Abstract
Accessing enantiomerically enriched amines often demands oxidation-state adjustments, protection/deprotection processes, and purification procedures that increase cost and waste, and limit applicability. When diastereomers can be formed, one isomer is attainable. Here, we show that nitriles, largely viewed as insufficiently reactive, can be transformed directly to multifunctional unprotected homoallylic amines by enantioselective addition of a carbon-based nucleophile and diastereodivergent reduction of the resulting ketimine. Successful implementation requires that competing copper-based catalysts be present simultaneously, and that the slower-forming and less- reactive one engages first. This challenge was addressed by incorporation of a nonproductive side- cycle, fueled selectively by inexpensive additives, to delay the function of the more active catalyst. Utility of the approach is highlighted by its application to the efficient of anti-cancer agent (+)- tangutorine.
Facile and economical access to amines in high diastereomeric and enantiomeric purity is crucial to future advance in chemistry, biology, and medicine. Despite substantial progress (1), however, several key limitations remain. There are just a handful of methods for catalytic enantioselective preparation of unprotected α-secondary amines RNH2 (2, 3, 4, 5). N-Activated/protected starting materials must otherwise be used, resulting in manipulations that escalate waste generation and diminish efficiency. Synthesizing an unprotected α-secondary amine frequently necessitates (at times strongly) acidic, oxidizing, or reducing conditions that can be low/moderate yielding (6); in some instances, up to three chemical steps must be dedicated to protecting group manipulations alone, (7, 8, 9) and/or excess amounts of a costly reagent (e.g., SmI2) might be required (10). The conditions needed for removal of an N-protected unit can lead to side reactions occurring at other sites in a multifunctional molecule (11). Another major issue is that alkyl-substituted N-protected imines, relevant to synthesis of numerous bioactive molecules, tend to be unstable (vs. aryl- or heteroaryl variants) and difficult to purify (on account of fast hydrolysis upon exposure to moisture and/or enamine/aminal formation), and must therefore be used as mixtures, which spells low yields. Some of these complications may, in principle, be resolved by the use of N-H aldimines, but these are also unstable and reports regarding their productive reactivity are scarce and restricted in scope (12). What is more, when diastereomers can be generated, often only one of them can be accessed (13, 14, 15, 16). An enantioselective strategy should ideally be diastereodivergent (17).
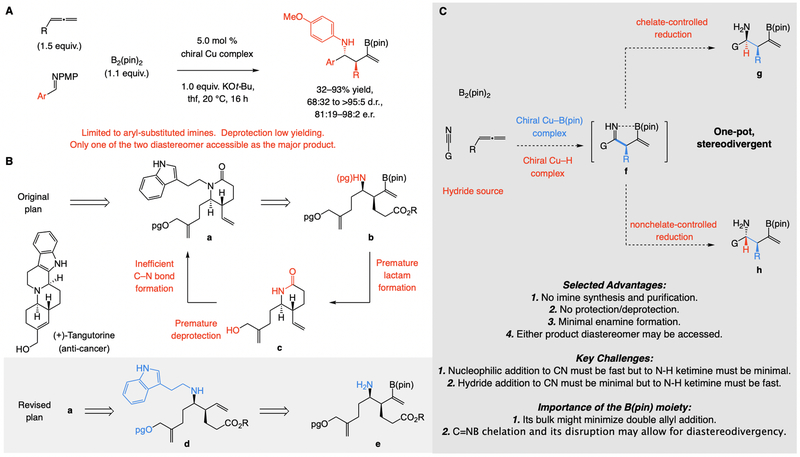
An attractive way to generate enantiomerically enriched amine would be by converting a readily accessible unsaturated hydrocarbon to a reactive intermediate, which can then react in situ with an imine. An example is the recent protocol (Fig. 1A) for synthesis of N-p-methoxyphenyl-protected homoallylic α-secondary amines (15). However, the method is confined to aryl imines (18), and conversion to an N-H amine not only demands harsh oxidative conditions, it is low yielding as well (19).
Fig. 1. The state-of-the-art in enantioselective multicomponent synthesis of secondary amines, the key problems, and a possible general solution.
(A) Synthesis of homoallylic α-secondary amines from N-protected imines and allenes as latent nucleophiles, while highly enantioselective, is limited in scope, requires inefficient N-deprotection, and is not stereodivergent. (B) (+)-Tangutorine might be prepared concisely without N-protection. (C) One-pot nucleophilic addition/ketimine reduction involving a nitrile would be more efficient. (D) While posing several challenges, enantioselective addition and diastereoselective C=N reduction would be effected in situ by simultaneously present Cu–B(pin) and Cu–H catalysts. PMP, p-methoxyphenyl; Fmoc, fluorenylmethyloxycarbonyl; pg, protecting group; pin, pinacolato.
The above shortcomings recently thwarted our efforts to conceive a scalable, diastereo- and enantioselective synthesis of tangutorine, a naturally occurring anti-cancer agent. Our initial plan involved the intermediacy of tertiary lactam a (Fig. 1B), to be derived from a protected form of homoallylic amine b. Removal of an N-protecting group, however, under acidic or basic conditions, proved to be compromising for two reasons: first, untimely and rapid cyclization to secondary amide c, which rendered subsequent N-alkyl formation severely inefficient; second, premature unmasking of the allylic alcohol, seriously complicating downstream chemoselective amide activation needed for generation of the polycyclic framework. It might therefore not be surprising that the only reported enantioselective synthesis of tangutorine (20) is 26 steps long (21), with seven operations relating to protecting group manipulations and another nine devoted to oxidation state alterations. We needed to access secondary amine d, probably through merger of unprotected amine e with the appropriate aldehyde; d could then be easily converted to cyclic lactam a, en route to tangutorine. The question thus became: how does one generate N-H amine e efficiently and with high distereo- and enantioselectivity?
Sequential additions to a nitrile.
An unprotected α-secondary amine might be synthesized by nucleophilic addition to a nitrile followed by reduction of the resulting N-H ketimine (Fig. 1C). Many nitriles are either commercially available (unlike aldimines) or can be accessed by an increasing number of protocols (22, 23). Additionally, aldehyde synthesis by oxidation or reduction of more readily available and robust starting materials, N-protection to generate a suitable aldimine, and subsequent unmasking would be obviated. Without an N-activating unit, enamine formation would be a much less likely pitfall.
The prerequisite for high diastereo- and enantioselectivity aside, the idea of synthesizing unprotected amines by sequential, one-pot, addition to a nitrile presented several challenges. Nitriles are generally considered inert; they are better known as ligands (24) for transition metals (including copper) than as electrophiles. After all, acetonitrile is a common solvent and the preferred medium for catalytic allyl additions to N-phenyl aldimines (25). Carboxylic esters, even sterically hindered ones, can be reduced selectively in the presence of a cyanide unit (26). To the best of our knowledge, nitriles are yet to be utilized in a catalytic enantioselective reaction with a carbon-based nucleophile.
Pursuant to our original studies with aldehydes and ketones serving as electrophiles, and as recently adopted for additions to N-protected aldimines (Fig. 1A), we chose to focus on multifunctional Cu–allyl complexes, conveniently accessed in situ by the addition of a Cu–B(pin) (pin, pinacolato) species to a monosubstituted allene (27, Fig. 1C). A related transformation would be applicable to the projected tangutorine synthesis (Fig. 1B). It would be preferable for the nucleophile to add first, as the alternative sequence of nitrile reduction/Cu–allyl addition would proceed via problematic N-H aldimines. It would be equally advantageous for the ketimine to be reduced before its reaction with another Cu–allyl molecule to give an achiral α-tertiary amine. There was furthermore the question of finding a way to reduce the ketimine intermediates diastereodivergently (f to g or h, Fig. 1C). We suspected that the boryl and the C=N units would be internally chelated, and the attendant structural rigidity would be conducive to diastereoselective reduction (addition from the less hindered C=N face to give g). Still, it was unclear whether we would be able to retain N→B chelation during ketimine reduction and, if so, how the alternative diastereomer h would then be accessed. Besides, although the large B(pin) (pin, pinacolato) moiety could help reduce the rate of addition of a second Cu–allyl complex, we were concerned that the Lewis acidic boryl group might facilitate epimerization at the sensitive α-stereogenic center of the β,γ-unsaturated ketimine (f, Fig. 1C).
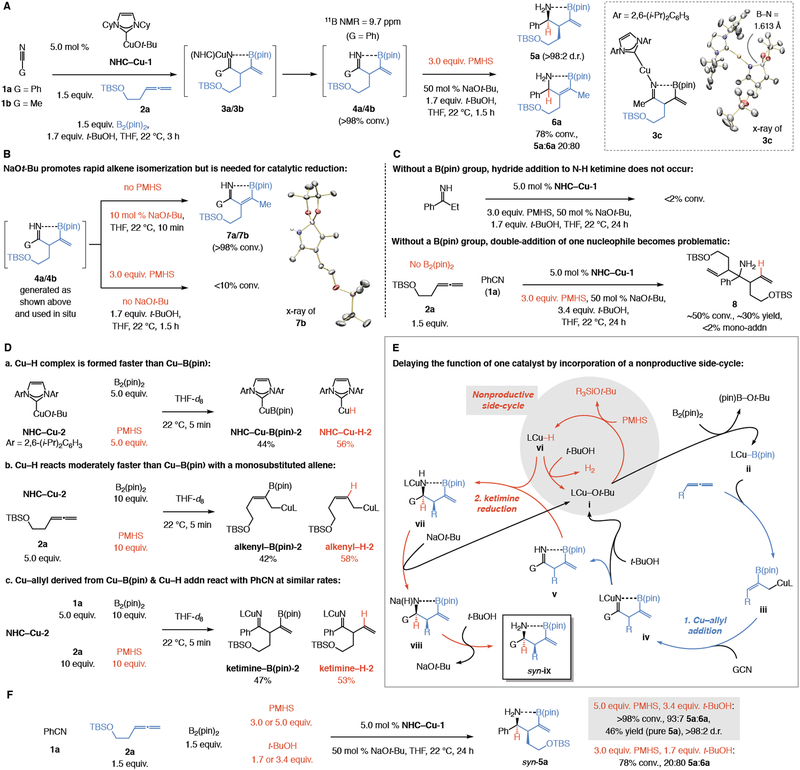
We began by examining the reactions involving phenyl cyanide or acetonitrile (1a or 1b; Fig. 2A), monosubstituted allene 2a, B2(pin)2, and t-BuOH together with 5.0 mol % of an NHC–Cu (NHC, N- heterocyclic carbene) copper complex (NHC–Cu-1). Ketimine 4a was formed after just three hours, indicating that a Cu–allyl complex can react readily with an aryl or an alkyl nitrile (1b). We later obtained the x-ray structure of Cu–ketimide 3c by reacting equivalent amounts of acetonitrile, NHC-Cu-2 (Ar = 2,6-(i-Pr)2 C6H3), B2(pin)2, and 2a (see Supplementary Materials for details). The 11B NMR spectrum for β-boryl ketimine 4a bears an upfield peak (9.7 ppm), which suggests a largely sp3-hybridized boron atom and C=N→B(pin) coordination.
Fig. 2. Managing the reactivity order of competing catalysts.
(A) Addition of a Cu–allyl complex to a nitrile is efficient, but ketimine reduction causes alkene isomerization/byproduct formation. (B) A metal alkoxide promotes olefin isomerization but is required for CuH-catalyzed ketimine reduction. (C) Without a neighboring boryl group, ketimine reduction is slow and double addition dominates. (D) Reactivities of a Cu–B(pin) and Cu–H complex are competitive, with Cu–H forming and reacting somewhat faster. (E) By incorporating a nonproductive side cycle, homoallylic amines might be generated more efficiently. (F) Relatively efficient formation of syn-5a is achieved by incorporating a side cycle. NHC, N-heterocyclic carbene; pin, pinacolato; TBS, t-butyldimethylsilyl; PMHS, polymethylhydrosiloxane. See the Supplementary Materials for details.
To probe the feasibility of efficient and diastereoselective ketimine reduction, we treated a sample of catalytically generated 4a (not isolable, detectable by spectroscopy) with inexpensive polymethylhydrosiloxane (PMHS; 3.0 equiv.), NaOt-Bu (50 mol%), and t-BuOH (1.7 equiv.; Fig. 2A), thus generating the corresponding Cu–H complex in situ. There was 78% conversion to a mixture of compounds after 1.5 hours, among which we detected a 20:80 mixture of the desired homoallylic amine 5a, generated as a single diastereomer (>98% syn), and allylic amine 6a (>98% E). In all likelihood, due to higher acidity of the allylic C–H in a β,γ-unsaturated ketimine, alkene isomerization occurs prior to C=N reduction; this is supported by swift isomerization of 4a and 4b (Fig. 2B) to α,β-unsaturated ketimines 7a and 7b, respectively (10 min., 22 °C; >98% E) with just 10 mol % NaOt-Bu. The identity of ketimine 7b was confirmed by X-ray crystallography (Fig. 2B). The latter findings imply that ketimine reduction must occur readily and under mild conditions, otherwise olefin isomerization becomes dominant. Another key finding was that there was minimal imine reduction when 4a or 4b were subjected to PMHS without any NaOt-Bu, showing that a metal alkoxide must be available for efficient regeneration of a Cu–H catalyst [i.e., LCu(H)N–C → LCu(Ot-Bu) → LCuH; see: vii → i → vi, Fig. 2E]. Follow-up investigations confirmed that, although Cu–H addition to a ketimine is fast, a metal alkoxide must be present for Cu–N bond cleavage and product release (see the Supplementary Materials for details).
Several other findings merit note. Unlike β-boryl ketimines 4a-b (or 7a-b), which underwent reduction easily, there was no reaction with the N-H ketimine derived from propiophenone (<2% under the same conditions, 24 h; Fig. 2C). This means that C=N→B(pin) chelation must be retained for facile reduction. Also, reaction of 1a and allene 2a in the absence of B2(pin)2 led to the formation of α-tertiary amine 8 (Fig. 2C); none of the single-addition product was generated (<2%). Consequently, without a B(pin) moiety double-addition becomes dominant, indicating that processes with only a Cu–H complex (that is, no Cu–B(pin) species; 28) cannot be used to synthesize these and related homoallylic amines (more on this later).
Delayed catalysis.
The capacity of a metal alkoxide to facilitate olefin isomerization (Fig. 2A–B) together with the necessity for CuH-catalyzed ketimine reduction (Fig. 2A) posed the question of how to obtain the desired homoallylic amines with minimal olefin isomerization. Early investigations (Fig. 2A) had shown that generating a Cu–H complex after the C–C bond is formed, despite NaOt-Bu being added last, leads to significant amounts of the allylic amine (6a; ~80%). However, the latter experiment involved a ten-fold excess of NaOt-Bu compared to the Cu–H catalyst (50 vs. 5.0 mol %), rendering alkene isomerization more competitive (vs. C=N reduction). Additionally, exploratory studies revealed that subjection of the intermediate ketimine to stoichiometric amounts of different reducing agents does not afford the desired syn isomer with high diastereoselectivity (vs. >98:2 diastereomeric ratio, d.r., with NHC–Cu–H) and/or is only moderately efficient (~50% yield; see the Supplementary Materials for additional details). We surmised that the low d.r. is probably because syn selectivity is possible only if hydride addition does not require the disruption of the N→B chelation; subsequent studies later verified this hypothesis (see below). In searching for a solution, we posited that if the Cu–B(pin) and Cu–H complexes were generated concurrently, the relatively rapid CuH-catalyzed ketimine reduction could occur as soon as the β,γ-unsaturated ketimine was formed. Ketimine reduction would then have to be faster than alkene isomerization, despite increased metal alkoxide availability.
It was unclear whether the relative rates of formation and reactivity of a Cu–B(pin) and a Cu–H complex would favor the desired sequence of events. To understand these factors better, we performed the experiments shown in Fig 2D (conditions reflect the catalytic process in Fig. 2A). We determined that with equal amounts of B2(pin)2 and PMHS, a Cu–H complex is generated somewhat faster than a Cu–B(pin) species (Fig. 2D, a) and that it adds somewhat more readily to a monosubstituted allene (Fig. 2D, b). Neither factor is favorable to the desired sequence of events (i.e., Cu–B(pin) addition to allene, followed by CuH-catalyzed reduction). Still more discouraging, the Cu–allyl species derived from Cu–H addition to 2a added to PhCN (1a) at nearly the same rate as the Cu–allyl intermediate formed by Cu- B(pin) addition to the same allene (Fig. 2D, c). Similar observations were later made when the same investigations involved a bis-phosphine-Cu complex (see below).
We therefore needed to conceive of a strategy that would favor Cu–B(pin) addition to the allene even in the presence of the Cu–H species. Despite many examples of multi-catalytic transformations (29), those involving catalysts that possess competing functions are relatively uncommon (30, 31), especially when the one that is formed more slowly and is less reactive must nonetheless participate first.
Use of excess B2(pin)2 (vs. PMHS) would not favor Cu–B(pin) (vs. Cu–H) addition to an allene; this would only slow down ketimine reduction and cause more alkene isomerization. Instead, we envisioned modulating the relative rate of Cu–H versus Cu–B(pin) addition to an allene by exploiting the efficiency with which the metal hydride can react not only with a ketimine but also – in a nonproductive manner – with an alcohol. By feeding the reaction mixture sufficient amounts of inexpensive t-BuOH and PMHS, neither of which react rapidly with Cu–B(pin), we sought to engage the Cu–H complex in a side-cycle (see i → vi → i, Fig. 2E). We argued that this could raise the relative Cu–B(pin) concentration sufficiently to favor its addition to allene (ii → iii) and onwards to nitrile (→ iv). The success of this plan hinged on whether reaction of Cu–H with the alcohol would preempt its undesired addition to the allene but still allow its reaction with ketimine to out-compete alkene isomerization. Otherwise, higher PMHS concentration would simply favor reaction between the allene and the Cu–H complex. Subsequent protonolysis and CuH-catalyzed ketimine reduction would give vii followed by viii en route to syn-ix (Fig. 2E).
Treatment of PhCN (1a), 2a, and B2(pin)2, with 5.0 mol % NHC–Cu-1 and 50 mol % NaOt-Bu along with 3.4 equivalents of t-BuOH (vs. 1.7 equiv.) and 5.0 equivalents of PMHS (vs. 3.0 equiv.) afforded homoallylic amine syn-5a in 46% yield and >98:2 d.r. (Fig. 2F). There was just 7% (vs. 80%) of the isomerized byproduct formed (6a, readily separable from 5a; see below for discussion on diastereoselectivity). Although more PMHS leads to higher Cu–H concentration, concomitant increase in alcohol concentration stimulates the nonproductive side cycle, allowing Cu–B(pin) addition to the allene to compete effectively. The combined influence of higher PMHS and t-BuOH amounts was confirmed by the fact that with only excess silane (5.0 equiv. vs 1.7 equiv. t-BuOH), nearly 50% of the product mixture consisted of 6a, and there was 30 to 35% of unreacted nitrile (insufficient alcohol to feed the entire process). When only more t-BuOH was used but the amount of silane was kept the same (3.4 equiv.; 3.0 equiv. PMHS), a 66:34 ratio of allylic:homoallylic amine was observed (5a:6a); under these conditions the process slows down, allowing for increased olefin isomerization (more on this later). A control experiment corresponding to that shown in Fig. 2Da, but involving larger amounts of PMHS (vs. B2(pin)2 to emulate the catalytic conditions) showed that formation of a Cu–H complex is significantly more efficient under such conditions (15:85 Cu–B(pin):Cu–H; see the Supplementary Materials for details). That is, the nonproductive side cycle delays the action of a Cu-based complex that is clearly formed faster.
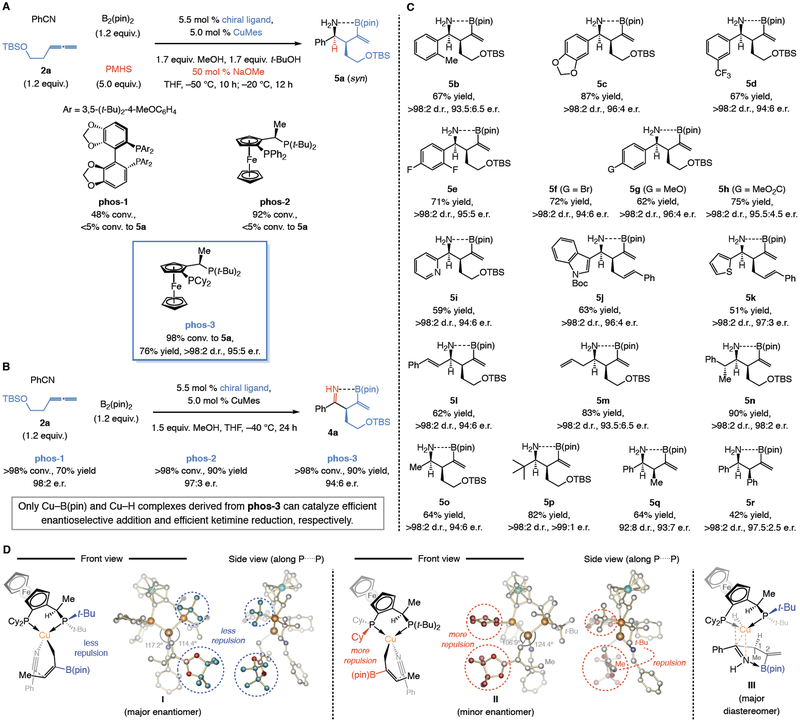
We then set out to identify an effective chiral ligand with the hopes that we would also improve efficiency (vs. NHC–Cu-1, Fig. 2F). With benzodioxole-based phos-1 (Fig. 3A) and ferrocenyl-based phos- 2 there was appreciable nitrile consumption (48% and 92% conv., respectively), but only different byproducts, principally α,β-unsaturated ketimine 7a, were formed (<5% 5a). In contrast, with phos-3, which bears a PCy2 coordination site (vs. PPh2) but is otherwise identical to phos-2, 5a was isolated in 76% yield (vs. 46% yield with NHC–Cu-1), >98:2 syn:anti ratio, and 95:5 e.r. (enantiomeric ratio). We could detect only 2% allylic amine 6a.
Fig. 3. Multi-catalytic four-component diastereo- and enantioselective processes.
(A) Synthesis of unprotected homoallylic α-secondary amines is most efficient with phos-3. (B) Whereas other chiral Cu complexes promote C–C bond formation enantioselectively, only phos-3–Cu–H is efficient in ketimine reduction. (C) The catalytic process has considerable scope. (D) DFT calculations (MN15/def2-TZVPP//M06L/def2-SVP) provide a stereochemical model for the observed diastereo- and enantioselectivities. pin, pinacolato; TBS, t-butyldimethylsilyl; PMHS, polymethylhydrosiloxane; Mes, 2,4,6-trimethyphenyl (mesityl). *Catalyst loading was 2.0 mol %. See the Supplementary Materials for details.
Most achiral or chiral NHC- and bisphosphine–Cu–B(pin) complexes facilitated C–C bond formation, but those that also gave rise to a Cu–H complex capable of efficient ketimine reduction were far less common; the boryl and hydride species derived from NHC–Cu-1 and phos-3 are two such instances. The three bisphosphine–Cu complexes catalyze C–C bond generation efficiently and enantioselectively (to give 4a; Fig. 3B), but it is only with phos-3–Cu–H that catalytic reduction is efficient (to give 5a). This may be attributed to higher Lewis basicity of dialkylphosphine moieties and enhanced reducing ability of the derived Cu–H species (vs. one dialkylphosphine and one diarylphosphine, phos-2).
The method is widely applicable (Fig. 3C). Aryl-substituted homoallylic amines (5b-h) were isolated in 62–87% yield with complete syn selectivity (>98:2 d.r.), and 93.5:6.5 to 96:4 e.r. Nitriles containing a sterically demanding o-tolyl moiety (5b), one or more halides (5e-f), or a strongly electron donating or withdrawing group (5g and 5h, respectively) proved to be suitable substrates. In contrast to a carboxylic ester, however, an aldehyde competitively reacts with a Cu–allyl species; a ketone, while remaining intact during the first stage of the process, is competitively reduced. Heterocyclic nitriles may be used (5i-k). A Lewis basic pyridyl ring, even with the ring nitrogen at the ortho position (see 5i), where it can serve as part of a bidentate Cu-based chelate with the C=N unit, does not negatively impact yield or stereoselectivity. Other starting materials of note consisted of an α,β-unsaturated nitrile (5l), a β,γ-unsaturated nitrile (5m), and an enantiomerically pure benzyl (5n) compound; in the latter instance, reaction with the alternative (mis-matched) substrate enantiomer was less efficient and stereoselective (53% yield of partially pure homoallylic amine, 91:9 d.r., 83:17 e.r. vs. 90% yield, >98:2 d.r., 98:2 e.r. for 5n). Reactions with acetonitrile (5o) or the much larger tert-butyl nitrile (5p) were similarly high yielding and selective. Allenes containing a smaller methyl group could be used (5q), and although the reaction with phenylallene afforded 5r in 42% yield, diastereoselectivity was complete (>98:2 d.r.) and enantioselectivity high (97:3 e.r.). In most cases, ≤8% of the allylic amine products were observed (% yield values in Fig. 3 correspond to pure homoallylic products). Accessing 5l-n is especially useful, as the corresponding (N-protected or otherwise) aldimines are difficult to use due to their instability (4, 32), namely, dimerization by cycloaddition (33) for 5l, alkene isomerization for 5m, and enamine formation for 5n. We know of just two examples of catalytic enantioselective nucleophilic addition to a benzyl- substituted aldimine (34, 35).
A rationale for the high diastereo- and enantioselectivity may be proposed based on stereochemical models derived from DFT calculations (Fig. 3D). In the transition state leading to the major ketimine enantiomer (I), while the nitrile associates from the rear of the copper-based complex, the allyl nucleophile is oriented such that the sterically demanding B(pin) group can be situated below a t-butyl group of the P(t-Bu)2 moiety. As shown in the side view projection of I (Fig. 3D), this causes the t-butyl group that is closer to the (pin)B-substituted allyl unit to tilt upwards. The less favorable transition state II suffers from steric repulsion between the B(pin) group and a cyclohexyl (Cy) substituent of the PCy2 moiety. Further destabilizing II is the interaction between the alkenyl substituent (Me) and a proximal t-butylphosphine moiety, which causes contraction of the C-Cu-P angles (106.9° and 124.2° vs. 117.2° and 114.2° in I). The major diastereomer arises from Cu–H addition to the less hindered face of a boryl-chelated ketimine, similar to the way such a complex would react with an alkene, namely without any interaction between the transition metal and the nitrogen atom (iii, Fig. 3D).
Achieving stereodivergency.
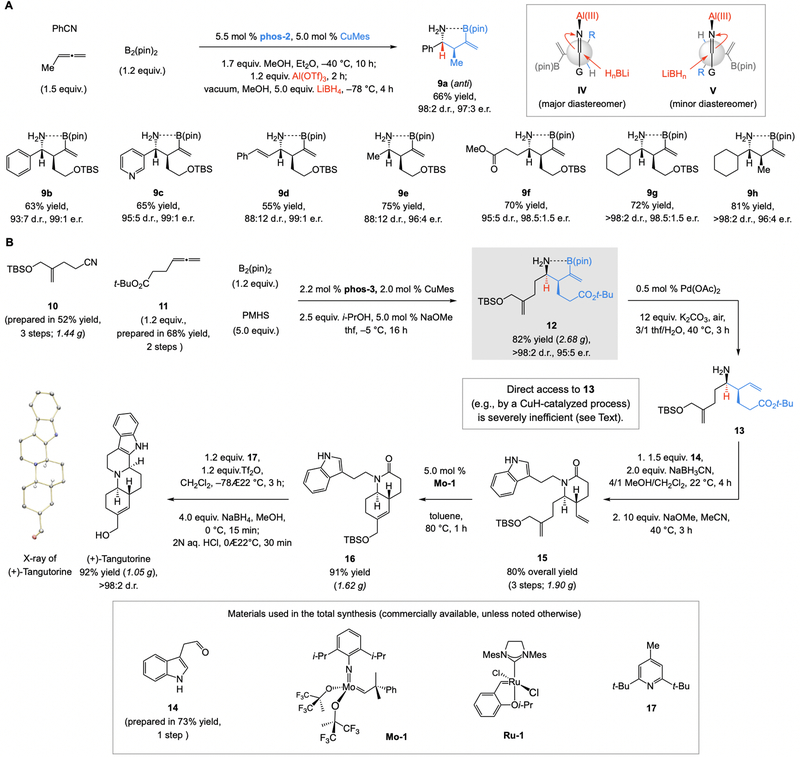
Since syn-selective ketimine reduction proceeds via an internally chelated ketimine, the alternative anti diastereomer would have to be generated by disruption of the same interaction. We reasoned that by using a Lewis acid capable of rupturing N→B chelation with ensuing ketimine reduction, we might access the desired homoallylic amine diastereomers. The same B(pin) group, this time as the largest substituent in a Felkin-Anh type addition mode (IV, Fig. 4A), would again play a key role. Screening studies with phos-2 as the chiral ligand (for maximal efficiency and e.r. vs. phos-3; see Fig. 3B) led us to determine that Al(OTf)3 and LiBH4 are an effective Lewis acid/metal hydride combination. Accordingly, anti-homoallylic amines 9a-h (Fig. 4A) were obtained in 55 to 81% yield, 88:12 to >98:2 d.r., and 96:4 to 99:1 e.r. While the high yield for 9f is striking, considering the ability of LiBH4 to reduce a carboxylic ester, the lower d.r. for the reaction of the less bulky nitriles might be due to a competing mode of addition (see V, Fig. 4A). There were no isomerized allylic amine byproducts detected (<2%).
Fig. 4. Scope and utility.
(A) By altering the conditions for ketimine reduction, the anti-allylic N-H amines can be accessed. (B) Utility is highlighted by a nine-step diastereo- and enantioselective gram-scale synthesis of tangutorine, affording the natural product in 28% overall yield (vs. 26 steps and 10% overall yield, previously). pin, pinacolato; TBS, t-butyldimethylsilyl; PMHS, polymethylhydrosiloxane, Mes, 2,4,6-trimethyphenyl (mesityl). See the Supplementary Materials for details.
Use of CuMes (Mes, mesityl) (36), a robust and commercially available organometallic reagent that may be used without purification, in this protocol is preferable partly because of its higher solubility (vs. copper halides) in organic solvents, allowing for greater reproducibility. Additionally, since copper alkoxide is generated by reaction of CuMes with an alcohol, there is no need for additional amounts of sodium alkoxide that could give rise to alkene isomerization. This is particularly important in synthesis of anti-homoallylic amines, where formation of β,γ-unsaturated N-H ketimines must be completed before C=N reduction (see 9a-h, Fig. 4A). 2) Use of a combination of alcohols led to better yields of the syn-homoallylic amines (Fig. 2; vs. when only MeOH or t-BuOH was used). The smaller MeOH can induce faster protonolysis of the Cu–N bond (leading to the release of the N-H ketimine intermediate), but it can also react faster with the Cu–H complex, decreasing its concentration too rapidly in the side-cycle (Fig. 2E). The latter hypothesis is supported by the observation that there was partial ketimine reduction (~50%) when only MeOH was used. 3) For the synthesis of anti-homoallylic amines, it was necessary to remove Et2O and replace it with MeOH before adding LiBH4 in order to ensure optimal diastereoselectivity.
Application to natural product synthesis.
The final question was whether the advances described above might enable concise synthesis of gram quantities of diastereo- and enantiomerically enriched tangutorine. Multi-gram amounts of nitrile 10 (Fig, 4B) and allene 11 were prepared in three (52% overall yield) and two steps (68% overall yield), respectively, from commercially available starting materials. The two-catalyst, four-component process, carried out at reduced loading (2.0 mol %), afforded 2.68 grams of syn homoallylic amine 12 in 82% yield, >98:2 d.r., and 95:5 e.r. This transformation was performed at −5 °C (vs. −50 °C) because reactions of alkyl-substituted nitriles are generally less sensitive to variations in temperature; control experiments indicate that this might be because the derived ketimines are less prone to epimerization (vs. aryl- or heteroaryl-substituted cases). Furthermore, 5.0 mol % NaOMe, which can still cause considerable alkene isomerization if ketimine reduction is not facile, was sufficient under these conditions. Use of i-PrOH proved to be as effective as a MeOH/t-BuOH mixture (see above).
The boryl group was excised catalytically to furnish monosubstituted alkene 13 (Fig. 4B). Subsequent reductive coupling with aldehyde 14, prepared in a single step from tryptophol (80% yield), gave the desired alkylamine, which was used directly (without purification) to access 1.9 grams of 15 (80% overall yield for three steps from 12). Catalytic ring-closing metathesis with Mo-1 afforded 1.62 grams of 16 (91% yield). Several issues regarding these latter transformations (12 →16) merit brief note. 1) At first glance, it might appear that a more economical strategy for synthesis of 13 would entail a multicomponent process that includes Cu–H addition (vs. Cu–B(pin)) to a monosubstituted allene (i.e., no C–B-to-C–H conversion (i.e., 12→13)). In practice, however, this is not the case. Apart from inhibiting the addition of two identical allyl moieties (see Fig. 2C), without the sizeable B(pin) group and the internal N→B chelation, not only would ketimine reduction not occur alkene isomerization would dominate (see Fig. 2C, 3, and 4). In brief, the C–B bond is much more than a mere C–H placeholder. 2) The high efficiency of catalytic C–B-to-C–H conversion indicates that other types of cross-coupling are feasible and unlikely to be adversely affected by the presence of Lewis basic amine and/or adventitious lactam formation. We have already shown that oxidation of the alkenyl–B(pin) to the corresponding β-amino ketone in the presence of an N-H amine is high yielding (32). 3) As was noted (Fig. 1C), generating primary amine 13 under relatively neutral conditions – without cyclic lactam formation – was crucial (inefficient subsequent N-alkylation). The intermediacy of an N-protected variant of 13 would thus be undesirable. 4) Ring-closing metathesis proceeded to completion with Ru-1, but repeated purification of 16 was needed (to remove residual transition metal salts), resulting in diminished yields (65% vs. 91% yield with Mo-1).
Subjection of 16 to 17 and triflic anhydride (37, 38) and then NaBH4 in MeOH delivered 1.05 grams of the anti-cancer agent (92% yield) as a single diastereomer. The stereochemical identity of synthetic (+)-tangutorine was confirmed by x-ray crystallography (formerly only that of racemic material was reported, despite the absolute stereochemical identity being known). The nine-step route, involving commercially available ligands, catalysts and reagents, provided the final target in high diastereo- and enantiomeric purity and in 28% overall yield. This route, which is readily amenable to gram-scale synthesis, is nearly three times more concise and efficient than the formerly disclosed alternative (26-steps, 10% overall yield (21)). Although tangutorine belongs to an alkaloid family that occurs in nature typically in the racemic form, higher activity has been observed with enantiomerically enriched material (21). Moreover, separation of tangutorine enantiomers requires HPLC (high performance liquid chromatography) techniques (39), and is therefore non-trivial, especially on larger scale.
Outlook.
The ability to prepare multifunctional homoallylic amines efficiently, stereodivergently, and enantioselectively, by the use of readily available catalysts, directly from nitriles, and without any protection/deprotection steps will have substantial impact on the accessibility of bioactive N-containing compounds. We expect the approach to be applicable to protocols for synthesis of other types of unprotected amines through the use of alternative classes of organocopper compounds. For instance, diastereodivergent additions of carbon-based nucleophiles to enantiomerically enriched ketimines would provide easy access to α-tertiary homoallylic amines, valuable entities that can be generated by only a small number of protocols (32, 40). Ketimine hydrolysis may lead to otherwise difficult-to-prepare enantiomerically enriched β,γ-unsaturated ketones. In a broader sense, this may be the first step towards designing transformations that involve multiple catalysts, where the sequence with which they are required to enter the reaction fray differs from what would be expected on the basis of their inherent reactivity. In a broader sense, controlling the order in which different catalysts react productively by selectively delaying one of them with a nonproductive side-cycle is a concept that could find widespread use in future research in reaction development.
Supplementary Material
ACKNOWLEDGMENTS
Funding: Financial support was provided by the NIH (grants GM-47480 and GM-57212) and the Alfonso Martin Escudero Foundation (postdoctoral fellowship to J. d. P.). We thank Dr. B. Li for his invaluable assistance in securing X-ray structures.
Footnotes
Competing Interests: The authors declare no competing financial interest.
Data and materials availability: X-ray crystallographic data for compounds 3b, 3c, 6a, 7b, 9e, and synthetic (+)-tangutorine, are freely available from the Cambridge Crystallographic Data Centre (CCDC 1885227, 1885257, 1885259, 1885233, 1885258, and 1885261, respectively). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are available in the main text or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
Materials and Methods
Figs S1–S8
References (42–85)
X-ray structures
NMR spectra
References
- 1.Yus M, González-Gómez J, Foubelo F, Chem. Rev 111, 7774–7854 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Defieber C, Ariger MA, Moriel P, Carreira EM, Angew. Chem. Int. Ed 46, 3139–3143 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Nagano T, Kobayashi S, J. Am. Chem. Soc 131, 4200–4201 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Hou at al G. J. Am. Chem. Soc 131, 9882–9883 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Pouy MJ, Stanley LM, Hartwig JF, J. Am. Chem. Soc 131, 11312–11313 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison RJ, Hoveyda AH, Angew. Chem. Int. Ed 57, 11654–11661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger R, Rabbat RPMA, Leighton JL, J. Am. Chem. Soc 125, 9596–9597 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Wada R, et al. , J. Am. Chem. Soc 128, 7687–7691 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Mandai H, Mandai K, Snapper ML, Hoveyda AH, J. Am. Chem. Soc 130, 17961–17969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakrabarti A, Konishi H, Yamaguchi M, Schneider U, Kobayashi S, Angew. Chem. Int. Ed 49, 1838–1841 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Crossley SWM, Shenvi RA, Chem. Rev 115, 9465–9531 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Sugiura M, Hirano K, Kobayashi S, J. Am. Chem. Soc 126, 7182–7183 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Sebelius S, Wallner OA, Szabó KJ, Org. Lett 5, 3065–3068 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Hepburn HB, Chotsaeng N, Lam HW, Angew. Chem. Int. Ed 51, 8309–8313 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Yeung K, Ruscoe RE, Rae J, Pulis AP, Procter DJ, Angew. Chem. Int. Ed 55, 11912–11916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S-L, Wong ZL, Buchwald SL, Nature 532, 353–356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krautwald S, Carreira EM, J. Am. Chem. Soc 139, 5627–5639 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Shimizu M, Kimura M, Watanabe T, Tamaru Y, Org. Lett 7, 637–640 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Yeung K, Talbot FJT, Howell GP, Pulis AP, Procter DJ, ACS Catal. 9, 1655–1661 (2019). [Google Scholar]
- 20.Duan J-A, Williams ID, Che C-T, Zhou R-H, Zhao S-X, Tetrahedron Lett. 40, 2593–2596 (1999). [Google Scholar]
- 21.Nemoto T, et al. Org. Lett 12, 872–875 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Anbarasan P, Schareina T, Beller M, Chem. Soc. Rev 40, 5049–5067 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Najam T, et al. Inorg. Chim. Acta 469, 408–423 (2018). [Google Scholar]
- 24.Rach SF, Kühn FE, Chem. Rev 109, 2061–2080 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Iwamoto S, Nagayama S, Synlett 1099–1101 (1997). [Google Scholar]
- 26.White JD, Quaranta L, Wang G, J. Org. Chem 72, 1717–1728 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Meng F, Jang H, Jung B, Hoveyda AH, Angew. Chem. Int. Ed 52, 5046–5051 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu RY, Yang Y, Buchwald SL, Angew. Chem. Int. Ed 55, 14077–14080 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooperative Catalysis: Designing Efficient Catalysts for Synthesis, Peters R, Ed. (Wiley–VCH: New York, 2015). [Google Scholar]
- 30.Manville N, Alite H, Haeffner F, Hoveyda AH, Snapper ML, Nat. Chem 5, 768–774 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang M, et al. J. Am. Chem. Soc 140, 10593–10601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang H, Romiti F, Torker S, Hoveyda AH, Nat. Chem 9, 1269–1275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassberger MA, Horvath A, Schulz G, Tetrahedron Lett. 32, 7393–7396 (1991). [Google Scholar]
- 34.Wu H, Haeffner F, Hoveyda AH, J. Am. Chem. Soc 136, 3780–3783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shikora JM, Chemler SR, Org. Lett 20, 2133–2137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stollenz M, Meyer F, Organometallics 31, 7708–7727 (2012). [Google Scholar]
- 37.Movassaghi M, Hill MD, Org. Lett 10, 3485–3488 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie C, et al. Org. Lett 20, 2386–2390 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Putkonen T, Tolvanen A, Jokela R, Caccamese S, Parrinello N, Tetrahedron 59, 8589–8595 (2003). [Google Scholar]
- 40.Tran DN, Cramer N, Angew. Chem. Int. Ed 49, 8181–8184 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Laitar DS, Müller P, Sadighi JP, J. Am. Chem. Soc 127, 17196–17197 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Mankad NP, Laitar DS, Sadighi JP, Organometallics 23, 3369–3371 (2004). [Google Scholar]
- 43.Crabbé P, Fillion H, André D, Luche JL, J. Chem. Soc. Chem. Commun 859–860 (1979). [Google Scholar]
- 44.Meng F, McGrath KP, Hoveyda AH, Nature 513, 367–374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung B, Hoveyda AH, J. Am. Chem. Soc 134, 1490–1493 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemmen TH, et al. Inorg. Chem 29, 3680–3685 (1990). [Google Scholar]
- 47.Tsuda T, Hashimoto T, Saegusa T, J. Am. Chem. Soc 94, 658–659 (1972). [Google Scholar]
- 48.Tsuda T, Yazawa T, Watanabe K, Fujii T, Saegusa T, J. Org. Chem 46, 192–194 (1981). [Google Scholar]
- 49.Lee J, Radomkit S, Torker S, del Pozo J, Hoveyda Nat AH. Chem. 10, 99–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrara SJ, Burton JW, Chem. Eur. J 22, 11597–11600 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miura K, Itoh D, Hondo T, Hosomi A, Tetrahedron Lett. 35, 9605–9608 (1994). [Google Scholar]
- 52.Zang Z-L, et al. Org. Lett 18, 5014–5017 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Werkhoven PR, et al. Org. Biomol. Chem 14, 701–710 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Younai A, Zeng B-S, Meltzer HY, Scheidt KA, Angew. Chem. Int. Ed 54, 6900–6904 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Cramer CJ, Truhlar DG, Phys. Chem. Chem. Phys 11, 10757–10816 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Peverati R, Truhlar DG, Phil. Trans. R. Soc. A 372, 20120476 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Mardirossian N, Head-Gordon M, Chem J. Theory Comput. 12, 4303–4325 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Mardirossian N, Head-Gordon M, J. Chem. Phys 144, 214110 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Brauer B, Kesharwani MK, Kozuch S, Martin JM, Phys. Chem. Chem. Phys 18, 20905–20925 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Weymuth T, Couzijn EPA, Chen P, Reiher M, Chem J. Theory Comput. 10, 3092–3103 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Truhlar DG, Tang M, Chem J. Theory Comput. 9, 3965–3977 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Yu HS, He X, Li SL, Truhlar DG, Chem. Sci 7, 5032–5051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinmetz M, Grimme S, ChemistryOpen 2, 115–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goerigk L, Kruse H, Grimme S, ChemPhysChem 12, 3421–3433 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Frisch MJ, et al. Gaussian 09, Revision D.01, Gaussian, Inc, Wallingford CT, 2009. [Google Scholar]
- 66.Zhao Y, Truhlar DG, Acc. Chem. Res 41, 157–167 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Weigend F, Ahlrichs R, Phys. Chem. Chem. Phys 7, 3297–3305 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Weigend F, Phys. Chem. Chem. Phys 8, 1057–1065 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Marenich AV, Cramer CJ, Truhlar DG, J. Phys. Chem. B 113, 6378–6396 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Page M, McIver JW Jr., J. Chem. Phys 88, 922–935 (1988). [Google Scholar]
- 71.Page M, M.; Doubleday C Jr. Jr., McIver JW Jr., J. Chem. Phys 93, 5634–5642 (1990). [Google Scholar]
- 72.Torker S, Merki D, Chen P, J. Am. Chem. Soc 130, 4808–4814 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Minenkov Y, Occhipinti G, Singstad A, Jensen VR, Dalton Trans. 41, 5526–5541 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Minenkov Y, Occhipinti G, Jensen VR, Organometallics 32, 2099–2111 (2011). [Google Scholar]
- 75.Khan RKM, Torker S, Hoveyda AH, J. Am. Chem. Soc 136, 14337–14340 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Torker S, Koh MJ, Khan RKM, Hoveyda AH, Organometallics 35, 543–562 (2016). [Google Scholar]
- 77.Mikus MS, Torker S, Hoveyda AH, Angew. Chem. Int. Ed 55, 4997–5002 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Xu C, Liu Z, Torker S, Shen X, Xu D, Hoveyda AH, J. Am. Chem. Soc 139, 15640–15643 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Zhou Y, Shi Y, Torker S, Hoveyda AH, J. Am. Chem. Soc 140, 16842–16854 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Huang Y, del Pozo J, Torker S, Hoveyda AH, J. Am. Chem. Soc 140, 2643–2655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chai J-D, Head-Gordon M, Phys. Chem. Chem. Phys 10, 6615–6620 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Lichtenberger DL, Gladysz JA, Organometallics 33, 835–835 (2014). [Google Scholar]
- 83.Wagner JP, Schreiner PR, Angew. Chem. Int. Ed 54, 12274–12296 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Chen L, Ren P, Carrow BP, J. Am. Chem. Soc 138, 6392–6395 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Albers L, Rathjen S, Baumgartner J, Marschner C, Müller T, J. Am. Chem. Soc 138, 6886–6892 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.