Abstract
Background:
Bacterial sexually transmitted infections may facilitate HIV transmission. Bacterial sexually transmitted infection testing is recommended for sexually active HIV-infected patients annually and more frequently for those at elevated sexual risk. We estimated percentages of HIV-infected patients in the United States receiving at least one syphilis, gonorrhea, or chlamydia test, and repeat (≥2 tests, ≥3 months apart) tests for any of these sexually transmitted infections from mid-2008 through mid-2010.
Design:
The Medical Monitoring Project collects behavioral and clinical characteristics of HIV-infected adults receiving medical care in the United States using nationally representative sampling.
Methods:
Sexual activity included self-reported oral, vaginal, or anal sex in the past 12 months. Participants reporting more than 1 sexual partner or illicit drug use before/during sex in the past year were classified as having elevated sexual risk. Among participants with only 1 sex partner and no drug use before/during sex, those reporting consistent condom use were classified as low risk; those reporting sex without a condom (or for whom this was unknown) were classified as at elevated sexual risk only if they considered their sex partner to be a casual partner, or if their partner was HIV-negative or partner HIV status was unknown. Bacterial sexually transmitted infection testing was ascertained through medical record abstraction.
Results:
Among sexually active patients, 55% were tested at least once in 12 months for syphilis, whereas 23% and 24% received at least one gonorrhea and chlamydia test, respectively. Syphilis testing did not vary by sex/sexual orientation. Receipt of at least 3 CD4+ T-lymphocyte cell counts and/or HIV viral load tests in 12 months was associated with syphilis testing in men who have sex with men (MSM), men who have sex with women only, and women. Chlamydia testing was significantly higher in sexually active women (30%) compared with men who have sex with women only (19%), but not compared with MSM (22%). Forty-six percent of MSM were at elevated sexual risk; 26% of these MSM received repeat syphilis testing, whereas repeat testing for gonorrhea and chlamydia was only 7% for each infection.
Conclusions:
Bacterial sexually transmitted infection testing among sexually active HIV-infected patients was low, particularly for those at elevated sexual risk. Patient encounters in which CD4+ T-lymphocyte cell counts and/or HIV viral load testing occurs present opportunities for increased bacterial sexually transmitted infection testing.
Bacterial sexually transmitted infections (BSTIs) may facilitate HIV transmission1 by increasing plasma HIV viremia2,3 and HIV shedding in the genital tract.4,5 Incident BSTIs among HIV-infected persons also indicate recent risky sexual behavior that may result in HIV transmission.6,7 Thus, early detection and treatment of BSTI may contribute to reducing HIV transmission,7 and afford opportunities for risk reduction counseling.8
National guidelines recommend annual BSTI testing for sexually active HIV-infected patients and more frequent testing for those at elevated risk for BSTI.9–11 The Infectious Diseases Society of America (IDSA) primary care guidelines for management of HIV-infected persons were first published in 200412 and were updated in 200913 and 2013.9 In the 2004 and 2009 guidelines, annual testing was recommended for all sexually active patients and repeat testing at 3- to 6-month intervals for patients with risk behaviors including multiple or anonymous sex partners and “other behaviors associated with transmission” of HIV and BSTI. The 2013 IDSA guidelines recommend annual BSTI testing for patients “at risk for infection” and repeat screening “depending on symptoms and signs, behavioral risk, and possible exposures.”9 A separate set of guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents, published by the Centers for Disease Control and Prevention (CDC), the National Institutes of Health, and IDSA in April 2009,14 specifically recommended syphilis screening every 3 to 6 months for those reporting multiple sex partners, condomless sex, or sex in conjunction with illicit drug use, and for those using methamphetamines or with partners participating in these activities.
Studies conducted in a limited number of clinical settings indicate that BSTI screening in HIV-infected patients may be suboptimal, particularly for gonorrhea and chlamydia,15–19 and these studies, which typically were conducted in large, primarily urban HIV clinics, may not sufficiently account for geographic variation or represent BSTI testing practices for patients receiving care in suburban or rural communities. There are no national estimates of BSTI testing among HIV-infected persons; thus, we estimated percentages of HIV-infected patients in the United States (US) receiving at least one test for syphilis, gonorrhea, or chlamydia, and repeat tests for these infections, during a 12-month period, and examined differences in testing by demographic, sexual, and other factors.
METHODS
The Medical Monitoring Project (MMP) is a complex-sample, cross-sectional survey designed to produce nationally representative estimates of behavioral and clinical characteristics of HIV-infected adults receiving medical care in the United States.20 States and territories were sampled first, followed by facilities providing HIV care, and then by HIV-infected adults (aged ≥18 years) who had at least 1 medical care visit from January to April 2009 at participating facilities. Data were collected using face-to-face interviews about self-reported patient experiences and behaviors for the past 12 months; interviews were conducted between June 2009 and May 2010. Medical record abstraction was used to collect data on clinical care received in the 12 months preceding interview.
All sampled states and territories participated in MMP.20 Of 603 sampled facilities, 461 participated (facility-level response rate, 76%). Of 9338 sampled patients, 4217 completed interviews and had their medical records abstracted (patient-level response rate, 51%). Data were weighted based on known probabilities of selection at state/territory, facility, and patient levels, and to adjust for nonresponse.21,22 Participants in the 2009 data collection cycle were estimated to represent a population of 421,186 (95% confidence interval [CI], 378,187–464,186) HIV-infected adults receiving medical care in the United States from January to April 2009.
We defined syphilis testing in 2 ways: (1) medical record documentation of at least one syphilis (treponemal or nontreponemal) test within the 12 months before interview and (2) documentation of repeat syphilis tests (≥2 tests, >3 months apart) in the same period. The same criteria were used to define gonorrhea and chlamydia testing, regardless of anatomical site of specimen collection. We also created a variable for medical record documentation of at least 3 CD4+ T-lymphocyte cell count and/or HIV viral load (CD4/VL) tests, all 3 months apart or longer, within the 12 months before interview.
Sex, sexual behavior, and sexual orientation were self-reported. We created 4 mutually exclusive sex/sexual orientation categories: gay, bisexual, and other men who have sex with men (MSM), men who have sex with women only (MSW), women, and transgender or other participants. Results reported for all participants include those for transgender/other participants; however, because of the small number of these participants (n = 62), this group was excluded from sex/sexual orientation–specific estimates. Demographic variables for age group and race/ethnicity were self-reported.
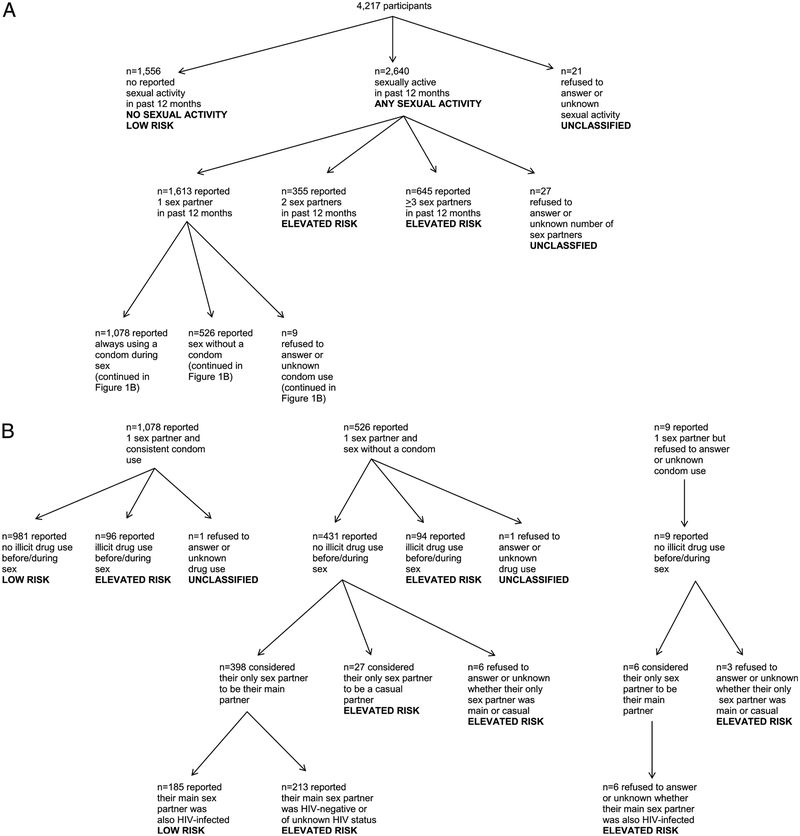
Interview questions on sexual activity and sexual risk behaviors in the past 12 months were used to create 2 indicator variables: any sexual activity (oral, vaginal, and/or anal; Fig. 1A) and sexual activity associated with elevated risk of BSTI or HIV transmission. For the latter variable, participants reporting more than 1 sexual partner or illicit drug use before/during sex in the past 12 months were classified as having elevated sexual risk (Fig. 1A, B). Among participants with only 1 sex partner and no drug use before/during sex, those reporting consistent condom use were classified as low risk; those reporting sex without a condom (or for whom this was unknown) were classified as at elevated risk if they considered their sex partner to be a casual partner, or if their partner was HIV negative or of unknown HIV status (Fig. 1B). Twenty-one participants did not respond to questions assessing sexual activity, and 29 sexually active participants did not provide sufficient information to classify their sexual activity as low or elevated risk.
Figure 1.
A, Sexual activity and risk classification—MMP, United States. B, Sexual risk classification among those reporting 1 sex partner in the past 12 months—MMP, United States.
Analyses were conducted using SAS 9.3 (SAS Institute Inc, Cary, NC; http://www.sas.com/en_us/home.html) and SAS-callable SUDAAN 11.0 (Research Triangle Institute, Research Triangle Park, NC; http://www.rti.org/sudaan/) to account for increased variance from the complex-sample design.
We conducted descriptive analyses and estimated weighted percentages of those receiving at least one and repeat syphilis, gonorrhea, and chlamydia tests by sex/sexual orientation for all patients, for those reporting any sexual activity, and for those at elevated sexual risk. Among those reporting any sexual activity and those at elevated risk, we conducted bivariate analyses of percentages tested by demographic and other factors. Multivariable logistic regression was used to generate prevalence ratios. Tests of significance (including CI) were adjusted for multiple comparisons using the Bonferroni method; different P values, for different BSTI, were used as thresholds for statistical significance (see Supplementary Material, http://links.lww.com/OLQ/A103).23
The MMP was determined by CDC to be a nonresearch, public health surveillance activity24 and was reviewed and approved by CDC accordingly. Participating states/territories and facilities obtained local institutional review board approval to conduct MMP if required locally, and informed consent was obtained from all interviewed participants.
RESULTS
Demographic, Sexual, and Other Attributes by Sex/Sexual Orientation
Patients were 47% MSM, 23% MSW, 27% women, and 3% transgender/other. Most patients (86%) were 35 years of age or older (Table 1). Age and race/ethnicity distributions varied significantly between MSM, MSW, and women. Most patients (62%) had at least 3 CD4/VL tests in the past 12 months.
TABLE 1.
Patient Demographics, Sexual Activity and Risk, and CD4/VL Testing by Sex/Sexual Orientation—MMP, United States
| Total§ | MSM | MSW | Women | |||||
|---|---|---|---|---|---|---|---|---|
| n¶ (%) | 95% CI | n¶ (%) | 95% CI | n¶ (%) | 95% CI | n¶ (%) | 95% CI | |
| Age group, y | ||||||||
| 18–34 | 607 (14) | 13–16 | 299 (15) | 13–17 | 81 (8) | 6–11 | 212 (18) | 15–21 |
| ≥35 | 3610 (86) | 84–87 | 1682 (85) | 83–87 | 917 (92‖) | 89–94 | 925 (82) | 79–85 |
| Race/Ethnicity | ||||||||
| White non-Hispanic | 1395 (35) | 28–41 | 1016 (53**) | 47–60 | 160 (16) | 12–23 | 193 (18) | 14–22 |
| Black non-Hispanic | 1740 (41) | 34–50 | 455 (23) | 18–29 | 555 (57) | 46–67 | 694 (62) | 53–69 |
| Hispanic | 881 (19) | 15–25 | 400 (19) | 16–22 | 244 (22) | 14–33 | 212 (17) | 11–25 |
| Other, including multiracial | 199 (5) | 4–6 | 109 (5) | 4–6 | 38 (5) | 3–7 | 38 (4) | 3–5 |
| Any sexual activity* | ||||||||
| Yes | 2640 (62) | 59–64 | 1411 (70††) | 67–73 | 566 (56) | 52–61 | 628 (55) | 52–58 |
| No | 1556 (38) | 36–41 | 560 (30) | 27–33 | 428 (44) | 39–48 | 503 (45) | 42–48 |
| Elevated sexual risk† | ||||||||
| Yes | 1445 (34) | 31–37 | 937 (46‡‡) | 43–50 | 232 (23) | 20–27 | 250 (22) | 19–25 |
| No | 2722 (66) | 63–69 | 1017 (54) | 50–57 | 754 (77) | 73–80 | 879 (78) | 75–81 |
| No. CD4/VL tests in past 12 mo‡ | ||||||||
| 0–2 | 1622 (38) | 35–43 | 768 (39) | 35–44 | 377 (36) | 30–41 | 437 (39) | 34–44 |
| ≥3 | 2595 (62) | 57–65 | 1213 (61) | 56–65 | 621 (64) | 59–70 | 700 (61) | 56–66 |
Defined as persons reporting any oral, vaginal, or anal sex partners during the past 12 months.
Defined as persons reporting more than 1 sexual partner, or illicit drug use before/during sex during the past 12 months, or persons with only 1 sexual partner who reported sex without a condom with either a casual sexual partner, HIV-negative partner, or partner of unknown HIV status.
Defined as 0–2 vs. ≥3 tests at least 3 months apart during the past 12 months.
All HIV-infected adults receiving medical care, including transgender and other sex/sexual orientation.
Unweighted counts of participants in each category.
Age distribution differed significantly for MSW compared with MSM (P < 0.001) and women (P < 0.001).
Race/ethnicity distribution differed significantly for MSM compared with MSW (P < 0.001) and women (P < 0.001).
Distribution of those reporting having engaged in any sexual activity differed significantly for MSM compared to MSW (P < 0.001) and women (P < 0.001).
Distribution of those at elevated sexual risk differed significantly for MSM compared to MSW (P < 0.001) and women (P < 0.001).
Most patients (62%) engaged in sexual activity in the past 12 months; this was significantly higher for MSM (70%) compared with MSW (56%, P < 0.001) and women (55%, P < 0.001). Overall, 34% of patients were classified as at elevated sexual risk: 46% of MSM, compared with 23% of MSW (P < 0.001) and 22% of women (P < 0.001). Of those engaging in sex, 66% of MSM were at elevated risk, compared with 42% of MSW (P < 0.001) and 40% of women (P < 0.001).
At Least One BSTI Test and Repeat BSTI Testing, by Sexual Risk and Sex/Sexual Orientation
Fifty-two percent of all patients received at least one syphilis test in 12 months; this increased only slightly for those reporting any sex (55%) and those at elevated sexual risk (58%), and did not vary significantly by sex/sexual orientation (Table 2). Among those reporting no sex, 45% of MSM, 50% of MSW, and 45% of women were tested for syphilis. Rates of receiving at least one gonorrhea or chlamydia test were lower than for syphilis; only 26% of patients at elevated sexual risk received either test. Rates of receiving at least one chlamydia test were significantly higher among women reporting any sex (30%) compared with sexually active MSW (19%, P < 0.001), but these rates did not differ from those for sexually active MSM (22%).
TABLE 2.
Percentages* of HIV-Infected Adults Receiving Medical Care in the United States Who Were Tested for BSTIs Within the Past 12 Months, by Sexual Risk Category and Sex/Sexual Orientation—MMP
| Total†, % (95% CI) | MSM, % (95% CI) | MSW, % (95% CI) | Women, % (95% CI) | |
|---|---|---|---|---|
| Syphilis | ||||
| At least 1 test in the past 12 mo‡ | ||||
| All HIV-infected patients | 52 (49–55) | 54 (50–57) | 52 (47–57) | 47 (42–53) |
| Patients reporting no sexual activity | 47 (43–51) | 45 (40–51) | 50 (44–56) | 45 (40–50) |
| Patients reporting any sexual activity§ | 55 (51–58) | 57 (54–61) | 53 (47–60) | 49 (42–56) |
| Patients at elevated sexual risk¶ | 58 (54–61) | 59 (55–63) | 59 (51–66) | 52 (44–60) |
| Repeat testing in past 12 mo‖ | ||||
| All HIV-infected patients | 17 (14–20) | 20** (17–24) | 14 (10–19) | 12 (8–17) |
| Patients reporting no sexual activity | 12 (9–15) | 12 (9–17) | 11 (8–15) | 11 (7–16) |
| Patients reporting any sexual activity§ | 19 (16–23) | 23†† (20–27) | 16 (11–23) | 12 (8–19) |
| Patients at elevated sexual risk¶ | 23 (20–27) | 26†† (23–31) | 19 (13–27) | 13 (8–22) |
| Gonorrhea | ||||
| At least 1 test in past 12 mo‡ | ||||
| All HIV-infected patients | 20 (18–23) | 20 (17–24) | 17 (13–23) | 24 (20–28) |
| Patients reporting no sexual activity | 16 (13–19) | 14 (11–18) | 15 (10–22) | 18 (14–23) |
| Patients reporting any sexual activity§ | 23 (20–27) | 23 (19–27) | 18 (13–25) | 28 (23–33) |
| Patients at elevated sexual risk¶ | 26 (22–30) | 26 (22–31) | 21 (14–29) | 29 (21–39) |
| Repeat testing in past 12 mo‖ | ||||
| All HIV-infected patients | 4 (3–5) | 5 (3–6) | 2 (1–4) | 4 (3–6) |
| Patients reporting no sexual activity | 2 (1–3) | 1 (1–3) | 1 (1–3) | 2 (1–5) |
| Patients reporting any sexual activity§ | 5 (4–7) | 6 (4–8) | 3 (1–6) | 6 (4–9) |
| Patients at elevated sexual risk¶ | 7 (5–9) | 7 (5–10) | 4 (2–10) | 8 (4–14) |
| Chlamydia | ||||
| At least 1 test in past 12 mo‡ | ||||
| All HIV-infected patients | 21 (18–24) | 20 (17–24) | 18 (13–23) | 26 (22–29) |
| Patients reporting no sexual activity | 16 (13–20) | 14 (11–18) | 16 (11–23) | 19 (16–24) |
| Patients reporting any sexual activity§ | 24 (21–27) | 22 (19–27) | 19 (14–25) | 30‡‡ (25–36) |
| Patients at elevated sexual risk¶ | 26 (23–30) | 26 (22–30) | 21 (16–28) | 32 (25–40) |
| Repeat testing in past 12 mo‖ | ||||
| All HIV-infected patients | 4 (3–5) | 4 (3–6) | 2 (1–1) | 4 (3–6) |
| Patients reporting no sexual activity | 2 (1–3) | 2 (1–3) | 1 (1–3) | 2 (1–4) |
| Patients reporting any sexual activity§ | 5 (4–6) | 6 (4–7) | 3 (1–6) | 6 (3–9) |
| Patients at elevated sexual risk¶ | 6 (5–8) | 7 (5–9) | 4 (2–10) | 8 (4–14) |
Percentages are national estimates of HIV-infected adults receiving medical care in the United States who were tested for the specified BSTI within the 12-month surveillance period.
Defined as 0 vs. ≥1 tests during the past 12 months.
Defined as persons reporting any oral, vaginal, or anal sex partners during the past 12 months.
Defined as persons reporting more than 1 sexual partner or illicit drug use before/during sex during the past 12 months, or persons with only 1 sexual partner who reported sex without a condom with either a casual sexual partner, HIV-negative partner, or partner of unknown HIV status.
Defined as 0–1 vs. ≥2 tests at least 3 months apart during the past 12 months.
All HIV-infected adults receiving medical care, including transgender and other sex/sexual orientation.
Prevalence for MSM was significantly higher compared with MSW (P < 0.001) and women (P < 0.001).
Prevalence for MSM was significantly higher compared with women (P < 0.001).
Prevalence for women was significantly higher compared with MSW (P < 0.001).
Rates of repeat syphilis testing were much lower than rates of receipt of at least one test; only 19% of those reporting any sex and 23% of those at elevated sexual risk received repeat testing. Among all patients, repeat syphilis testing was significantly higher in MSM (20%) compared with MSW (14%, P < 0.001) and women (12%, P < 0.001). Among those reporting any sex and those at elevated sexual risk, repeat syphilis testing was significantly higher for MSM (23% and 26%, respectively) compared with women (12% [P < 0.001] and 13% [P < 0.001]), but not compared with MSW (16% and 19%). Repeat testing for gonorrhea (7%) and chlamydia (6%) was extremely low among all patients at elevated risk and did not vary by sex/sexual orientation.
Receipt of at Least One BSTI Test Among Patients Reporting any Sexual Activity, by Sex/Sexual Orientation and Demographic and Other Attributes
Although the percentage of patients receiving at least one test for each BSTI was somewhat higher among sexually active patients aged 18 to 34 years, rates were not significantly different from those 35 years or older (Table 3). There were no significant differences in BSTI testing across sexes/sexual orientations within either age group. Among women, receipt of at least one chlamydia test was significantly higher for very young women aged 18 to 24 years (63%) compared with those 25 years or older (29%, P < 0.001); although testing was dissimilar by these age groups for gonorrhea (52% and 27%, respectively) and syphilis (61% and 49%), these differences were not statistically significant.
TABLE 3.
Percentages* of HIV-Infected Adults Receiving Medical Care in the United States Who Reported any Sexual Activity and Were Tested for BSTIs Within the Past 12 Months, by Sex/Sexual Orientation, Demographics, and Other Attributes—MMP
| At Least 1 Test in 12 mo†, Restricted to Participants Reporting Any Sexual Activity‡ | Syphilis | Gonorrhea | Chlamydia | |||
|---|---|---|---|---|---|---|
| % (95% CI) | Prevalence Ratio (99.4% CI)¶ |
% (95% CI) | Prevalence Ratio (99.7% CI)‖ |
% (95% CI) | Prevalence Ratio (99.7% CI)‖ |
|
| MSM | ||||||
| Age group, y | ||||||
| 18–34 | 66 (59–73) | 1.2 (1.0–1.5) | 31 (23–39) | 1.5 (1.0–2.1) | 30 (23–38) | 1.5 (1.0–2.1) |
| ≥35 | 55 (51–59) | 1.0 | 21 (17–25) | 1.0 | 21 (17–25) | 1.0 |
| Race/Ethnicity | ||||||
| White non-Hispanic | 52** (48–56) | 1.0 | 20 (16–24) | 1.0 | 19 (15–23) | 1.0 |
| Black non-Hispanic | 59 (52–66) | 1.1 (0.9–1.4) | 21 (16–28) | 1.1 (0.7–1.6) | 21 (16–28) | 1.1 (0.8–1.7) |
| Hispanic | 69 (61–75) | 1.3 (1.1–1.6)‡‡ | 30 (24–37) | 1.5 (1.1–2.2)§§ | 30 (24–36) | 1.6 (1.1–2.2)§§ |
| Other, including multiracial | 61 (49–73) | 1.2 (0.9–1.6) | 36 (24–50) | 1.9 (1.1–3.1)§§ | 35 (23–49) | 1.8 (1.1–3.1)§§ |
| No. CD4/VL tests in the past 12 mo§ | ||||||
| 0–2 | 46 (41–50) | 1.0 | 16 (12–21) | 1.0 | 15 (11–21) | 1.0 |
| ≥3 | 65 (61–69) | 1.4 (1.2–1.7)‡‡ | 27 (23–32) | 1.7 (1.2–2.4)§§ | 27 (23–32) | 1.8 (1.2–2.6)§§ |
| MSW | ||||||
| Age group, y | ||||||
| 18–34 | 50 (40–61) | 0.9 (0.7–1.3) | 21 (12–35) | 1.2 (0.5–3.0) | 23 (13–37) | 1.3 (0.5–3.0) |
| ≥35 | 54 (47–61) | 1.0 | 18 (13–25) | 1.0 | 19 (14–25) | 1.0 |
| Race/Ethnicity | ||||||
| White non-Hispanic | 41 (31–51) | 1.0 | 14 (7–27) | 1.0 | 15 (8–28) | 1.0 |
| Black non-Hispanic | 60 (52–68) | 1.5 (1.0–2.2) | 18 (12–26) | 1.3 (0.5–3.4) | 20 (14–27) | 1.3 (0.5–3.3) |
| Hispanic | 50 (38–63) | 1.2 (0.8–2.0) | 23 (14–35) | 1.6 (0.5–5.8) | 22 (13–33) | 1.4 (0.4–4.8) |
| Other, including multiracial | 34 (17–57) | 0.8 (0.3–2.0) | 7 (2–25) | 0.5 (0.1–4.4) | 7 (2–25) | 0.4 (0.1–4.0) |
| No. CD4/VL tests in the past 12 mo§ | ||||||
| 0–2 | 39 (32–47) | 1.0 | 12 (8–19) | 1.0 | 14 (9–20) | 1.0 |
| ≥3 | 62 (53–70) | 1.6 (1.1–2.2)‡‡ | 22 (15–30) | 1.7 (0.9–3.4) | 22 (16–29) | 1.6 (0.8–2.9) |
| Women | ||||||
| Age group, y | ||||||
| 18–34 | 50 (40–60) | 1.0 (0.8–1.3) | 37 (28–7) | 1.5 (1.0–2.2) | 39 (30–50) | 1.5 (1.0–2.1) |
| ≥35 | 49 (42–56) | 1.0 | 25 (20–30) | 1.0 | 27 (22–32) | 1.0 |
| Race/ethnicity | ||||||
| White non-Hispanic | 30 (23–39) | 1.0 | 16 (10–24) | 1.0 | 21 (14–31) | 1.0 |
| Black non-Hispanic | 55 (47–63) | 1.8 (1.2–2.8)‡‡ | 32 (25–39) | 2.0 (1.1–3.7)§§ | 34 (27–41) | 1.6 (0.9–2.7) |
| Hispanic | 50 (37–62) | 1.7 (1.0–2.7) | 24 (17–33) | 1.5 (0.6–3.4) | 28 (19–38) | 1.3 (0.6–2.9) |
| Other, including multiracial | 55 (24–82) | 1.8 (0.7–4.7) | 42 (25–61) | 2.6 (0.9–7.3) | 30 (15–51) | 1.4 (0.4–4.7) |
| No. CD4/VL tests in the past 12 mo§ | ||||||
| 0–2 | 36 (27–47) | 1.0 | 18 (12–25) | 1.0 | 20 (14–28) | 1.0 |
| ≥3 | 57 (50–64) | 1.6 (1.1–2.3)‡‡ | 34 (28–41) | 1.9 (1.1–3.5)§§ | 37†† (31–43) | 1.8 (1.1–3.2)§§ |
Percentages are national estimates of HIV-infected adults receiving medical care in the United States who reported any sexual activity and were tested for the specified BSTIs within the 12-month surveillance period.
Annual testing was defined as 0 vs. ≥1 tests during the past 12 months.
Defined as persons reporting any oral, vaginal, or anal sex partners during the past 12 months.
Defined as 0–2 vs. ≥3 tests at least 3 months apart during the past 12 months.
Confidence intervals for syphilis testing prevalence ratios were adjusted for 8 multiple statistical comparisons within each gender/sexual orientation group.
Confidence intervals for gonorrhea and chlamydia testing prevalence ratios were adjusted for 16 multiple statistical comparisons within each gender/sexual orientation group.
Among white non-Hispanic, prevalence was significantly higher for MSM compared to women (P < 0.001).
Among those receiving ≥3 CD4/VL tests, prevalence was significantly higher for women compared to MSW (P < 0.001).
Statistically significant after adjusting for multiple comparisons for syphilis tests (P ≤ 0.006).
Statistically significant after adjusting for multiple comparisons for gonorrhea and chlamydia tests (P ≤ 0.003).
Among sexually active MSM, receipt of at least one test for each BSTI was significantly higher for Hispanics compared with non-Hispanic whites; gonorrhea and chlamydia testing also was higher for those of other race/ethnicity compared with whites. BSTI testing did not vary by race/ethnicity for MSW. Among women, syphilis and gonorrhea testing was higher for sexually active non-Hispanic blacks (55% and 32%, respectively) compared with whites (30% and 16%, respectively; P < 0.001 for each comparison). Among non-Hispanic whites, receipt of at least one syphilis test was significantly higher for MSM compared with women (P < 0.001), but BSTI testing did not differ by sex/sexual orientation within other race/ethnicities.
Receipt of at least 3 CD4/VL tests in the past 12 months was associated with significantly higher rates of at least one syphilis test among sexually active MSM (65%), MSW (62%), and women (57%), compared with 0 to 2 CD4/VL tests (46%, 39%, and 36%, respectively; P < 0.001 for each comparison). Rates of having at least one test for gonorrhea and chlamydia, while lower than for syphilis, were also higher for those with at least 3 annual CD4/VL tests among MSM and women, but not MSW. Among those with at least 3 CD4/VL tests, comparisons across sex/sexual orientation for each BSTI showed that only chlamydia testing was significantly higher in women (37%) compared with MSW (22%, P < 0.001).
Repeat BSTI Testing Among Patients at Elevated Sexual Risk, by Sex/Sexual Orientation and Demographic and Other Attributes
Among those at elevated sexual risk, repeat testing for each BSTI varied significantly by age group only among women; those aged 18 to 34 years had higher testing rates than older women (Table 4). Comparisons across sex/sexual orientation showed that repeat syphilis testing was higher in older MSM (25%) compared with older women (8%, P < 0.001). Gonorrhea and chlamydia testing did not vary by sex/sexual orientation within either age group.
TABLE 4.
Percentages* of HIV-Infected Adults Receiving Medical Care in the United States at Elevated Sexual Risk Who Were Repeatedly Tested for BSTIs Within the Past 12 Months, by Sex/Sexual Orientation, Demographic, and Other Attributes—MMP
| Repeat Testing†, Restricted to Participants at Elevated Sexual Risk‡ | Syphilis | Gonorrhea | Chlamydia | |||
|---|---|---|---|---|---|---|
| % (95% CI) | Prevalence Ratio (99.4% CI)§ |
% (95% CI) | Prevalence Ratio (99.7% CI)¶ |
% (95% CI) | Prevalence Ratio (99.7% CI)¶ |
|
| MSM | ||||||
| Age group, y | ||||||
| 18–34 | 30 (24–38) | 1.2 (0.8–1.8) | 10 (6–15) | 1.5 (0.7–3.2) | 9 (5–14) | 1.4 (0.7–3.1) |
| ≥35 | 25‖ (21–30) | 1.0 | 6 (4–9) | 1.0 | 6 (4–8) | 1.0 |
| Race/Ethnicity | ||||||
| White non-Hispanic | 22 (17–28) | 1.0 | 6 (4–8) | 1.0 | 5 (3–7) | 1.0 |
| Black non-Hispanic | 26 (19–34) | 1.2 (0.7–2.1) | 8 (5–15) | 1.5 (0.6–4.0) | 9 (5–15) | 2.0 (0.8–4.8) |
| Hispanic | 37** (27–48) | 1.7 (1.0–2.9) | 7 (4–13) | 1.3 (0.6–2.9) | 8 (4–14) | 1.7 (0.7–3.9) |
| Other, including multiracial | 34 (24–46) | 1.6 (0.8–2.9) | 14 (7–26) | 2.4 (0.8–7.2) | 11 (6–20) | 2.5 (0.9–6.8) |
| No. CD4/VL tests in the past 12 mo†† | ||||||
| 0–2 | 12‡‡ (8–18) | 1.0 | 2 (1–6) | 1.0 | 3 (1–6) | 1.0 |
| ≥3 | 37 (31–43) | 3.1 (1.7–5.8)§§ | 10 (7–14) | 4.1 (1.2–14.3)¶¶ | 9 (7–13) | 3.6 (1.1–12.3)¶¶ |
| MSW | ||||||
| Age group, y | ||||||
| 18–34 | 11 (4–31) | 0.6 (0.1–2.9) | 4 (1–18) | 1.1 (0.1–18.6) | 4 (1–18) | 1.1 (0.1–15.1) |
| ≥35 | 20 (13–29) | 1.0 | 4 (1–11) | 1.0 | 4 (1–11) | 1.0 |
| Race/Ethnicity | ||||||
| White non-Hispanic | 18 (7–40) | 1.0 | 0 | — | 0 | — |
| Black non-Hispanic | 17 (10–27) | 0.9 (0.2–3.7) | 3 (1–8) | 1.0 | 3 (1–8) | 1.0 |
| Hispanic | 28 (17–42) | 1.5 (0.4–5.5) | 10 (2–32) | 2.9 (0.4–24.2) | 10 (2–32) | 2.9 (0.4–24.2) |
| Other, including multiracial | 13 (2–55) | 0.7 (0.0–11.4) | 0 | — | 0 | — |
| No. CD4/VL tests in past 12 mo†† | ||||||
| 0–2 | 3 (1–7) | 1.0 | 2 (0–12) | 1.0 | 2 (0–12) | 1.0 |
| ≥3 | 29 (20–40) | 9.8 (2.7–36.2)§§ | 5 (2–12) | 3.1 (0.3–33.1) | 5 (2–12) | 3.1 (0.3–33.1) |
| Women | ||||||
| Age group, y | ||||||
| 18–34 | 25 (14–39) | 3.1 (1.5–6.6)§§ | 20 (10–34) | 8.0 (1.6–40.9)¶¶ | 19 (10–34) | 7.8 (1.5–40.6)¶¶ |
| ≥35 | 8 (4–14) | 1.0 | 2 (1–6) | 1.0 | 2 (1–6) | 1.0 |
| Race/Ethnicity | ||||||
| White non-Hispanic | 14 (7–26) | 1.0 | 3 (1–12) | 1.0 | 3 (1–12) | 1.0 |
| Black non-Hispanic | 15 (8–25) | 1.1 (0.4–3.1) | 10 (5–20) | 3.1 (0.4–26.5) | 10 (5–20) | 3.1 (0.4–26.5) |
| Hispanic | 6 (1–24) | 0.5 (0.0–4.3) | 6 (1–26) | 1.8 (0.1–42.4) | 5 (1–28) | 1.4(0.0–50.9) |
| Other, including multiracial | 9 (1–43) | 0.7 (0.1–7.7) | 0 | — | 0 | — |
| No. CD4/VL tests in past 12 mo†† | ||||||
| 0–2 | 1 (0–5) | 1.0 | 1 (0–7) | 1.0 | 1 (0–7) | 1.0 |
| ≥3 | 20 (12–32) | 14.3 (2.3–90.1)§§ | 12 (7–21) | 12.5 (0.5–293.2) | 12 (6–21) | 12.3 (0.5–289.3) |
Percentages are national estimates of HIV-infected adults receiving medical care in the United States who reported risky sexual activity‡ and were repeatedly tested for the specified BSTIs within the 12-month surveillance period.
Repeat testing was defined as 0–1 vs. ≥2 tests at least 3 months apart during the past 12 months.
Defined as persons reporting more than 1 sexual partner or illicit drug use before/during sex during the past 12 months, or persons with only 1 sexual partner who reported sex without a condom with either a casual sexual partner, HIV-negative partner, or partner of unknown HIV status.
Defined as 0–2 vs. ≥3 tests at least 3 months apart during the past 12 months.
CIs for syphilis testing prevalence ratios were adjusted for 8 multiple statistical comparisons within each sex/sexual orientation group.
CIs for gonorrhea and chlamydia testing prevalence ratios were adjusted for 16 multiple statistical comparisons within each sex/sexual orientation group.
Among those aged 35 years or older, prevalence was significantly higher for MSM compared with women (P < 0.001).
Among Hispanics, prevalence was significantly higher for MSM compared with women (P < 0.001).
Among those receiving 0 to 2 CD4/VL tests, prevalence was significantly higher for MSM compared with MSW (P = 0.001) and women (P < 0.001).
Statistically significant after adjusting for multiple comparisons for syphilis tests (P ≤ 0.006).
Statistically significant after adjusting for multiple comparisons for gonorrhea and chlamydia tests (P ≤ 0.003).
Repeat testing did not differ by race/ethnicity for MSM, MSW, or women for any BSTI (Table 4). Comparisons across sex/sexual orientation indicated that among Hispanics at elevated risk, repeat syphilis testing was higher in MSM (37%) compared with women (6%, P < 0.001).
Repeat testing for each BSTI was significantly higher among MSM at elevated sexual risk who received at least 3 annual CD4/VL tests, compared with those with 0 to 2 tests. Among MSW and women, having at least 3 CD4/VL tests was only significantly associated with increased repeat syphilis testing. Comparisons across sexes/sexual orientations showed that among those with 0 to 2 CD4/VL tests, MSM had higher rates of repeat syphilis testing (12%) than MSW (3%, P = 0.001) and women (1%, P < 0.001).
DISCUSSION
Most HIV-infected patients in the United States engaged in sexual activity during the past 12 months; almost half of MSM and almost one quarter of MSW and women were at elevated sexual risk. Among those engaging in sex, 66% of MSM and approximately 40% of MSW and women were at elevated risk. However, testing for BSTI among HIV-infected patients was low and did not substantially increase for those at elevated risk. Only 55% of sexually active patients received at least one test for syphilis, and approximately one quarter received at least one gonorrhea or chlamydia test. Repeat BSTI testing was low for each BSTI examined, particularly gonorrhea and chlamydia.
The 200412 and 200913 IDSA primary care guidelines for management of HIV-infected persons were available when participants in this study received medical care (mid-2008 through mid-2010). In both of these guidelines, annual testing was recommended for all sexually active patients and repeat testing at 3- to 6-month intervals for patients with risk behaviors including multiple or anonymous sex partners and “other behaviors associated with transmission” of HIV and BSTI. However, in other sections of these guidelines and in the guidelines published in 2013,9 BSTI screening recommendations after the initial patient encounter were based on behavioral risk and other factors, including infection with other BSTI and community prevalence of BSTI; these sections only specified that “more frequent testing may be indicated” for those at elevated risk for BSTI. It is possible that more consistent recommendations within and across guidelines would have resulted in higher testing rates than we observed.
Higher rates of syphilis testing, compared with gonorrhea and chlamydia testing, may be partially due to provider awareness of increasing primary and secondary syphilis rates among US MSM since 2001. A high percentage of syphilis-infected MSM are coinfected with HIV,25 which may have influenced testing for all HIV-infected patients. Also, guidelines for the prevention and treatment of opportunistic infections in HIV-infected persons14 emphasized syphilis testing, which may have contributed to provider awareness. However, these were first published in April 2009 and thus were only available for part of our data collection period.
Provider awareness of increasing syphilis infections among MSM also may partially account for the higher rates of repeat syphilis testing in MSM, compared with MSW and women. Early syphilis infection continued to increase among MSM in 2007 to 2012; among MSW and women, infection rates increased in 2007 to 2008, but declined from 2008 to 2012.26 Surveillance data from 2005 to 2008 in 27 states indicate that increases in syphilis infections have been highest among MSM aged 15 to 29 years27; however, this information, which was published in 2011, would not have been widely disseminated during our study period. We did not find significant variation in receipt of at least one syphilis test, or repeat syphilis testing, among sexually active HIV-infected MSM aged 18 to 34 years compared with those 35 years or older.
Receipt of at least one chlamydia test among sexually active women was significantly higher than among comparable MSW. The US Preventive Services Taskforce recommends annual chlamydia screening for all sexually active women younger than 25 years28; gonorrhea screening is recommended only for women at increased risk for infection.29 We found that more sexually active women aged 18 to 24 years received at least one chlamydia test (63%) compared with those aged 25 years or older (29%). The chlamydia testing rate we observed in young women was much higher than the 2006 to 2008 national estimate of 38% for sexually active women aged 15 to 25 years, derived from self-report.30 Provider awareness of the need for chlamydia screening in sexually active younger women may have partially accounted for this finding.
BSTI screening guidelines and counseling cannot be appropriately implemented without periodically conducting a sexual risk assessment.7 Less than half of MMP participants reported receiving individual-level HIV or BSTI prevention counseling from health care providers.31 It is possible that the proportion of patients receiving sexual risk assessment was similarly low because approximately half of patients reporting no sexual activity received a syphilis test. Barriers to conducting sexual histories may include time constraints, language and cultural barriers, patient confidentiality concerns, and provider and/or patient hesitancy to discuss sexuality.32 The MMP patient interview collected detailed information on patient sexual history using protocols to ensure strict confidentiality. It is possible that some patients we identified as having any sex or elevated sexual risk were not similarly identified by their clinicians, and therefore, appropriate BSTI testing was not performed. Some patients may have been offered BSTI testing but declined.
Other barriers to conducting BSTI testing may include provider disagreement with national guidelines or low prioritization of screening by providers. Interventions to increase BSTI screening such as provider adoption of standard testing protocols, or alerts and prompts incorporated into electronic medical records, may be effective additions to national guidelines. Clinical decision support systems that use software algorithms to generate patient-specific recommendations have been shown to improve HIV provider practice33 and increase BSTI testing in MSM.34
Repeat CD4/VL testing was associated with increased receipt of at least one syphilis test and with repeat syphilis testing in MSM, MSW, and women. Syphilis tests are serologic and thus can be easily ordered during the same encounter in which CD4/VL tests are ordered; gonorrhea and chlamydia testing requires additional specimen collection, which may partially account for lower levels of testing. However, we did observe increases in receipt of at least one gonorrhea or chlamydia test with increased CD4/VL testing in MSM and women, and with repeat testing for these BSTIs in MSM. This may be a function of more frequent patient encounters, and therefore more opportunities to order these tests. More simply, CD4/VL and BSTI tests may both indicate high-quality HIV care.
This analysis was subject to several limitations. The population of inference is HIV-infected adults receiving medical care and therefore does not reflect rates of BSTI testing among all HIV-infected adults, including those not engaged in medical care, which are likely to be lower. Moreover, our patient response rate was moderate; however, samples with moderate response rates that are selected from well-constructed frames have reduced overall risk of bias.21 We assessed and adjusted for nonresponse using widely accepted techniques,22 but the possibility of residual bias in either direction remains. These data, reflecting patient care from mid-2008 through mid-2010, also may not represent current BSTI testing rates.
It is possible that some patients sought BSTI testing at a sexually transmitted disease clinic or community-based health care organization, rather than from their HIV care provider, perhaps due to concerns regarding confidentiality or cost of testing and/or treatment; tests conducted at these facilities would not have been included in our data. We also were unable to distinguish follow-up BSTI tests (e.g., tests of cure) from screening/diagnostic tests. It is possible that tests occurring early in an individual’s 12-month surveillance period were follow-up tests for previously diagnosed BSTI, and therefore our findings may somewhat overestimate true screening or diagnostic testing. This may be of less concern for repeat BSTI tests, which we defined as being at least 3 months apart.
We also could not examine gonorrhea and chlamydia testing for rectal and pharyngeal specimens because information on anatomical site of specimen collection was missing for 38% and 37% of participants who received at least one gonorrhea or chlamydia test, respectively. This information may be missing for so many participants because these data often would have been abstracted from laboratory request and/or report forms; it is possible that some of these forms may not have included fields for anatomical site because nucleic acid amplification tests are not Food and Drug Administration cleared for use on rectal and pharyngeal specimens (although some laboratories have established performance specifications for using nucleic acid amplification tests with these specimens).35 Even if a field (or fields) for specification of anatomical site was included on the laboratory request form, this information may not have been provided by some clinicians for extragenital specimens for this reason.
We could not use multivariable modeling to determine which demographic and other factors were most strongly associated with BSTI testing, due to differences in rates of testing by age group, race/ethnicity, and sex/sexual orientation (i.e., effect modification); after the data were stratified by these factors, insufficient numbers of records were available in several strata to provide sufficient power for such statistical analysis. However, we minimized spurious associations by using more conservative significance levels for our statistical comparisons (and consequently increasing the width of confidence limits for these comparisons) to account for the number of comparisons we conducted.
This study also had several strengths. We were able to estimate population rates of BSTI testing among HIV-infected adults in care in the US and to account for differences by sex/sexual orientation and other factors. Other studies of BSTI testing among HIV-infected patients have been conducted in a small number of clinics15–19 or limited geographic areas,16–19 and, thus may not represent BSTI testing in the population of HIV-infected patients. Our rates of receipt of at least one syphilis test were lower than syphilis screening rates of 66% to 76% for HIV-infected MSM from a 2004 to 2006 retrospective review of medical records in 8 large, urban HIV clinics in 6 cities,15 although testing rates for gonorrhea and chlamydia in these clinics were similar to ours. It is likely that provider awareness of the importance of syphilis screening was higher in these clinics, as most of them received Ryan White funding, for which syphilis screening is required as a quality-of-care measure.
In conclusion, rates of BSTI testing among sexually active HIV-infected patients were low, and repeat testing rates among those at elevated sexual risk were particularly low. Patient encounters in which CD4/VL testing is conducted present opportunities for BSTI testing. Additional interventions, such as alerts and prompts incorporated into electronic medical records, may be effective additions to national guidelines for improving BSTI testing.
Supplementary Material
Funding:
This work was supported by the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: None declared.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarzebowski W, Caumes E, Dupin N, et al. Effect of early syphilis infection on plasma viral load and CD4 cell count in human immunodeficiency virus–infected men. Arch Intern Med. 2012;172: 1237–1243. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–949. [DOI] [PubMed] [Google Scholar]
- 5.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–110. [DOI] [PubMed] [Google Scholar]
- 6.Golden MR, Wood RW, Buskin SE, et al. Ongoing risk behavior among persons with HIV in medical care. AIDS Behav. 2007;11: 726–735. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, et al. Recommendations for incorporating human immunodeficiency virus (HIV) prevention into the medical care of persons living with HIV. Clin Infect Dis 2004;38:104–121. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JL, Milam J, McCutchan A, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: A multi-clinic assessment. AIDS. 2004;18:1179–1186. [DOI] [PubMed] [Google Scholar]
- 9.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1–e34. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed December 26, 2013.
- 11.Marrazzo JM, del Rio C, Holtgrave DR, et al. HIV prevention in clinical care settings. 2014 Recommendations of the International Antiviral Society—USA Panel. JAMA. 2014;312:390–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberg JA, Gallant JE, Anderson J, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39:609–629. [DOI] [PubMed] [Google Scholar]
- 13.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009;58(No. RR-4):41–45. [PubMed] [Google Scholar]
- 15.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37:771–776. [DOI] [PubMed] [Google Scholar]
- 16.Kahle E, Zhang Q, Golden M, et al. Trends in evaluation for sexually transmitted infections among HIV-infected people, King County, Washington. Sex Transm Dis. 2007;37:940–946. [DOI] [PubMed] [Google Scholar]
- 17.Phipps W, Stanley H, Kohn R, et al. Syphilis, chlamydia and gonorrhea screening in HIV-infected patients in primary care, San Francisco, California, 2003. AIDS Patient Care. 2005;19:495–498. [DOI] [PubMed] [Google Scholar]
- 18.Klausner JD, Stanley H, Stansell J. STD screening among HIV-infected patients in care, San Francisco. AIDS Patient Care. 2001;15:73–76. [DOI] [PubMed] [Google Scholar]
- 19.Mimiaga MJ, Helms DJ, Reisner SL, et al. Gonococcal, chlamydia, and syphilis infection positivity among MSM attending a large primary care clinic, Boston, 2003 to 2004. Sex Transm Dis. 2009;36:507–511. [DOI] [PubMed] [Google Scholar]
- 20.Frankel MR, McNaghten AD, Shapiro MF, et al. A probability sample for monitoring the HIV-infected population in care in the U.S. and in selected states. Open AIDS J 2012;6(suppl 1:M2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn EL, Graubard BI. Analysis of Health Surveys. New York, NY: John Wiley and Sons, Inc., 1999. [Google Scholar]
- 22.Groves RM. Nonresponse rates and nonresponse bias in household surveys. Publ Opin Q. 2006;70:646–674. [Google Scholar]
- 23.Neter J, Wasserman W, Kutner MH. Applied Linear Statistical Models Chapter 5: Simultaneous Inferences and Other Topics in Regression Analysis—I, 2nd ed. Homewood, IL: Richard D. Irwin, Inc., 1985. [Google Scholar]
- 24.Centers for Disease Control and Prevention. Distinguishing Public Health Research and Public Health Nonresearch. Available at: http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf. Accessed May 21, 2014.
- 25.Heffelfinger JD, Swint EB, Berman SM, et al. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health. 2007;97:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2012. Atlanta, GA: U.S. Department of Health and Human Services, 2013. [Google Scholar]
- 27.Su JR, Beltrami JF, Zaidi AA, et al. Primary and secondary syphilis among black and Hispanic men who have sex with men: Case report data from 27 states. Ann Intern Med. 2011;155:145–151. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007;147:128–134. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Preventive Services Task Force. Screening for gonorrhea: Recommendation statement. Ann Fam Med 2005;3:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao G, Hoover KW, Leichliter JS, et al. Self-reported chlamydia testing rates of sexually active women aged 15–25 years in the United States, 2006–2008. Sex Transm Dis. 2012;39:605–607. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno Y, Zhu J, Crepaz N, et al. Receipt of HIV/sexually transmitted disease prevention counseling by HIV-infected adults receiving medical care in the United States, Medical Monitoring Project, 2009. AIDS. 2014;28:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter JW, Hart-Cooper GD, Butler MO, et al. Provider barriers prevent recommended sexually transmitted disease screening of HIV-infected men who have sex with men. Sex Transm Dis. 2014;41:127–142. [DOI] [PubMed] [Google Scholar]
- 33.Robbins GK, Lester W, Johnson KL, et al. Efficacy of a clinical decision-support system in an HIV practice. A randomized trial. Ann Intern Med. 2012;157:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou H, Fairley CK, Guy R, et al. The efficacy of clinic-based interventions aimed at increasing screening for bacterial sexually transmitted infections among men who have sex with men:A systematic review. Sex Transm Dis. 2012;39:382–387. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2010. MMWR Recomm Rep 2010;59(No. RR-12):45–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.