The avian pathogen Mycoplasma gallisepticum, the etiological agent of chronic respiratory disease in chickens, exhibits enhanced pathogenesis in the presence of a copathogen such as low-pathogenic avian influenza virus (LPAIV).
KEYWORDS: LPAIV, Mycoplasma gallisepticum, RNA-seq, avian influenza A virus, chicken, immune response
ABSTRACT
The avian pathogen Mycoplasma gallisepticum, the etiological agent of chronic respiratory disease in chickens, exhibits enhanced pathogenesis in the presence of a copathogen such as low-pathogenic avian influenza virus (LPAIV). To further investigate the intricacies of this copathogenesis, chickens were monoinfected or coinfected with either virulent M. gallisepticum strain Rlow or LPAIV H3N8 (A/duck/Ukraine/1963), with assessment of tracheal histopathology, pathogen load, and transcriptomic host responses to infection by RNA sequencing. Chickens coinfected with M. gallisepticum Rlow followed by LPAIV H3N8 exhibited significantly more severe tracheal lesions and mucosal thickening than chickens infected with LPAIV H3N8 alone and greater viral loads than chickens infected first with H3N8 and subsequently with M. gallisepticum Rlow. Recovery of live M. gallisepticum was significantly higher in chickens infected first with LPAIV H3N8 and then with M. gallisepticum Rlow, compared to chickens given a mock infection followed by M. gallisepticum Rlow. The transcriptional responses to monoinfection and coinfection with M. gallisepticum and LPAIV highlighted the involvement of differential expression of genes such as Toll-like receptor 15, Toll-like receptor 21, and matrix metallopeptidase 1. Pathway and gene ontology analyses of these differentially expressed genes suggest that coinfection with virulent M. gallisepticum and LPAIV induces decreases in the expression of genes related to ciliary activity in vivo and alters multiple immune-related signaling cascades. These data aid in the understanding of the relationship between M. gallisepticum and LPAIV during copathogenesis in the natural host and may contribute to further understanding of copathogen infections of humans and other animals.
INTRODUCTION
The bacterial pathogen Mycoplasma gallisepticum causes significant diseases such as sinusitis in turkeys, conjunctivitis in passerines, and chronic respiratory disease in chickens (1, 2). Pathogenesis of M. gallisepticum in chickens involves severe inflammation in components of the respiratory system, as well as deleterious effects on egg production in these birds, making M. gallisepticum a pathogen of great relevance and concern to the agricultural industry (1). Infection of chickens with the virulent M. gallisepticum strain Rlow was shown to induce a dysregulated immune response in the chicken host, with differential gene expression in the trachea peaking 3 days postinfection in the first 7 days (3). Toll-like receptor (TLR) responses, most notably TLR4 and TLR15, were significantly upregulated in the trachea during infection, as were interleukin-1β (IL-1β), matrix metallopeptidase 7 (MMP7), and an array of inflammatory cytokines and chemokines (3). An additional report demonstrated that the lipoproteins of M. gallisepticum stimulated a TLR2-mediated response in primary chicken tracheal epithelial cells (4).
Another avian pathogen of great importance is avian influenza A virus. Avian influenza viruses are classified into one of two pathotypes according to the severity of disease in gallinaceous birds, namely, highly pathogenic avian influenza virus (HPAIV) or low-pathogenic avian influenza virus (LPAIV) (5). HPAIV causes severe disease in a multitude of avian species, with an extremely high mortality rate (6). In contrast, LPAIV causes little to no disease in birds and, without rigorous surveillance, goes unnoticed in wild or domestic populations of birds (6).
Coinfections consisting of M. gallisepticum and LPAIV in chickens exacerbate disease, compared to either pathogen in isolation (7). Chickens challenged with both M. gallisepticum strain 1226 and LPAIV H3N8 (A/mallard/Hungary/19616/07) showed more severe clinical signs and increased recovery of M. gallisepticum (7). Coinfection with these two pathogens also yielded significantly more severe clinical scores for gross and histopathological lesions of infected chickens (8). Ex vivo experiments with M. gallisepticum strain S6 and H9N2 (A/chicken/Saudi Arabia/CP7/1998) in an avian tracheal organ culture system showed significant differences in replication of M. gallisepticum and H9N2 between monoinfected and coinfected tracheas. Ciliostasis was observed at a significantly higher rate in the coinfected tracheal cultures than in monoinfected tracheas (9).
In the case of M. gallisepticum and LPAIV coinfection, there is strong evidence that the presence of both pathogens enhances various markers of disease, compared with infection with either pathogen alone. However, the host-pathogen dynamics involved in this copathogenesis relationship remain unexplored. With the knowledge that this coinfection results in enhanced pathogenesis, we hypothesized that host genes critical for differential copathogenesis of M. gallisepticum and LPAIV would be differentially expressed in monoinfected and coinfected states. To examine this, we utilized an in vivo infection model in chickens, a natural host of both M. gallisepticum and LPAIV. In addition, we hypothesized that coinfection with LPAIV H3N8 would cultivate an environment to sustain the chronic persistence of an attenuated M. gallisepticum mutant in vivo. To explore this, we utilized the attenuated M. gallisepticum mutant P1H9, which has a transposon insertion inactivation of the Mycoplasma-specific lipoprotein A (mslA) gene, significantly diminishing its virulence in chickens and resulting in the mutant being cleared from the animals 14 days postinfection during monoinfection (10).
The copathogenesis of M. gallisepticum and LPAIV is of great relevance to the agricultural industry for the prevention and treatment of viral and bacterial infections in avian flocks (11). Either pathogen alone may go undetected in a population of birds for a sufficient period to set the stage for severe losses due to later infection with the other pathogen. The dynamics involved in this system of coinfection may also prove beneficial for understanding the factors contributing to instances of viral and bacterial copathogenesis in other animals and humans.
RESULTS
Tracheal thickness and lesion scores.
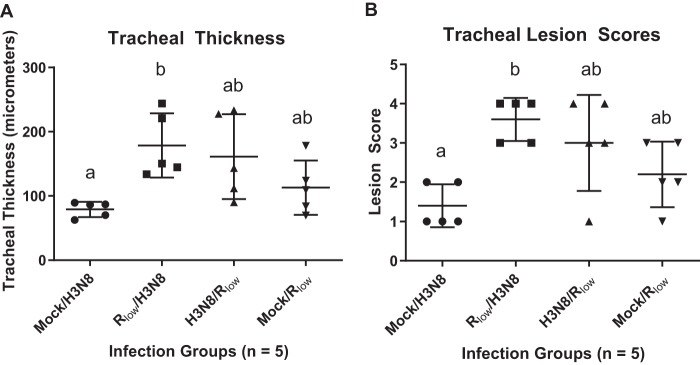
Tracheal mucosal thickness was significantly increased in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, compared to mock/LPAIV H3N8-infected chickens (P < 0.05 in experiment 1, one-way analysis of variance [ANOVA] with Tukey’s post hoc test). This pattern was replicated in the significant differences observed in histopathological tracheal lesion scores (P < 0.05) between mock/LPAIV H3N8-infected and M. gallisepticum Rlow/LPAIV H3N8-infected chickens in experiment 1 (Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test) (Fig. 1A and B). Although not statistically significant at this time point, tracheal thickness (P = 0.3901) exhibited a trend of increased severity in the H3N8/M. gallisepticum Rlow group, compared to the mock/M. gallisepticum Rlow group (Fig. 1A and B).
FIG 1.
Tracheal thickness measurements (A) and tracheal lesion scores (B) for chickens monoinfected and coinfected with M. gallisepticum and LPAIV H3N8. Statistically significant differences among group tracheal lesion scores were calculated using Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test. Statistically significant differences among group tracheal thickness measurements were calculated using one-way ANOVA with Tukey’s post hoc test. Significant (P < 0.05) differences between groups are denoted using a and b.
Mycoplasma and viral load.
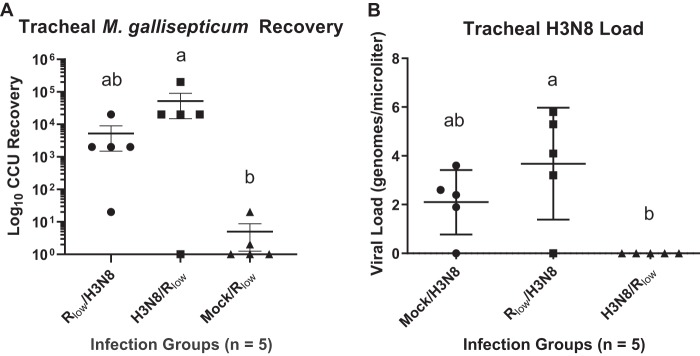
M. gallisepticum was recovered from birds in all groups that received an M. gallisepticum inoculation in experiment 1 (Fig. 2A). The bacterial loads recovered from LPAIV H3N8/M. gallisepticum Rlow-infected chickens were significantly higher than those in the mock/M. gallisepticum Rlow group (P < 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test) (Fig. 2A). Although tracheal pathology did not differ significantly between the H3N8/M. gallisepticum Rlow group and the mock/M. gallisepticum Rlow group (Fig. 1A and B), M. gallisepticum was recovered from 80% of chickens in the H3N8/M. gallisepticum Rlow group (Fig. 2A), 20% of chickens in the mock/M. gallisepticum Rlow group, and 100% of chickens in the M. gallisepticum Rlow/LPAIV H3N8 group (Fig. 2A).
FIG 2.
Pathogen loads from the tracheas of monoinfected and coinfected chickens of experiment 1. (A) M. gallisepticum recovery, quantified using CCU dilutions. Statistically significant differences were calculated using Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test, and significantly (P < 0.05) different groups are denoted using a and b. (B) LPAIV H3N8 loads, estimated using qRT-PCR. Statistically significant differences were determined using one-way ANOVA with Tukey’s post hoc test, and significantly (P < 0.01) different groups are denoted using a and b.
In a similar vein, LPAIV H3N8 was detected in chickens in all groups that received an inoculation with LPAIV H3N8 in experiment 1, with the exception of chickens in the LPAIV H3N8/M. gallisepticum Rlow infection group (Fig. 2B). LPAIV H3N8 was detected in 80% of chickens in the mock/LPAIV H3N8 group and the M. gallisepticum Rlow/LPAIV H3N8 group, both having been challenged with LPAIV H3N8 only 3 days before the end of the experiment. LPAIV H3N8 was not detected in the LPAIV H3N8/M. gallisepticum Rlow group after 6 days of H3N8 infection. The LPAIV H3N8 load was significantly higher in the M. gallisepticum Rlow/LPAIV H3N8 group than in the LPAIV H3N8/M. gallisepticum Rlow group in experiment 1 (P < 0.01, one-way ANOVA with Tukey’s post hoc test) (Fig. 2B).
Attenuated M. gallisepticum mutant recovery.
Experiment 2 demonstrated that M. gallisepticum was recovered from all M. gallisepticum P1H9-infected chickens, with the exception of P1H9/mock-infected birds sacrificed at 14 days postinfection (Fig. 3). The difference in M. gallisepticum recovery between these two groups was statistically significant (P < 0.001, Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test). P1H9 was previously shown to fail to persist at 14 days postinfection (10); however, these data showed that the attenuated P1H9 mutant did survive in the chicken trachea at 7 days postinfection in 90% of infected chickens. Persistence of P1H9 in the host trachea to 14 days postinfection was partially rescued only in the presence of coinfection with LPAIV H3N8 in 40% of infected chickens (Fig. 3).
FIG 3.
Tracheal M. gallisepticum recovery in experiment 2. Bacterial recovery was quantified using CCU 10-fold dilutions. Significant differences in bacterial loads were found between the P1H9/mock group at day 7 postinfection and the P1H9/mock group at day 14 postinfection, using Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test. Significantly (P < 0.01) different groups are denoted using a and b.
Significantly differentially expressed gene analysis.
Differential gene expression analysis between challenge groups of experiment 1 indicated that there were 260 significant differentially expressed genes (DEGs) increased in expression and 286 significant DEGs decreased in expression in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, relative to mock/LPAIV H3N8-infected chickens. Lists of DEGs for experimental groups of experiment 1 with >2-log2-unit fold changes can be found in Tables S5 through S7 in the supplemental material.
Two TLR genes were significantly differentially expressed in groups of infected chickens in experiment 1. TLR15 transcription was decreased in mock/M. gallisepticum Rlow-infected chickens, relative to mock/LPAIV H3N8-infected chickens (Table 1). TLR15, a bird- and reptile-specific TLR, is shown to be upregulated in response to yeast and bacterial pathogen-associated molecular patterns (PAMPS) (12). TLR21 is an avian functional homolog of human TLR9 and recognizes CpG oligodeoxynucleotides (13). TLR21 is also decreased in expression in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, relative to mock/LPAIV H3N8-infected chickens (Table 1). The suppression of TLR15 and TLR21 transcripts during monoinfection or coinfection with M. gallisepticum Rlow, relative to monoinfection with LPAIV H3N8, suggests potential immune signaling dysregulation induced by M. gallisepticum in this model.
TABLE 1.
Significant DEGsa
| Comparison | Ensembl gene identification | Gene name | Fold change | q |
|---|---|---|---|---|
| Mock/H3N8 vs mock/Rlow | ENSGALG00000008166 | TLR15 | −2.028 | 0.0115 |
| Mock/H3N8 vs Rlow/H3N8 | ENSGALG00000000774 | TLR21 | −2.100 | 0.0184 |
| Mock/H3N8 vs mock/Rlow | ENSGALG00000019061 | MMP1 | −2.054 | 0.0115 |
DEGs had changes of >2 log2 units.
Additionally, MMP1 expression was decreased in mock/M. gallisepticum Rlow-infected birds, relative to mock/LPAIV H3N8-infected chickens (Table 1). Differences in the transcription of MMP1 may indicate alterations in the rates of tissue damage and repair between LPAIV H3N8- and M. gallisepticum Rlow-infected chickens.
Functional gene ontology.
Between mock/LPAIV H3N8- and M. gallisepticum Rlow/LPAIV H3N8-infected chickens, DEGs could be grouped into an array of functional pathways. Significant DEGs that were decreased in expression in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, relative to mock/LPAIV H3N8-infected chickens, belonged primarily to functional pathways involved in cilium organization and function and macromolecule metabolic processes (Table S1). Significant DEGs that were increased in expression in M. gallisepticum Rlow/LPAIV H3N8-infected chickens belonged primarily to positive regulation of immune system processes (Table S2).
Pathway analysis.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of significant DEGs between experimental groups illuminated key pathways that were differentially regulated during monoinfection and coinfection. The TLR, phagosome, and cytokine-cytokine receptor pathways were significantly decreased in expression in mock/M. gallisepticum Rlow-infected chickens, relative to mock/LPAIV H3N8-infected chickens (Table S3). Metabolic pathways were significantly decreased in expression between the groups stated above, as well as in Rlow/LPAIV H3N8-infected chickens, relative to mock/LPAIV H3N8-infected chickens (Table S4).
Expression was notably increased in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, relative to mock/H3N8-infected chickens, for pathways including the TLR, phagosome, metabolic, apoptosis, RIG-I-like receptor signaling, focal adhesion, and extracellular matrix-receptor interaction pathways (Table S4). The influenza A response pathway was significantly increased in expression in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, relative to mock/LPAIV H3N8-infected chickens (Table S4), and was significantly altered across multiple comparisons. Regulation of the actin cytoskeleton was also significantly increased in expression in M. gallisepticum Rlow/LPAIV H3N8-infected chickens, relative to mock/LPAIV H3N8-infected chickens (Table S4).
DISCUSSION
These data contribute to our understanding of the copathogenesis of Mycoplasma gallisepticum and LPAIV. The exacerbated disease phenotype produced by these copathogens has been described ex vivo using a model system and in vivo at a later time point of 15 days postinfection with alternate strains of M. gallisepticum and LPAIV (7–9). In our in vivo model examining this copathogenesis at an early time point, tracheal histopathology was significantly more severe in M. gallisepticum Rlow/LPAIV H3N8-coinfected chickens than in mock/LPAIV H3N8-monoinfected chickens, both infected with LPAIV H3N8 for a total of 3 days (Fig. 1A and B).
In contrast, the inverse order of infection yielded no significant differences in tracheal histopathology between LPAIV H3N8/M. gallisepticum Rlow-coinfected chickens and mock/M. gallisepticum Rlow-monoinfected chickens, both infected with M. gallisepticum Rlow for 3 days (Fig. 1A and B). Although we could not discern copathogenic exacerbation of pathological lesions using the experimental conditions and kinetics of our virus/mycoplasma infection model, the recovery of live M. gallisepticum Rlow from the tracheas of LPAIV H3N8/M. gallisepticum Rlow-coinfected chickens was notably higher than that in mock/M. gallisepticum Rlow-monoinfected chickens (Fig. 2A). Previous in vivo studies by Stipkovits et al. similarly yielded significant differences in tracheal lesions between monoinfected and coinfected chickens in a bacterium/virus model of M. gallisepticum and LPAIV H3N8 copathogenesis (7, 8); however, our model examines coinfection at a more acute stage (6 days postinfection), as seen in experiment 1. The increased persistence of M. gallisepticum Rlow in LPAIV H3N8/M. gallisepticum Rlow-coinfected chickens at 6 days postinfection may contribute to more dramatic differences in tracheal pathology between monoinfected and coinfected birds over a longer period. In addition, our in vivo model of M. gallisepticum infection in chickens showed a peak in differential gene expression in the trachea of Rlow-infected chickens 3 days postinfection, which decreased over time (3). Our use of a 6-day infection period in this study encompasses this window of peak differential host response to monoinfection or coinfection.
Although previous studies utilized the transcriptomic approach to monitor M. gallisepticum infection of chickens, the present study furthers the use of these techniques in an M. gallisepticum coinfection experiment in vivo (3, 14–16). This method of capturing the global response to infection in the airway highlighted a number of critical host responses in this coinfection model. Although the experiments presented in this report did not include uninfected control chickens for direct comparisons with monoinfected or coinfected chickens, previous studies utilizing our in vivo model of M. gallisepticum infection of chickens demonstrated differential expression of inflammatory, metabolic, and signaling genes and pathways between uninfected control chickens and virulent M. gallisepticum Rlow-infected chickens over time, early in infection (3, 16). As further examples in this discussion indicate, some of these genes and pathways are also differentially expressed in the data from monoinfected and coinfected chickens presented herein (3, 16); this indicates that changes in gene expression captured in these experiments would likely not be seen in uninfected control animals.
One of the prominent host responses described was the significant decrease in expression of genes in pathways contributing to ciliary activity and integrity in the trachea. Although prior works examined this phenotype ex vivo (9), the data presented here provide evidence associated with the deleterious impact on genes and functional pathways associated with the ciliary mucosa function in the natural chicken host. This reduction of ciliary activity was shown between mock/H3N8-infected and M. gallisepticum Rlow/LPAIV H3N8-infected chickens (see Table S1 in the supplemental material). Loss of the integrity of the mucociliary elevator could contribute significantly to the exacerbated copathogenesis, by inhibiting pathogen clearing and promoting the inflammatory immune response due to damage in the tracheal mucosa.
Another novel aspect of these experiments was the differential expression of TLR genes in response to monoinfection or coinfection with M. gallisepticum and LPAIV. TLR15, which was significantly decreased in expression in mock/M. gallisepticum Rlow-infected chickens, compared to mock/LPAIV H3N8-infected chickens (Table 1), has been shown to be abundantly expressed in response to M. gallisepticum Rlow infection in chickens and specifically in response to diacylated lipopeptides of another avian pathogen, Mycoplasma synoviae (3, 17). TLR15 has also been shown to be upregulated in the lungs of chickens infected with the HPAIV H5N1 (A/duck/India/02CA10/2011) (18). Our current findings suggest that modulation of the expression of TLR15 remains critical in response to M. gallisepticum; however, TLR15 is more highly expressed in response to LPAIV H3N8 alone. This finding could indicate that a TLR15-mediated response is more relevant in response to influenza virus monoinfection and is not enhanced during coinfection with M. gallisepticum.
TLR21 expression is suppressed during coinfection; between mock/LPAIV H3N8 infection and M. gallisepticum coinfection, TLR21 was significantly decreased in expression (Table 1). This suppression of TLR21 during M. gallisepticum coinfection negatively correlates with the significantly enhanced tracheal pathology and viral loads in coinfected chickens (Fig. 1A and B and Fig. 2B). In chicken bone marrow macrophages, TLR21 and TLR4 costimulation induces increases in the expression of proinflammatory genes such as IL-1β, IL-12p40, and IL-10, as well as nitric oxide production (19). TLR4 is dramatically increased in expression in the tracheas of chickens infected with M. gallisepticum Rlow, within the first 7 days of infection (3). Therefore, suppression of TLR21 signaling in response to CpG deoxynucleotides and potential disruption of the association with TLR4 during coinfection with Mycoplasma gallisepticum may also be a critical factor in our model of copathogenesis.
MMP1 was also differentially expressed between mock/LPAIV H3N8-infected and mock/M gallisepticum Rlow-infected groups in experiment 1. These data can be associated with the increased tracheal pathology seen in the mock/M. gallisepticum Rlow group, relative to the mock/LPAIV H3N8 group, although the increase was not statistically significant (Fig. 1A and B). MMP7 was among the most highly expressed genes in the trachea in response to M. gallisepticum Rlow infection of chickens (3). MMP1 and MMP7 have both been implicated as biomarkers of human idiopathic pulmonary fibrosis and are overexpressed in the lungs of patients with chronic pulmonary disease (20). Therefore, it appears that a precedent exists for these MMP genes being involved in tissue remodeling in response to ongoing inflammatory challenges such as that induced by M. gallisepticum infection, with potential association with the manifestation of pathological lesions in the airway.
Functional pathway analysis also determined the differential regulation of the response to influenza A virus in comparisons between monoinfected and coinfected chickens, illustrating the importance of modulation of this specific response mechanism. Coinfection with virulent M. gallisepticum appears to have a unique effect on this response, as the influenza A response pathway was increased in expression in mock/LPAIV H3N8- and M. gallisepticum Rlow/LPAIV H3N8-infected chickens (Table S4). Although this influenza A response pathway is increased in expression, it may not yield a productive immune response to clear the virus during coinfection, as evidenced by the highest tracheal viral load being present in the M. gallisepticum Rlow/LPAIV H3N8-infected group of experiment 1 (Fig. 2B).
Another novel finding of these experiments was the persistence of attenuated M. gallisepticum mutant P1H9 in the chicken trachea 7 days postinfection in experiment 2 (Fig. 3). Previously, the P1H9 mutant was shown to be cleared from chickens 14 days postinfection and to bind polynucleotides in vitro (10, 21). The ability of the attenuated P1H9 mutant to persist in the shorter term in vivo illustrates that the absence of the Mycoplasma-specific lipoprotein A (mslA) in M. gallisepticum does not prohibit early colonization and replication in the chicken trachea. Notably, the attenuated mutant P1H9 was able to persist in the trachea to a chronic state at 14 days postinfection only in the presence of LPAIV H3N8 coinfection (Fig. 3). P1H9/mock-infected chickens in this experiment yielded no viable M. gallisepticum P1H9 recovery at 14 days postinfection (Fig. 3). Coinfection with H3N8 likely induces changes in the host environment, such as alterations in the immune response and changes in the tracheal architecture that partially rescue the mutant phenotype to allow for the its persistence. The capacity of LPAIV to rescue the persistence of an attenuated mutant indicate that populations of chickens infected with a subclinical M. gallisepticum field isolate are still susceptible to potential deleterious effects of copathogenesis. Furthermore, these findings have profound implications for potential consequences of LPAIV outbreaks in flocks recently immunized with live attenuated M. gallisepticum vaccines (e.g., TS-11 and 6/85).
In addition to effects on commercial flocks, these findings are relevant to backyard free-range flocks exposed to primary infection with either M. gallisepticum or LPAIV isolates of various virulence, which may evade common clinical surveillance measures and could result in severe economic losses to farmers and increased loads of the secondary pathogen if not properly addressed. Overall, our findings contribute to the understanding of host-microbe interactions during the copathogenesis of M. gallisepticum and LPAIV in chickens and indicate that the copathogenesis of M. gallisepticum and LPAIV is a complex dynamic that warrants further experimental analysis.
MATERIALS AND METHODS
Animals.
For each study, 4-week-old, specific-pathogen-free (SPF), female White Leghorn chickens (SPAFAS, North Franklin, CT, USA) were procured, separated randomly into experimental groups, and placed in HEPA-filtered isolator units. These chickens were allowed to acclimate for 1 week before the start of the experiment. For this acclimation period, as well as the remainder of the experiment, the chickens were maintained on nonmedicated food and water provided ad libitum. These animal experiments were performed with approval from the University of Connecticut Institutional Animal Care and Use Committee (protocol A18-057).
Mycoplasma gallisepticum and LPAIV preparation.
Influenza A virus isolate H3N8 (A/duck/Ukraine/1963) (BEI Resources) was used to inoculate 10-day-old, SPF, embryonated chicken eggs (SPAFAS) at 10-fold dilutions of viral stock (22, 23). After 48 h, allantoic fluid was collected from these eggs and viral titers were determined by 50% tissue culture infective dose (TCID50) assays on Madin-Darby canine kidney (MDCK) cells, using the Reed-Meunch method (22, 23). Viral stocks were frozen at –80°C and diluted in complete Hayflick’s medium to a concentration of 5 × 106 times TCID50/200 μl at the time of use as an experimental inoculum.
Mycoplasma gallisepticum strain Rlow passage 17 was propagated overnight at 37°C in complete Hayflick’s medium, until mid-log phase, from stock cultures that had been frozen at –80°C. The M. gallisepticum mutant P1H9 was also grown from frozen stock cultures, in complete Hayflick’s medium with the addition of 150 μg/ml gentamicin to maintain the position of the Tn4001 transposon insertion (10). Mycoplasma cultures were quantified by the optical density at 620 nm and then were centrifuged for 10 min at 10,000 × g to pellet the cultures. Mycoplasma pellets were then resuspended in fresh complete Hayflick’s medium to a density of 1 × 108 CFU/200 μl, to create the experimental inoculum for each of the chicken infection experiments.
Chicken infection experiments.
At 5 weeks of age, chickens were challenged intratracheally with 200 μl of either 1 × 108 CFU M. gallisepticum Rlow or 5 × 106 times TCID50 LPAIV H3N8 or were mock challenged with fresh complete Hayflick’s medium, according to the schedule shown in Table 2 (n = 5 per group); the chickens were sacrificed 6 days postinfection. In a companion study, additional sets of chickens (n = 10) were challenged with the attenuated M. gallisepticum mutant P1H9 as described above, in the groups shown in Table 3, and were sacrificed either 7 or 14 days postinfection.
TABLE 2.
Protocol for experiment 1
| Primary infection (day 0) | Secondary infection (day 3) | Sacrifice |
|---|---|---|
| Mock (Hayflick’s medium) | M. gallisepticum Rlow | Day 6 |
| LPAIV H3N8 | M. gallisepticum Rlow | Day 6 |
| M. gallisepticum Rlow | LPAIV H3N8 | Day 6 |
| Mock (Hayflick’s medium) | LPAIV H3N8 | Day 6 |
TABLE 3.
Protocol for experiment 2
| Primary infection (day 0) | Secondary infection (day 3) | Sacrifice |
|---|---|---|
| M. gallisepticum P1H9 | Mock (Hayflick’s medium) | Day 7 (n = 10) or day 14 (n = 10) |
| M. gallisepticum P1H9 | LPAIV H3N8 | Day 14 (n = 10) |
Sample collection, mycoplasma recovery, and RNA extraction.
In each of the experiments described above, all chickens from each of the groups were humanely sacrificed by cervical dislocation and the tracheas were excised. Tracheal rings from each chicken were taken from the proximal, middle, and distal portions of each trachea and fixed in neutral buffered formalin for pathological assessments. An additional ring was taken from the distal end of the trachea of each bird, placed in complete Hayflick’s medium for mycoplasma recovery, and incubated for 3 h at 37°C. After incubation, the cultures were filtered through 0.45-μm filters and diluted 10-fold for quantification of color changing units (CCUs), as performed previously (14). Statistically significant differences among groups in M. gallisepticum CCU recovery were calculated using Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test (GraphPad Prism 8.0).
The remaining sections of excised tracheas from each bird were washed with 1 ml of TRIzol (Zymo Research, Carlsbad, CA, USA) pipetted four times through the lumen for RNA collection, as described previously (15). RNA was extracted from these TRIzol washes using the Zymo DirectZol RNA miniprep kit (Zymo Research) and quantified using a Qubit 2.0 fluorometer (Thermo Fisher Scientific).
Tracheal thickness and lesion scoring.
Tracheal rings were embedded and sectioned for hematoxylin and eosin staining as described previously (15). These rings were measured for tracheal thickness and lesions were scored by an American Association of Veterinary Parasitologists board-certified veterinary pathologist. The lesions were scored on a scale of 0 to 4, denoting no inflammation, mild inflammation, moderate inflammation, marked inflammation, or severe inflammation, as described previously (24). Significant differences among challenge groups in tracheal thickness measurements were calculated using one-way ANOVA and Tukey’s post hoc test, and differences in tracheal lesion scores were calculated using Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test (GraphPad Prism 8.0).
Viral load quantification.
RNA collected from the tracheas of infected chickens in experiment 1 was assayed using quantitative reverse transcription-PCR (qRT-PCR) of the influenza matrix gene (25), in a 25-μl reaction volume (Applied Biosystems 75000 fast real-time PCR system), by the Connecticut Veterinary Medical Diagnostic Laboratory. Cycling conditions for this reaction were 45.0°C for 10 min, 95.0°C for 10 min, and 94.0°C for 1 s, with cycling at this step 45 times before a final step at 60.0°C for 30 s. A standard curve of LPAIV H3N8 (A/duck/Ukraine/1963) RNA was used in 10-fold dilutions, ranging from 1.4 × 100 times TCID50 to 1.4 × 106 times TCID50, for calculation of viral genomes per microliter.
Illumina sequencing.
RNA extracted from tracheal washes from each bird of experiment 1 was used as the template for cDNA library synthesis using the Illumina TruSeq stranded mRNA library preparation kit (Illumina, San Diego, CA). Each library was quantified and assessed for quality using the Agilent TapeStation 2200 (Agilent Technologies). These libraries were then normalized and pooled for sequencing on the Illumina NextSeq 500 system (Illumina) (paired-end, 75-bp reads, with 5 to 10 million reads per sample [3]), as performed by the University of Connecticut Center for Genome Innovation.
RNA sequencing analysis.
Read data from each sample (in the Fastq format) were mapped to the Gallus gallus reference genome using the TopHat package and Bowtie2 engine, to produce sequence alignments (26). Alignment files were pooled according to experimental infection group by using Cufflinks to map to reference transcript files. Cuffdiff was utilized to determine expression values and statistically significant differences in these values between experimental infection groups. The expression values per transcript were normalized within groups via calculation of fragments per kilobase per exon per million fragments mapped (FPKM). Benjamini-Hochberg analysis then generated a P value for statistical significance, accounting for a false-discovery rate of <5%, to yield a q value for significance. Fold changes between experimental infection groups were calculated by log2 calculation of FPKM values for each group (26). Significant DEGs with positive or negative fold changes of ≥2 log2 units were displayed for between-group comparisons.
Pathway and functional gene ontology analyses.
Significant DEGs were used as the input for analysis using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (version 6.8), to assemble the DEGs into biological pathways defined by the KEGG and functional gene ontologies, using GO Term BP Direct (27).
Supplementary Material
ACKNOWLEDGMENTS
We thank Kirklyn Kerr for his expertise in veterinary pathology and for assistance during necropsy and Katherine Pflaum, Arlind Mara, and Tyler Gavitt for assistance during the chicken experiments and for helpful experimental discussions. We also thank the Connecticut Veterinary Medical Diagnostic Laboratory for assistance in avian influenza virus testing.
This work was supported by the USDA National Institute of Food and Agriculture, Animal Health and Disease project 1010590 (S.J.G.), and the Center of Excellence for Vaccine Research. This work was also supported by the USDA National Institute of Food and Agriculture, Hatch project 1010739 (S.M.S.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Razin S, Yogev D, Naot Y. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 62:1094–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley DH, Berkhoff JE, McLaren JM. 1996. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis 40:480–483. doi: 10.2307/1592250. [DOI] [PubMed] [Google Scholar]
- 3.Beaudet J, Tulman ER, Pflaum K, Liao X, Kutish GF, Szczepanek SM, Silbart LK, Geary SJ. 2017. Transcriptional profiling of the chicken tracheal response to virulent Mycoplasma gallisepticum strain R low. Infect Immun 85:e00343-17. doi: 10.1128/IAI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majumder S, Zappulla F, Silbart LK. 2014. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-κB dependent pathway. PLoS One 9:e112796. doi: 10.1371/journal.pone.0112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capua I, Alexander DJ. 2004. Avian influenza: recent developments. Avian Pathol 33:393–404. doi: 10.1080/03079450410001724085. [DOI] [PubMed] [Google Scholar]
- 6.Umar S, Guerin JL, Ducatez MF. 2017. Low pathogenic avian influenza and coinfecting pathogens: a review of experimental infections in avian models. Avian Dis 61:3–15. doi: 10.1637/11514-101316-Review. [DOI] [PubMed] [Google Scholar]
- 7.Stipkovits L, Egyed L, Palfi V, Beres A, Pitlik E, Somogyi M, Szathmary S, Denes B. 2012. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol 41:51–57. doi: 10.1080/03079457.2011.635635. [DOI] [PubMed] [Google Scholar]
- 8.Stipkovits L, Glavits R, Palfi V, Beres A, Egyed L, Denes B, Somogyi M, Szathmary S. 2012. Pathologic lesions caused by coinfection of Mycoplasma gallisepticum and H3N8 low pathogenic avian influenza virus in chickens. Vet Pathol 49:273–283. doi: 10.1177/0300985811415702. [DOI] [PubMed] [Google Scholar]
- 9.Sid H, Hartmann S, Petersen H, Ryll M, Rautenschlein S. 2016. Mycoplasma gallisepticum modifies the pathogenesis of influenza A virus in the avian tracheal epithelium. Int J Med Microbiol 306:174–186. doi: 10.1016/j.ijmm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Szczepanek SM, Frasca S, Schumacher VL, Liao X, Padula M, Djordjevic SP, Geary SJ. 2010. Identification of lipoprotein MslA as a neoteric virulence factor of Mycoplasma gallisepticum. Infect Immun 78:3475–3483. doi: 10.1128/IAI.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelwhab E, Hafez H. 2012. Insight into alternative approaches for control of avian influenza in poultry, with emphasis on highly pathogenic H5N1. Viruses 4:3179–3208. doi: 10.3390/v4113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd AC, Peroval MY, Hammond JA, Prickett MD, Young JR, Smith AL. 2012. TLR15 is unique to avian and reptilian lineages and recognizes a yeast-derived agonist. J Immunol 189:4930–4938. doi: 10.4049/jimmunol.1101790. [DOI] [PubMed] [Google Scholar]
- 13.Brownlie R, Zhu J, Allan B, Mutwiri GK, Babiuk LA, Potter A, Griebel P. 2009. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol Immunol 46:3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Pflaum K, Tulman ER, Beaudet J, Canter J, Geary SJ. 2018. Variable lipoprotein hemagglutinin A gene (vlhA) expression in variant Mycoplasma gallisepticum strains in vivo. Infect Immun 86:e00524-18. doi: 10.1128/IAI.00524-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pflaum K, Tulman ER, Beaudet J, Liao X, Geary SJ. 2016. Global changes in Mycoplasma gallisepticum phase-variable lipoprotein gene vlhA expression during in vivo infection of the natural chicken host. Infect Immun 84:351–355. doi: 10.1128/IAI.01092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaudet J, Tulman ER, Pflaum K, Canter JA, Silbart LK, Geary SJ. 2018. Immunologic pathways in protective versus maladaptive host responses to attenuated and pathogenic strains of Mycoplasma gallisepticum. Infect Immun 87:e00613-18. doi: 10.1128/IAI.00613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oven I, Resman Rus K, Dušanić D, Benčina D, Keeler CL, Narat M. 2013. Diacylated lipopeptide from Mycoplasma synoviae mediates TLR15 induced innate immune responses. Vet Res 44:99. doi: 10.1186/1297-9716-44-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranaware PB, Mishra A, Vijayakumar P, Gandhale PN, Kumar H, Kulkarni DD, Raut AA. 2016. Genome wide host gene expression analysis in chicken lungs infected with avian influenza viruses. PLoS One 11:e0153671. doi: 10.1371/journal.pone.0153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Kaiser P, Borowska D, Vervelde L. 2018. Synergistic effect of co-stimulation of membrane and endosomal TLRs on chicken innate immune responses. Vet Immunol Immunopathol 199:15–21. doi: 10.1016/j.vetimm.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, MacDonald SD, Pardo A, Sciurba F, Dauber J, Selman M, Gochuico BR, Kaminski N. 2008. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masukagami Y, Tivendale KA, Mardani K, Ben-Barak I, Markham PF, Browning GF. 2013. The Mycoplasma gallisepticum virulence factor lipoprotein MslA is a novel polynucleotide binding protein. Infect Immun 81:3220–3226. doi: 10.1128/IAI.00365-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balish AL, Katz JM, Klimov AI. 2013. Influenza: propagation, quantification, and storage. Curr Protoc Microbiol 29:15G.1.1–15G.1.24. doi: 10.1002/9780471729259.mc15g01s29. [DOI] [PubMed] [Google Scholar]
- 23.Eisfeld AJ, Neumann G, Kawaoka Y. 2014. Influenza A virus isolation, culture and identification. Nat Protoc 9:2663–2681. doi: 10.1038/nprot.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gates AE, Frasca S, Nyaoke A, Gorton TS, Silbart LK, Geary SJ. 2008. Comparative assessment of a metabolically attenuated Mycoplasma gallisepticum mutant as a live vaccine for the prevention of avian respiratory mycoplasmosis. Vaccine 26:2010–2019. doi: 10.1016/j.vaccine.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. 2002. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol 40:3256–3260. doi: 10.1128/jcm.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.