Staphylococcus aureus has evolved different strategies to evade the immune response, which play an important role in its pathogenesis. The bacteria express and shed various cell wall components and toxins during different stages of growth that may affect the protective T cell responses to extracellular and intracellular S. aureus. However, if and how the dendritic cell (DC)-mediated T cell response against S. aureus changes during growth of the bacterium remain elusive.
KEYWORDS: growth phase, Staphylococcus aureus, T cells, dendritic cells, peptidoglycan
ABSTRACT
Staphylococcus aureus has evolved different strategies to evade the immune response, which play an important role in its pathogenesis. The bacteria express and shed various cell wall components and toxins during different stages of growth that may affect the protective T cell responses to extracellular and intracellular S. aureus. However, if and how the dendritic cell (DC)-mediated T cell response against S. aureus changes during growth of the bacterium remain elusive. In this study, we show that exponential-phase (EP) S. aureus bacteria were endocytosed very efficiently by human DCs, and these DCs strongly promoted production of the T cell polarizing factor interleukin-12 (IL-12). In contrast, stationary-phase (SP) S. aureus bacteria were endocytosed less efficiently by DCs, and these DCs produced small amounts of IL-12. The high level of IL-12 production induced by EP S. aureus led to the development of a T helper 1 (Th1) cell response, which was inhibited after neutralization of IL-12. Furthermore, preincubation with the staphylococcal cell wall component peptidoglycan (PGN), characteristically shed during the exponential growth phase, modulated the DC response to EP S. aureus. PGN preincubation appeared to inhibit IL-12p35 expression, leading to downregulation of IL-12 and an increase of IL-23 production by DCs, enhancing Th17 cell development. Taken together, our data indicate that exponential-phase S. aureus bacteria induce a stronger IL-12-dependent Th1 cell response than stationary-phase S. aureus and that this Th1 cell response shifted toward a Th17 cell response in the presence of PGN.
INTRODUCTION
Staphylococcus aureus normally colonizes the human skin and mucosal surfaces as a commensal bacterium but is also capable of causing a wide range of infections when passing the epithelial barriers in case of injury or implantation of medical devices (1, 2). In hospital-acquired infections, staphylococci are among the most important pathogens (3, 4), persisting by adapting to an extracellular or an intracellular lifestyle. The high infection rate may be due to the fact that S. aureus has evolved a variety of strategies to evade the immune response when persisting extracellularly and intracellularly, by producing a wide array of secreted and cell surface-associated virulence factors (5–7). The bacterial growth phase is highly important for the regulation and expression of these virulence factors (8). During the exponential growth phase, corresponding to the time when an infection is first being established, extracellular S. aureus predominantly expresses adhesion molecules, which enable attachment to host tissues (1, 9, 10). In the stationary growth phase, resembling the situation when the infection is established, S. aureus secretes toxins and produces antiphagocytic capsular polysaccharides (1, 11). The potential adaptation of the nature of the immune response to S. aureus bacteria in these different growth phases has received little attention, and available information is restricted to the responses of innate immune cells (12). Whether human adaptive CD4+ T cells respond differentially to S. aureus in the exponential phase (EP) or stationary growth phase (SP) is not known.
The induction of the T cell response to S. aureus is driven by dendritic cells (DCs). DCs play an important role in activating and coordinating antistaphylococcal CD4+ T helper (Th) cell responses by producing cytokines such as interleukin-12 (IL-12) and IL-23 (13–16). IL-12 is an essential polarizing cytokine for the development of interferon gamma (IFN-γ)-producing Th1 cells and IL-23 for the development of IL-17-producing Th17 cells (17). Th1 and Th17 cell responses play an important role in protective immune responses against intracellular and extracellular S. aureus, respectively (15, 18, 19). Previous work with mouse DCs showed that EP S. aureus bacteria were more potent inducers of IL-12 secretion by DCs than SP S. aureus (12). In this murine system, the EP S. aureus-induced IL-12 production by DCs was downregulated in the presence of peptidoglycan (PGN), a cell wall component naturally shed in high concentrations by S. aureus during exponential growth (20). These findings indicate a possible role of S. aureus growth phase and PGN in Th1 cell polarization. Therefore, we aimed to study such a potential differential Th cell polarization in a human cell system. We compared the human monocyte-derived DC response to EP or SP S. aureus and the consequences for the ensuing human Th1/Th17 cell polarization and assessed how PGN influences the responses to EP S. aureus.
Our results show that EP S. aureus induced a stronger IL-12-dependent human Th1 cell polarization than SP S. aureus. Exposure to PGN dampened the DC IL-12 response, resulting in a shift of the Th1-Th17 cell balance in favor of Th17 cells. This shift appeared to be caused by inhibition of IL-12p35 expression. The differential response to EP and SP S. aureus may represent an adaptation to optimize the immune response to extracellular and intracellular S. aureus, respectively.
RESULTS
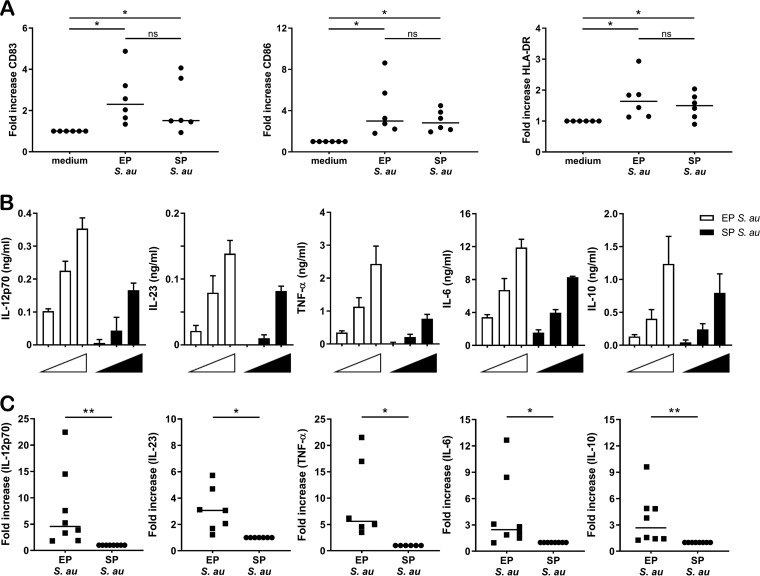
EP and SP S. aureus induced DC maturation marker expression and cytokine production.
The expression of surface molecules and secretion of proteins and polysaccharides by S. aureus are growth phase dependent. Here, we set out to determine if EP and SP S. aureus differently affect the activation of human DCs. First, we studied the effect of S. aureus growth phase on the maturation status of immature DCs. For this, DCs were incubated with EP S. aureus or SP S. aureus or were left untouched (immature DCs). Both EP and SP S. aureus were able to upregulate HLA-DR, CD83, and CD86 expression compared to the levels of immature DCs (Fig. 1A), but no differences were observed between EP and SP S. aureus in their capability to induce expression of these maturation markers (Fig. 1A). Next, we compared the ability of EP and SP S. aureus to induce cytokine production by DCs (Fig. 1B). DCs were incubated with multiplicities of infection (MOIs) of 5, 10, and 20 of S. aureus, and IL-12p70, IL-23, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) levels were measured (Fig. 1B). For all cytokines tested, the data revealed dose-dependent responses that differed for EP and SP S. aureus (Fig. 1B). EP S. aureus induced higher cytokine production by DCs than SP S. aureus did. The combined data of DCs derived from at least six donors stimulated with S. aureus at an MOI of 20 clearly showed that DCs incubated with EP S. aureus produced significantly higher levels of cytokines than those incubated with SP S. aureus (Fig. 1C). Moreover, this S. aureus growth phase-dependent cytokine response was not strain specific, since in addition to our standard S. aureus test strain, a panel of different S. aureus strains in the exponential growth phase also induced stronger cytokine responses than these strains in stationary growth phase (see Fig. S1 and S2 in the supplemental material). These results demonstrated that EP and SP S. aureus are equally potent stimulators of DC maturation, while EP S. aureus induces higher levels of DC cytokines.
FIG 1.
DC marker and cytokine response upon stimulation by EP or SP S. aureus. (A) Fold difference in surface expression of CD83, CD86, and HLA-DR on DCs stimulated with EP or SP S. aureus relative to unstimulated DCs (medium). The data come from 6 independent experiments. Each dot represents one donor, and the horizontal lines represent the median value. (B) DCs were incubated with EP (white) or SP (black) S. aureus (MOI of 5, 10, or 20), and concentrations of IL-12p70, IL-23, TNF-α, IL-6, and IL-10 in the supernatant were measured using ELISA. The data shown are the mean + standard deviation (SD) of three technical replicates from one representative experiment out of three experiments with different donors. (C) Fold-increase in cytokine production after stimulation of immature DCs with EP bacteria relative to SP bacteria at an MOI of 20. Each dot represents one donor, and the horizontal lines represent the median value. *, P < 0.05; **, P < 0.01; ns, not significant (Wilcoxon signed rank test).
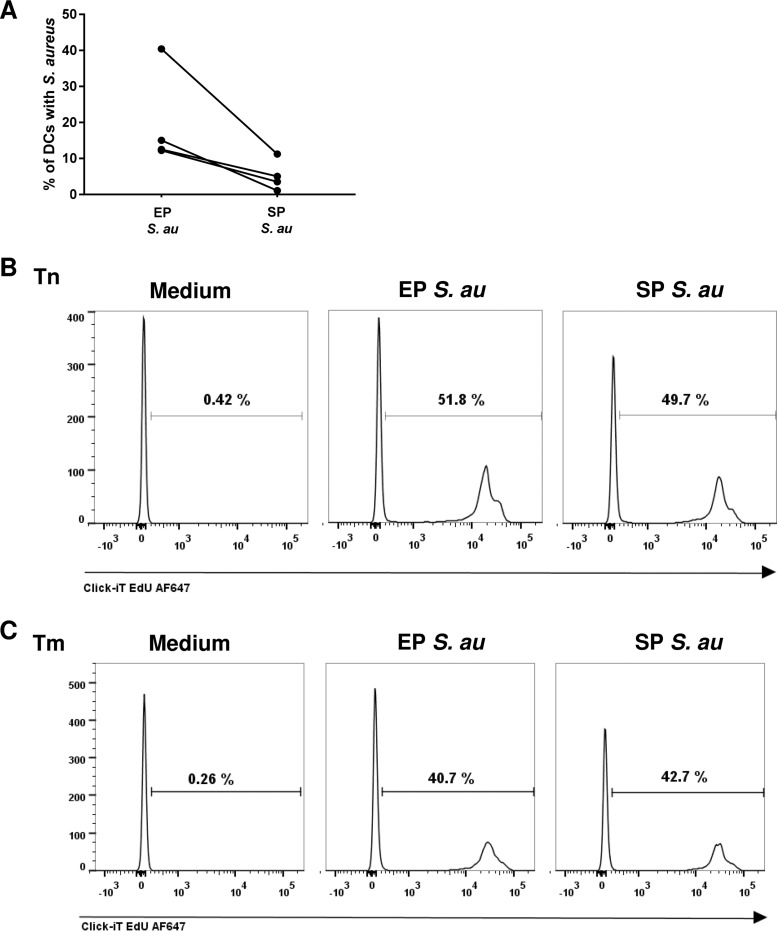
Enhanced endocytosis of EP S. aureus by DCs does not affect T cell proliferation.
In addition to HLA-DR and CD86 expression, bacterial uptake and antigen presentation by DCs are of major importance for the induction of antigen-specific T cell proliferation (21). To determine the bacterial uptake of EP or SP S. aureus by DCs, we incubated DCs with fluorescently labeled S. aureus and quantified the percentage of DCs which internalized S. aureus by flow cytometry. Although DCs internalized both EP and SP S. aureus, the percentage of DCs that internalized EP S. aureus was significantly higher than the percentage of DCs that internalized SP S. aureus (Fig. 2A). Subsequently, we determined the T cell stimulatory capacity of DCs activated by EP or SP S. aureus. Surprisingly, we did not find any differences in the induction of naive and memory T cell proliferation by DCs matured with S. aureus from either exponential or stationary growth phase (Fig. 2B and C). Apparently, the efficiency of endocytosis by DCs did not influence the induction of naive and memory T cell proliferation. Together, these data demonstrated that, despite the different levels of endocytosis efficiency by DCs, EP and SP S. aureus are equally potent stimulators of DC-induced antigen-specific CD4+ T cell proliferation.
FIG 2.
Enhanced endocytosis of EP S. aureus by DCs does not affect T cell proliferation. (A) Endocytosis of fluorescently labeled EP or SP S. aureus by DCs. Percentages of DCs which endocytosed fluorescently labeled S. aureus were assessed by flow cytometry. Each pair of dots represents values from DCs of one donor. (B) Proliferation of naive (Tn) or (C) memory (Tm) T cells upon coculture with unstimulated (medium) DCs and EP or SP S. aureus-stimulated DCs. The data are from one representative experiment out of three experiments with cells of different donors. The percentage of proliferated T cells is indicated in each histogram.
IL-12 induction is essential for Th1 development from both naive and memory T cells.
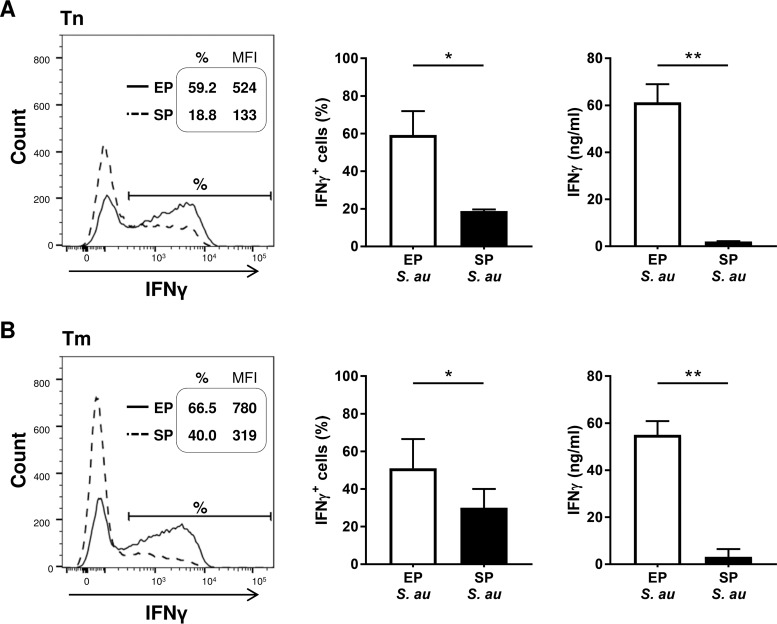
As the S. aureus growth phase strongly influenced the cytokine production by DCs, which are important T cell polarizing factors, we investigated if the S. aureus growth phase would influence CD4+ T cell polarization. DCs incubated with EP S. aureus induced more IFN-γ+ naive and memory T cells, which also secreted higher concentrations of IFN-γ than did DCs stimulated with SP S. aureus, indicating polarization toward Th1 cells (Fig. 3A and B). Since the DC and Th cell responses to EP and SP S. aureus were of the same nature, but more pronounced in response to EP S. aureus, we performed the additional experiments described below with EP S. aureus only.
FIG 3.
Enhanced Th1 cell polarization by S. aureus in EP. DCs were cocultured with EP or SP S. aureus and (A) naive (Tn) or (B) memory T cells (Tm). Fluorescence-activated cell sorter (FACS) histograms of one representative experiment out of three with the intracellular levels of IFN-γ (left panels). The percentages of IFN-γ-producing T cells and the mean fluorescence intensity (MFI) of IFN-γ production are indicated in the histograms. The percentages of IFN-γ+ T cells (middle panels) and the concentration of secreted IFN-γ (right panels) of three independent experiments with cells of three different donors were analyzed using flow cytometry and ELISA, respectively. The data shown are the median + range. *, P < 0.05; **, P < 0.01 (Wilcoxon signed rank test).
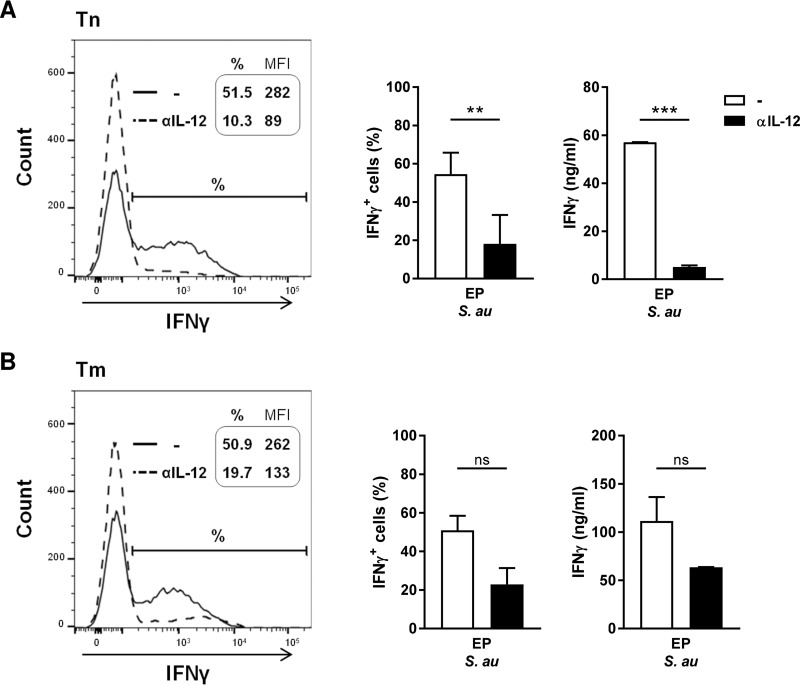
To determine the role of IL-12 in the observed Th1 cell polarization, we added neutralizing antibodies against IL-12p70 and assessed the effect on the induction of IFN-γ-producing Th1 cells (Fig. 4A and B). Neutralization of IL-12p70 in cocultures with EP S. aureus-primed DCs severely reduced Th1 polarization of naive T cells (Fig. 4A). The percentage of IFN-γ+ T cells and the concentration of secreted IFN-γ in these cultures were reduced to similar levels as those induced by SP S. aureus (Fig. 3A). IFN-γ production by memory T cells stimulated with EP S. aureus-primed DCs showed a slightly lower IL-12 dependency (Fig. 4B). Together, these results demonstrate that the polarization of, in particular, naive CD4+ T cells into Th1 cells is highly dependent on IL-12 produced by DCs in response to EP S. aureus.
FIG 4.
Th1 cell polarization is driven by IL-12. DCs were cocultured with EP S. aureus and (A) naive (Tn) or (B) memory (Tm) T cells in the absence or presence of neutralizing antibodies against IL-12p70. FACS histograms of one representative experiment out of three with the intracellular levels of IFN-γ (left panels) are shown. The percentages of IFN-γ-producing T cells and the mean fluorescence intensity (MFI) of IFN-γ production are indicated in the histograms. Percentages of IFN-γ+ T cells (middle panels) and the concentration of secreted IFN-γ (right panels) of three independent experiments with cells of three different donors were analyzed using flow cytometry and ELISA, respectively. The data shown are the median + range. **, P < 0.01; ***, P < 0.001; ns, not significant (Wilcoxon signed rank test).
Preincubation with PGN impairs Th1 cell polarization in favor of Th17 cell polarization.
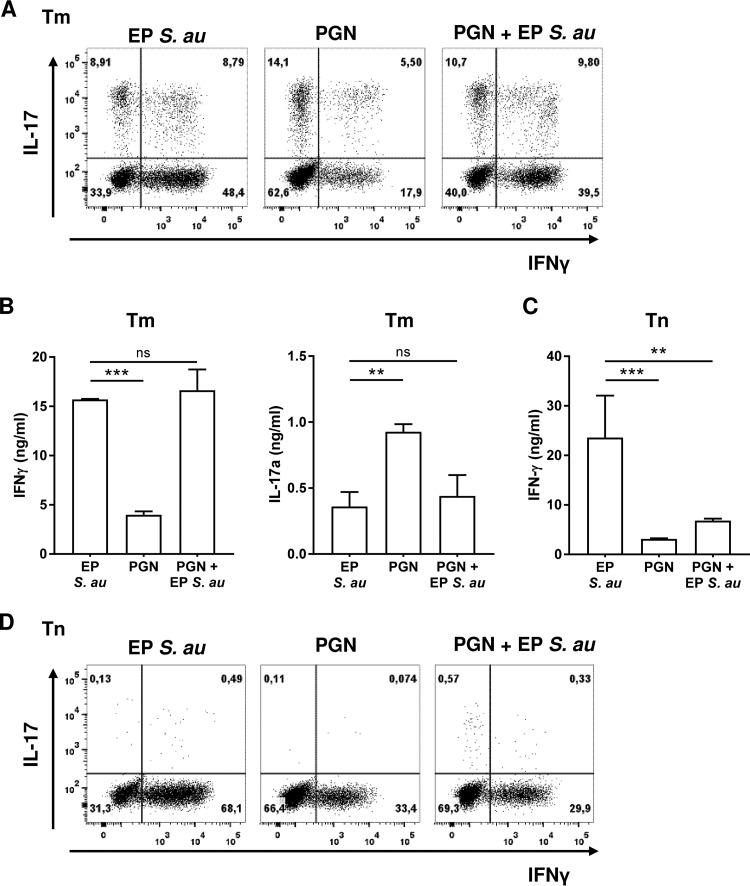
Since the S. aureus cell wall component PGN is naturally shed in large amounts during exponential growth and since stimulation with PGN-derived muramyl dipeptide (MDP) has been shown to promote Th17 skewing in memory T cells (22), we hypothesized that PGN might affect the immunological response against whole EP S. aureus. To test this hypothesis, we preincubated DCs for 30 min with PGN prior to coculture with EP S. aureus and either memory or naive Th cells. Th17 and Th1 cell polarization was determined by measuring the intracellular and secreted cytokines IL-17 and IFN-γ, respectively (Fig. 5). PGN alone induced a stronger Th17 response than intact EP S. aureus in memory T cells but was less potent in inducing a Th1 response (Fig. 5A). The same tendency was observed with secreted IL-17 and IFN-γ in the supernatants (Fig. 5B). Interestingly, preincubation with PGN reduced Th1 cell induction mediated by EP S. aureus in favor of Th17 cell induction in memory T cells (Fig. 5A). This effect of preincubation with PGN was not observed with secreted IL-17 and IFN-γ in the supernatants (Fig. 5B). When naive T cells were stimulated, PGN induced lower levels of IFN-γ secretion than EP S. aureus (Fig. 5C). Furthermore, PGN preincubation significantly reduced the IFN-γ secretion induced by EP bacteria (Fig. 5C). Naive T cells did not secrete IL-17 (data not shown) and also did not produce detectable intracellular amounts of IL-17 (Fig. 5D). EP S. aureus induced twice as many Th1 cells as PGN (Fig. 5D). Furthermore, coculturing with PGN and EP bacteria resulted in reduction in the relative numbers of IFN-γ-positive cells, corresponding to those induced by PGN alone (Fig. 5D). In summary, the presence of PGN impaired the development of naive and memory T cells into Th1 cells in favor of Th17 cells.
FIG 5.
Staphylococcal PGN modulates EP S. aureus-primed DC instruction of naive and memory T cells. DCs were cocultured with memory (Tm) or naive (Tn) T cells upon stimulation with EP S. aureus or PGN or upon preincubation with PGN and subsequent stimulation with EP S. aureus. Intracellular IFN-γ and IL-17 levels were measured in (A) memory (Tm) or (D) naive (Tn) T cells by flow cytometry. FACS dot plots of one representative experiment out of three are shown. Secreted IFN-γ and IL-17 were detected in the supernatants after restimulation of (B) Tm and (C) Tn cells. Data of three independent experiments are presented as median + range. **, P < 0.01; ***, P < 0.001; ns not significant (Wilcoxon signed rank test).
Preincubation with PGN attenuates IL-12 production while enhancing IL-23 production in DCs.
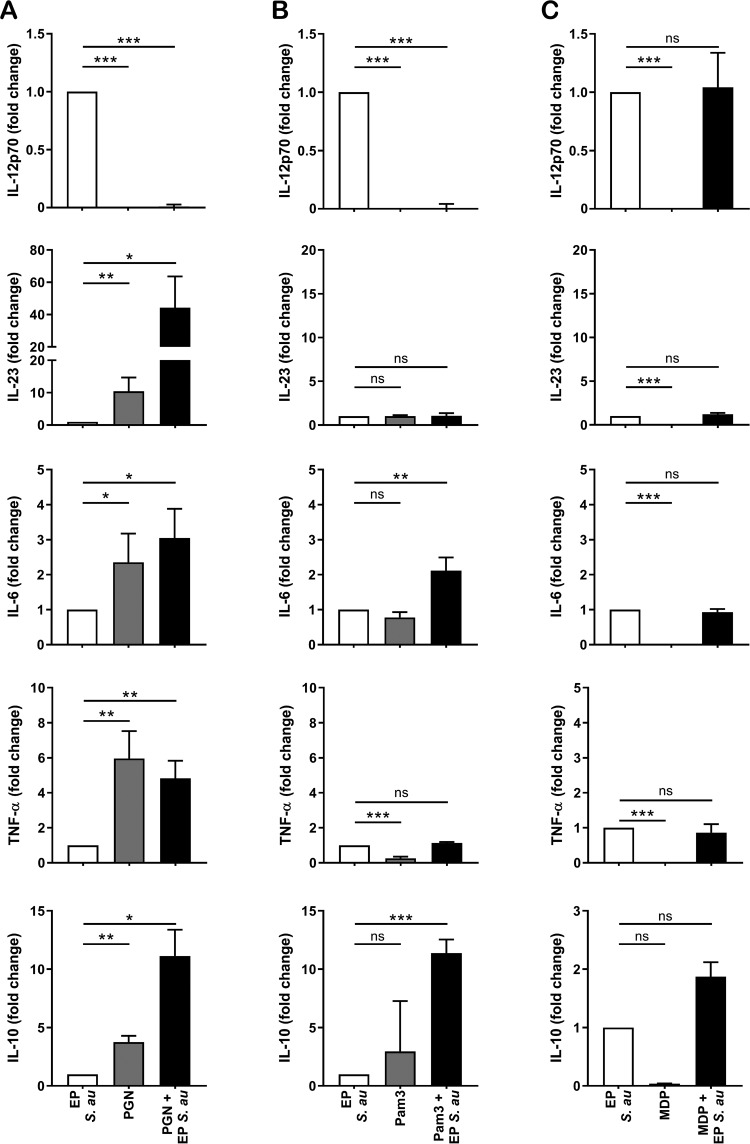
To assess whether the shift in Th cell polarization upon PGN preincubation was mediated by an alteration in the production of polarizing cytokines by DCs, we preincubated DCs with PGN and subsequently stimulated them with EP S. aureus. PGN by itself failed to induce IL-12 production by DCs (Fig. 6A). Preincubation with PGN completely abrogated the IL-12 induction by EP S. aureus, indicating an inhibitory role of PGN in Th1 polarization. In contrast, such DCs produced significantly higher levels of IL-23, IL-6, TNF-α, and IL-10 than DCs stimulated with EP S. aureus alone. The inhibition of IL-12 production and the increase in IL-23 production by DCs upon PGN preincubation may play a role in the strong polarization toward Th17 cells.
FIG 6.
Cell wall components or synthetic ligands modulate S. aureus-induced DC cytokine production. DCs were stimulated with EP S. aureus, PGN (A), Pam3CSK4 (B), and MDP (C) or preincubated with PGN, Pam3CSK4, or MDP and subsequently stimulated with EP S. aureus. Cytokine levels were measured in the supernatant using ELISA. Data represent the fold-change relative to EP bacteria from one experiment out of three. Data shown are the mean + SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant (Student’s t test).
The profile of cytokine production by DCs largely depends on the activation of (multiple) pattern recognition receptors (PRRs). PGN is a ligand for Toll-like receptor 2 (TLR2), and PGN-derived MDP is a ligand for nucleotide-binding oligomerization domain 2 (NOD2) (23–25). To assess the relative importance of TLR2 and NOD2 triggering in the observed cytokine response upon PGN preincubation, we analyzed the production of cytokines by DCs upon preincubation with the synthetic TLR2 ligand Pam3CSK4 or with the NOD2 ligand MDP. Similarly to PGN, Pam3CSK4 by itself did not induce IL-12 production by DCs, and preincubation with Pam3CSK4 also abrogated IL-12 production and enhanced the IL-6 and IL-10 production induced by EP S. aureus (Fig. 6B). In contrast to PGN, Pam3CSK4 preincubation did not affect the EP S. aureus-induced IL-23 and TNF-α production by DCs. MDP by itself failed to induce any of the tested cytokines and had no effect on induction of cytokines by EP S. aureus (Fig. 6C). Together, these data suggest that preincubation with PGN alters the DC cytokine response to EP S. aureus via triggering of both TLR2 and the NOD2 receptor.
Preincubation with PGN downregulates IL-12p35 expression in DCs.
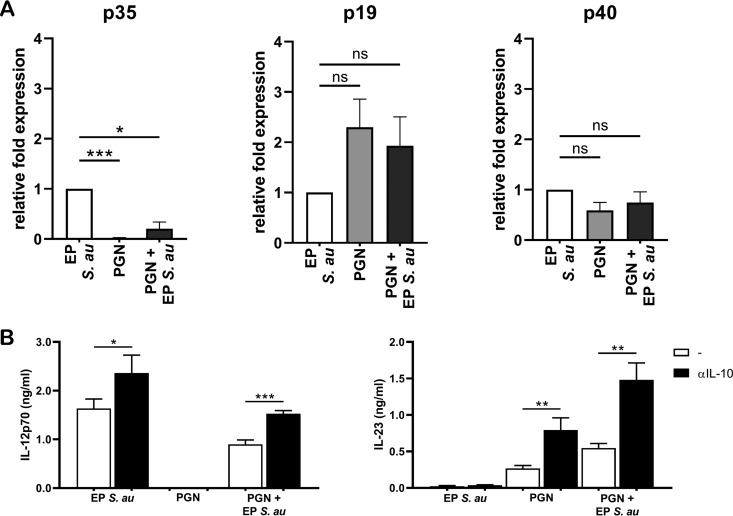
IL-12 and IL-23 are heterodimeric cytokines which share the IL-12p40 subunit but have a unique IL-12p35 and IL-23p19 subunit, respectively (26). A possible explanation for changes in IL-12 and IL-23 levels upon PGN preincubation could be the differential synthesis of the unique subunits. Therefore, we determined whether preincubation with PGN affected the balance between p19 and p35 expression induced by EP S. aureus by measuring mRNA encoding the p19, p35, and p40 subunits in stimulated DCs. In line with the expectations, PGN-stimulated DCs expressed high levels of p19 and low levels of p35 compared to DCs stimulated with EP S. aureus (Fig. 7A). Interestingly, the high levels of p35 induced by EP S. aureus were significantly reduced when the DCs were preincubated with PGN, while the levels of p19 and p40 were not affected (Fig. 7A). Thus, PGN preincubation affects the balance of the EP S. aureus-induced IL-12/IL-23 production via downregulation of p35 expression in DCs.
FIG 7.
PGN inhibits IL-12p35 expression and abrogates IL-12 production in an IL-10-dependent manner. (A, B) DCs were stimulated with EP S. aureus or PGN or preincubated with PGN and subsequently stimulated with EP S. aureus. (A) After 9 h, DCs were collected and processed for mRNA quantification by RT-PCR of the indicated genes. Data are mRNA levels normalized to GAPDH, 18S, and β2M expression, and the fold-change was calculated compared to expression by DCs stimulated with only EP S. aureus. Data are representative of three experiments with different donors. (B) DCs were preincubated for 30 min with or without neutralizing antibodies against IL-10 (αIL-10), after which cells were stimulated as described above. IL-12p70 and IL-23 levels were measured using ELISA after 24 h. Data are representative of three experiments with different donors. The data shown are the mean + SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant (Student’s t test).
DCs (pre)incubated with PGN induced significantly higher levels of IL-10 than DCs stimulated with EP S. aureus alone (Fig. 6). IL-10 is a well-reported autocrine negative regulator of TLR-induced IL-12 production in DCs (27–29), suggesting that the PGN-induced IL-10 production may be responsible for the inhibition of EP S. aureus-induced IL-12 production. To assess the role of IL-10 in suppression of IL-12, we used neutralizing antibodies against IL-10 (anti-IL-10). Indeed, neutralization of IL-10 restored the IL-12 levels of DCs preincubated with PGN to the levels induced by EP S. aureus in the absence of PGN and without IL-10 neutralization (Fig. 7B). The presence of anti-IL-10 also enhanced IL-12 production in DCs stimulated only with EP S. aureus, indicating that IL-10 also partially suppresses IL-12 production in response to EP S. aureus (Fig. 7B). In addition, PGN-induced levels of IL-23 also increased in the presence of anti-IL-10. These data suggest that IL-10 produced by DCs in response to PGN suppresses both the EP S. aureus-induced IL-12 production and the PGN-induced IL-23 production by DCs.
DISCUSSION
S. aureus expresses a number of virulence factors in a growth phase-dependent way, which can compromise the effectiveness of host immunity. In many studies of immunity against S. aureus, stationary-phase (SP) or a mixture of exponential-phase (EP) and SP bacteria have been used as a stimulant (13, 30, 31), while EP and SP bacteria may differently influence the immune response (12). In this study, we investigated whether the S. aureus growth phase influences human DC responses and the ensuing Th cell polarization and how the bacterial cell wall component PGN influences these immune responses. We show that DCs stimulated with EP or SP S. aureus were equally maturated in terms of HLA-DR, CD80, and CD86 expression and gave rise to similar proliferation patterns of both naive and memory CD4+ T cells. However, DCs stimulated with EP S. aureus had a greater endocytosis efficiency and were stronger inducers of the Th1 polarizing factor IL-12 and other proinflammatory cytokines than DCs stimulated with SP S. aureus. This EP S. aureus-induced DC cytokine response led to the development of Th1 cells, which was clearly reduced after neutralization of IL-12. Furthermore, PGN modulated the EP S. aureus-induced DC cytokine response by downregulating IL-12 and enhancing IL-23 production, leading to a shift in the Th1-Th17 cell balance in favor of Th17 cell polarization. These data indicate that the growth phase of S. aureus, as well as the presence of PGN, affects the DC-mediated induction of Th cell polarization.
Polarization of Th cells depends on the profile of cytokines produced by DCs upon ligation of individual or multiple DC PRRs (32, 33). EP and SP S. aureus both contain PRR ligands for TLR2 and NOD2 receptors. However, the accessibility of PRR ligands for TLRs and NOD2 receptors may be different when the bacteria are in the exponential or stationary phase of growth. Endocytosis of S. aureus and endosome acidification, required for degradation of S. aureus, in murine DCs were necessary for IL-12 secretion (12). Endocytosis of S. aureus enables intracellular sensing by the intracellular NOD2 receptor, and NOD2 activation enhances the cytokine response mediated by TLR activation (22, 34, 35). In addition, triggering of TLR2 by endocytosed bacteria in the endosomal compartment can induce TLR2-specific signaling, leading to the production of polarizing cytokines (e.g., IL-12) (36, 37), which may be different from the cytokines produced upon surface TLR2 triggering. We observed that higher percentages of human DCs endocytosed EP S. aureus, and these DCs produced high levels of the Th1 cell polarizing factor IL-12 compared to DCs stimulated with SP S. aureus. This suggests that the differential DC cytokine and Th1 cell polarization response to EP and SP S. aureus is the result of higher levels of EP S. aureus endocytosis and ensuing intracellular activation of both TLR2 and NOD2 receptors.
Interestingly, DCs were less efficient in endocytosing SP S. aureus than EP S. aureus, while the T cell proliferation induced by these DCs did not differ. Low endocytosis of SP S. aureus can be related to the expression of capsular polysaccharides (CPs), such as poly-N-acetylglucosamine (PNAG), and of capsule types CP5 or CP8, which accumulate during the stationary growth phase (38). However, since the S. aureus strain NCTC8325-4 used in this study did not express CP5 or CP8 (39, 40) and the PNAG-deficient variant of this strain indirectly showed PNAG to have no effect on endocytosis (12), it is likely that factors other than capsular polysaccharides frustrate the process of endocytosis, e.g., EfB (41) or ClfA (42). Although the efficiency of SP S. aureus endocytosis was low compared to endocytosis of EP S. aureus, this low level of endocytosis was high enough to induce sufficiently high levels of DC maturation marker expression and antigen presentation to activate antigen-specific T cells. A role of superantigens in the observed T cell proliferation is not likely, since we selected an S. aureus strain which is negative for genes encoding staphylococcal T cell superantigens (23). Previous results also indicate that DC maturation marker expression and antigen presentation, rather than high bacterial endocytosis, are prominent in the induction of T cell proliferation (P. P. Balraadjsing, E. C. de Jong, W. J. van Wamel, and S. A. J. Zaat, submitted for publication). Apparently, the levels of DC activation and antigen presentation are more appropriate measures for the potency of human DCs to induce T cell proliferation than the levels of bacterial endocytosis.
Our results suggest that PGN, which is naturally shed during exponential growth of S. aureus (20), can determine the balance between DC-induced Th1/Th17 cell responses. Th1 and Th17 cells both play a role in protective responses against S. aureus infection (19, 43), with Th17 cells being responsible for driving neutrophil recruitment to combat extracellular S. aureus and Th1 cells being responsible for the eradication of intracellular S. aureus by enhancing macrophage intracellular bactericidal responses. In our experimental procedure, we washed the bacteria before coculture with DCs, thereby removing any shed PGN. We showed that the presence of PGN strongly inhibited the EP S. aureus-induced IL-12 production by human DCs, leading to a reduction of Th1 cell development from naive T cells. DCs stimulated with PGN induced high-level secretion of IL-23 and IL-6, and interestingly, the presence of PGN strongly upregulated EP S. aureus-induced IL-23 and IL-6 secretion. These cytokines support Th17 cell development in humans (17, 44, 45), which may explain why the presence of PGN induced the development of IL-17-producing Th cells. Of note, we observed DC-induced T cell activation by PGN alone, which may be explained by T cell recognition of PGN-derived glycopeptides, lipoproteins, or zwitterionic polysaccharides (e.g., teichoic acids), which are presented by DCs (46, 47).
The differential response to EP and SP S. aureus and PGN can be considered an adaptation of the immune response to the pathogenesis of S. aureus infection. During the early course of infection, exponentially and extracellularly growing S. aureus shed large amounts of PGN, which stimulates DC-induced Th17 cell polarization, generating an immune response directed against extracellular bacteria. During later phases of infection, when S. aureus may reside intracellularly inside host cells (48), less PGN will be available to interfere with DC-induced T cell polarization. This will lead to skewing toward Th1 cell polarization, reinforcing intracellular killing of internalized bacteria. This way, the immune response covers both the extracellular and intracellular phases of S. aureus infection.
Our data indicate that the possible mechanism by which PGN influences the DC cytokine response to EP S. aureus involves at least two levels of regulation. First, simultaneous ligation of TLR2 and NOD2 by PGN is necessary to enhance the EP S. aureus-induced IL-23 production by DCs. Solely TLR2 or NOD2 ligation by Pam3CSK4 or MDP, respectively, was not sufficient to enhance the EP S. aureus-induced IL-23 response by DCs. Additionally, PGN inhibited the EP S. aureus-induced expression of IL-12p35 while the expression of IL-12p40 and IL-23p19 remained unchanged upon PGN preincubation. The PGN-induced shift in IL-12p35 expression resulted in IL-23 rather than IL-12 production by DCs. As a second mechanism, IL-10, which is produced in large amounts by DCs upon PGN stimulation, negatively regulated the IL-12 production in DCs. Neutralization of IL-10 increased the IL-12 levels but also the IL-23 levels of DCs preincubated with PGN. This indicates that IL-10 contributes to the suppression of IL-12 production but that other IL-10-independent signaling mechanisms participate in the enhancement of IL-23 production. Hence, interfering at the transcriptional level of IL-12p35 and negatively regulating IL-12 via autocrine IL-10 are part of the mechanism by which PGN modulates the DC response to EP S. aureus.
Taken together, our data indicate that human DCs respond more strongly to exponentially growing than to stationary growing S. aureus, resulting in different levels of Th1 cell development. PGN which is naturally shed from the cell wall of exponentially growing S. aureus interferes with the DC IL-12 response to EP S. aureus, leading to enhanced Th17 cell development. In light of the pathogenesis of S. aureus, the shift in the Th1-Th17 cell balance may be of importance for efficient bacterial clearance during the different extracellular and intracellular phases of S. aureus.
MATERIALS AND METHODS
Bacteria.
The laboratory S. aureus strain NCTC8325-4 (49), positive for the icaADBC gene cluster (50) and negative for enterotoxin genes (51), was used in this study. The bacteria were grown aerobically overnight at 37°C on sheep blood agar plates (bioMérieux). Next, the bacteria were cultured overnight in tryptic soy broth (TSB; BD Difco), while shaking, to the stationary phase (SP) (optical density at 600 nm [OD600], >1). Subsequently, 0.5% of the overnight culture was inoculated into fresh TSB and grown to the exponential phase (EP) (OD600, <1). Bacteria were washed (removing TSB components, cell debris, and bacterial secreted factors) and adjusted to 1 × 109 CFU/ml in sterile PBS and kept on ice until use. The bacterial concentration was verified by quantitative culture on blood agar plates. For internalization experiments, S. aureus was fluorescently labeled using Alexa Fluor 647 conjugated succinimidyl-ester (Molecular Probes).
Generation of immature DCs and purification of autologous CD4+ T lymphocytes.
Human peripheral blood was collected after obtaining written informed consent in accordance with the approval of the Medical Ethical Committee of the Amsterdam UMC, location AMC, Amsterdam, The Netherlands. Peripheral blood mononuclear cells (PBMCs) were isolated from healthy individuals by density centrifugation on Lymphoprep (Nycomed). Immature monocyte-derived dendritic cells were generated as previously described (52). Briefly, monocytes were isolated from PBMCs by density centrifugation on Percoll (GE Healthcare), and monocytes (4 × 105 cells/ml) were cultured for 6 days in Iscove’s modified Dulbecco medium (IMDM; Gibco) containing gentamicin (86 μg/ml; Duchefa) and 5% fetal calf serum (FCS) (Lonza) supplemented with 500 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Schering-Plough) and 10 U/ml rec-IL-4 (Miltenyi Biotec). CD4+ T cells were isolated from PBMCs and purified with a magnetically activated cell sorting (MACS) isolation kit for CD4+ cells (Miltenyi Biotec). Next, naive and memory CD4+ T cells were purified using anti-CD45RO-phycoerythrin (anti-CD45RO-PE) beads (Dako) and anti-PE MACS beads (Miltenyi Biotech). The purity of naive and memory CD4+ was >97% for naive and >95% for memory T cells (data not shown) as measured by flow cytometry (Canto II; BD Biosciences).
Cytokine production and maturation molecule expression by DCs.
Immature DCs were harvested, and 4 × 104 DCs/well were stimulated for 24 h in 96-well round-bottom culture plates (Costar; Corning) with staphylococcal PGN (10 μg/ml; Sigma), staphylococcal MDP (10 μg/ml; Sigma), Pam3Cys-Ser-(Lys)4 (Pam3CSK4; 10 μg/ml; Sigma), or EP or SP S. aureus at an MOI of 20, unless stated otherwise. For preincubation experiments, EP S. aureus bacteria were added 30 min after incubation of DCs with PGN, MDP, or Pam3CSK4. To block endogenous IL-10, neutralizing anti-IL-10 antibodies (10 μg/ml; BD Pharmingen) were added to the DCs 30 min before stimulation with PGN or EP S. aureus. Supernatants were harvested and stored at –20°C for later measurement of levels of IL-12p70 (U-Cytech), IL-23 (U-Cytech), IL-10 (BD Pharmingen), IL-6 (U-Cytech), and TNF-α (eBioscience) using sandwich enzyme-linked immunosorbent assay (ELISA). For the analysis of cell surface molecules, immature DCs were stimulated for 48 h in 24-well plates (Costar; Corning) in medium supplemented with 500 U/ml GM-CSF and with EP or SP S. aureus (MOI of 20). The expression of maturation molecules was analyzed by flow cytometry (Canto II; BD Biosciences) after staining with fluorescent antibodies against CD83-allophycocyanin (CD83-APC), CD86-PE, and HLA-DR-peridinin chlorophyll protein (HLA-DR-PerCP) (all purchased from BD Biosciences).
Internalization of S. aureus by DCs.
Immature DCs were cocultured with fluorescently labeled EP or SP S. aureus (MOI of 50) at 37°C for 1 h. The DCs were washed twice with ice-cold PBS to stop uptake of bacteria and analyzed using flow cytometry. The endocytosis efficiency of the DCs was expressed as the percentage of DCs which internalized fluorescently labeled S. aureus.
T cell proliferation assessed using click-iT EdU.
Immature DCs were stimulated for 48 h with EP or SP S. aureus (MOI of 20), washed and cocultured with either autologous CD4+ naive or memory T cells (5 × 103 DCs:2 × 104 T cells) in IMDM (Lonza) supplemented with 5% heat-inactivated human AB serum (Lonza) and gentamicin (86 μg/ml). After 5 days, cells were incubated with 10 μM EdU (5-ethynyl-2′-deoxyuridine; Invitrogen) for 16 h. Next, cells were washed with PBS, fixated with 4% formaldehyde (Sigma) for 15 min, washed in PBS, and permeabilized with 0.5% saponin (Sigma) in PBS containing 1% FCS. T cell proliferation was detected using a Click-iT EdU Alexa Fluor 647 imaging kit (Invitrogen) and analyzed using flow cytometry.
Polarization of CD4+ T cells.
For Th cell polarization, either autologous CD4+ naive or memory T cells were cocultured with immature DCs (5 × 104:5 × 104 cells) and EP or SP S. aureus (MOI of 20). For experiments investigating the role of IL-12, 10 μg/ml of neutralizing anti-hIL-12p70 (U-Cytech) was added to the culture. For preincubation experiments, DCs were preincubated for 30 min with normal medium or medium containing PGN (10 μg/ml) and subsequently cocultured with EP S. aureus and T cells. After 3 days, cells were transferred to 96-well round-bottom plates (Greiner Bio-One), and every second day half of the medium was replaced by fresh IMDM containing 5% human serum (Sigma) and 20 U/ml IL-2 (Chiron). The cell suspensions with proliferating antigen-specific T cells were diluted when cells became confluent until T cell cultures were resting (at day 12). For detection of intracellular production of the Th1 and Th17 cell signature cytokines IFN-γ and IL-17, resting T cells were restimulated with 100 ng/ml phorbol myristate acetate (PMA), 1 μg/ml ionomycin, and 10 μg/ml brefeldin A (all purchased from Sigma) for 5 h. The T cells were analyzed using flow cytometry after 15 min fixation in 3.7% formaldehyde, permeabilization with 0.5% saponin, and intracellular staining with anti-hIFN-γ-fluorescein isothiocyanate (FITC) and anti-hIL17A-APC (both from BD Biosciences). In parallel, secreted IFN-γ and IL-17A in the supernatant were measured using ELISA after restimulation of resting T cells (1 × 105 cells/well) for 24 h with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) (both obtained from Sanquin).
Quantitative real-time PCR.
To determine the mRNA levels of subunits IL-23p19, IL-12p35, and IL-12p40, DCs were lysed 9 h after stimulation. RNA was extracted from DCs using a NucleoSpin RNA isolation kit (Macherey-Nagel), and cDNA was synthesized using a RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific). Quantitative real-time PCR (RT-PCR) (CFX Connect real-time PCR detection system; Bio-Rad) was performed using iQ SYBR green Supermix (Bio-Rad) and primer pairs as listed in Table 1. mRNA levels were normalized to the geometric mean of threshold cycle (CT) values of three housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 18S rRNA, and β2-microglobulin (β2M) [2Ct(housekeeping)-Ct(target)], and fold changes were calculated compared to those in DCs stimulated with EP S. aureus.
TABLE 1.
Primers for quantitative RT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| IL-12p35 | AGTGCCGGCTCAGCATGTGTC | GTGGCCACGGGGAGGTTTCT |
| IL-12p40 | ACGTTTCACCTGCTGGTGGCT | CTCCGCACGTCACCCCTTGG |
| IL-23p19 | GTGGGACACATGGATCTAAGAGAAG | TTTGCAAGCAGAACTGACTGTTG |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTT |
| 18S | CGGCTACCACATCCAAGGAA | GCTGGAATTACCGCGGCT |
| β2M | AAGATTCAGGTTTACTCACGTC | TGATGCTGCTTACATGTCTCG |
Statistics.
Data were compared using the Wilcoxon signed rank test or Student’s t test with GraphPad Prism 7.03 (GraphPad Software). Differences were considered significant when P was <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by the Dutch Technology Foundation STW (grant 10725), and L.D.L. was supported by the Danish Council for Independent Research, Natural Sciences (grant 0602-01522B).
We thank Femke Muller, Toni van Capel, Daisy Picavet, Esther Taanman, and Leonie de Boer for technical assistance.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell BG, Shaban RZ, MacBeth D, Wood C-J, Russo PL. 2017. The burden of healthcare-associated infection in Australian hospitals: a systematic review of the literature. Infect Dis Health 22:117–128. doi: 10.1016/j.idh.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 5.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraunholz M, Sinha B. 2012. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol 2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sendi P, Proctor RA. 2009. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol 17:54–58. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 8.George EA, Muir TW. 2007. Molecular mechanisms of agr quorum sensing in virulent staphylococci. Chembiochem 8:847–855. doi: 10.1002/cbic.200700023. [DOI] [PubMed] [Google Scholar]
- 9.Foster TJ, Hook M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 10.Wann ER, Gurusiddappa S, Hook M. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem 275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]
- 11.Cunnion KM, Lee JC, Frank MM. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immun 69:6796–6803. doi: 10.1128/IAI.69.11.6796-6803.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund LD, Ingmer H, Frøkiær H. 2016. D-alanylation of teichoic acids and loss of poly-N-acetyl glucosamine in Staphylococcus aureus during exponential growth phase enhance IL-12 production in murine dendritic cells. PLoS One 11:e0149092. doi: 10.1371/journal.pone.0149092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Roderiquez G, Norcross MA. 2012. Control of adaptive immune responses by Staphylococcus aureus through IL-10, PD-L1, and TLR2. Sci Rep 2:606. doi: 10.1038/srep00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haileselassie Y, Navis M, Vu N, Qazi KR, Rethi B, Sverremark-Ekström E. 2016. Lactobacillus reuteri and Staphylococcus aureus differentially influence the generation of monocyte-derived dendritic cells and subsequent autologous T cell responses. Immun Inflamm Dis 4:315–326. doi: 10.1002/iid3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laborel-Préneron E, Bianchi P, Boralevi F, Lehours P, Fraysse F, Morice-Picard F, Sugai M, Sato’o Y, Badiou C, Lina G, Schmitt A-M, Redoulès D, Casas C, Davrinche C. 2015. Effects of the Staphylococcus aureus and Staphylococcus epidermidis secretomes isolated from the skin microbiota of atopic children on CD4+ T cell activation. PLoS One 10:e0141067. doi: 10.1371/journal.pone.0141067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindler D, Gutierrez MG, Beineke A, Rauter Y, Rohde M, Foster S, Goldmann O, Medina E. 2012. Dendritic cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection. Am J Pathol 181:1327–1337. doi: 10.1016/j.ajpath.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. 2008. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev 226:112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haraguchi S, Day NK, Nelson RP Jr, Emmanuel P, Duplantier JE, Christodoulou CS, Good RA. 1998. Interleukin 12 deficiency associated with recurrent infections. Proc Natl Acad Sci U S A 95:13125–13129. doi: 10.1073/pnas.95.22.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borisova M, Gaupp R, Duckworth A, Schneider A, Dalugge D, Muhleck M, Deubel D, Unsleber S, Yu W, Muth G, Bischoff M, Gotz F, Mayer C. 2016. Peptidoglycan recycling in Gram-positive bacteria is crucial for survival in stationary phase. mBio 7:e00923-16. doi: 10.1128/mBio.00923-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 22.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. 2006. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem 281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 24.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 25.Dziarski R, Gupta D. 2005. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect Immun 73:5212–5216. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 27.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. 2001. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol 166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 28.Re F, Strominger JL. 2004. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol 173:7548–7555. doi: 10.4049/jimmunol.173.12.7548. [DOI] [PubMed] [Google Scholar]
- 29.Amar Y, Rizzello V, Cavaliere R, Campana S, De Pasquale C, Barberi C, Oliveri D, Pezzino G, Costa G, Meddah AT, Ferlazzo G, Bonaccorsi I. 2015. Divergent signaling pathways regulate IL-12 production induced by different species of Lactobacilli in human dendritic cells. Immunol Lett 166:6–12. doi: 10.1016/j.imlet.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 30.den Dunnen J, Vogelpoel LT, Wypych T, Muller FJ, de Boer L, Kuijpers TW, Zaat SA, Kapsenberg ML, de Jong EC. 2012. IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcγRIIa in human dendritic cells. Blood 120:112–121. doi: 10.1182/blood-2011-12-399931. [DOI] [PubMed] [Google Scholar]
- 31.Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML. 2004. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol 34:1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 32.Geijtenbeek TB, Gringhuis SI. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Foley NM, Wang J, Redmond HP, Wang JH. 2015. Current knowledge and future directions of TLR and NOD signaling in sepsis. Mil Med Res 2:1. doi: 10.1186/s40779-014-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapetanovic R, Nahori MA, Balloy V, Fitting C, Philpott DJ, Cavaillon JM, Adib-Conquy M. 2007. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect Immun 75:830–837. doi: 10.1128/IAI.01199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boye L, Welsby I, Lund LD, Goriely S, Frokiaer H. 2016. Plasma membrane Toll-like receptor activation increases bacterial uptake but abrogates endosomal Lactobacillus acidophilus induction of interferon-beta. Immunology 149:329–342. doi: 10.1111/imm.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt KJ, Fickentscher C, Kruithof EK, de Moerloose P. 2013. TLR2 ligands induce NF-κB activation from endosomal compartments of human monocytes. PLoS One 8:e80743. doi: 10.1371/journal.pone.0080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Gotz F, Goldmann DA, Pier GB. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun 73:6868–6876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC. 2006. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol Microbiol 59:948–960. doi: 10.1111/j.1365-2958.2005.04978.x. [DOI] [PubMed] [Google Scholar]
- 40.Wann ER, Dassy B, Fournier JM, Foster TJ. 1999. Genetic analysis of the cap5 locus of Staphylococcus aureus. FEMS Microbiol Lett 170:97–103. doi: 10.1111/j.1574-6968.1999.tb13360.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuipers A, Stapels DA, Weerwind LT, Ko YP, Ruyken M, Lee JC, van Kessel KP, Rooijakkers SH. 2016. The Staphylococcus aureus polysaccharide capsule and Efb-dependent fibrinogen shield act in concert to protect against phagocytosis. Microbiology 162:1185–1194. doi: 10.1099/mic.0.000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins J, Loughman A, van Kessel KP, van Strijp JA, Foster TJ. 2006. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol Lett 258:290–296. doi: 10.1111/j.1574-6968.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- 43.Brown AF, Murphy AG, Lalor SJ, Leech JM, O’Keeffe KM, Mac Aogáin M, O’Halloran DP, Lacey KA, Tavakol M, Hearnden CH, Fitzgerald-Hughes D, Humphreys H, Fennell JP, van Wamel WJ, Foster TJ, Geoghegan JA, Lavelle EC, Rogers TR, McLoughlin RM. 2015. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog 11:e1005226. doi: 10.1371/journal.ppat.1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. 2008. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol 9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 45.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 46.Avci FY, Kasper DL. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu Rev Immunol 28:107–130. doi: 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- 47.Schrijver IA, Melief MJ, Markusse HM, Van Aelst I, Opdenakker G, Hazenberg MP, Laman JD. 2001. Peptidoglycan from sterile human spleen induces T-cell proliferation and inflammatory mediators in rheumatoid arthritis patients and healthy subjects. Rheumatology (Oxford) 40:438–446. doi: 10.1093/rheumatology/40.4.438. [DOI] [PubMed] [Google Scholar]
- 48.Horn J, Stelzner K, Rudel T, Fraunholz M. 2018. Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol 308:607–624. doi: 10.1016/j.ijmm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Novick R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 50.Haaber J, Cohn MT, Frees D, Andersen TJ, Ingmer H. 2012. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PLoS One 7:e41075. doi: 10.1371/journal.pone.0041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalinski P, Vieira P, Schuitemaker JHN, Cai Q, Kapsenberg M. 2003. Generation of human type 1- and type 2-polarized dendritic cells from peripheral blood. Methods Mol Biol 215:427–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.