018;103(1):e10–e12. doi: 10.3324/haematol.2017.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L.P., Xu Z.L., Zhang X.H., Chen H., Chen Y.H., Han W. Allogeneic stem cell transplantation for patients with T315I BCR-ABL mutated chronic myeloid leukemia. Biol Blood Marrow Transpl. 2016;22(6):1080–1086. doi: 10.1016/j.bbmt.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Innes A.J., Milojkovic D., Apperley J.F. Allogeneic transplantation for CML in the TKI era: striking the right balance. Nat Rev Clin Oncol. 2016;13(2):79–91. doi: 10.1038/nrclinonc.2015.193. [DOI] [PubMed] [Google Scholar]
- 23.Nicolini F.E., Basak G.W., Kim D.W., Olavarria E., Pinilla-Ibarz J., Apperley J.F. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer. 2017;123(15):2875–2880. doi: 10.1002/cncr.30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iurlo A., Cattaneo D., Orofino N., Bucelli C., Molica M., Breccia M. Low-dose ponatinib in intolerant chronic myeloid leukemia patients: a safe and effective option. Clin Drug Investig. 2018;38(5):475–476. doi: 10.1007/s40261-018-0623-7. [DOI] [PubMed] [Google Scholar]
- 25.Tefferi A. Upfront low-dose ponatinib (15 mg/day) for multi-TKI resistant chronic myeloid leukemia. Hematol Oncol. 2018;36(4):718–720. doi: 10.1002/hon.2517. [DOI] [PubMed] [Google Scholar]
- 26.Marchesi F., Salvatorelli E., Renzi D., Mengarelli A., Minotti G., Menna P. Efficacy and safety of low dose ponatinib in a case of Ph-positive acute lymphoblastic leukaemia. Br J Haematol. 2019;187(1):e15–e17. doi: 10.1111/bjh.16132. [DOI] [PubMed] [Google Scholar]
- 27.Mian A.A., Rafiei A., Haberbosch I., Zeifman A., Titov I., Stroylov V. PF-114, a potent and selective inhibitor of native and mutated BCR/ABL is active against Philadelphia chromosome-positive (Ph+) leukemias harboring the T315I mutation. Leukemia. 2015;29(5):1104–1114. doi: 10.1038/leu.2014.326. [DOI] [PubMed] [Google Scholar]
- 28.Turkina A.G., Vinogradova O., Lomaia E., Shatokhina E., Shukhov O., Chelysheva E. Phase-1 study of PF-114 mesylate in CML failing prior tyrosine kinase-inhibitor therapy. Blood. 2018;132(Suppl 1):790. [Google Scholar]
- 29.Rea D., Lang F., Kim D., Cortes J.E., Hughes T.P., Minami H. Asciminib, a specific allosteric BCR-ABL1 inhibitor, in patients with chronic myeloid leukemia carrying the T315I mutation in a phase 1 trial. Blood. 2018;132(Suppl 1):792. [Google Scholar]
- 30.Kennedy B.J. The evolution of hydroxyurea therapy in chronic myelogenous leukemia. Semin Oncol. 1992;19(3 Suppl 9):21–26. [PubMed] [Google Scholar]
- 31.Hehlmann R., Heimpel H., Hasford J., Kolb H.J., Pralle H., Hossfeld D.K. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: prolongation of survival by hydroxyurea. The German CML Study Group. Blood. 1993;82(2):398–407. [PubMed] [Google Scholar]
- 32.Hehlmann R., Berger U., Pfirrmann M., Hochhaus A., Metzgeroth G., Maywald O. Randomized comparison of interferon alpha and hydroxyurea with hydroxyurea monotherapy in chronic myeloid leukemia (CML-study II): prolongation of survival by the combination of interferon alpha and hydroxyurea. Leukemia. 2003;17(8):1529–1537. doi: 10.1038/sj.leu.2403006. [DOI] [PubMed] [Google Scholar]
- 33.Spanoudakis E., Bazdiara I., Kotsianidis I., Margaritis D., Goutzouvelidis A., Christoforidou A. Hydroxyurea (HU) is effective in reducing JAK2V617F mutated clone size in the peripheral blood of essential thrombocythemia (ET) and polycythemia vera (PV) patients. Ann Hematol. 2009;88(7):629–632. doi: 10.1007/s00277-008-0650-1. [DOI] [PubMed] [Google Scholar]
- 34.Wong T.E., Brandow A.M., Lim W., Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124(26):3850–3857. doi: 10.1182/blood-2014-08-435768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J.I., Choi H.S., Jeong J.S., Han J.Y., Kim I.H. Involvement of p38 kinase in hydroxyurea-induced differentiation of K562 cells. Cell Growth Differ. 2001;12(9):481–486. [PubMed] [Google Scholar]
- 36.Bruchova H., Borovanova T., Klamova H., Brdicka R. Gene expression profiling in chronic myeloid leukemia patients treated with hydroxyurea. Leuk Lymphoma. 2002;43(6):1289–1295. doi: 10.1080/10428190290026358. [DOI] [PubMed] [Google Scholar]
- 37.Baccarani M., Deininger M.W., Rosti G., Hochhaus A., Soverini S., Apperley J.F. European Leukemianet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steegmann J.L., Baccarani M., Breccia M., Casado L.F., García-Gutiérrez V., Hochhaus A. European Leukemianet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648–1671. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller M.C., Cross N.C., Erben P., Schenk T., Hanfstein B., Ernst T. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23(11):1957–1963. doi: 10.1038/leu.2009.168. [DOI] [PubMed] [Google Scholar]
- 40.Soverini S., Hochhaus A., Nicolini F.E., Gruber F., Lange T., Saglio G. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European Leukemianet. Blood. 2011;118(5):1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 41.Preuner S., Denk D., Frommlet F., Nesslboeck M., Lion T. Quantitative monitoring of cell clones carrying point mutations in the BCR-ABL tyrosine kinase domain by ligation-dependent polymerase chain reaction (LD-PCR) Leukemia. 2008;22(10):1956–1961. doi: 10.1038/leu.2008.97. [DOI] [PubMed] [Google Scholar]
- 42.Yuan H., Wang Z., Gao C., Chen W., Huang Q., Yee J.K. BCR-ABL gene expression is required for its mutations in a novel KCL-22 cell culture model for acquired resistance of chronic myelogenous leukemia. J Biol Chem. 2010;285(7):5085–5096. doi: 10.1074/jbc.M109.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Rosee P., Corbin A.S., Stoffregen E.P., Deininger M.W., Druker B.J. Activity of the BCR-ABL kinase inhibitor PD180970 against clinically relevant BCR-ABL isoforms that cause resistance to imatinib mesylate (Gleevec, STI571) Cancer Res. 2002;62:7149–7153. [PubMed] [Google Scholar]
- 44.Byrgazov K., Lucini C.B., Berkowitsch B., Koenig M., Haas O.A., Hoermann G. Transposon-mediated generation of BCR-ABL1-expressing transgenic cell lines for unbiased sensitivity testing of tyrosine kinase inhibitors. Oncotarget. 2016;7(47):78083–78094. doi: 10.18632/oncotarget.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gleixner K.V., Ferenc V., Peter B., Gruze A., Meyer R.A., Hadzijusufovic E. Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Res. 2010;70(4):1513–1523. doi: 10.1158/0008-5472.CAN-09-2181. [DOI] [PubMed] [Google Scholar]
- 46.Stefanzl G., Berger D., Cerny-Reiterer S., Blatt K., Eisenwort G., Sperr W.R. The pan-BCL-2-blocker obatoclax (GX15-070) and the PI3-kinase/mTOR-inhibitor BEZ235 produce cooperative growth-inhibitory effects in ALL cells. Oncotarget. 2017;28(40):67709–67722. doi: 10.18632/oncotarget.18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou T.C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.Legros L., Hayette S., Nicolini F.E., Raynaud S., Chabane K., Magaud J.P. BCR-ABLT315I transcript disappearance in an imatinib-resistant CML patient treated with homoharringtonine: a new therapeutic challenge. Leukemia. 2007;21(10):2204–2206. doi: 10.1038/sj.leu.2404772. [DOI] [PubMed] [Google Scholar]
- 49.De Lavallade H., Khorashad J.S., Davis H.P., Milojkovic D., Kaeda J.S., Goldman J.M. Interferon-alpha or homoharringtonine as salvage treatment for chronic myeloid leukemia patients who acquire the T315I BCR-ABL mutation. Blood. 2007;110(7):2779–2780. doi: 10.1182/blood-2007-06-094508. [DOI] [PubMed] [Google Scholar]
- 50.Sokal J.E., Gockerman J.P., Bigner S.H. Evidence for a selective antileukemic effect of cytosine arabinoside in chronic granulocytic leukemia. Leuk Res. 1988;12(6):453–458. doi: 10.1016/0145-2126(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 51.Wylie A.A., Schoepfer J., Jahnke W., Cowan-Jacob S.W., Loo A., Furet P. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543(7647):733–737. doi: 10.1038/nature21702. [DOI] [PubMed] [Google Scholar]
- 52.Al-Khalaf H.H., Lach B., Allam A., Hassounah M., Alkhani A., Elkum N. Expression of survivin and p16(INK4a)/Cdk6/pRB proteins and induction of apoptosis in response to radiation and cisplatin in meningioma cells. Brain Res. 2008;1188:25–34. doi: 10.1016/j.brainres.2007.10.074. [DOI] [PubMed] [Google Scholar]
- 53.O'Leary B., Finn R.S., Turner N.C. Treating cancer with selective CDK4/CDK6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 54.Baughn L.B., Di Liberto M., Wu K., Toogood P.L., Louie T., Gottschalk R. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66(15):7661–7667. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- 55.Hanfstein B., Müller M.C., Kreil S., Ernst T., Schenk T., Lorentz C. Dynamics of mutant BCR-ABL-positive clones after cessation of tyrosine kinase inhibitor therapy. Haematologica. 2011;96(3):360–366. doi: 10.3324/haematol.2010.030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griswold I.J., MacPartlin M., Bumm T., Goss V.L., O'Hare T., Lee K.A. Kinase domain mutants of BCR-ABL exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26(16):6082–6093. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timson J. Hydroxyurea. Mutat Res. 1975;32(2):115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- 58.Singh A., Xu Y.J. The cell killing mechanisms of hydroxyurea. Genes. 2016;7(11) doi: 10.3390/genes7110099. pii:E99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aleem E., Arceci R.J. Targeting cell cycle regulators in hematologic malignancies. Front Cell Dev Biol. 2015;3:16. doi: 10.3389/fcell.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheicher R., Hoelbl-Kovacic A., Bellutti F., Tigan A.S., Prchal-Murphy M., Heller G. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125(1):90–101. doi: 10.1182/blood-2014-06-584417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savona M., Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8(5):341–350. doi: 10.1038/nrc2368. [DOI] [PubMed] [Google Scholar]
- 62.Nemoto A., Saida S., Kato I., Kikuchi J., Furukawa Y., Maeda Y. Specific antileukemic activity of PD0332991, a CDK4/6 inhibitor, against Philadelphia chromosome-positive lymphoid leukemia. Mol Cancer Ther. 2016;15(1):94–105. doi: 10.1158/1535-7163.MCT-14-1065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.