Abstract
Poly-l-lactide-co-ε-caprolactone (PLCL) is a unique polymer containing both polylactic acid and poly-ε-caprolactone (PCL) chain units, and thus it has better flexible and biodegradable properties. Based on these unique properties of PLCL, we have developed balloons that are now widely used in treating major medical problems [Biomaterials2016,105, 109–116]. One of the most important considerations needed for balloons is to ensure that the material properties remain similar after undergoing ethylene oxide (EtO) or gamma (γ-) sterilization treatments. From the biotechnological point of view, we focused on analyzing the vital molecular properties of the PLCL material after sterilization, such as changes in crystallinity, molecular weight distributions (Mw, Mn, and polydispersity index), and inherent viscosity (η). Analysis of the data reveals that EtO sterilization does not engender any change in crystallinity, melting temperature (Tm), molecular weights, and η of the polymer. On the contrary, γ-radiations induce chain scission and consequential decrease of ∼33 and ∼15% in molecular weights and η values, respectively. Based on our observations, we recommend EtO sterilization instead of γ-radiation for PLCL. This ensures prolonged stability of the polymer against degradation in a biological environment, long-shelf life, and absolute assurance that balloon failures do not occur after implantation.
1. Introduction
In recent years, biodegradable polymers have become one of the most frequently used materials for the development of a variety of biodegradable polymer-based medical devices. One example is poly-l-lactide-co-ε-caprolactone (PLCL) balloons used widely by orthopedic surgeons for rotor-cuff muscular injury.1−4 These balloons are inflated with a phosphate buffer to their required shape and thickness. Flexibility in inflated balloons to withhold extreme muscular pressures relies on the physical properties of PLCL materials.5,6 The composition of the PLCL consists of a soft matrix of a poly-ε-caprolactone (PCL) chain that provides a stable environment for the cellular matrix surrounding the balloons.7−9 This helps to create a smooth balloon surface that reduces friction between the joints, providing improved shoulder movements and guaranteed pain reduction.10 The elastic mechano-property of the PLCL material is also expected to prevent premature failure of the balloons.
Because of the inherent flexible and biocompatible properties, PLCL is a highly valued material used for medical applications. Examples of success because of PLCL are11,12 bone regeneration and bone xenografts,13,14 implants for pancreatic islet transplantation,15−17 and carriers for immunosuppressive drugs.18
Despite the wide variety of PLCL biomedical applications, physical aging is a common drawback that reduces shelf-life and efficiency. According to Hutchinson, physical aging is a change in the physical properties in polymers as a function of storage time at constant temperature, at zero stress, and without influence from any other external condition.19 During physical aging, the physical properties of the polymer material modify the chain conformation of the polymer. These modifications accompany changes in molecular weight distribution and intrinsic viscosity. This shortcoming is primarily associated with a decrease in the viscoelasticity of the polymer material, thus reducing product stability/durability, reliability, and safety.20−22 In this context, PLCL has been reported to be unstable, undergoing changes in its physical properties such as ductile deformation.23 Supporting this, Tsuji et al.24 and Saha and Tsuji25 have demonstrated that the ductile properties of the PLCL material are due to changes in crystalline properties during storage.
Factors that are mainly responsible for changes in the polymer material include process conditions and sterilization methods. The most widely used sterilization techniques for biodegradable polymers include treatment with ethylene oxide (EtO) and γ-irradiation.26,27 These sterilization conditions increase stiffness of the polymer.28 Farrar and Gillson29 show that decrease in the strength of the polymer material is due to the decrease in its polymer molecular weight. The drawbacks of EtO sterilization are that the EtO gas can induce chemical reactions potentially damaging the polymer structure.30−32 On the other hand, γ-irradiation induces changes in the polymer structure through chain scission reactions.33 Valente et al.34 and Pietrzak35 show that after sterilization, the changes in the molecular properties of the bioabsorbable polymers can completely impact biodegradation kinetics and performance time of the polymer device.
The decrease in the polymer molecular weight during sterilization hydrolyzes the device, making it be reabsorbed faster than predicted. Changes in the molecular weight of the polymer also disturb chain segment mobility in the polymer microstructure.36 Thus, any change in the molecularity of the polymer changes the life span, and efficiency of the device affects its mechanical properties. Changes in the dimension of the polymer device may also occur.37 It is, therefore, extremely necessary to examine and determine suitable sterilization methods for polymeric bioimplants.38
Predicting the consequences of sterilization methods on the polymer material is complex. Alteration of PLCL material over time is obvious, and the analysis of the device performance in real-time is probably impractical. Before end-use, the balloons need to be sterilized and subjected to long-term shelf-storage under varying conditions. Given these difficulties, a fundamental understanding of polymer structure and behavior is essential, examining physical properties such as crystallinity, thermal behavior, molecular weight, and inherent viscosity (η) of the final product before and after the manufacture processes. This offers the possibility to obtain the most reliable predictions of device or polymer-based scaffold performances in biomedical tissue engineering.39,40 In this report, we examine the changes in the structural properties of the PLCL that may occur in the balloon material during the sterilization processes. Focus is on determining properties such as crystallinity, thermal properties, molecular weight distribution, and η before and after EtO and γ-sterilization.
2. Results and Discussion
The aim of the experiments is to screen commonly used procedures for polymer sterilization such as EtO and gamma (γ-) sterilization on PLCL balloons. Our goal is to determine whether any of the sterilization methods can cause undesired alterations in the PLCL material. The effect of EtO and γ-sterilization on the weight of balloons, molecular weight, and η of PLCL material is examined in Table 1.
Table 1. Average Weight, Molecular Weight Distribution, and Inherent Viscosity (η) of the PLCL Material in Balloons before and after Sterilizationa.
| sterilization method | average weight [g] | Mw [g/mol] | Mn [g/mol] | PDI | viscosity (η) [dL/g] |
|---|---|---|---|---|---|
| control | 1.319 ± 0.388 | 133 000 ± 4900 | 81 000 ± 2400 | 1.65 ± 0.05 | 1.23 ± 0.06 |
| EtO | |||||
| cycle 1 | 1.065 ± 0.160 | 147 000 ± 6700 | 93 000 ± 5400 | 1.58 ± 0.01 | 1.20 ± 0.06 |
| cycle 2 | 1.253 ± 0.027 | 138 000 ± 6500 | 86 000 ± 5000 | 1.60 ± 0.02 | 1.25 ± 0.02 |
| cycle 3 | 1.268 ± 0.022 | 136 000 ± 7000 | 83 000 ± 4600 | 1.63 ± 0.01 | 1.24 ± 0.02 |
| β-radiation | |||||
| 19.5 kGy | 1.220 ± 0.032 | 110 000 ± 1700 | 65 000 ± 1900 | 1.68 ± 0.03 | 1.09 ± 0.04 |
| 21.5 kGy | 1.236 ± 0.049 | 128 000 ± 1300 | 82 000 ± 2900 | 1.54 ± 0.04 | 1.08 ± 0.02 |
| 25 kGy | 1.291 ± 0.036 | 89 000 ± 6500 | 45 000 ± 4900 | 1.96 ± 0.07 | 1.05 ± 0.06 |
Mw = weight average molecular weight; Mn = number average molecular weight; PDI = polydispersity index.
2.1. EtO Sterilization
The weight of the balloons was measured before and after each EtO sterilization cycle. Gel permeation chromatography (GPC) was performed to determine if sterilization by EtO influences the molecular weight of PLCL material compared to the balloons not treated with EtO. GPC data were always obtained in triplicate and for samples from three different balloons. We also examined the effect of repetitive EtO sterilization (up to three continuous sterilization cycles) conditions on the balloons.
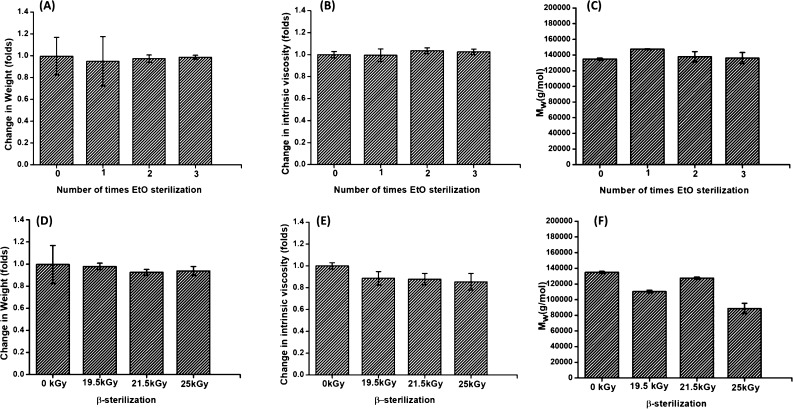
No significant changes in the weight of the balloons were observed (Figure 1). Visual appearance of EtO-treated balloon samples remained unchanged. It is interesting to note that even the repetitive sterilization cycles have an insignificant effect on the balloon weights. This means that EtO is not absorbed within the PLCL material during sterilization.
Figure 1.
Effect of EtO sterilization on the (A) weight, (B) intrinsic viscosity, and (C) molecular weight (Mw) of the PLCL material in balloons. The effect of β-radiation on the (D) weight, (E) intrinsic viscosity, and (F) molecular weight (Mw) of the PLCL material in balloons.
Changes in molecular weight [Mw, Mn, and polydispersity index (PDI)] for samples sterilized by EtO are shown in Table 1. We also analyzed changes in the molecular weight of the PLCL material, when the balloons were treated with multiple cycles of EtO. The molecular weight data indicate that the EtO sterilization processes have no significant influence on the molecular integrity of PLCL material. As represented in Table 1 and Figure 1, no significant difference between the Mw and Mn of the control and EtO-sterilized balloons is observed. Following the first sterilization cycles, the Mw shows a slight increase with a difference of less than 10% (Table 1) from the nonsterile PLCL material. However, the PDI values for the treated and untreated samples are nearly constant to ∼1.6. This indicates that EtO does not promote chain scission nor does it cause any chemical changes in the PLCL material.
The η measurements support the GPC data that the physical and chemical integrity of the PLCL material after EtO sterilization is not compromised, and that sterilization due to EtO does not cause any changes in the chain conformation of the PLCL material. The variations in η of neat and sterilized samples are provided in Figure 1. No change in the η was observed in the PLCL material for the balloons that were treated with EtO.
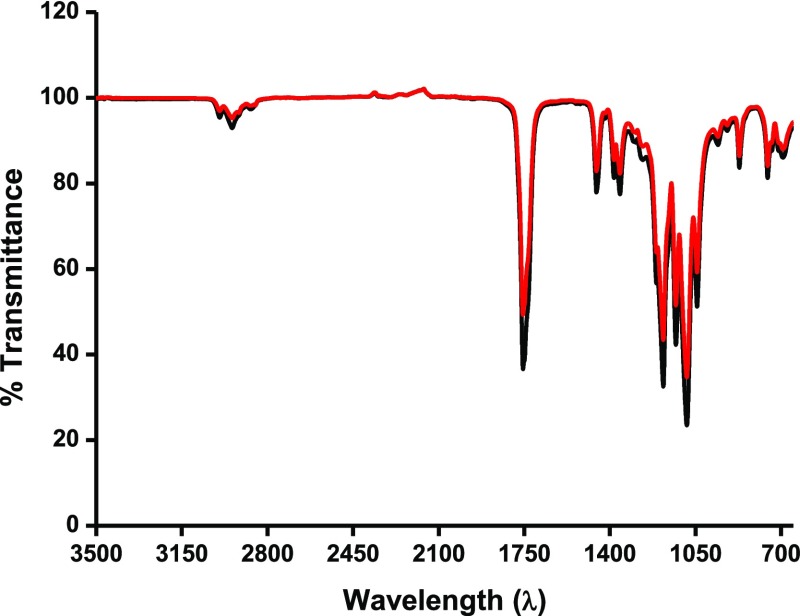
These results coincide with the results of Horakova et al.,41 who report that EtO does not interact with the PLCL material. This also confirms that EtO does not interact with parent polymeric chains such as of PCL. No difference in the Fourier transform infrared (FTIR) spectra of the native polymer with the EtO-treated balloon was observed (Figure 2). Therefore, we focused on determining the changes in the melting point (Tm) and changes in the crystalline properties (X) of the sterilized samples. The results are provided in the sections below.
Figure 2.
FTIR spectra of nonsterilized (black) and three cycle EtO-sterilized (red) PLCL balloon material.
2.2. γ-Sterilization
Radiation sterilization was performed at 19.5, 21.5, and 25 kGy for balloons. The average weight of the balloons did not change after the sterilization process. GPC data indicate that γ-irradiation breaks PLCL polymer chains. γ-Sterilization markedly decreases the molecular weight and η of the PLCL in the balloons. An approximately 33% decrease in the Mw of the balloons was observed under 25 kGy. As presented in Table 1, both Mw and Mn in PLCL are decreased after radiation sterilization. Undoubtedly, the γ-radiation induces the chain scission in the PLCL, which is also reflected in the increase in the PDI from 1.5 to 2.0. The result is consistent with observations in the literature, where exposure of radiation during the sterilization process induces reduction in the polymer chains.42 Chaochanchaikul and Harnnarongchai42 show that γ-irradiation decreases the Mw and Mn in the polylactic acid (PLA) material. Obviously, Table 1 shows that the changes in the molecular weights of the polymer material are dependent on the amount of radiation exposure.43 Nugroho et al.44 illustrate that a chain degradation in PLA can occur in any atmosphere, whether in air or in vacuum during γ-sterilization.
The η of the PLCL material significantly decreases to ∼15% after a radiation exposure of 25 kGy. The η values in sterilized balloons decrease from 1.23 (control) to 1.09 and 1.08 for radiation strengths of 19.5 and 21.5 kGy, respectively. Decrease in the η in PLCL material after radiation treatment is a direct indication of polymer chain scission. It is known that the concept of η relates directly to polymer molecular weight.45 Based on our observations, it is obvious that for a bioabsorbable polymer PLCL, reduction in the molecular weight is reflected in the decrease in its η.
The obtained results are consistent with Nuutinen et al.,46 who illustrate that the η of the PLA polymer decreases to 64% with γ-sterilization. Similarly, Suuronen et al.47 report a decrease in the η of PLA after a sterilization dose of 25 kGy. Additionally, we observed that the decrease in the η is dependent on the strength of the radiation. The η of PLCL decreases gradually with an increase in radiation strength from 1.97 to 2.57 kGy. The answer lies in the observations of Nugroho et al.48 that with an increase in radiation strength, the polymer is more susceptible and easily undergoes oxidative chain-scission.
2.3. Thermal Analysis and Crystallinity after Sterilization
The PLCL material crystalline properties were measured before and after the sterilization treatments using differential scanning calorimetry (DSC) (Figure 3). The relationship between enthalpy of fusion (ΔH) of polymers with its crystallinity is provided in eq 1. Generally, an increase in the crystalline properties of the polymer leads to an increase in the ΔH. We observed that the melting temperature (Tm) of the raw PLCL material (Resomer LC703S) is 168 °C, whereas the Tm of the solvent-casted balloon films was reduced to 160 °C. The reason for such variation is totally dependent on the crystallization properties of the polymer and the method of preparing the polymer films. The literature illustrates that the crystallinity of the polymer films is widely affected by solvent-induced conformational changes.49,50 Apart from the type of solvent used in the polymer film preparation, Runt and Rim51 emphasize that processing conditions such as polymer film thickness, casting temperature, evaporation rate, and solvent retention time are the additional factors that control the crystalline properties of the polymer.
Figure 3.
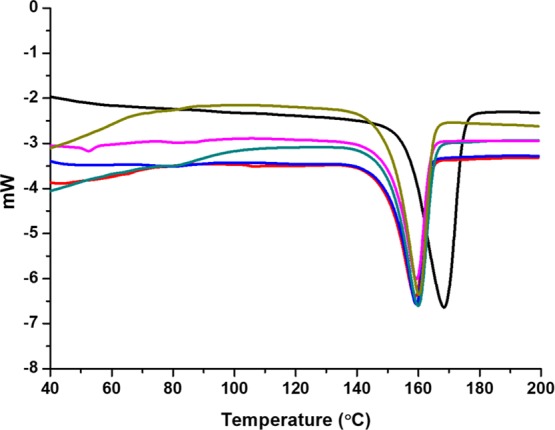
DSC curve of, PLCL raw material (Resomer LC703S) (black), balloon films: nonsterilized (red), EtO treated [cycle 1 (blue), cycle 2 (green), cycle 3 (magenta)], and 25 kGy treated (dark yellow).
It should be noted from Figure 3 that samples treated with EtO and 25 kGy radiation also exhibited the Tm of 160 °C. Because the ratio of monomers (PLA and PCL) in PLCL is 70:30, the observation of Tm at 160 °C for all the balloons is because of randomization of the PLA with the PCL blocks in the polymer. Han et al.52 illustrate that when the PLA segment is the major component within the PLCL, it is possible that during polymerization, PLA chains become bonded to the PCL chains, hence being in excess, only the crystalline part of the PLA appears in DSC. This is an indication that the crystallinity of the PCL is drastically reduced in the presence of PLA chains in PLCL. Patel et al.53 provide bibliographic references reporting covalent conjugation between PLA and PCL significantly reducing the crystallinity of the PCL. The crystallinity in PCL is far less than the PLA. Possibly, the earlier crystallization of the PLA chains strongly restricts the crystallization of the PCL segments, which remain undetectable in DSC.54,55
The degree of crystallinity was determined from the enthalpy of fusion (ΔH) which was calculated from the area under the endotherm (eq 1).
| 1 |
where X is the extent of crystallinity, ΔH is the enthalpy of fusion measured at the melting point (Tm), and ΔH0 is the enthalpy of fusion of the completely crystalline polymer measured at equilibrium melting temperature (Tm0). The presence of PCL in PLCL makes it a semicrystalline material. We, therefore, determined the percentage crystallinity in PLCL using the reported values of PLA. A literature value of 95 J g–1 for ΔH0 was considered, assuming 100% crystalline form of the PLA as determined by Sosnowski.56 The ΔH and crystallinity of the nonsterilized balloons were −24.5 J g–1 and ∼26%, respectively. This value is comparatively lower than the value of 65% crystallinity for the pure PLCL reported in the literature.73 This decrease in the crystallinity, as stated earlier, is because of the solvent medium chosen for the balloon film preparation. In support, Byun et al.57 observe a decrease in the crystallinity of the PLA films treated with dichloromethane (DCM) using DSC measurements. This is in agreement with our observed crystallinity for nonsterilized PLCL films in balloons casted using DCM. The ΔH value for the PLCL after up to three continuous EtO sterilization cycles almost remains constant compared to the untreated balloon sample (−24.5 J g–1). The average ΔH values were −24.3, −24.4, and −24.0 for balloons exposed under 1, 2, and 3 EtO cycles. The insignificant changes in the ΔH after EtO treatment are an indication that the polymer properties remain unaffected after sterilization. The crystallinity of the PLCL after EtO sterilization cycles remained constant at ∼26%. This indicates that EtO sterilization has no effect on the polymer properties of the balloon films.
On the other hand, an increase in the ΔH of the polymer is seen after treatment with a 25 kGy radiation dose. The ΔH and the crystallinity in the radiation-treated balloon sample were 27.5 J g–1 and ∼29%, respectively. This increase in the crystallinity in PLCL is attributed to the mobility and rearrangement of the smaller chain segments upon radiation. In support to our observation, Mansouri et al.58 observe an increase in the crystallinity of the PLA upon radiation treatment to 45 kGy, which they ascribe to the reorientation of shorter polymer chains. Suljovrujić et al.59 suggested that during the radiation treatment scission of the PLA molecules occur, leading to the growth of thin-crystal structures. Possibly, the formation of low molecular weight degradation products nucleates together internally, exaggerating crystallinity of the polymer material. This formation of the thin crystal structures induces an overall increase in polymer crystallinity. However, Walo et al.60 illustrate that there is no advantageous relationship between the radiation-induced increased crystallinity with the stability of the polymers. They explored the phenomenon that the increase in the crystallinity is not only observed in PLA but also with PCL. However, this increase in crystallinity in both the PLA and PCL is temporary and decreases with time. We believe that the observed increase in the crystallinity in PLCL when treated with 25 kGy radiation is simply because of the early stage self-nucleation of the degraded byproducts such as short polymer chains.
3. Discussion
It has been widely observed that bioabsorbable polymers are more susceptible to degradation because of their hydrolytic instability during sterilization.61−63 The polymer undergoes chain scission when treated under 25 kGy radiation. However, the short chain-fragmented macromolecule initially tends to reorient itself over the already existing polymer crystals, resulting in a more crystalline form of the polymer. With an increase in the radiation dose, this reorientation is destroyed because of the recombination of free radicals within crystalline regions.60 Such changes were detected by DSC while analyzing our balloons. Experiments showed an increase in the ΔH of the radiation-treated samples. On the other hand, EtO treatment did not promote any chain mobilization or reorientation, therefore no change in the ΔH and the degree of crystallinity was obtained in the balloons.
We presume that the wide difference between the EtO and γ-sterilization results for PLCL is because of the sensitivity of its PLA and PCL chain components. A comparative analysis confirms that radiation sterilization initiates degradation in both polymers, however the effect in PCL is smaller than in PLA.60 Several studies report that γ-radiation causes chain scission in PLA, leading to a decrease in molecular weight and mechanical properties.64,65 This effect has been studied using a mathematical model, which reveals that sterilization hydrolysis of PLCL after radiation is about 3.5 times faster than polymer samples without sterilization.73
It is interesting that EtO sterilization does not alter the molecular structure or molecular weight of the polymers.66 These results are in agreement with Steripro67 and Tipnis,68 who report that EtO sterilization is considered compatible with polymers used in implantable medical devices. Middleton et al.69 studied the effects of γ- and EtO-sterilization on the intrinsic viscosities of molded PLA parts. They report that physical properties of the material sterilized using γ-radiation are considerably less than material sterilized by EtO.
It is now clear that the PLA chain segment in PLCL is the major component sensitive to γ-ration treatment. Because 70% of the total chain segments in PLCL are composed of PLA chains, it is obvious that any change in the PLA segment is sufficient to cause major changes in the physical properties of the PLCL material during sterilization. We conclude that for PLCL balloons, sterilization treatment using EtO is much more beneficial, as it has an insignificant effect on molecular properties. Undoubtedly, EtO-sterilized PLCL balloons will have self-stability and high performance as polymer implants compared to the balloons treated with γ-radiation.
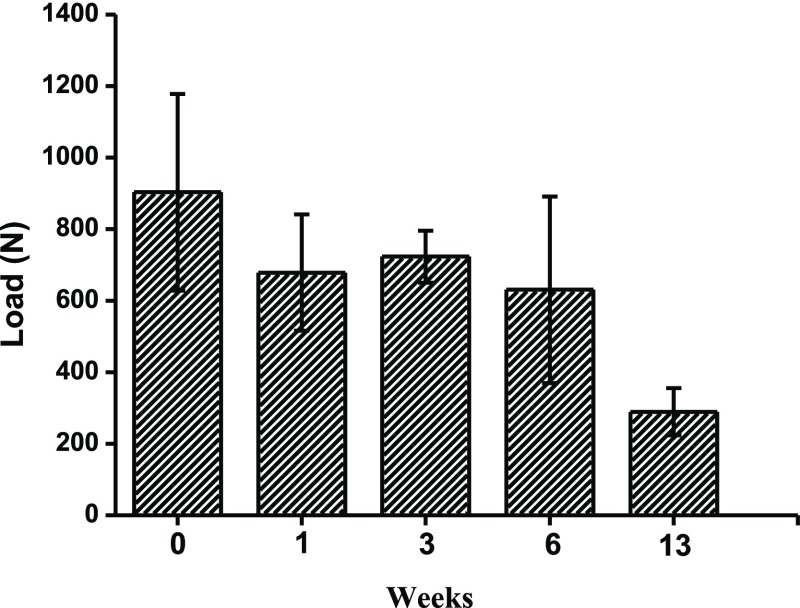
To evaluate the mechanical stability of PLCL balloon implants after EtO sterilization, we examined the stability of the balloons under external loading using an INSTRON fatigue testing machine. Saline-filled balloons were immersed into a buffer medium for 1, 3, 6, and 13 weeks and then taken out from the aqueous medium to check their mechanical stability. The load on the balloons was increased until the balloon leaked. Figure 4 summarizes the mean of the maximum loads until the balloon ruptured or started leaking. Balloon resistance to external loads: the test reveals that at least until week 6 (42 days in the incubator), there was no statistically significant difference between the external loads applied on the balloons until failure. At week 13 (91 days), the balloons sprang a leak under external loads. External loading tests demonstrate that EtO-sterilized balloons withstand higher loads of more than 900 N. Variability of the results was high within each group, but no statistically significant difference was found between the groups from 0 to 6 weeks. Although it appears that there is a slight decrease in the maximum load applied on the balloons until failure, the correlation is not good (R2 = 0.56), and analysis of variance tests using Excel reveal that there is no statistically significant difference between the results of any of the groups 2, 4, 6, and 8 (0, 1, 3, and 6 weeks, respectively) (min. p value was 0.11). When considering week 6 versus week 13, there is a significant difference (F(1,8) = 6.317, p = 0.0362). The results demonstrate that the mechanical properties of the balloons are not influenced by exposure to EtO. This also correlates with results from the in vitro fatigue test, which shows that the mechanical properties of the balloons remain the same when subjected under a constant cyclic loading for more than 8 weeks.70
Figure 4.
Maximum load applied on the balloons until failure. The balloons were kept in closed glass containers (separate container per balloons), and pH levels were monitored to be 7.4 ± 0.2 throughout the test period. After a certain time period, the balloons were taken out from the aqueous medium and further examined under applied loading.
4. Conclusions
Our study clarifies the impacts of EtO and γ-radiation sterilization on the safety and performance of absorbable PLCL-based balloon implants. γ-Radiation sterilization results in a change in the material properties in balloons. Apparently, the molecular weight (Mw, Mn, and PDI) and η for the PLCL material decrease significantly after radiation sterilization. Because most medical devices are constructed from biodegradable polymers, a decrease in the molecular weight distribution increases the risk of early degradation after γ-sterilization. On the other hand, EtO sterilization does not disturb polymer material properties. No change in the molecular weight distribution and η was observed for EtO-treated PLCL.
In conclusion, we recommend EtO sterilization for PLCL-based medical devices because it has been compatible with the polymer in this experimental study. These considerations are critical to balloon performance because complete assurance that failures will not occur during the defined time-period is a major concern.
5. Material and Methods
5.1. Materials
PLCL (70:30) (Resomer LC703S) was obtained from Evonik, Germany. Water and DCM HPLC grade were purchased from Bio-Lab, Jerusalem, Israel. Agarose was obtained from Merck, Israel.
5.2. Balloon Preparation
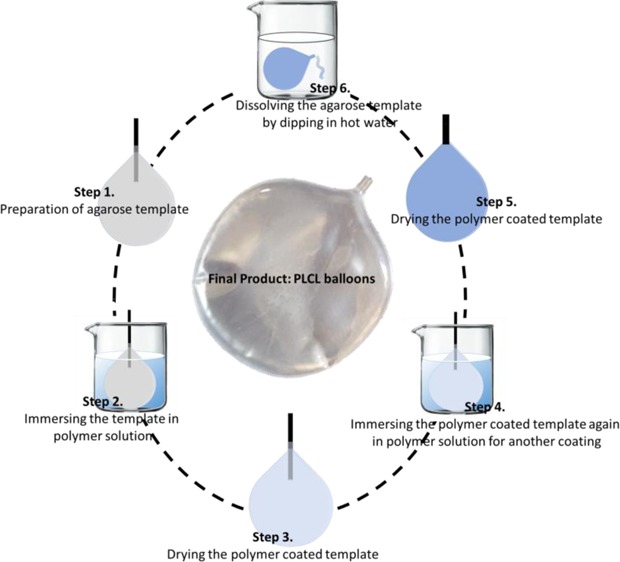
PLCL balloons were prepared using a multicycle dip-coating process.1 As shown in Scheme 1, the balloons were prepared by dipping a balloon-shaped agarose template into 14% w/v PLCL (70:30) in DCM solution and drying the polymer-coated template under a nitrogen atmosphere. The agar was then removed by immersing the coated cast into hot water and drying again. The final balloons were kept under sealed dry conditions.
Scheme 1. Steps Involved in the Preparation of PLCL Balloons.
5.3. Methods
PLCL balloons were tested for their molecular stability using different terminal sterilization methods. In this study, the control balloons were those without sterilization.
5.4. EtO Sterilization
EtO sterilization is a low-temperature sterilization procedure that can be applied to a wide range of materials. It is the preferred alternative to irradiation for heat-labile medical devices and pharmaceutical-packaging components. The EtO sterilization process utilizes EtO gas that has bactericidal, sporicidal, and virucidal effects. The basic EtO sterilization cycle was followed according to the standard guidelines consisting of five stages: preconditioning and humidification, gas introduction, exposure, evacuation, and air washes.71 Mendes et al.72 provide detailed guidelines for the safe and effective use of EtO sterilization for medical devices. In this study, the EtO sterilizations were performed in Mediplast Israel Ltd. (Yavne, Israel) following EN ISO-11135:2007, EN ISO-13485, and ISO-9001:2008 guidelines. ISO 11135 specifies the requirements for the development, validation, and routine control of the EtO sterilization process for medical devices in both the industrial and health care facility settings. Cingolani et al.73 describe the details of the EtO sterilization of PLCL material based on the guidelines of ISO 1135.
Briefly, the samples were preconditioned for 15 h at 45 ± 5 °C in 50–80% of relative humidity in a separate chamber. Thereafter, the samples were placed in the EtO sterilization chamber. During the sterilization cycle, inside the sterilization chamber, preconditioning is done for 3 h at 45 ± 5 °C and a vacuum of −0.8 ± 0.05 bar. Then, the product is exposed to a gas mixture of 90% EtO and 10% CO2 for 6 h. The quantity of sterilant is 10.04 ± 0.315 kg. The degassing step is followed by seven cycles of vacuuming to −0.8 to 0.8 ± 0.05 bar with each vacuuming cycle involving 10–15 min.
5.5. Radiation Sterilization
Radiation sterilization, a γ-sterilization, is perhaps the most popular procedure for the terminal sterilization of bioabsorbable polymer-based medical devices that can occur at low temperature. Radiation sterilization utilizes ionizing radiation from a cobalt-60 (60Co) isotope source or machine-generated accelerated electrons. The most commonly validated dose used to sterilize medical devices is 25 kGy.74−76 In this study, γ-sterilization was performed at Sorvan Radiation Ltd. (Yavne, Israel), according to the international standards ISO 11137 and EN 552. The samples were subjected to 60Co γ-irradiation at a dose of 25 kGy (2.5 Mrad). Details of the radiation sterilization of PLCL material based on the guidelines of ISO 1137 are provided elsewhere.73
5.6. Differential Scanning Calorimetry
The thermal properties of PLCL in the EtO and radiation-treated balloons were measured by a DSC-1, Mettler Toledo instrument under a nitrogen atmosphere. Each sample of about 8 mg in an aluminum crucible was heated from 25 to 200 °C at a scan rate of 10 °C/min. Each sample was run in triplicate. The results are presented as the average data.
5.7. Gel Permeation Chromatography
Molecular weight distributions of the PLCL before and after sterilization were determined by GPC equipped with a Waters 1515 isocratic HPLC pump, L-7490 refractive index detector (Hitachi), and a Rheodyne (Cotati, CA) injection valve with a 20 μL loop. Polymer samples (5 mg) were dissolved in 2 mL chloroform. Samples were tested in triplicate. Each sample was filtered through a 0.45 μm filter directly into the GPC vials. Samples were eluted with chloroform (HPLC grade) through a linear Styragel column, HR4 (Waters, MA) at a flow rate of 1 mL/min. Molecular weights were determined relative to polystyrene standards (PolyScience, Warrington, PA). Calibration curve and calculations were performed using Empower Software (Murrieta, CA, USA).
5.8. Inherent Viscosity (η) Measurements
One full-thickness section from each balloon was dissolved in chloroform to a concentration of 1.0% (w/v). Viscosity was measured using Cannon Ubbelohde Semi-Micro N223 with 50 μm capillary diameter at 25.0 ± 1.0 °C.
The η was calculated according to the following equation (eq 2)
| 2 |
where η = inherent viscosity, ηrel = relative viscosity, and C = mass concentration of the polymer (g/dL).
The authors declare no competing financial interest.
References
- Basu A.; Haim-Zada M.; Domb A. J. Biodegradable inflatable balloons for tissue separation. Biomaterials 2016, 105, 109–116. 10.1016/j.biomaterials.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Szöllösy G.; Rosso C.; Fogerty S.; Petkin K.; Lafosse L. Subacromial Spacer Placement for Protection of Rotator Cuff Repair. Arthrosc. Tech. 2014, 3, e605–e609. 10.1016/j.eats.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senekovic V.; Poberaj B.; Kovacic L.; Mikek M.; Adar E.; Dekel A. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 311–316. 10.1007/s00590-012-0981-4. [DOI] [PubMed] [Google Scholar]
- Rosa D.; Balato G.; Ciaramella G.; Di Donato S.; Auletta N.; Andolfi C. Treatment of massive irreparable rotator cuff tears through biodegradable subacromial InSpace Balloon. BMC Surg. 2013, 13, A43. 10.1186/1471-2482-13-s1-a43. [DOI] [Google Scholar]
- Yuan M. W.; Qin Y. Y.; Yang J. Y.; Wu Y.; Yuan M. L.; Li H. L. Preparation and Characterization of Poly(l-Lactide-co-ε-Caprolactone) Copolymer for Food Packaging Application. Adv. Mater. Res. 2013, 779–780, 231–234. 10.4028/www.scientific.net/amr.779-780.231. [DOI] [Google Scholar]
- Park J.; Lee B.; Park S.; Kim M.; Lee J.; Lee H.; Lee H.; Kim J.; Kim M. Preparation of Biodegradable and Elastic Poly(ε-caprolactone-co-lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier. Int. J. Mol. Sci. 2017, 18, 671. 10.3390/ijms18030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi N.; Hashemi S. M.; Salehi M.; Jahani H.; Mowla S. J.; Soleimani M.; Hosseinkhani H. Influence of oriented nanofibrous PCL scaffolds on quantitative gene expression during neural differentiation of mouse embryonic stem cells. J. Biomed. Mater. Res., Part A 2016, 104, 155–164. 10.1002/jbm.a.35551. [DOI] [PubMed] [Google Scholar]
- Jahani H.; Jalilian F. A.; Wu C. Y.; Kaviani S.; Soleimani M.; Abassi N.; Ou K. L.; Hosseinkhani H. Controlled surface morphology and hydrophilicity of polycaprolactone toward selective differentiation of mesenchymal stem cells to neural like cells. J. Biomed. Mater. Res., Part A 2015, 103, 1875–1881. 10.1002/jbm.a.35328. [DOI] [PubMed] [Google Scholar]
- Jeong S. I.; Kim B.-S.; Lee Y. M.; Ihn K. J.; Kim S. H.; Kim Y. H. Morphology of Elastic Poly(l-lactide-co-ε-caprolactone) Copolymers and in Vitro and in Vivo Degradation Behavior of Their Scaffolds. Biomacromolecules 2004, 5, 1303–1309. 10.1021/bm049921i. [DOI] [PubMed] [Google Scholar]
- Szöllösy G.; Rosso C.; Fogerty S.; Petkin K.; Lafosse L. Subacromial Spacer Placement for Protection of Rotator Cuff Repair. Arthrosc. Tech. 2014, 3, e605–e609. 10.1016/j.eats.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartoneva R.; Haimi S.; Miettinen S.; Mannerström B.; Haaparanta A.-M.; Sándor G. K.; Kellomäki M.; Suuronen R.; Lahdes-Vasama T. Comparison of a poly-l-lactide-co-ε-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J. R. Soc., Interface 2011, 8, 671–677. 10.1098/rsif.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roato I.; Belisario D. C.; Compagno M.; Verderio L.; Sighinolfi A.; Mussano F.; Genova T.; Veneziano F.; Pertici G.; Perale G.; Ferracini R. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018, 2018, 4126379. 10.1155/2018/4126379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro D.; Perale G.; Milazzo M.; Moscato S.; Stefanini C.; Pertici G.; Danti S. Bovine bone matrix/poly(l-lactic-co-ε-caprolactone)/gelatin hybrid scaffold (SmartBone) for maxillary sinus augmentation: A histologic study on bone regeneration. Int. J. Pharm. 2017, 523, 534–544. 10.1016/j.ijpharm.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Gu P.; Wang Z.; Lin M.; Xie Q.; Sun H.; Huang Y.; Zhang D.; Yu Z.; Bi X.; Chen J.; Wang J.; Shi W.; Fan X. Electrospun silk fibroin/poly(lactide-co-ε-caprolactone) nanofibrous scaffolds for bone regeneration. Int. J. Nanomed. 2016, 11, 1483–1500. 10.2147/ijn.s97445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smink A. M.; de Haan B. J.; Wolters A. H. G.; Kuipers J.; Giepmans B. N. G.; Schwab L.; Engelse M. A.; van Apeldoorn A. A.; de Koning E.; Faas M. M.; de Vos P. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed. Mater. 2016, 11, 035006. 10.1088/1748-6041/11/3/035006. [DOI] [PubMed] [Google Scholar]
- Smink A. M.; Hertsig D. T.; Schwab L.; van Apeldoorn A. A.; de Koning E.; Faas M. M.; de Haan B. J.; de Vos P. A retrievable, efficacious polymeric scaffold for subcutaneous transplantation of rat pancreatic islets. Ann. Surg. 2017, 266, 149–157. 10.1097/sla.0000000000001919. [DOI] [PubMed] [Google Scholar]
- Smink A. M.; Li S.; Hertsig D. T.; de Haan B. J.; van Apeldoorn L.; de Koning E.; Faas M. M.; Lakey J. R. T.; de Vos P. The Efficacy of a Prevascularized, Retrievable Poly(d,l,-lactide-co-ε-caprolactone) Subcutaneous Scaffold as Transplantation Site for Pancreatic Islets. Transplantation 2017, 101, e112–e119. 10.1097/tp.0000000000001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai O.; Panchagnula R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- Hutchinson J. M. Physical aging of polymers. Prog. Polym. Sci. 1995, 20, 703–760. 10.1016/0079-6700(94)00001-i. [DOI] [Google Scholar]
- Odegard G. M.; Bandyopadhyay A. Physical aging of epoxy polymers and their composites. J. Polym. Sci., Part B: Polym. Phys. 2011, 49, 1695–1716. 10.1002/polb.22384. [DOI] [Google Scholar]
- Pan P.; Zhu B.; Inoue Y. Enthalpy Relaxation and Embrittlement of Poly(l-lactide) during Physical Aging. Macromolecules 2007, 40, 9664–9671. 10.1021/ma071737c. [DOI] [Google Scholar]
- Pan P.; Zhu B.; Dong T.; Yazawa K.; Shimizu T.; Tansho M.; Inoue Y. Conformational and microstructural characteristics of poly(L-lactide) during glass transition and physical aging. J. Chem. Phys. 2008, 129, 184902. 10.1063/1.3010368. [DOI] [PubMed] [Google Scholar]
- Pan H.; Na B.; Lv R.; Zou S. Embrittlement of poly (L-lactide)/poly (ε-caprolactone) blendsupon physical aging. J. Polym. Res. 2012, 19, 9936. 10.1007/s10965-012-9936-z. [DOI] [Google Scholar]
- Tsuji H.; Mizuno A.; Ikada Y. Enhanced crystallization of poly(l-lactide-co-ε-caprolactone) during storage at room temperature. J. Appl. Polym. Sci. 2000, 76, 947–953. . [DOI] [Google Scholar]
- Saha S. K.; Tsuji H. Effects of rapid crystallization on hydrolytic degradation and mechanical properties of poly(l-lactide-co-ε-caprolactone). React. Funct. Polym. 2006, 66, 1362–1372. 10.1016/j.reactfunctpolym.2006.03.020. [DOI] [Google Scholar]
- McKeen L.Effect of Sterilization Method on Plastics and Elastomers, 3rd ed.; Elsevier Inc.: New York, NY, USA, 2012. [Google Scholar]
- Tipnis N. P.; Burgess D. J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. 10.1016/j.ijpharm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Leonard D.; Buchanan F. J.. The Effect of Gamma Sterilisation on the Mechanical Properties and Bioresorption Rate of Poly(l-Lactide). 7th World Biomaterials Congress, 2004.
- Farrar D. F.; Gillson R. K. Hydrolytic degradation of polyglyconate B: the relationship between degradation time, strength and molecular weight. Biomaterials 2002, 23, 3905–3912. 10.1016/s0142-9612(02)00140-0. [DOI] [PubMed] [Google Scholar]
- Clayton J. L. Decontamination, sterilization, and disinfection. Minim. Invasive Surg. Nurs. 1996, 10, 13–20. [PubMed] [Google Scholar]
- Guidoin R.; Snyder R.; King M.; Martin L.; Botzko K.; Awad J.; Marois M.; Gosselin C. A compound arterial prosthesis: the importance of the sterilization procedure on the healing and stability of albuminated polyester grafts. Biomaterials 1985, 6, 122–128. 10.1016/0142-9612(85)90075-4. [DOI] [PubMed] [Google Scholar]
- Shintani H. Ethylene Oxide Gas Sterilization of Medical Devices. Biocontrol Sci. 2017, 22, 1–16. 10.4265/bio.22.1. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Bjursten L. M.; Freij-Larsson C.; Kober M.; Wesslen B. Tissue response to commercial silicone and polyurethane elastomers after different sterilization procedures. Biomaterials 1996, 17, 2265–2272. 10.1016/0142-9612(96)00055-5. [DOI] [PubMed] [Google Scholar]
- Valente T. A. M.; Silva D. M.; Gomes P. S.; Fernandes M. H.; Santos J. D.; Sencadas V. Effect of Sterilization Methods on Electrospun Poly(lactic acid) (PLA) Fiber Alignment for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 3241–3249. 10.1021/acsami.5b10869. [DOI] [PubMed] [Google Scholar]
- Pietrzak W. S. Effects of Ethylene Oxide Sterilization on 82:18 PLLA/PGA Copolymer Craniofacial Fixation Plates. J. Craniofac. Surg. 2010, 21, 177–181. 10.1097/scs.0b013e3181c50e9c. [DOI] [PubMed] [Google Scholar]
- Saeidlou S.; Huneault M. A.; Li H.; Park C. B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. 10.1016/j.progpolymsci.2012.07.005. [DOI] [Google Scholar]
- Mendes G. C. C.; Brandão T. R. S.; Silva C. L. M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 2007, 35, 574–581. 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Bernkopf M. Sterilisation of bioresorbable polymer implants. Med. Device Technol. 2007, 26, 28–29. [PubMed] [Google Scholar]
- Saberianpour S.; Heidarzadeh M.; Geranmayeh M. H.; Hosseinkhani H.; Rahbarghazi R.; Nouri M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng. 2018, 12, 36. 10.1186/s13036-018-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghitalab F.; Hosseinkhani H.; Shokrgozar M. A.; Mao C.; Yang M.; Farokhi M. Silk as a potential candidate for bone tissue engineering. J. Controlled Release 2015, 215, 112–128. 10.1016/j.jconrel.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Horakova J.; Mikes P.; Saman A.; Jencova V.; Klapstova A.; Svarcova T.; Ackermann M.; Novotny V.; Suchy T.; Lukas D. The effect of ethylene oxide sterilization on electrospun vascular grafts made from biodegradable polyesters. Mater. Sci. Eng., C 2018, 92, 132–142. 10.1016/j.msec.2018.06.041. [DOI] [PubMed] [Google Scholar]
- Chaochanchaikul K.; Harnnarongchai W. Influence of Multifunctional Monomers on Gamma Irradiated Polylactic Acid. Appl. Mech. Mater. 2015, 804, 59–62. 10.4028/www.scientific.net/amm.804.59. [DOI] [Google Scholar]
- Cairns M.-L.; Dickson G. R.; Orr J. F.; Farrar D.; Hawkins K.; Buchanan F. J. Electron-beam treatment of poly(lactic acid) to control degradation profiles. Polym. Degrad. Stab. 2011, 96, 76–83. 10.1016/j.polymdegradstab.2010.10.016. [DOI] [Google Scholar]
- Nugroho P.; Mitomo H.; Yoshii F.; Kume T. Degradation of poly(l-lactic acid) by γ-irradiation. Polym. Degrad. Stab. 2001, 72, 337–343. 10.1016/s0141-3910(01)00030-1. [DOI] [Google Scholar]
- Pietrzak W. S.; Kumar M.; Eppley B. L. The influence of temperature on the degradation rate of LactoSorb copolymer. J. Craniofac. Surg. 2003, 14, 176–183. 10.1097/00001665-200303000-00008. [DOI] [PubMed] [Google Scholar]
- Nuutinen J.-P.; Clerc C.; Virta T.; Törmälä P. Effect of gamma, ethylene oxide, electron beam, and plasma sterilization on the behaviour of SR-PLLA fibres in vitro. J. Biomater. Sci., Polym. Ed. 2002, 13, 1325–1336. 10.1163/15685620260449723. [DOI] [PubMed] [Google Scholar]
- Suuronen R.; Pohjonen T.; Hietanen J.; Lindqvist C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J. Oral Maxillofac. Surg. 1998, 56, 604–614. 10.1016/s0278-2391(98)90461-x. [DOI] [PubMed] [Google Scholar]
- Nugroho P.; Mitomo H.; Yoshii F.; Kume T. Degradation of poly(l-lactic acid) by γ-irradiation. Polym. Degrad. Stab. 2001, 72, 337–343. 10.1016/s0141-3910(01)00030-1. [DOI] [Google Scholar]
- Thanki Paragkumar N.; Edith D.; Jean-Luc S. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. 10.1016/j.apsusc.2006.05.047. [DOI] [Google Scholar]
- Sato S.; Gondo D.; Wada T.; Kanehashi S.; Nagai K. Effects of Various Liquid Organic Solvents on Solvent-Induced Crystallization of Amorphous Poly(lactic acid) Film. J. Appl. Polym. Sci. 2013, 129, 1607–1617. 10.1002/app.38833. [DOI] [Google Scholar]
- Runt J.; Rim P. B. Effect of preparation conditions on the development of crystallinity in compatible polymer blends: poly(styrene-co-acrylonitrile)/poly(ε-caprolactone). Macromolecules 1982, 15, 1018–1023. 10.1021/ma00232a013. [DOI] [Google Scholar]
- Han W.; Liao X.; Yang Q.; Li G.; He B.; Zhu W.; Hao Z. Crystallization and morphological transition of poly(l-lactide)-poly(ε-caprolactone) diblock copolymers with different block length ratios. RSC Adv. 2017, 7, 22515–22523. 10.1039/c7ra03496b. [DOI] [Google Scholar]
- Patel S. P.; Vaishya R.; Pal D.; Mitra A. K. Novel Pentablock Copolymer-Based Nanoparticulate Systems for Sustained Protein Delivery. AAPS PharmSciTech 2015, 16, 327–343. 10.1208/s12249-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein T.; Nasser-Eddine M.; Delaite C.; Bistac S.; Dumas P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. 10.1016/j.jcis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Tsuji H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- Sosnowski S. Poly(l-lactide) microspheres with controlled crystallinity. Polymer 2001, 42, 637–643. 10.1016/s0032-3861(00)00362-1. [DOI] [Google Scholar]
- Byun Y.; Whiteside S.; Thomas R.; Dharman M.; Hughes J.; Kim Y. T. The effect of solvent mixture on the properties of solvent cast polylactic acid (PLA) film. J. Appl. Polym. Sci. 2012, 124, 3577–3582. 10.1002/app.34071. [DOI] [Google Scholar]
- Mansouri M.; Berrayah A.; Beyens C.; Rosenauer C.; Jama C.; Maschke U. Effects of electron beam irradiation on thermal and mechanical properties of poly(lactic acid) films. Polym. Degrad. Stab. 2016, 133, 293–302. 10.1016/j.polymdegradstab.2016.09.005. [DOI] [Google Scholar]
- Suljovrujić E.; Ignjatović N.; Uskoković D. Gamma irradiation processing of hydroxyapatite/poly-L-lactide composite biomaterial. Radiat. Phys. Chem. 2003, 67, 375–379. 10.1016/s0969-806x(03)00070-7. [DOI] [Google Scholar]
- Walo M.; Przybytniak G.; Nowicki A.; Świeszkowski W. Radiation-Induced Effects in Gamma-Irradiated PLLA and PCL at Ambient and Dry Ice Temperatures. J. Appl. Polym. Sci. 2011, 122, 375–383. 10.1002/app.34079. [DOI] [Google Scholar]
- Smit T. H.; Thomas K. A.; Hoogendoorn R. J. W.; Strijkers G. J.; Helder M. N.; Wuisman P. I. J. M. Sterilization and strength of 70/30 polylactide cages: e-beam versus ethylene oxide. Spine 2007, 32, 742–747. 10.1097/01.brs.0000259057.94986.3b. [DOI] [PubMed] [Google Scholar]
- Holy C. E.; Cheng C.; Davies J. E.; Shoichet M. S. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 2000, 22, 25–31. 10.1016/s0142-9612(00)00136-8. [DOI] [PubMed] [Google Scholar]
- Weir N. A.; Buchanan F. J.; Orr J. F.; Farrar D. F.; Boyd A. Processing, annealing and sterilisation of poly-l-lactide. Biomaterials 2004, 25, 3939–3949. 10.1016/j.biomaterials.2003.10.076. [DOI] [PubMed] [Google Scholar]
- Middleton J. C.; Tipton A. J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- Araújo A.; Botelho G. L.; Silva M.; Machado A. V. UV Stability of Poly(Lactic Acid) Nanocomposites. J. Mater. Sci. Eng. B 2013, 3, 75–83. 10.17265/2161-6221/2013.02.001. [DOI] [Google Scholar]
- Choi Y.; Yoon Kim S.; Moon M.-H.; Hee Kim S.; Lee K.-S.; Byun Y. Poly(ethylene glycol)-poly(l-lactide) diblock copolymer prevents aggregation of poly(l-lactide) microspheres during ethylene oxide gas sterilization. Biomaterials 2001, 22, 995–1004. 10.1016/s0142-9612(00)00265-9. [DOI] [PubMed] [Google Scholar]
- Steripro M.; Emea C.. EO Sterilization in Plastic and Polymers, June, 2017.
- Tipnis N. P.; Burgess D. J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. 10.1016/j.ijpharm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Middleton J.; Kines P.; Rogers A.; Strong J.. Proceedings Society of Biomaterials Conference, Society for Biomaterials 27th Annual Meeting Transactions: St. Paul, MN, 2001; p 457.
- Haim Zada M.; Kumar A.; Elmalak O.; Markovitz E.; Icekson R.; Machlev E.; Mechrez G.; Domb A. J. Biodegradable implantable balloons: Mechanical stability under physiological conditions. J. Mech. Behav. Biomed. Mater. 2019, 100, 103404. 10.1016/j.jmbbm.2019.103404. [DOI] [PubMed] [Google Scholar]
- Shintani H. Ethylene Oxide Gas Sterilization of Medical Devices. Biocontrol Sci. 2017, 22, 1–16. 10.4265/bio.22.1. [DOI] [PubMed] [Google Scholar]
- Mendes G. C. C.; Brandão T. R. S.; Silva C. L. M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 2007, 35, 574–581. 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Cingolani A.; Casalini T.; Caimi S.; Klaue A.; Sponchioni M.; Rossi F.; Perale G. A Methodologic Approach for the Selection of Bio-Resorbable Polymers in the Development of Medical Devices: The Case of Poly(l-lactide-co-ε-caprolactone). Polymers 2018, 10, 851. 10.3390/polym10080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. F.Sterilization of Medical Devices; Interpharm Press: Buffalo Grove, Illinois, 1999. [Google Scholar]
- Simmons A.; Hyvarinen J.; Poolewarren L. The effect of sterilisation on a poly(dimethylsiloxane)/poly(hexamethylene oxide) mixed macrodiol-based polyurethane elastomer. Biomaterials 2006, 27, 4484–4497. 10.1016/j.biomaterials.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Pharmaceutical Technical Procedures; Methods for Sterilization Organization TWH, 2013.