Human α-defensins are 3- to 5-kDa disulfide-bridged peptides with a multitude of antimicrobial activities and immunomodulatory functions. Recent studies show that human enteric α-defensin 5 (HD5), a host defense peptide important for intestinal homeostasis and innate immunity, aids the highly infectious enteropathogen Shigella in breaching the intestinal epithelium in vitro and in vivo.
KEYWORDS: human defensins, Shigella, macrophages
ABSTRACT
Human α-defensins are 3- to 5-kDa disulfide-bridged peptides with a multitude of antimicrobial activities and immunomodulatory functions. Recent studies show that human enteric α-defensin 5 (HD5), a host defense peptide important for intestinal homeostasis and innate immunity, aids the highly infectious enteropathogen Shigella in breaching the intestinal epithelium in vitro and in vivo. Whether and how HD5 influences Shigella infection of resident macrophages following its invasion of the intestinal epithelium remain poorly understood. Here, we report that HD5 greatly promoted phagocytosis of Shigella by macrophages by targeting the bacteria to enhance bacterium-to-cell contacts in a structure- and sequence-dependent fashion. Subsequent intracellular multiplication of phagocytosed Shigella led to massive necrotic cell death and release of the bacteria. HD5-promoted phagocytosis of Shigella was independent of the status of the type 3 secretion system. Furthermore, HD5 neither inhibited nor enhanced phagosomal escape of Shigella. Collectively, these findings confirm a potential pathogenic role of HD5 in Shigella infection of not only epithelial cells but also macrophages, illuminating how an enteropathogen exploits a host protective factor for virulence and infection.
INTRODUCTION
Human α-defensins are small (3 to 5 kDa) cationic peptides with broad antimicrobial activities against bacteria, fungi, and viruses. Based on their expression patterns and gene structures, the six human α-defensins identified to date are divided into two major classes: four myeloid defensins or human neutrophil peptides 1 to 4 (HNP1 to -4) and two enteric defensins, HD5 and HD6 (1–4). Despite their small size, α-defensins are active against microbes through multiple mechanisms (5–10). In addition to their direct antimicrobial activities, immunomodulatory effects of defensins are believed to play an important role in both innate and adaptive immunity, where they interact with various immune cells such as monocytes, T cells, dendritic cells, and macrophages under physiological and pathological conditions (11–14).
Shigella is a highly contagious Gram-negative enteropathogen that causes severe dysentery in humans at very low infectious doses (<100 bacteria) (15, 16). One critical step in Shigella pathogenesis is phagocytosis by resident macrophages after the bacterium has breached the intestinal epithelium. Rather than being killed by macrophages, phagocytosed Shigella manages to quickly escape from the phagosome through activation of the type III secretion system (T3SS) (17, 18). An activated T3SS then injects the invasion plasmid antigens IpaB, C, and D into the cytoplasm, resulting in the death of macrophages and secretion of interleukin (IL)-1β and IL-1, two major cytokines for recruitment of polymorphonuclear neutrophils (PMN) (19–21). These two cytokines and PMN are critical mediators of an acute and intense inflammatory response elicited by Shigella (22, 23). Once released from dying macrophages, Shigella subsequently invades intestinal epithelial cells from the basolateral side, followed by phagosomal escape, intracellular proliferation, and intra- and intercellular spread instigated by the virulence factor IcsA-mediated cytoskeleton rearrangement (17, 24–28).
Several recent reports have demonstrated that exogenous defensins can control infection of macrophages by intracellular pathogens such as Listeria monocytogenes, Mycobacterium tuberculosis, and Legionella pneumophila by enhancing phagocytosis, promoting reactive oxygen species (ROS) production, preventing phagosomal escape, and inhibiting cytoplasmic replication (29–35). While these studies collectively suggest a beneficial role of defensins in the fight of macrophages against some intracellular pathogens, whether this mode of action of defensins prevails in Shigella pathogenesis remains unclear. This uncertainty comes against the backdrop of our recent discovery that HD5 promotes Shigella infection of epithelial cells in vitro and in vivo by enhancing bacterial adhesion and invasion, conferring Shigella’s host selectivity and pathogenicity (36). Similar observations have also been made for HNP1 (37).
In light of these opposing effects of defensins and the fact that HD5 is abundantly expressed by Paneth cells of the small intestine proximal to the site of Shigella infection, we investigated the influence of HD5 on the pathogenesis of Shigella in macrophages. Our results demonstrated that HD5 greatly promoted the phagocytosis of Shigella by macrophages by enhancing their contact with the bacteria. However, HD5 had little effect on subsequent phagosomal escape and activation of the T3SS, resulting in unrestrained intracellular bacterial multiplication and accelerated death of infected macrophages accompanied by a massive bacterial release. Thus, HD5 exacerbates the pathogenicity of Shigella in macrophages, contrasting the findings made with other intracellular pathogens (30, 32, 35).
RESULTS
HD5 promotes Shigella internalization into macrophages by enhancing bacterial adhesion.
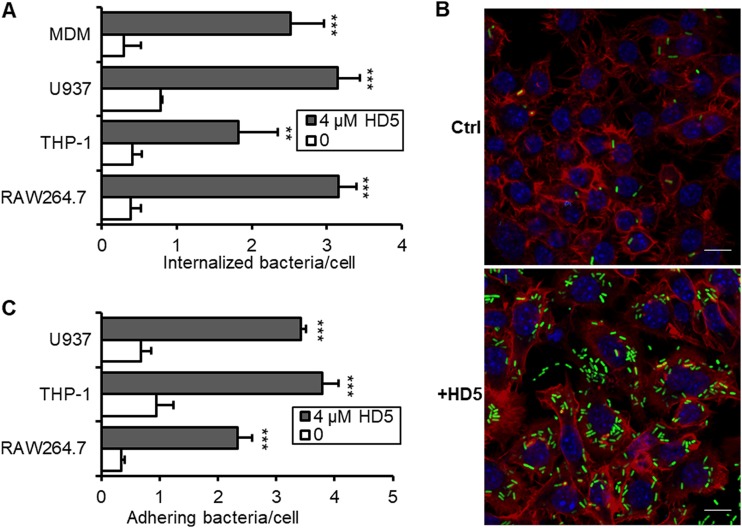
To investigate the influence of HD5 on Shigella pathogenesis in macrophages, we used Shigella flexneri Sf301 to infect human primary monocyte-derived macrophage (MDM) and three cultured macrophages: human U937 cells and THP-1 cells differentiated with phorbol 12-myristate 13-acetate (PMA) and murine RAW264.7 cells. CD11b, a typical macrophage differentiation marker, was examined by immunoblot during the differentiation. U937 cells differentiated for 4 days and THP-1 cells differentiated for 6 days with stable CD11b expression were used in the in vitro assays (see Fig. S1A and B in the supplemental material). In the classical gentamicin killing assay (17), we recovered significantly more intracellular bacteria from macrophages 1 h after infection in the presence of 4 μM HD5 than in the absence of HD5 (Fig. 1A). Fluorescence microscopic analysis verified the colony counting results, showing that 4 μM HD5 greatly increased the number of green fluorescent protein (GFP)-expressing Shigella bacteria inside macrophages compared to that in the mock-treated control (Fig. 1B), due, presumably, to HD5-enhanced bacterial internalization.
FIG 1.
HD5 promotes Shigella internalization into macrophages by enhancing bacterial adherence. (A) The effects of HD5 on the internalization of Shigella flexneri 2a strain Sf301 into primary monocyte-derived macrophages (MDMs) and three cultured macrophages, namely, PMA-differentiated human U937 cells, PMA-differentiated human THP-1 cells, and murine RAW264.7 cells, within 60 min. (B) Fluorescence microscopy analysis of GFP-expressing Sf301 internalization by HeLa cells in the absence or presence of 4 μM HD5 (MOI = 50:1). GFP-expressing bacteria are green, F-actin is red, and nuclei are blue (DAPI). Bars, 10 μm. (C) The effects of HD5 on the adhesion of Sf301 on macrophages within 10 min. Statistical significance was calculated (for HD5-treated samples compared to vehicle controls [0 μM]) using a Student's t test. **, P < 0.01; ***, P < 0.001.
To dissect the infection process of Shigella, we examined the effect of the defensin on bacterial adhesion, defined as the CFU of cell-associated bacteria 10 min after centrifugation to synchronize infection. While HD5 treatment significantly promoted initial bacterial adhesion to macrophages (Fig. 1C), the addition of the defensin after bacterial entry (30 min after) had little effect on the intracellular CFU in Shigella-infected macrophages (Fig. S1C). Thus, HD5 likely facilitated Shigella internalization into macrophages by enhancing bacterial adhesion. Since all four macrophages used in our work, MDM, U937, and THP-1 and RAW264.7 cells, behaved similarly in the in vitro infection assays, we focused on RAW264.7 cells in the subsequent studies unless indicated otherwise.
HD5 enhances phagocytosis of other enteric bacteria into macrophages.
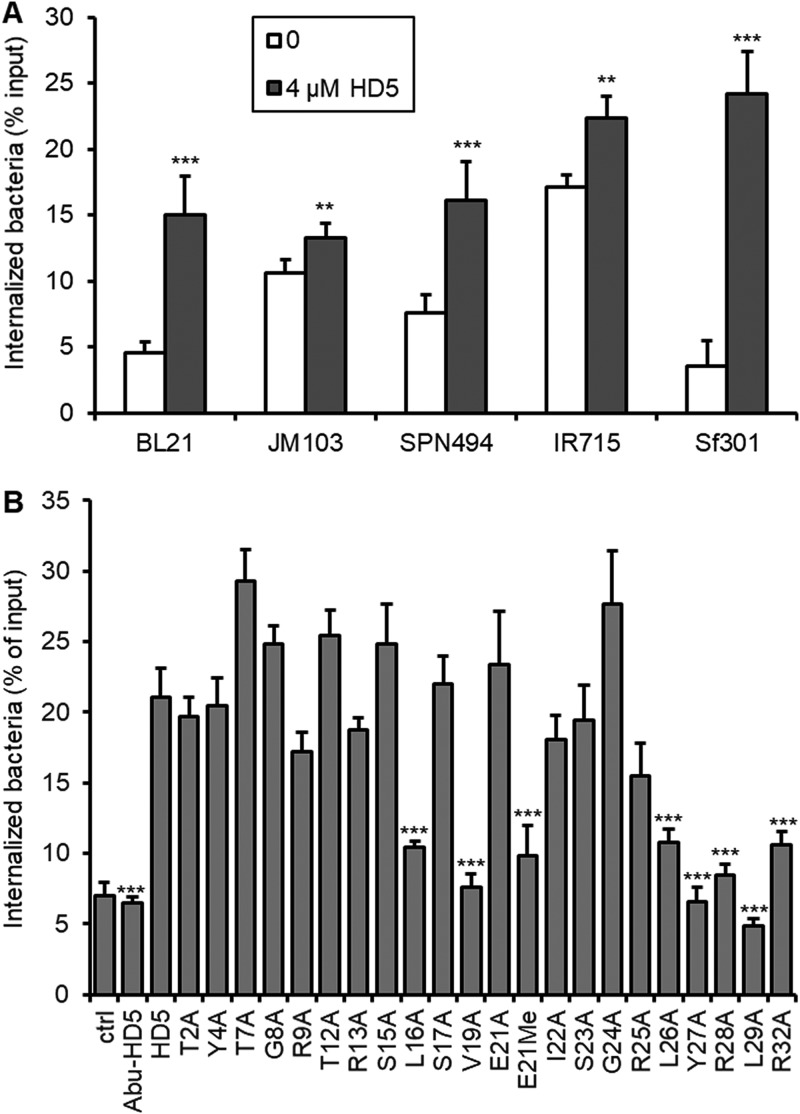
We previously demonstrated that the lack of fimbria expression on Shigella confers its sensitivity to HD5-enhanced infection (36). To understand whether HD5-enhanced phagocytosis is specific to Shigella, we examined four additional enteric bacteria with macrophages: wild-type Escherichia coli JM103 and its nonfimbriated mutant strain BL21 and wild-type Salmonella enterica serovar Typhimurium IR715 and its mutant strain SPN494 lacking both fimbria and flagella (7). As shown in Fig. 2A, both the invasive and noninvasive Salmonella and E. coli strains were efficiently internalized into RAW264.7 macrophages in the absence of HD5, with wild-type E. coli JM103 and Salmonella IR715 internalized at a higher level than their mutant strains. However, HD5 treatment only marginally improved internalization of E. coli JM103 and Salmonella IR715 by 25% to 35% while significantly enhancing internalization of their fimbria- and/or flagellum-deficient mutant strains by 2- to 3-fold. For Shigella Sf301, the difference of internalization between HD5 and mock treatment was 7-fold. These results suggest that HD5-enhanced bacterial uptake by macrophages, although not specific to Shigella, is strongly dependent on the expression or lack thereof of fimbria and/or flagella, consistent with the previous findings (36). Nevertheless, our subsequent studies focused on Shigella for two reasons: (i) its phagocytosis is highly sensitive to HD5 treatment in vitro, and (ii) its site of infection of macrophages is proximal to the site of expression of HD5 in vivo.
FIG 2.
(A) HD5 promotes internalization of E. coli and Salmonella strains into macrophages. Internalization assays were performed on nonfimbriated E. coli strain BL21, fimbriated E. coli strain JM103, wild-type Salmonella Typhimurium strain IR715, and its flagellum/fimbria-dual mutant strain SNP494; Sf301 was included for comparison. Data are shown as means ± SDs from at least three independent experiments. Statistical significance was calculated (for HD5-treated groups compared to vehicle controls [0]) using a Student's t test. **, P < 0.01; ***, P < 0.001. (B) Structural determinants of HD5 for its activity of promoting bacterial internalization into macrophages. Linear HD5 analog Abu-HD5, dimerization-debilitating analog E21Me, and a panel of alanine-substitution mutants were analyzed in the Shigella internalization assay. Wild-type HD5 and mock-treated control were included for comparison. Data are shown as means ± SDs from at least three independent experiments. Statistical significance was calculated using one-way analysis of variance (ANOVA) in comparison with wild-type HD5. ***, P < 0.001.
Critical molecular determinants of HD5 for its phagocytosis-enhancing activity.
It is known that hydrophobicity and selective cationicity segregated on a stable dimeric structure of HD5 dictate its functionality (8, 38, 39). We elucidated the critical molecular determinants of HD5 for its phagocytosis-enhancing activity in macrophages by analyzing a linear analog, a dimerization-debilitating analog, and a panel of alanine-substitution mutants of HD5. As shown in Fig. 2B, the linear HD5 with its six Cys residues replaced by isosteric aminobutyric acid (Abu), thus devoid of the three intramolecular disulfide bonds, had no activity in promoting phagocytosis of Shigella; the dimerization-debilitating analog E21Me-HD5, whose amide peptide bond at Glu21 is methylated to impair backbone H-bond-mediated HD5 dimerization (40), was largely inactive. Ala replacement of bulky hydrophobic residues, particularly those in the C-terminal region of HD5 such as Leu26, Tyr27, and Leu29, was functionally detrimental. Arg28 was a key residue for activity despite the fact that the other cationic Arg residues were functionally dispensable. These results from macrophages are in agreement with the findings from epithelial cells (36), indicative of similar molecular determinants underlying the ability of HD5 to promote phagocytosis of Shigella into macrophages and its adhesion to and invasion of epithelial cells.
HD5 targets Shigella to facilitate its phagocytosis independently of Shigella’s type 3 secretion system.
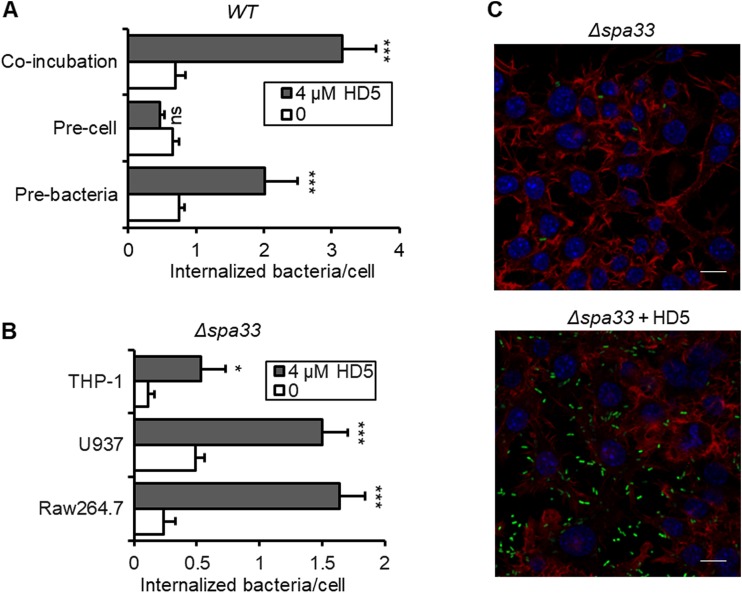
To elucidate the molecular and cellular mechanisms by which HD5 promotes Shigella internalization into macrophages, we pretreated bacteria or macrophages separately with HD5 before the gentamicin killing assay. As presented in Fig. 3A and Fig. S2, preexposure of Shigella to HD5 for 15 min boosted its intracellular CFU by a factor of 2.3 compared with that in the mock-treated control, whereas incubation of macrophages with HD5 for up to 1 h prior to infection had no effect. These results suggest that HD5-enhanced bacterial internalization is attributable to the interaction of the defensin with Shigella rather than with macrophages.
FIG 3.
HD5 mainly targets bacteria to promote internalization of Shigella into macrophages, independent of Shigella T3SS activity. (A) Either Sf301 bacteria or the RAW264.7 cells were pretreated with HD5 at the indicated concentration for 15 min and washed once with DMEM; gentamicin killing assays were performed in the absence of HD5. Internalized bacteria were determined as intracellular viable bacteria within 60 min. Colony counting analysis (B) and fluorescence microscopy analysis (C) of the effects of HD5 on the internalization of T3SS-deficient Shigella flexneri Δspa33 strain by macrophages within 60 min: F-actin is red, and nuclei are blue (DAPI). Bars, 10 μm. Data are shown as means ± SDs from at least three independent experiments. Statistical significance was calculated (for HD5-treated samples compared to vehicle controls [0 μM]) using a Student's t test. ns, not significant (P > 0.05); *, P < 0.05; ***, P < 0.001.
Contrasting Shigella’s entry into epithelial cells, a cellular event dependent on the activation of the type 3 secretion system (T3SS) (41), Shigella’s internalization into macrophages largely depends on the phagocytic machinery (24). To examine the role, if any, of the T3SS in HD5-augmented phagocytosis, we constructed a T3SS-null Shigella strain by deleting the spa33 gene that encodes the basal C-ring component of the T3SS (42). This mutant strain lacked the ability to invade epithelial cells in the presence of HD5 as previously described (36). As shown in Fig. 3A to C, although the deletion of spa33 reduced Shigella internalization into macrophages compared to that of wild-type Sf301 in the absence of HD5, the defensin significantly promoted the phagocytosis of Δspa33 bacteria at least to the same extent as Sf301. These data imply that HD5-enhanced phagocytosis of Shigella is independent of the T3SS.
HD5 does not inhibit or promote phagosomal escape of Shigella.
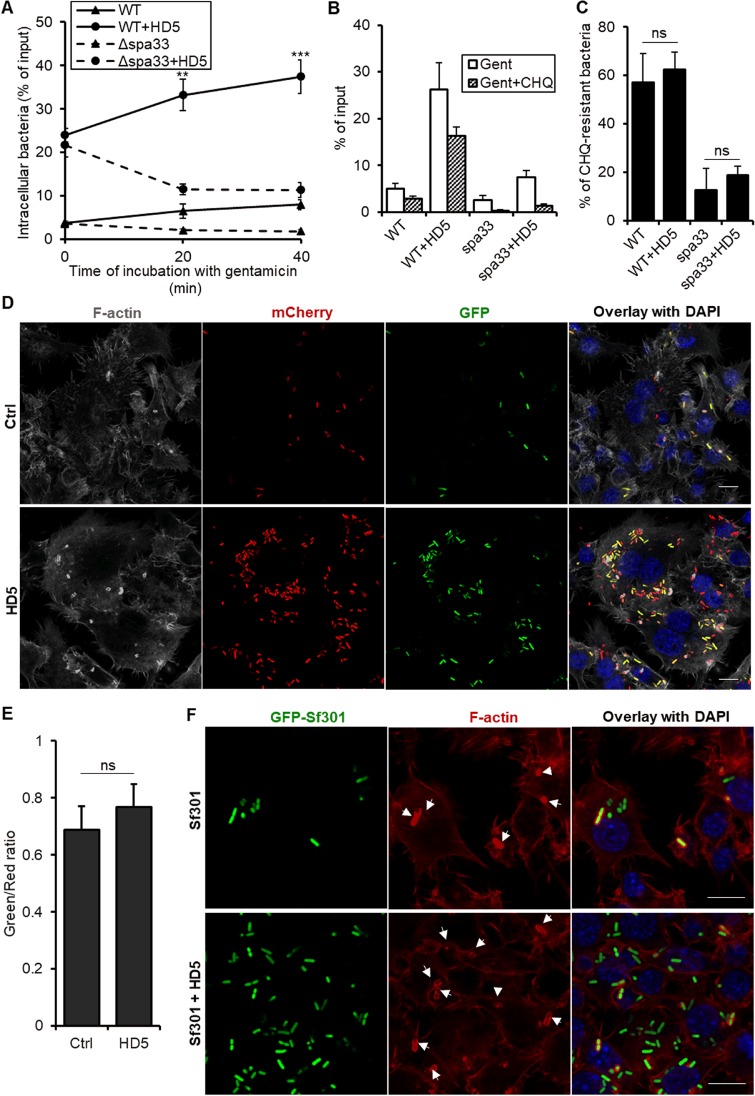
Internalized S. flexneri rapidly escapes from phagosomes in order to avoid phagosome-lysosome fusion and subsequent degradation; the escape is mediated by an activated T3SS that secretes virulence factors such as IpaC to facilitate the disruption of the phagosomal membrane (24). To examine the influence of HD5 on phagosomal escape of Shigella, we monitored dynamic changes in intracellular CFU at 0, 20, and 40 min postinfection, a time period when efficient phagosomal escape and the first round of bacterial multiplication ensued. As shown in Fig. 4A, wild-type Shigella increasingly proliferated intracellularly over time regardless of HD5 treatment. In contrast, the Δspa33 strain, deficient in phagosomal escape due to the loss of activity of the T3SS, failed to multiply in the absence of HD5. Although HD5 treatment boosted its initial uptake, intracellular CFU of Δspa33 Sf301 progressively tapered off over time.
FIG 4.
HD5 fails to inhibit phagosomal escape of Shigella. (A) Dynamic changes of intracellular bacteria in RAW264.7 cells infected with wild-type Sf301 or Δspa33 strains in the presence or absence of 4 μM HD5. Data are shown as means ± SDs from at least three independent experiments. Statistical significance was calculated (for samples at later time points compared to time point zero of the same treatment) using a one-way ANOVA. **, P < 0.01; ***, P < 0.001. (B and C) Analysis of chloroquine (CHQ) resistance of internalized Sf301 and Δspa33 bacteria in the presence or absence of 4 μM HD5. Chloroquine was added along with gentamicin to discriminate the bacteria trapped in phagosomes (chloroquine sensitive) and bacteria that escaped into the cytosol (chloroquine resistant). Percentages of chloroquine-resistant bacteria of total internalized bacteria in panel B were calculated and presented in panel C. Data are shown as means ± SDs from at least three independent experiments. Statistical significance was calculated using Student's t test. ns, not significant (P > 0.05). (D and E) Fluorescence microscopy analysis of activation of Shigella T3SS in the presence or absence of 4 μM HD5 40 min after infection. RAW264.7 cells were infected by Sf301 harboring a transcription-based secretion activity reporter (TSAR) plasmid which produces GFP upon activation of Shigella T3SS. Constitutive expression of mCherry fluorescence from the plasmid enabled us to identify all the phagocytosed bacteria. F-actin is gray and nuclei are blue (DAPI). Bars, 10 μm. The ratios of green activated bacteria to total red bacteria in panel D were calculated and presented in panel E. Ten randomly chosen fields of each group were analyzed, and the individual bacteria were automatically enumerated. Results are representative of three independent experiments and are shown as means ± SDs. Statistical significance of the HD5-treated group compared with mock treatment was calculated using Student's t test, and no significance was found. (F) Fluorescence microscopy analysis of actin polymerization by intracellular Sf301. GFP-expressing Sf301 cells are green, F-actin is red, and nuclei are blue (DAPI). Arrows indicated F-actin recruitment around bacteria. Bars, 10 μm.
Phagosomal escape was further verified by chloroquine resistance assays, which distinguish the bacteria trapped in phagosomes (chloroquine sensitive) from those that escaped (chloroquine resistant). As shown in Fig. 4B and C, ∼60% of wild-type Shigella escaped phagocytic vacuoles within 1 h, whereas the spa33 mutant bacteria were mostly killed in the vacuole by chloroquine during the same time. HD5 treatment, while boosting phagocytosis, did not alter the percentage of chloroquine-resistant or chloroquine-sensitive bacteria of either strain.
We further examined the activation of T3SS of Shigella by introducing a plasmid harboring a transcription-based secretion activity reporter (TSAR), which senses recent activation of the T3SS through MxiE-dependent promoter-controlled expression of a fast-maturing GFP (43). Phagocytosed bacteria that constitutively express the reporter gene emit red fluorescence of mCherry, which turns green upon activation of the T3SS. As demonstrated in Fig. 4D, 40 min after their initial uptake by macrophages, internalized bacteria in both mock-treated and HD5-treated groups exhibited extensive T3SS activation as evidenced by the green fluorescence. As expected, HD5 treatment substantially enhanced bacterial uptake by macrophages. However, quantification of the ratio of green fluorescent bacteria to phagocytosed total bacteria with red fluorescence indicated that HD5 treatment did not contribute to T3SS activation (Fig. 4E).
Upon escaping into the cytosol, Shigella recruits F-actin through its surface autotransporter IcsA to facilitate bacterial motility (44). Fluorescence microscopic analysis of polymerization of F-actin around the wild-type bacteria confirmed actin recruitment (Fig. 4F). However, no significant difference in actin recruitment was observed between HD5- and mock-treated groups (Fig. 4F). As expected, actin polymerization was not detected with the Δspa33 mutant bacteria, as they were trapped in the phagosomes (see Fig. S3). Taken together, our data demonstrated that HD5 enhances Shigella uptake by macrophages but does not prevent or promote its phagosomal escape, a process tightly dependent on T3SS activation.
Direct and indirect roles of HD5 in the death of macrophages induced by Shigella.
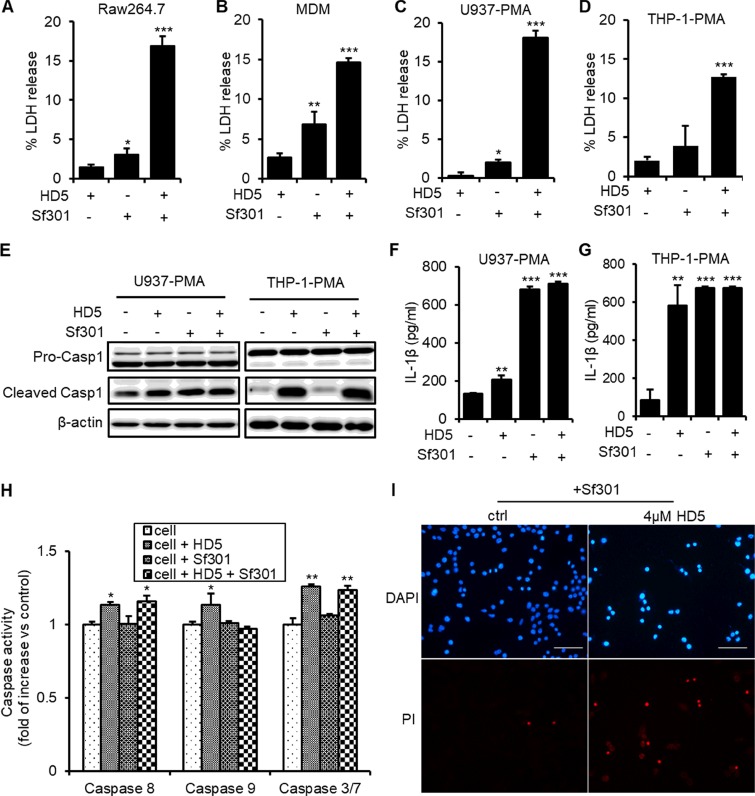
Upon escaping from the phagosome, Shigella begins to kill the macrophage, which is mainly mediated by the T3SS translocator/effectors (21). Following the killing of macrophages, released Shigella invades epithelial cells from the basolateral side of the human intestine (24). As evidenced by an elevated release of the cytoplasmic lactate dehydrogenase (LDH) (Fig. 5A to D) and by morphological changes (Fig. S2A), HD5 substantially accentuated the death of Shigella-infected macrophages within 1 h after enhanced phagocytosis, even though the defensin itself had no effect on T3SS activation. To investigate the release of bacteria from dying macrophages, we modified the conventional gentamicin killing assay by removing antibiotics after an initial 20-min treatment, followed by the addition of fresh medium without gentamicin and quantification of released bacteria, at 20 min intervals, from 40 min to 3 h after infection. As shown in Fig. S4B and C, the total released bacteria in the HD5-treated group were 7-fold more than that in the control group.
FIG 5.
HD5 exacerbates Shigella-induced macrophage death. (A to D) Release of the cytoplasmic lactate dehydrogenase (LDH) from the Sf301-infected RAW264.7, MDM, PMA-differentiated U937, and PMA-differentiated THP-1 cells in the presence or absence of 4 μM HD5. (E) Immunoblotting analysis of cleavage of caspase-1 in PMA-differentiated U937 and THP-1 cells infected with Sf301 in the presence or absence of 4 μM HD5. β-Actin was set as the internal control. (F and G) ELISA analysis of secretion of IL-1β from PMA-differentiated U937 and THP-1 cells infected with Sf301 in the presence or absence of 4 μM HD5. (H) Caspase activities of PMA-differentiated U937 cells infected with Sf301 in the presence or absence of 4 μM HD5 as analyzed by the Caspase-Glo assay system from Promega. (I) PI/DAPI staining of PMA-differentiated U937 cells after being infected with Sf301 for 1 h in the presence or absence of 4 μM HD5. Results are representative of three independent experiments and are shown as means ± SDs. Statistical significance was calculated (for samples compared to mock treatment) using a one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Since both caspase-1-mediated apoptosis (pyroptosis) and necrosis can be involved in Shigella-induced death of macrophages (19–21, 45–48), to better understand mechanisms of cell death, we first analyzed caspase-1 activation using an antibody that recognizes both uncleaved pro-caspase-1 and the p10 subunit of cleaved caspase-1. As shown in Fig. 5E, although caspase-1 activation was initiated within 1 h after phagocytosis of wild-type Shigella by macrophages, HD5 treatment did not further intensify it. Secretion of IL-1β triggered by activated caspase-1, one of the main proinflammatory responses during Shigella pathogenesis (23), was also analyzed using an enzyme-linked immunosorbent assay (ELISA). While Shigella infection elicited secretion of IL-1β in both PMA-differentiated U937 and THP-1 cells, addition of HD5 did not affect this proinflammatory response (Fig. 5F). Our results demonstrate that HD5-enhanced Shigella-mediated macrophage death is independent of caspase-1 activation.
We then examined necrosis by propidium iodide (PI)/4′,6-diamidino-2-phenylindole (DAPI) staining, which showed that at merely 1 h after initial infection, ∼80% of infected macrophages in the HD5-treated group lost integrity of the cytoplasmic membrane (Fig. 5H [not I]), a typical feature of necrosis. In contrast, in the absence of HD5, the virulent wild-type Shigella caused death of less than 10% infected macrophages, whereas the avirulent Δspa33 strain did not kill any macrophage regardless of the presence of HD5 during the same time period (Fig. 5I; see also Fig. S4D). These findings confirm that HD5 indirectly enhances macrophage necrosis instigated by intensified intracellular proliferation of virulent Shigella.
It is worth pointing out that HD5 itself under our experimental conditions also showed modest cytotoxic effects against macrophages (Fig. 5A to D). In fact, HD5 alone stimulated caspase-1 activation in PMA-differentiated U937 and THP-1 macrophages, consistent with its proinflammatory properties reported in our previous studies (49, 50). We previously showed that HD5 induced macrophage death through interactions with the extracellular domain of tumor necrosis factor receptor 1 (TNFR1) and targeting the mitochondrial membrane (49, 51). To examine the direct role of HD5 in inducing macrophage death, we quantified canonical caspase activity in PMA-differentiated U937 macrophages exposed to Shigella in the presence or absence of HD5. As shown in Fig. 5I (not H), HD5 treatment of macrophages slightly increased (10% to 15%) the activity of the two initiator caspases 8 and 9 and the executor caspase-3/7. However, Shigella infection had little or no effect on caspase activation. These data indicate that HD5 contributes modestly to macrophage death through its induction of both caspase-1-mediated and classical apoptotic pathways.
DISCUSSION
Defensins communicate with their environment and modulate the function of the neighboring cells (12, 13, 39). Rather than previously implied beneficial influences of defensins on macrophages in fight with pathogens, here we demonstrated that human enteric defensin HD5 promoted phagocytosis of Shigella into macrophages yet failed to inhibit T3SS activity and the subsequent phagosomal escape and intracellular proliferation, which thereby contributed to exacerbating the destruction of Shigella in macrophages. Our study gives the first example that α-defensins also act as accomplices of certain pathogens, such as Shigella, during their interaction with macrophages.
Since their discovery in the early 1990s, the potent antimicrobial activities of defensins have been extensively characterized; hence, they were named defensins (1, 39). Composed of both cationic and hydrophobic residues, defensins are capable of interacting with and potentially disintegrating negatively charged bacterial membranes, which had long been thought the prevailing mechanism of their antimicrobial activity (1, 2). Nevertheless, more sophisticated antimicrobial mechanisms of defensins were deciphered in recent studies, including inhibition of bacterial cell wall synthesis, interference of membrane function, neutralization of bacterial toxins, and formation of nanonet “traps” to restrain pathogens in host intestine (7, 9, 10, 52, 53). Besides these direct antimicrobial effects, synergistic interactions between defensins and immune cells, especially with macrophages, are believed to provide assistance for the control of bacterial infection, including Mycobacterium tuberculosis, Legionella pneumophila, Staphylococcus aureus, and Listeria monocytogenes, through boosting the phagocytosis and further inhibiting phagosomal escape and intracellular multiplication (29, 31–35, 54). A recent study revealed HNP1 functions as a “molecular brake” on macrophage-driven inflammation caused by Salmonella by preventing protein translation, ensuring both pathogen clearance and the resolution of inflammation with minimal bystander tissue damage (55). Until now, all the studies described defensins as “the good guys” in helping macrophages deal with bacterial infection. However, our study gives the first demonstration that the involvement of defensins could also be devastating to macrophages when they encounter Shigella.
HD5, secreted by Paneth cells in the small intestine, is considered an important protective molecule at the intestinal lumen (56). While the local concentration of HD5 in the small intestine may be high enough to prohibit Shigella infection, the lower abundance of defensin in the colon might be co-opted by Shigella to promote its adhesion and infection, as implied in our previous study (36). Disruption of epithelial integrity during Shigella infection brings the real possibility that HD5 is present when Shigella encounters macrophages at the basolateral side of colonic epithelia. Although resident macrophages are supposed to engulf and degrade incoming pathogens, Shigella manages to evade the killing by escaping rapidly from phagosomes through T3SS translocator proteins IpaB, IpaC, and IpaD (18, 57, 58), which eventually results in the death of the macrophages and triggers an inflammatory response (24, 27). While increased phagocytosis alone is not necessarily favorable to invading pathogens, the failure of HD5 to prevent the subsequent phagosomal escape turns the boost in phagocytosis into a disaster for macrophages, which were eventually destroyed by Shigella.
The killing of macrophages is the critical step in Shigella pathogenesis for two reasons: first, released bacteria from dying macrophages get access to the basolateral side of intestinal epithelia, where further infection into epithelial cells occurs; second, the dying macrophages produce strong proinflammatory cytokines that recruit infiltrating PMN, which in turn destroy the integrity of intestinal epithelia and let in more bacteria (17, 24). All these events lead to the eventual eruption of shigellosis, characteristic of massive bacterial invasion and a strong inflammatory response. Shigella induces both apoptosis and necrosis in macrophage-like cells, as reported in previous studies (17, 45–47, 59, 60). Unlike typical apoptosis, macrophage apoptosis caused by Shigella infection, also referred to as pyroptosis in more recent reports, is uniquely characteristic of activation and thus cleavage of caspase-1, which triggers the cascade of inflammatory events (17, 24, 61). Whether the infected macrophage cells undergo necrosis or apoptosis depends on the virulence of bacteria, origins of the macrophages, and their expression level of CD11b. It has been verified that macrophage-like cells differentiated from U937 cells by treatment with all-trans-retinoic acid, which show higher CD11b expression levels than gamma-interferon-induced U937 cells, were more likely induced to necrosis rather than apoptosis by virulent Shigella (59). In our study, the heavier intracellular bacterial burden due to HD5 treatment accelerated the killing of macrophages by Shigella mainly through necrosis, evidenced by the rapid loss of membrane integrity and DNA fragmentation without nuclear condensation. Besides, HD5 also contributes modestly to macrophage death through its induction of both caspase-1-mediated and canonical apoptotic pathways, consistent with its proinflammatory and proapoptotic properties reported in our previous studies (49, 51).
In conclusion, our study gives the first demonstration that HD5 secreted from human intestinal Paneth cells boosted the phagocytosis of Shigella into macrophage cells yet failed to inhibit the following phagosomal escape and intracellular multiplication, leading to accelerated cell death and massive bacterial release. HD5, presumably present at the infection site during Shigella intrusion into human intestine, promoted the Shigella infection in macrophages and therefore facilitated its translocation through macrophages from the mucosal side to the basolateral side, posing a greater threat to the intestinal epithelium. While it is not unusual for a microbial pathogen to exploit host components to promote its pathogenicity, our findings contribute a new example that the interaction between defensins and macrophages could also be taken advantage of by a pathogen. Further studies are warranted to elucidate the physiological implications of the pathogenic role of HD5 in Shigella infection of macrophages.
MATERIALS AND METHODS
Reagents.
All peptides used in this study were synthetically prepared, purified, and characterized to confirm proper mass and disulfide connectivity, as previously described. All the routine chemicals and reagents were purchased from Sigma-Aldrich unless indicated otherwise.
Bacterial strains and culture.
Wild-type Shigella flexneri strain Sf301 and its spa33 mutant were cultured aerobically at 37°C in tryptic soy (TS) broth (Aoboxing, Beijing, China) or on TS agar plates with 0.1% Congo red. Antibiotics (Sigma) were used as follows: ampicillin, 100 μg/ml; kanamycin, 100 μg/ml.
In vitro generation of monocyte-derived macrophages.
The study was approved by the local ethics committee (Ethics Committee of Xi’an Jiaotong University) and subjects gave written informed consent. Peripheral blood mononuclear cells (PBMCs) were enriched from whole blood by density gradient centrifugation using Ficoll-Paque (GE Healthcare) according to the manufacturer’s instructions. Isolated monocytes were first cultured in RPMI 1640 containing 2% human AB serum (HuS) in 24-well plates at a concentration of 5 × 105 cells/well. After 2 h, nonadherent lymphocytes were removed by washing plates twice with phosphate-buffered saline (PBS). Monocyte-derived macrophages were generated by culturing monocytes for 5 to 6 days in RPMI 1640/10% fetal bovine serum (FBS) supplemented with 100 ng/ml rHuM-CSF (macrophage colony-stimulating factor).
Cell culture and differentiation.
RAW264.7 cells (ATCC TIB-71) were cultured in Dulbecco’s minimal essential medium (DMEM), and U937 (ATCC CRL-1593) and THP-1 (ATCC TIB-202) cell lines were cultured in RPMI 1640 containing 10% fetal bovine serum with 5% CO2 at 37°C. RAW264.7 cells were seeded in 24-well plates and cultured to ∼80% confluence 1 day before the assays. U937 and THP-1 cells were differentiated for 3 to 7 days with phorbol myristate acetate at 200 ng/ml and 60 ng/ml, respectively.
In vitro adhesion and invasion assay.
One hour before the infection, cell culture medium was changed to serum-free medium, and ∼106 CFU Sf301 from mid-exponential phase was added to the cells together with a titration of HD5 (0 to 8 μM). Bacteria were centrifuged (2,000 rpm, 10 min, room temperature [RT]) onto macrophage cells (multiplicity of infection [MOI] 10:1 unless otherwise indicated) to synchronize the infection. For the adhesion assay, after washing, the cells were lysed with 0.1% Triton-H2O, and the CFU was enumerated after plating. For invasion and proliferation assays, bacterial/cell mixtures were incubated for 40 min after centrifugation and then washed and treated with gentamicin-containing (50 μg/ml) medium for various times before being lysed for plating. Adhesion was defined as the total number of macrophage cell-associated bacteria and is shown as the percentage of input. Internalization was defined as the total number of intracellular bacteria in cells (extracellular bacteria were killed by gentamicin, a cell-impermeable antibiotic). Average results from three independent experiments are shown as means ± standard deviations (SDs).
For pretreatment experiments, cells or bacteria were preincubated at various concentrations for 30 min, washed once with DMEM, and then mixed to allow infection. Adhesion assays and internalization assays were performed as mentioned above.
Immunofluorescence microscopy.
Macrophages were plated onto glass coverslips, and adhesion or internalization assays were performed as described using Sf301 harboring the GFP-expressing plasmid. Cells were fixed in 3% paraformaldehyde-PBS at room temperature for 15 min, washed in PBS, and permeabilized with 0.1% Triton-PBS for 5 min at room temperature. The coverslips were washed, mounted with Anti-Fade solution (Invitrogen) containing DAPI onto glass slides, and visualized under a Zeiss confocal microscope.
Analysis of T3SS secretion activity by transcription-based secretion activity reporter plasmids.
The TSAR plasmids were kindly gifted by F.-X. Campbell-Valois, who designed and constructed these plasmids (43). pTSAR1Ud2.4s plasmid, harboring a constitutively expressed mCherry fluorescent protein gene and an inducible GFP protein gene, whose expression is activated upon secretion of T3SS, was transformed into Sf301. Macrophage cells were seeded onto glass coverslips, and internalization assays were performed as described using Sf301 harboring pTSAR1Ud2.4s plasmid for 60 min, which allowed the full activation of GFP expression. Cells were then fixed and analyzed by immunofluorescence microscopy as described above.
Statistical analysis.
The data were collected from at least three independent experiments in triplicates or quadruplicates, unless otherwise indicated. Data were combined and are presented as means ± standard errors of the means (SEMs) or means ± SDs. Results were analyzed by various statistical tests as indicated in figure legends using GraphPad Prism version 7. A P value of <0.05 was considered statistically significant. Microscopy images are representative of at least two independent experiments.
Data availability.
The data that support the findings of this study are available from the corresponding author upon request.
Supplementary Material
ACKNOWLEDGMENTS
We thank Olga S. Latinovic, Institute of Human Virology, University of Maryland School of Medicine, for technical assistance with confocal fluorescence microscopic analysis.
This work was supported by grants from the Natural Science Foundation of China (31401211 & 31770146 to D.X.), China Postdoctoral Science Foundation (2015T81014 to D.X.), and China Scholarship Council (201806280363 to C.L.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 3.Selsted ME, Ouellette AJ. 2005. Mammalian defensins in the antimicrobial immune response. Nat Immunol 6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer RI. 2004. Primate defensins. Nat Rev Microbiol 2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 5.Bevins CL, Martin-Porter E, Ganz T. 1999. Defensins and innate host defence of the gastrointestinal tract. Gut 45:911–915. doi: 10.1136/gut.45.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chileveru HR, Lim SA, Chairatana P, Wommack AJ, Chiang IL, Nolan EM. 2015. Visualizing attack of Escherichia coli by the antimicrobial peptide human defensin 5. Biochemistry 54:1767–1777. doi: 10.1021/bi501483q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu H, Pazgier M, Jung G, Nuccio S-P, Castillo PA, de Jong MF, Winter MG, Winter SE, Wehkamp J, Shen B, Salzman NH, Underwood MA, Tsolis RM, Young GM, Lu W, Lehrer RI, Bäumler AJ, Bevins CL. 2012. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 337:477–481. doi: 10.1126/science.1218831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gounder AP, Wiens ME, Wilson SS, Lu W, Smith JG. 2012. Critical determinants of human α-defensin 5 activity against non-enveloped viruses. J Biol Chem 287:24554–24562. doi: 10.1074/jbc.M112.354068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C, Gajendran N, Mittrücker H-W, Weiwad M, Song Y-H, Hurwitz R, Wilmanns M, Fischer G, Kaufmann S. 2005. Human α-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc Natl Acad Sci U S A 102:4830–4835. doi: 10.1073/pnas.0500508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudryashova E, Quintyn R, Seveau S, Lu W, Wysocki VH, Kudryashov DS. 2014. Human defensins facilitate local unfolding of thermodynamically unstable regions of bacterial protein toxins. Immunity 41:709–721. doi: 10.1016/j.immuni.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Chen Q, Chertov O, Oppenheim JJ. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol 68:9–14. [PubMed] [Google Scholar]
- 12.Lillard JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. 1999. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci U S A 96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigat J, Soruri A, Forssmann U, Riggert J, Zwirner J. 2007. Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human α-defensin family. J Immunol 179:3958–3965. doi: 10.4049/jimmunol.179.6.3958. [DOI] [PubMed] [Google Scholar]
- 14.Territo MC, Ganz T, Selsted ME, Lehrer R. 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest 84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Q, Yuan Z, Xu J, Wang Y, Shen Y, Lu W, Wang J, Liu H, Yang J, Yang F, Zhang X, Zhang J, Yang G, Wu H, Qu D, Dong J, Sun L, Xue Y, Zhao A, Gao Y, Zhu J, Kan B, Ding K, Chen S, Cheng H, Yao Z, He B, Chen R, Ma D, Qiang B, Wen Y, Hou Y, Yu J. 2002. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res 30:4432–4441. doi: 10.1093/nar/gkf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuPont HL, Levine MM, Hornick RB, Formal SB. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis 159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 17.Philpott DJ, Edgeworth JD, Sansonetti PJ. 2000. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos Trans R Soc Lond B Biol Sci 355:575–586. doi: 10.1098/rstb.2000.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zychlinsky A, Prevost MC, Sansonetti PJ. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 19.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. 1997. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun 65:5165–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Smith MR, Thirumalai K, Zychlinsky A. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J 15:3853–3860. doi: 10.1002/j.1460-2075.1996.tb00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder GN, Jann NJ, Hilbi H. 2007. Intracellular type III secretion by cytoplasmic Shigella flexneri promotes caspase-1-dependent macrophage cell death. Microbiology 153:2862–2876. doi: 10.1099/mic.0.2007/007427-0. [DOI] [PubMed] [Google Scholar]
- 22.Singer M, Sansonetti PJ. 2004. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol 173:4197–4206. doi: 10.4049/jimmunol.173.6.4197. [DOI] [PubMed] [Google Scholar]
- 23.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten L. 2011. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol 32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder GN, Hilbi H. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev 21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnenberg MS. 2000. Pathogenic strategies of enteric bacteria. Nature 406:768–774. doi: 10.1038/35021212. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. 2005. Escape of intracellular Shigella from autophagy. Science 307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 27.Sansonetti PJ, Arondel J, Cantey JR, Prévost MC, Huerre M. 1996. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun 64:2752–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sansonetti PJ, Arondel J, Cavaillon JM, Huerre M. 1995. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Invest 96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva MT, Silva MN, Appelberg R. 1989. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog 6:369–380. doi: 10.1016/0882-4010(89)90079-X. [DOI] [PubMed] [Google Scholar]
- 30.Silva MT. 2010. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol 87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 31.Soehnlein O, Kenne E, Rotzius P, Eriksson EE, Lindbom L. 2008. Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages. Clin Exp Immunol 151:139–145. doi: 10.1111/j.1365-2249.2007.03532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. 2008. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112:1461. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, Eriksson EE, Herwald H, Agerberth B, Lindbom L. 2008. Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest 118:3491–3502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrd TF, Horwitz MA. 1991. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Invest 88:1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, Stenger S. 2006. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol 177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Liao C, Zhang B, Tolbert WD, He W, Dai Z, Zhang W, Yuan W, Pazgier M, Liu J, Yu J, Sansonetti PJ, Bevins CL, Shao Y, Lu W. 2018. Human enteric α-defensin 5 promotes Shigella infection by enhancing bacterial adhesion and invasion. Immunity 48:1233.e6–1244.e6. doi: 10.1016/j.immuni.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao C, Fang K, Xiao J, Zhang W, Zhang B, Yuan W, Lu W, Xu D. 2019. Critical determinants of human neutrophil peptide 1 for enhancing host epithelial adhesion of Shigella flexneri. Cell Microbiol 21:e13069. doi: 10.1111/cmi.13069. [DOI] [PubMed] [Google Scholar]
- 38.Tenge VR, Gounder AP, Wiens ME, Lu W, Smith JG. 2014. Delineation of interfaces on human alpha-defensins critical for human adenovirus and human papillomavirus inhibition. PLoS Pathog 10:e1004360. doi: 10.1371/journal.ppat.1004360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehrer RI, Lu W. 2012. α-Defensins in human innate immunity. Immunol Rev 245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 40.Rajabi M, Ericksen B, Wu X, de Leeuw E, Zhao L, Pazgier M, Lu W. 2012. Functional determinants of human enteric α-defensin HD5: crucial role for hydrophobicity at dimer interface. J Biol Chem 287:21615–21627. doi: 10.1074/jbc.M112.367995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsot C. 2009. Shigella type III secretion effectors: how, where, when, for what purposes? Curr Opin Microbiol 12:110–116. doi: 10.1016/j.mib.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Morita-Ishihara T, Ogawa M, Sagara H, Yoshida M, Katayama E, Sasakawa C. 2006. Shigella Spa33 is an essential C-ring component of type III secretion machinery. J Biol Chem 281:599–607. doi: 10.1074/jbc.M509644200. [DOI] [PubMed] [Google Scholar]
- 43.Campbell-Valois F-X, Schnupf P, Nigro G, Sachse M, Sansonetti Philippe J, Parsot C. 2014. A fluorescent reporter reveals on/off regulation of the Shigella type III secretion apparatus during entry and cell-to-cell spread. Cell Host Microbe 15:177–189. doi: 10.1016/j.chom.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Bernardini ML, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti PJ. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A 86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koterski JF, Nahvi M, Venkatesan MM, Haimovich B. 2005. Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect Immun 73:504–513. doi: 10.1128/IAI.73.1.504-513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T, Nakanishi K, Tsutsui H, Iwai H, Akira S, Inohara N, Chamaillard M, Nuñez G, Sasakawa C. 2005. A novel caspase-1/Toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in Infected Macrophages. J Biol Chem 280:14042–14050. doi: 10.1074/jbc.M414671200. [DOI] [PubMed] [Google Scholar]
- 47.Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP-Y. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ, Zychlinsky A. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem 273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 49.Lu W, de Leeuw E. 2013. Pro-inflammatory and pro-apoptotic properties of human defensin 5. Biochem Biophys Res Commun 436:557–562. doi: 10.1016/j.bbrc.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Leeuw E, Burks SR, Li X, Kao JPY, Lu W. 2007. Structure-dependent functional properties of human defensin 5. FEBS Lett 581:515–520. doi: 10.1016/j.febslet.2006.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu W, de Leeuw E. 2014. Functional intersection of human defensin 5 with the TNF receptor pathway. FEBS Lett 588:1906–1912. doi: 10.1016/j.febslet.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yount NY, Yeaman MR. 2013. Peptide antimicrobials: cell wall as a bacterial target. Ann N Y Acad Sci 1277:127–138. doi: 10.1111/nyas.12005. [DOI] [PubMed] [Google Scholar]
- 53.Oeemig JS, Lynggaard C, Knudsen DH, Hansen FT, Nørgaard KD, Schneider T, Vad BS, Sandvang DH, Nielsen LA, Neve S, Kristensen H-H, Sahl H-G, Otzen DE, Wimmer R. 2012. Eurocin, a new fungal defensin: structure, lipid binding, and its mode of action. J Biol Chem 287:42361–42372. doi: 10.1074/jbc.M112.382028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnett E, Lehrer RI, Pratikhya P, Lu W, Seveau S. 2011. Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell Microbiol 13:635–651. doi: 10.1111/j.1462-5822.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 55.Brook M, Tomlinson GH, Miles K, Smith RWP, Rossi AG, Hiemstra PS, van ‘t Wout EFA, Dean JLE, Gray NK, Lu W, Gray M. 2016. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci U S A 113:4350–4355. doi: 10.1073/pnas.1601831113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bevins CL, Salzman NH. 2011. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 57.Zychlinsky A, Thirumalai K, Arondel J, Cantey JR, Aliprantis AO, Sansonetti PJ. 1996. In vivo apoptosis in Shigella flexneri infections. Infect Immun 64:5357–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cossart P, Sansonetti PJ. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 59.Nonaka T, Kuwabara T, Mimuro H, Kuwae A, Imajoh-Ohmi S. 2003. Shigella-induced necrosis and apoptosis of U937 cells and J774 macrophages. Microbiology 149:2513–2527. doi: 10.1099/mic.0.26341-0. [DOI] [PubMed] [Google Scholar]
- 60.Nonaka T, Kuwae A, Sasakawa C, Imajoh-Ohmi S. 1999. Shigella flexneri YSH6000 induces two types of cell death, apoptosis and oncosis, in the differentiated human monoblastic cell line U937. FEMS Microbiol Lett 174:89–95. doi: 10.1111/j.1574-6968.1999.tb13553.x. [DOI] [PubMed] [Google Scholar]
- 61.Lucchini S, Liu H, Jin Q, Hinton JCD, Yu J. 2005. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect Immun 73:88–102. doi: 10.1128/IAI.73.1.88-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.