Abstract
Background
Kinesin-related gene diversity among strains and species of Leishmania may impact the sensitivity and specificity of serodiagnostic tests for visceral leishmaniasis (VL).
Methods
In this study, we report on the recombinant expression of this novel Iranian Leishmania infantum (MCAN14/47) homologue of rK39 (Li-rK39), in L. tarentolae. The diagnostic potential of the Li-rK39 antigen was evaluated in an ELISA, using sera from 100 VL patients, 190 healthy endemic controls, 46 non-endemic healthy controls and 47 patients with other infections.
Results
The results showed a sensitivity of 96% and a specificity of 93.8%. A commercial rK39 immunochromatographic test (ICT) was 90% sensitive and 100% specific on the same cohort.
Conclusions
Here, we show that the K39 gene from an Iranian L. infantum isolate is heterozygous as compared to the sequence of the Brazilian L. infantum (former L. chagasi), whose antigen is incorporated in most rK39-based immunochromatographic tests. Therefore, Li-rK39 has the potential to be used as an alternative for VL diagnosis in Iran.
Keywords: rK39, Leishmania, Serodiagnosis, Visceral leishmaniasis, Eukaryotic expression
Background
Visceral leishmaniasis (VL) or kala-azar is a neglected parasitic disease with an annual worldwide incidence of 500,000 human cases, caused by an obligate intracellular parasite belonging to the Leishmania donovani complex [1–3]. Early diagnosis and intervention are critical for symptomatic patients since the disease is lethal if left untreated [4]. The definitive diagnosis of VL is based on the visualization of the parasite in Giemsa-stained smears, obtained from the spleen, liver, bone marrow or lymph node. The main problem with parasitological diagnosis is its variable sensitivity and invasiveness [5, 6]. During a VL infection, substantial amounts of specific antibodies are produced that are not protective but can serve as a marker of ongoing or past infection with Leishmania sp. Accordingly, different serological tests such as immunofluorescence assay (IFAT), direct agglutination test (DAT), and enzyme-linked immunosorbent assay (ELISA) have been developed. Problems with IFAT, DAT and some ELISAs that use whole Leishmania parasites or crude extracts are batch to batch variation, the need for equipment and, most importantly, limited sensitivity [7–10]. Previous meta-analysis reported a sensitivity of 88% for IFAT, 87% for ELISA and 94% for DAT [11]. However, a study conducted in Iran, the region of the present study, reported a sensitivity of 70.5% for DAT [12].
The development of the recombinant antigen rK39 by Burns et al. [13] significantly contributed to the improvement of VL diagnosis. rK39 is a protein containing 39 amino acid repeats derived from a conserved region within a gene coding for a kinesin-related protein of L. infantum (former L. chagasi). Either using ELISA or simple immunochromatographic tests, high levels of antibodies against K39 are detectable in the blood of VL patients but not in those with mucosal or cutaneous leishmaniasis [10, 14–16]. Compared to other serological tests and test formats, rapid diagnostic tests (RDT) based on rK39 have the advantages of being fast, simple without the need for equipment and visual reading of the result [8, 10]. Nonetheless, rK39 RDTs show varying diagnostic performance in different parts of the world and even within a single region if heterogeneous Leishmania parasite populations are present [17]. Meanwhile, polymorphisms of the kinesin-related gene in various strains of L. donovani in different regions of the world may explain the discrepancies in sensitivity of the rK39 antigen in different serodiagnostic tests [18, 19].
Recombinant protein expression systems have been developed in prokaryotic organisms like bacteria and in eukaryotic cells and organisms such as yeast, mammalian, insect and plant cells, and protozoa like L. tarentolae, yet all have their advantages and drawbacks [20]. A major shortcoming of heterologous recombinant expression is codon bias and the processing of post-translational modifications [21]. Leishmania tarentolae, a trypanosomatid protozoan parasite of the Moorish gecko Tarentola mauritanica, was genetically engineered as a eukaryotic expression system (Leishmania expression system, LEXSY) for the production of recombinant proteins [22]. So far, rK39 and rK39-like antigens have been produced in heterologous expression systems [13, 23, 24]. We hypothesized that using L. tarentolae for expression of rK39 antigens derived from endemic Leishmania species would be advantageous in terms of codon usage and post-translational processing of the recombinant protein. Thus, the present study was undertaken to develop an alternative rK39 (Li-rK39), derived from an Iranian L. infantum strain and expressed in L. tarentolae, for serological diagnosis of VL in endemic regions of Iran.
Methods
Specimen collection
A total of 383 sera from 100 VL patients, 190 endemic healthy controls, 46 non-endemic healthy controls and 47 non-VL patients were included. Five ml of venous blood were collected in plain tubes to prepare serum. VL patients consisted of 48 male and 52 female children of 1 month to 16 years-old, all referred from VL-endemic regions and admitted to Nemazee Hospital, Shiraz University of Medical Sciences, Shiraz, Iran. All patients had ≥ 14 days’ fever, 88 had hepatosplenomegaly and 92 had anaemia (Hb < 11g/l). They were all positive in IFAT (titer > 64). After treatment with either antimonial or amphotericin B therapy, they were all cured with decreased spleen size. Endemic healthy controls were 190 children (55% male and 45% female) with age range of 1–16 years from endemic areas for VL in southern Iran, with no clinical symptoms and no history of VL. Non-endemic healthy controls included 46 individuals (50% male and 50% female) with an age range of 15–35 years, originating from non-endemic regions. Non-VL patients (n = 47) suffered from other infections: toxoplasmosis (n = 10); malaria (n = 10); cutaneous leishmaniasis (n = 11); fascioliasis (n = 7); and hydatidosis (n = 9). All sera from the endemic healthy controls, non-endemic healthy controls and non-VL patients were negative in IFAT (titer < 64).
rK39 rapid diagnostic test
The test was conducted according to the manufacturer’s instructions (Kalazar Detect™ Rapid Test for VL, InBios, Seattle, USA).
Leishmania infantum strain, DNA isolation and PCR amplification
A L. infantum strain (MCAN/IR/14/M14) was isolated from a domestic dog in Meshkin-Shahr area from north-western Iran in 2015 [25]. Promastigotes were cultured in 10 ml of HOMEM medium (GE Healthcare) supplemented with 10% heat-inactivated fetal calf serum and incubated at 26 °C. Pellets of 109 L. infantum promastigotes were washed and suspended in PBS and genomic DNA was extracted using QIAamp Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. DNA quantity was measured using NanoDrop spectrophotometer (NanoDrop ND-1000 UV-Vis spectrophotometer, NanoDrop Technologies, Wilmington, DE, USA).
Construction of the recombinant expression vector
An analogous fragment of the K39 gene, as described by Burns et al. [13], was amplified from the Iranian L. infantum strain, using a forward primer selected to bind to the upstream non-repetitive part of GenBank sequence L07879, and a reverse primer able to target within most of the repetitive 117 bp regions (Table 1) [13]. As required for In-Fusion cloning, each primer was modified at the 5’-end by addition of a 15-bp sequence including a restriction site complementary to the place of integration in the expression vector, pLEXSY-hyg2 (Jena Bioscience, Jena, Germany). Using XbaI-rK39 F and KpnI-rK39 R primers, integration in pLEXSY-hyg2 provided a L. mexicana signal peptide at the N-terminal and added a hexa histidine-tag at the C-terminal, thus allowing IMAC purification. For cytoplasmic expression in pLEXSY-hyg2, the M14/47 sequence was re-cloned from pLEXSY-hyg2-M14/47 using NcoI-rK39 F and KpnI-rK39 R primers. This reconfiguration to pLEXSY-hyg2-CYTO-M14/47 cleaved off the existing signal peptide at the N-terminal, and provided a NcoI start codon. PCR reactions were performed in a final volume of 20 µl including 4 µl of 5× Phusion buffer (NEB), 200 µM of dNTPs (Eurogentec, Seraing, Belgium), 0.5 µM of each primer, 0.5 U of Phusion DNA polymerase (NEB), 4 µl of Q-solution 5× (Qiagen) and 10 ng genomic DNA. The final PCR program was run as follows: 1 cycle of 98 °C for 10 s; 35 cycles of 98 °C for 10 s, 67 °C for 30 s, 72 °C for 1 min; 1 cycle of 72 °C for 5 min. PCR products were separated on 1.5% agarose gels by electrophoresis and visualized, using ethidium bromide staining.
Table 1.
Primers used in the PCR
| Primer | Configuration | Sequence (5′–3′) |
|---|---|---|
| XbaI-rK39 F | Secreted | CTGGCGCCTCTCTAGAGCTCGCAACC |
| NcoI-rK39 F | Cytoplasmic | ACCAGATCTGCCATGGTCGCAACCGAGTGGGAGGA |
| KpnI-rK39 R | Hexa-histag | TGGTGATGGTGGTGGGTACCACTCGCCAGCTCC |
Notes: Nucleotides in italics, sequence complementary to pLEXSY-hyg2; nucleotides in bold, restriction site; nucleotides underlined, K39 sequence from GenBank (L07879)
PCR products were purified, using the NucleoSpin PCR Clean-Up Kit (Macherey-Nagel, GmbH & Co. KG, Germany), according to the manufacturer’s instructions. Next, the purified amplicons were integrated into a XbaI or NcoI and KpnI double digested pLEXSY-hyg2 vector, using In-Fusion recombinase. Finally, the completed vector was transformed into Stellar™ Competent Cells (Takara Bio Company, CA, USA) by heat shock and plated on Luria Bertani (LB) agar supplemented with 100 µg/ml carbenicillin at 37 °C overnight. At least 48 isolated Escherichia coli colonies were screened using colony PCR with primers that surround the site of integration in the pLEXSY vector (P1442 and A264). Using gel electrophoresis, all colonies that contained inserts larger than 300 bp were selected for further subculture in 5 ml LB medium overnight. Plasmids were harvested using the QIAprep Spin Miniprep (Qiagen) and sent for bi-directional sequencing (VIB Genetic Sequencing Facility, Antwerp, Belgium) using the P1442 and A246 primers. Next, translated protein sequences of the K39 gene were analysed by MUSCLE alignment with GenBank: L07879 reference sequence in CLC Sequence Viewer (Qiagen).
Cultivation and transfection of L. tarentolae
Leishmania tarentolae strain P10 was cultivated in brain heart infusion (BHI) medium supplemented with hemin and penicillin-streptomycin. pLEXSY-hyg2-CYTO-M14/47, was linearized by SwaI digestion and concentrated to 330 ng/µl by QIAquick PCR purification kit (Qiagen). Transfections were performed with 10 µg of linearized plasmid via electroporation using the high voltage protocol on freshly passaged promastigotes, according to the Jena Bioscience manual [22]. Transgenic LEXSY strains were generated by polyclonal selection in BHI suspension culture, using 100 µg/ml hygromycin, after they were maintained under hygromycin pressure.
Purification and characterization of recombinant protein
Production of recombinant protein Li-rK39 was carried out in 500 ml BHI medium, supplemented with 1.5% yeast extract [26], 2 g/l glucose [27], 100 µg/ml hygromycin and 0.2% hemin. After 72 h, when the OD600 reached 4, the cells were harvested by centrifugation for 10 min at 7500×g at 4 °C. The pellet was re-suspended in 50 ml of binding buffer (20 mM phosphate buffer pH 7.4, 500 mM NaCl, 20 mM imidazole), supplemented with 3 tablets of a protease inhibitor cocktail (Cat No. 05892791001, Roche Diagnostics, Mannheim, Germany), and lysed by five freeze-thaw cycles using liquid nitrogen, followed by 30 s sonication. After centrifugation for 30 min at 15,000×g at 4 °C, the supernatant was applied to an equilibrated 5 ml Ni-NTA agarose column. Bound proteins were eluted using 6 ml elution buffer (20 mM phosphate buffer pH 7.4, 500 mM NaCl, and 500 mM imidazole). Protein fractions were separated on 15% polyacrylamide gels under reducing conditions and stained with Coomassie brilliant blue G 250 (Merck, Darmstadt, Germany) or transferred onto a 0.45 μm nitrocellulose membrane (Bio-Rad), blocked with 5% non-fat dry milk in tris buffered saline (TBS, 0.1 M Tris-HCl, pH 7.5, 2.5 M NaCl), followed by incubation with anti-his-tag alkaline phosphatase conjugate (Cat No. 1396A, Bio-Rad, CA, US). The membrane was washed with TBS, containing 0.05% Tween-20 and incubated with substrate solution and developer (0.1 M Tris, pH 9.5, 0.1 M NaCl, and 5 mM MgCl2, NBT and BCIP). The reaction was stopped with H2O.
ELISA
Microplates (Maxi binding, SPL Life Sciences, Eumhyeon, South Korea) were coated with 100 µl/well of purified Li-rK39 at a concentration of 0.5 μg/ml in 0.1 M carbonate/bicarbonate buffer (pH 9.6) overnight at 4 °C. Blocking was performed for 1 h at ambient temperature with 400 μl of PBS-Blotto (0.01 M phosphate, 0.14 M NaCl, pH 7.4). Diluted serum (100 μl of 1:100 dilution in PBS-Blotto) was added to each well and incubated for 30 min at ambient temperature. After three times washing with 350 µl/well of PBS-Tween (0.01 M, pH 7.4), 100 μl of horseradish peroxidase-conjugated goat anti-human IgG (Cat No. 109-035-003, Jackson ImmunoResearch, West Grove, PA, USA), diluted 1:40,000 in PBS-Tween (0.01 M, pH 7.4), were applied to the wells and incubated for 30 min at ambient temperature. After five times washing with 350 µl/well of PBS-Tween (0.01 M, pH 7.4), 100 µl/well of TMB substrate solution (Cat No. 34029, Thermo Scientific, Rockford, IL, USA) were added. The reaction was stopped with 100 µl/well of 1 N H2SO4. The absorbance (optical density, OD) at 450 nm and 630 nm was measured with a microplate reader (Epoch, Biotek Instruments, Winooski, VT, USA). The cut-off value for positive was defined as the OD corresponding with the highest value of the Youden index (J = sensitivity + specificity − 1).
Statistical analysis
Sensitivity and specificity, with 95% confidence intervals (CI), of the rK39-ICT (Kalazar Detect™ Rapid Test for VL, InBios) were calculated using IBM® SPSS version 22 (IBM® SPSS Statistics, NY, USA).
Sensitivity and specificity at different cut-off points were calculated for the Li-rK39-ELISA and used to construct a receiver-operator characteristic (ROC) curve and to calculate the area under the curve (AUC) with its 95% CI.
Concordances between Li-rK39-ELISA and rK39-ICT, between Li-rK39-ELISA and IFAT, between rK39-ICT and IFAT were determined by calculating Cohen’s Kappa (k) and interpreted as follows: negligible (k = 0–0.20); weak (k = 0.21–0.40); moderate (k = 0.41–0.60); good (k = 0.61–0.80); and excellent (k = 0.81–1) [28, 29].
Results
Amplification and sequencing of the L. infantum K39 gene fragment
PCR amplification of the L. infantum K39 fragment using the XbaI-K39F and KpnI-K39R primers resulted in a multi-banded amplification product, which could not be reduced to single bands by varying the annealing temperature (Fig. 1) or by using a different cloning polymerase. Therefore, PCR products were co-purified and used for cloning in the pLEXSY-hyg2 expression vector. Individual recombinant clones screened by colony PCR revealed in each the integration of a single fragment; however, between the different clones these fragments had various sizes, usually differing in 117 bp from each other, corresponding with the size of one 39 aa repeat. In total, six plasmids yielded rK39 sequences from MCAN/IR/14/M14 with a length greater than 300 bp, for which the largest, MCAN14/47 and MCAN14/22 had a size of 550 bp. Sequences of these two genes were deposited in the GenBank database with accession numbers MN585730 (MCAN14/47) and MN585731 (MCAN14/22).
Fig. 1.
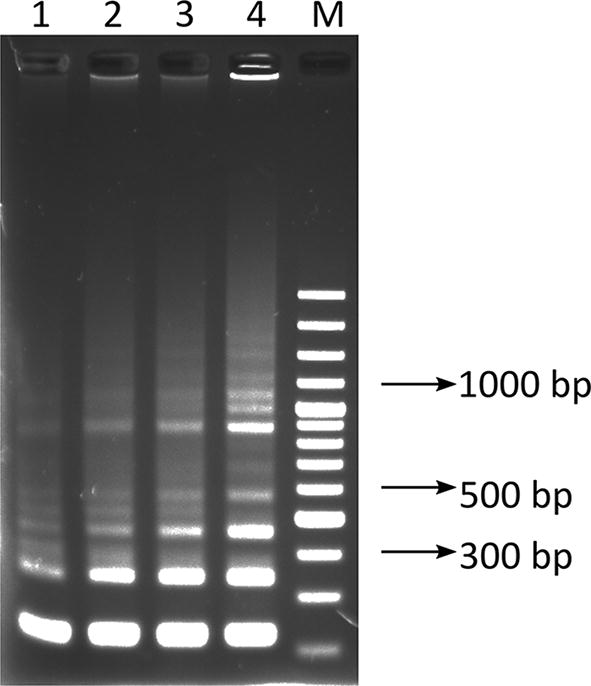
PCR amplified kinesin-like gene fragments from promastigotes of the Iranian L. infantum isolate MCAN/IR/14/M14 using XbaI-rK39F and KpnI-rK39R primers. Lanes 1–4: PCR products generated at annealing temperature of 64 °C, 65 °C, 66 °C and 67 °C, respectively; Lane M: GeneRuler 100 bp Plus DNA (Thermo Fisher Scientific)
When translated to protein, the sequences revealed that the Iranian L. infantum K39 appears to have at least 2 alleles. The first allele, from clone MCAN 14/22, had 100% identity at 180 amino acids positions of the corresponding translated L. chagasi kinesin-like gene (GenBank: L07879). In contrast, the second allele, from clone MCAN14/47 had no substitutions in the N-terminal non-repetitive part, 5 amino acids substitutions in the first repeat, 4 substitutions in the second repeat, none in the third repeat and 2 substitutions in a partial fourth repeat (Fig. 2), thus, resulting in only 94% protein similarity to the corresponding fragment from GenBank (L07879). The MCAN14/47 from pLEXSY-hyg2 was re-cloned using NcoI-K39 F and KpnI-K39 R primers into pLEXSY-hyg2-CYTO-MCAN14/47. Here, the artificial start codon provided by NcoI substituted the first amino acid, E, and the second, L, from MCAN14/47 to M and V, respectively in order to allow cytoplasmic expression of the 188 amino acid long Li-rK39 construct.
Fig. 2.
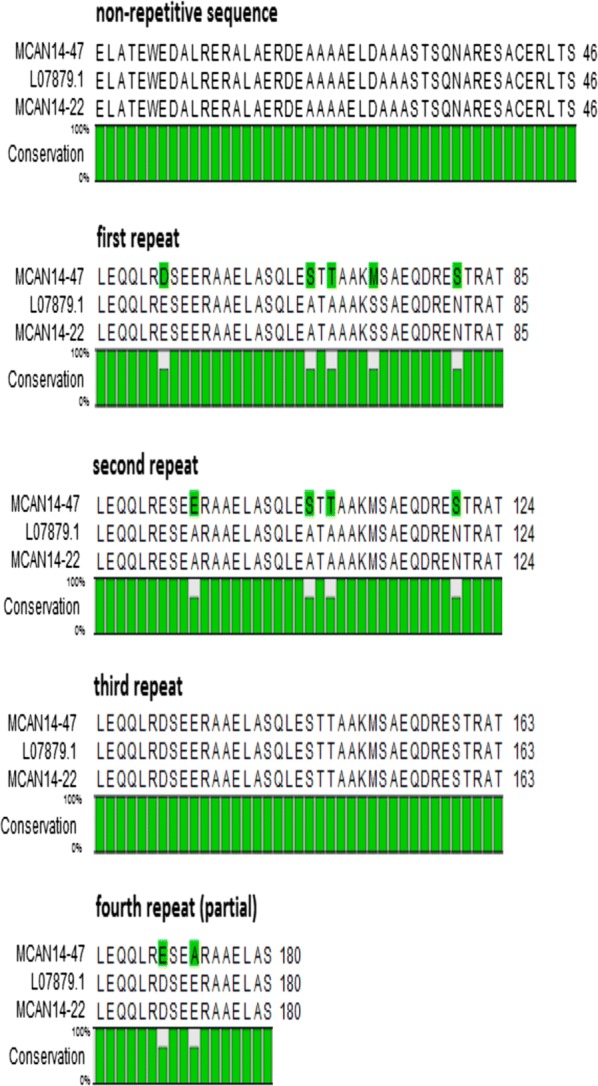
Amino acid alignment of the corresponding fragment of the translated GenBank reference sequence L07879 [13] with the two longest sequences from K39 clones from MCAN/IR/14/M14 obtained in pLEXSY-hyg2: MCAN14/47 (GenBank: MN585730) and MCAN14-22 (GenBank: MN585731)
Expression and purification
The pLEXSY-hyg2-MCAN14/47 plasmid was transfected to L. tarentolae and polyclonal selection using hygromycin yielded a recombinant population. This recombinant population expressed a cytosolic recombinant protein, Li-rK39, which could be purified on Ni-NTA resin. The yield of this purified protein was 250 µg/ml in 500 ml culture. The presence of the recombinant Li-rK39 protein and its purity was confirmed by SDS-PAGE and Coomassie blue staining (Fig. 3a) and by Western blot, using an anti-his-tag antibody (Fig. 3b). The apparent size of the protein was 25 kDa although the expected size was 21 kDa (including the 6 histidine residues), probably due to post-translational modification like glycosylation or due to the relatively high content in basic amino acids (34%).
Fig. 3.
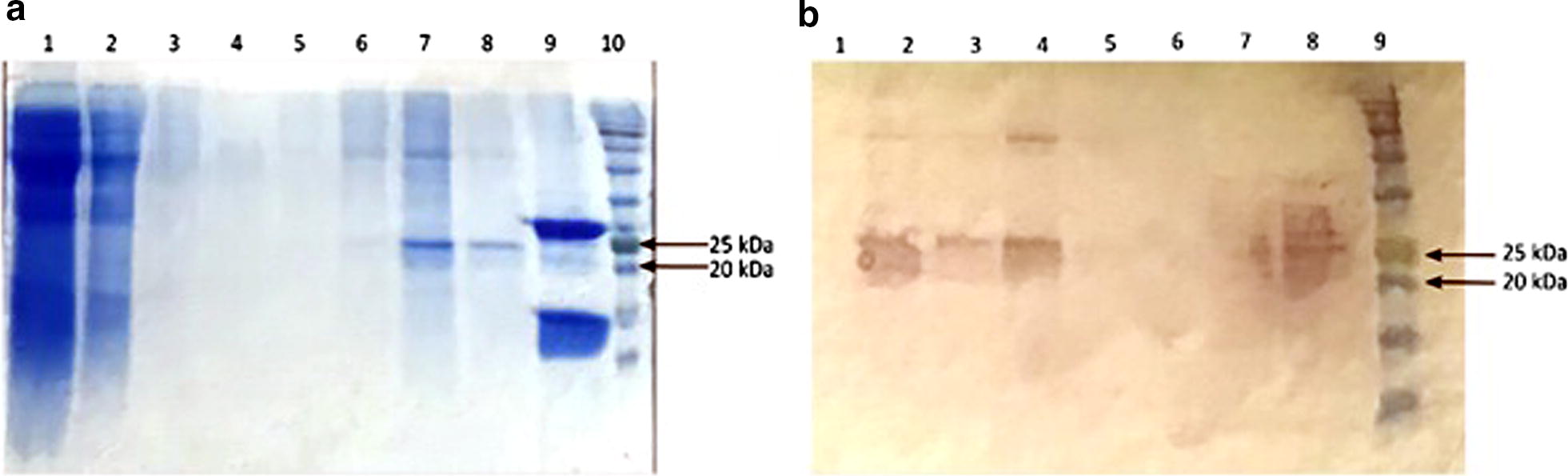
SDS-PAGE (a) and Western blot (b) showing the protein composition of the different fractions collected from L. tarentolae clone pLEXSY-hyg2-MCAN14/47, before and after the purification on the Ni-NTA. a Lane 1: whole cell lysate; Lane 2: flow-through from Ni-NTA column; Lanes 3 and 4: wash fractions; Lanes 5–8: elution fractions; Lane 9: recombinant His-tagged H2B (positive control); Lane 10: 11–245 kDa protein marker. b Lanes 1–6: elution fractions; Lane 7: flow-through; Lane 8: whole cell lysate; Lane 9: 11–245 kDa protein marker
Diagnostic performance of Li-rK39 in an ELISA
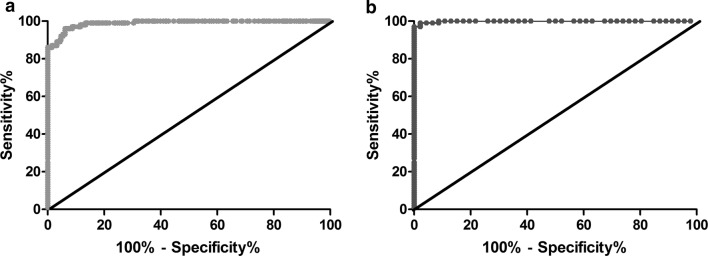
The diagnostic performance of the Li-rK39 antigen was assessed in an ELISA with sera from 100 VL patients, 190 endemic healthy controls, 46 non-endemic healthy controls and 47 non-VL patients. With IFAT as reference test and the cut-off OD of 0.312, corresponding with the highest Youden index (J = sensitivity +specificity − 1). The Li-rK39 antigen showed a sensitivity of 96.0% (95% CI: 90.1–98.9%) and a specificity of 93.8% (95% CI: 90.3–96.4%) yielding an AUC of 0.989 (95% CI: 0.981–0.997) by using endemic, non-endemic healthy controls and non-VL patients (Fig. 4a). Sensitivity and specificity using only non-endemic controls at cut-off OD of 0.295 was 97% (95% CI: 91.5–99.4%) and 100% (95% CI: 93–100%) yielding an AUC of 0.999 (95% CI: 0.996–1) (Fig. 4b). Two of the non-VL patients, including malaria and cutaneous leishmaniasis showed a positive reaction in the Li-rK39 ELISA.
Fig. 4.
Receiver operator characteristic curves constructed from ELISA results. a ROC curve obtained with sera from VL patients and all the samples. b ROC curve obtained with sera from VL patients and non-endemic controls
rK39 ICT (InBios) results
With IFAT as reference test, the rK39 ICT exhibited a sensitivity of 90.0% (95% CI: 82.4–95.1%) and a specificity of 100% (95% CI: 98.5–100%).
Concordance between different tests
By including all the samples including endemic, non-endemic and non-VL patients, the concordance between the Li-rK39 ELISA and the rK39-ICT was good with k = 0.80 and P < 0.0001. Concordance between the Li-rK39 ELISA and IFAT and between rK39-ICT and IFAT was excellent with k = 0.86 and 0.92, respectively, and P < 0.0001.
Discussion
Given the fact that VL has a fatal outcome if not treated, its early and accurate diagnosis is necessary. Immunochromatographic and ELISA tests, based on a recombinant antigen containing 39 amino acid repeats of a kinesin-related protein of a Brazilian L. infantum strain, are commercially available. Previous studies showed a considerable variation in the sensitivity and specificity of such tests, depending on test format and the geographical distribution of VL with reported sensitivities between 67.6–100% and specificities between 59–100% for rK39-ICTs. The rK39-ELISA has been shown to have sensitivity and specificity ranges between 88.6–100% and 84.5–100%, respectively [17]. A prospective study conducted in Iran on 17 VL patients and 137 patients with other diseases reported a sensitivity of 82.4% and specificity of 100% for the InBios rK39-ICT [28]. Such results imply that due to the suboptimal diagnostic accuracy of rK39-based tests, other diagnostic tests such as IFAT or quantitative PCR need to be performed, which are time-consuming, require equipment and well-trained personnel.
Bhattacharyya et al. [19] illustrated an extensive diversity of K39 among various strains of L. donovani which could affect the accuracy of rK39-ICTs. Indeed, in the present study, we confirm that there is also diversity in the K39 gene within an Iranian L. infantum isolate. Cloned 550-bp fragments of the K39 gene from the Iranian isolate MCAN/IR/14/M14 showed that this locus is heterozygous, carrying a sequence identical to the one described for Brazilian L. infantum, and also a variant that differed by 6% with the amino acid sequence of the Brazilian K39 sequence. Surprisingly, these amino acid substitutions were mostly found at the same positions to those noted in the L. donovani study by Bhattacharyya et al. [19], yet, not all substitutions were same as the ones in L. donovani. The observed difference in protein sequence within this limited range (3.5 repeats) might already affect the performance of ICTs. Therefore, we chose this variant, called Li-rK39, for expression in L. tarentolae.
In the present study, the ELISA with the purified recombinant Li-rK39 fragment was more sensitive (96%). One of the four false negative serum samples in the ELISA was also negative in ICT. The reason that some IFAT-positive VL patients remained negative in the Li-rK39 ELISA is not clear. Elfadi et al. [18] pointed out that it is hard to select a diagnostic test based on an antigen of a single strain of Leishmania in places where a population of heterogeneous parasites exists. This is illustrated by a study conducted in India, where a sensitivity of 100% was reported for an ELISA using an rK39 derived from an Indian isolate of L. donovani KE16 [24]. It may therefore be interesting to investigate the heterogeneity within the kinesin-related K39 gene of L. infantum isolated from a human patient in a given area, and to express the dominant sequence as a recombinant antigen for diagnostic purposes. Furthermore, it should be kept in mind that some cases of VL in Iran are caused by L. tropica [29, 30].
A study previously conducted in Iran on 12 parasitologically confirmed VL patients reported a sensitivity of 91% for ELISA with a recombinant antigen consisting of one 39 amino acid repeat of the K39 of an Iranian L. infantum strain, expressed in E. coli [31]. Compared to our study, the low sensitivity obtained in their study may be due to the use of only one repeat unit of K39, derived from a different L. infantum isolate and expressed in a prokaryotic host.
Another study showed that a higher copy number of K39 repeats in a recombinant antigen derived from L. infantum increased the affinity of antibodies to the antigen [32]. In the present study, the sensitivity of Li-rK39-ELISA is greater than the one conducted in Brazil and equal to the one in India which used a commercial rK39 of L. infantum in their ELISA system [24, 33]. Also, the sensitivity of the Li-rK39-ELISA was greater than that of rK39-ICT (InBios) (90%). This might be due to the higher analytical sensitivity of the ELISA compared to the ICT format. However, in contrast, we found three VL patients positive in the rK39-ICT that were negative in the Li-rK39-ELISA. This discordance may be due to: (i) small modifications of the original sequence by adding an N-terminal methionine for cytoplasmic expression; (ii) the different solid phases in ELISA (polystyrene) and ICT (nitrocellulose) that may influence the antigen conformation; (iii) the more stringent antibody-antigen binding conditions in the ELISA; and (iv) the fact that we expressed and evaluated only one of the two alleles of the kinesin related protein gene, which is different from the one used to produce the Brazilian L. infantum rK39 in the rK39-ICT. It follows that combining in one test recombinant proteins derived from both alleles may further increase the sensitivity of the ELISA.
The Li-rK39-ELISA revealed a specificity of 100% with the 46 non-endemic controls but 14 of the 190 endemic controls and two of the non-VL patients including cutaneous leishmaniasis and malaria were positive, although with OD below 0.5, resulting in a specificity of 93.8% which is not unexpected since in VL endemic regions a considerable number of infected people remain asymptomatic but develop a detectable antibody response [34].
In the present study, we used the L. tarentolae expression system (pLEXSY) to produce Li-rK39 and have evaluated its diagnostic performance. So far, the rK39 antigen has been produced in prokaryotic expression systems. Post-translational modification can affect an antigen in many aspects like stability, solubility, resistance against proteases etc. but doesn’t occur in prokaryotic expression systems. Also, codon bias is an important issue related to expression systems [21, 35]. The main advantage of prokaryotic expression systems is their low cost, easy handling and high yield [21, 35]. Leishmania tarentolae is a relatively easy eukaryotic expression system but compared to bacteria, it has a lower growth rate (doubling time of 6 h in agitated culture) and lower yield. On the other hand, it can be considered as a suitable expression system for rK39 of L. infantum, so the recombinantly produced protein may be more similar to its native counterpart. All these factors make this expression system suitable for the expression of proteins of kinetoplastid organisms as Rooney et al. [36] have shown in their study.
Conclusions
Considering the diversity of the kinesin-related gene encoding rK39 among the different strains of Leishmania species in different regions of the world, it can be suggested that the recombinant antigens be generated based on the dominant parasite strains circulating in a given region. Derived from the rK39 sequences found in an Iranian L. infantum strain isolated from a dog, a recombinant antigen showed satisfactory diagnostic accuracy when tested on sera from VL patients, endemic and non-endemic controls. Besides, we found that L. tarentolae is a good expression system for producing the rK39 antigen of L. infantum.
Acknowledgments
We would like to thank the technicians of the Diagnostic Parasitology Unit of the Institute of Tropical Medicine, Antwerp, Belgium for their assistance.
Abbreviations
- VL
visceral leishmaniasis
- Li-rK39
L. infantum-rK39
- ICT
immunochromatographic test
- IFAT
immunofluorescence assay
- DAT
direct agglutination test
- ELISA
enzyme-linked immunosorbent assay
- RDT
rapid diagnostic tests
- BHI
brain heart infusion
- TBS
tris-buffered saline
- CI
confidence intervals
- ROC
receiver-operator characteristic
- AUC
area under the curve
- k
kappa
Authors’ contributions
ZR, NV, BP, VK and AR performed the experiments, analyzed and interpreted the data and drafted the manuscript. PB, BP, BS and GHP contributed to the conception and supervision of the project and revised the manuscript. All authors read and approved the final manuscript.
Funding
This project, as ZR’s PhD thesis, was financially supported by Shiraz University of Medical Sciences, Iran (Grant No: 12833). VK received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 642609. NVR is supported by a Krediet aan Navorser No. 1509316N.
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The raw data obtained during the present study are available upon request.
Ethics approval and consent to participate
This study received ethical approval from the Ethics Committee of the Shiraz University of Medical Sciences (SUMS), Shiraz, Iran (No. IR.SUMS.REC.1396.S494). Informed consent was obtained from the parents or guardians of participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zahra Rezaei, Email: zahraa_rezaee@yahoo.com.
Nick Van Reet, Email: nvanreet@itg.be.
Gholamreza Pouladfar, Email: pouladfar_ghr@hotmail.com.
Vera Kühne, Email: vkuhne@itg.be.
Amin Ramezani, Email: aramezani@sums.ac.ir.
Bahador Sarkari, Email: sarkarib@sums.ac.ir.
Bahman Pourabbas, Email: pourabbasb@yahoo.com.
Philippe Büscher, Email: pbuscher@itg.be.
References
- 1.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 4.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 5.Siddig M, Ghalib H, Shillington DC, Petersen EA. Visceral leishmaniasis in the Sudan: comparative parasitological methods of diagnosis. Trans R Soc Trop Med Hyg. 1988;82:66–68. doi: 10.1016/0035-9203(88)90265-9. [DOI] [PubMed] [Google Scholar]
- 6.Zijlstra E, Ali MS, El-Hassan A, El-Toum IA, Satti M, Ghalib H, et al. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg. 1992;86:505–507. doi: 10.1016/0035-9203(92)90086-R. [DOI] [PubMed] [Google Scholar]
- 7.Boelaert M, El Safi S, Mousa H, Mbati J, Gurubacharya V, Shrestha J, et al. Multi-centre evaluation of repeatability and reproducibility of the direct agglutination test for visceral leishmaniasis. Trop Med Int Health. 1999;4:31–37. doi: 10.1046/j.1365-3156.1999.00348.x. [DOI] [PubMed] [Google Scholar]
- 8.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;21:563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 11.Maia Z, Lírio M, Mistro S, Mendes CMC, Mehta SR, Badaro R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: systematic review with meta-analysis. PLoS Negl Trop Dis. 2012;6:e1484. doi: 10.1371/journal.pntd.0001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikaeili F, Fakhar M, Sarkari B, Motazedian MH, Hatam G. Comparison of serological methods (ELISA, DAT and IFA) for diagnosis of visceral leishmaniasis utilizing an endemic strain. Iran J Immunol. 2007;4:116–121. [PubMed] [Google Scholar]
- 13.Burns JM, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badaro R, Benson D, Eulalio M, Freire M, Cunha S, Netto E, et al. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Pai K, Pathak K, Sundar S. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin Diagn Lab Immunol. 2001;8:1220–1224. doi: 10.1128/CDLI.8.6.1220-1224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zijlstra E, Nur Y, Desjeux P, Khalil E, El-Hassan AM, Groen J. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. Trop Med Int Health. 2001;6:108–113. doi: 10.1046/j.1365-3156.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- 17.Sarkari B, Rezaei Z, Mohebali M. Immunodiagnosis of visceral leishmaniasis: current status and challenges: a review article. Iran J Parasitol. 2018;13:331. [PMC free article] [PubMed] [Google Scholar]
- 18.Abass E, Kang C, Martinkovic F, Semião-Santos SJ, Sundar S, Walden P, et al. Heterogeneity of Leishmania donovani parasites complicates diagnosis of visceral leishmaniasis: comparison of different serological tests in three endemic regions. PloS ONE. 2015;10:e0116408. doi: 10.1371/journal.pone.0116408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharyya T, Boelaert M, Miles MA. Comparison of visceral leishmaniasis diagnostic antigens in African and Asian Leishmania donovani reveals extensive diversity and region-specific polymorphisms. PLoS Negl Trop Dis. 2013;7:e2057. doi: 10.1371/journal.pntd.0002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J, Li G, Ren X, Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol. 2007;127:335–347. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Clark EDB. Protein refolding for industrial processes. Curr Opin Biotechnol. 2001;12:202–207. doi: 10.1016/S0958-1669(00)00200-7. [DOI] [PubMed] [Google Scholar]
- 22.LEXSYcon2.1 Expression Kit. For constitutive expression of recombinant proteins in Leishmania tarentolae. https://www.jenabioscience.com. Accessed 6 Dec 2016.
- 23.Dhom-Lemos L, Viana AG, Cunha JLR, Cardoso MS, Mendes TAO, Pinheiro GRG, et al. Leishmania infantum recombinant kinesin degenerated derived repeat (rKDDR): a novel potential antigen for serodiagnosis of visceral leishmaniasis. PloS ONE. 2019;14:e0211719. doi: 10.1371/journal.pone.0211719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivakumar R, Sharma P, Chang K-P, Singh S. Cloning, expression, and purification of a novel recombinant antigen from Leishmania donovani. Protein Expr Purif. 2006;46:156–165. doi: 10.1016/j.pep.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Hajjaran H, Mohebali M, Teimouri A, Oshaghi MA, Mirjalali H, Kazemi-Rad E, et al. Identification and phylogenetic relationship of Iranian strains of various Leishmania species isolated from cutaneous and visceral cases of leishmaniasis based on N-acetylglucosamine-1-phosphate transferase gene. Infect Genet Evol. 2014;26:203–212. doi: 10.1016/j.meegid.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Lobato L, Chaptal V, Molle J, Falson P. Leishmania tarentolae as a promising tool for expressing polytopic and multi-transmembrane spans eukaryotic membrane proteins: the case of the ABC pump ABCG6. Methods Mol Biol. 2016;1432:119–131. doi: 10.1007/978-1-4939-3637-3_8. [DOI] [PubMed] [Google Scholar]
- 27.Fritsche C, Sitz M, Weiland N, Breitling R, Pohl HD. Characterization of the growth behavior of Leishmania tarentolae—a new expression system for recombinant proteins. J Basic Microbiol. 2007;47:384–393. doi: 10.1002/jobm.200710111. [DOI] [PubMed] [Google Scholar]
- 28.Alborzi A, Rasouli M, Nademi Z, Kadivar M, Pourabbas B. Evaluation of rK39 strip test for the diagnosis of visceral leishmaniasis in infants. East Mediterr Health J. 2006;12:94–99. [PubMed] [Google Scholar]
- 29.Alborzi A, Pouladfar GR, Fakhar M, Motazedian MH, Hatam GR, Kadivar MR. Isolation of Leishmania tropica from a patient with visceral leishmaniasis and disseminated cutaneous leishmaniasis, southern Iran. Am J Trop Med Hyg. 2008;79:435–437. doi: 10.4269/ajtmh.2008.79.435. [DOI] [PubMed] [Google Scholar]
- 30.Sarkari B, Ahmadpour NB, Moshfe A, Hajjaran H. Molecular evaluation of a case of visceral leishmaniasis due to Leishmania tropica in southwestern Iran. Iran J Parasitol. 2016;11:126. [PMC free article] [PubMed] [Google Scholar]
- 31.Taran M, Mohebali M, Modaresi M, Mamishi S, Mojarad M, Mahmoudi M. Preparation of a K39 subrecombinant antigen for the detection of Leishmania infantum antibodies in human: a comparative study with an immunochromatographic test and direct agglutination. Iran J Parasitol. 2007;2:25–33. [Google Scholar]
- 32.Goto Y, Carter D, Guderian J, Inoue N, Kawazu S-I, Reed SG. Upregulated expression of B-cell antigen family tandem repeat proteins by Leishmania amastigotes. Infect Immun. 2010;78:2138–2145. doi: 10.1128/IAI.01102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braz RF, Nascimento ET, Martins DR, Wilson ME, Pearson RD, Reed SG, et al. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. Am J Trop Med Hyg. 2002;67:344–348. doi: 10.4269/ajtmh.2002.67.344. [DOI] [PubMed] [Google Scholar]
- 34.Alborzi A, Pourabbas B, Shahian F, Mardaneh J, Pouladfar GR, Ziyaeyan M. Detection of Leishmania infantum kinetoplast DNA in the whole blood of asymptomatic individuals by PCR-ELISA and comparison with other infection markers in endemic areas, southern Iran. Am J Trop Med Hyg. 2008;79:839–842. doi: 10.4269/ajtmh.2008.79.839. [DOI] [PubMed] [Google Scholar]
- 35.Marston F. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986;240:1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rooney B, Piening T, Büscher P, Rogé S, Smales CM. Expression of Trypanosoma brucei gambiense Antigens in Leishmania tarentolae. Potential for use in rapid serodiagnostic tests (RDTs) PLoS Negl Trop Dis. 2015;9:0004271. doi: 10.1371/journal.pntd.0004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this article are included within the article. The raw data obtained during the present study are available upon request.