Abstract
The innovative plasmon-activated water (PAW) with reduced hydrogen bonds exhibits intrinsically distinct properties at room temperature, which are significantly different from the properties of untreated conventional deionized (DI) water. Examples of this are their ability to scavenge free radicals and higher vapor pressure. However, distinct properties of energetic PAW decay within the day after its creation in a metastable liquid state. In this work, we report a facile method for persisting its distinct activities by letting as-prepared PAW be quickly frozen in liquid nitrogen and letting the frozen PAW (for one month before further measurements) be quickly melted to room temperature in a warm-water bath (called treated PAW). Experimental results indicate that the activity of the higher evaporation rate of treated PAW compared to DI water can be maintained ca. 90% of magnitude, as compared to the as-prepared PAW. Also, its abilities to scavenge free hydroxyl and 2,2-diphenyl-1-picrylhydrazyl radicals can be maintained at ca. 70 and 80% of magnitudes, respectively. Moreover, this strategy of quickly freezing and melting treatments to PAW on persisting in distinct activity of PAW is effective in oxygen evolution reactions. This promises the stored energy and the distinct property of created liquid PAW being available in water-related fields after long-term storage.
Introduction
Water is the most abundant compound on the earth. It is involved in many physical processes, and in various chemical and biological reactions.1−5 The most notable characteristic of water is its ability to form intramolecular hydrogen bonds (HBs). The different strengths of HBs result in the presence of water in the solid, liquid, or gas state. Also, the network interactions of water molecules critically decide the properties of liquid water. Liquid water can play a central role in various physical processes and chemical reactions because of its imperfect tetrahedral symmetry of the HB network, in contrast to ice with a nearly perfect tetrahedral symmetry around each water molecule because of strong HBs. It is well known that the dynamic equilibrium of HBs in liquid water occurs at a picosecond time scale, making the knowledge on the local structure of water limited.6−9 Thus, all of the generally recognized properties of liquid water are related to inert bulk water, comprising the tetrahedral HB network. However, the properties of liquid water are indeed modified because of the significant change of its HB structure for confined liquid water,10−12 or liquid water in contact with hydrophobic surfaces.13−15 Moreover, functional engineered water can be prepared employing electro-spraying technology for inactivating food-borne microorganisms, as reported in the literature.16,17 Also, water with the desired functions was developed for actual applications. The most noticed study is related to hydrogen-rich water, a powerful hydroxyl radical scavenger, which can efficiently reduce oxidative damage.18 Meanwhile, acidic cosmetic water19 and sulfurous water possess similar anti-inflammatory and anti-oxidative functions.20 However, additional additives are necessary for these kinds of functional water, in which water just acts as a carrier without changing its original HB structure.
Recently, liquid plasmon-activated water (PAW) was first created by innovatively using the effect of hot electron transfer (HET)21,22 which occurred at noble metal nanoparticles for breaking HBs of bulk water, which can be achieved under illumination with wavelength-optimized resonant light.23 Also, most of PAW’s tunable activities are linearly proportional to its degree of nonhydrogen-bonded structure, which is derived from the O–H stretching in a deconvoluted Raman spectrum.24 So far, nineteen PAW-related papers have been published. Examples of this are its creation mechanism23,25 and its innovative applications in green energies of evolution reactions of oxygen and hydrogen, medicine (lung cancer, hemodialysis, and chronic sleep deprivation), chemical reactions, and physical processes.26−35 However, the activity of the resulting metastable PAW decayed after its creation for a couple of days.24,25,28 For expanding convenience in application of PAW, an innovative strategy on persisting PAW’s distinct activities is proposed by utilizing quickly freezing and melting methods in this work. The distinct properties and anti-oxidative activity, as compared to bulk water, of as-prepared and aged PAW are compared to each other in detail.
Results and Discussion
Persisting in the Ability on Scavenging Free Radicals of PAW
As shown in our previous studies,21−25 the innovative PAW with reduced HBs exhibits intrinsically distinct properties at room temperature, which are significantly different from the properties of untreated conventional deionized (DI) water. Examples of this are their ability to scavenge free radicals and higher evaporation rates. Figure S1 demonstrates the scavenging abilities of as-prepared and 7 day aged PAW, compared to DI water, on active hydroxyl radicals. The four electro-spin resonance (ESR) splitting signals shown in Figure S1A are characteristic of hydroxyl radicals.18Figure S1B shows the corresponding statistically significant results. Compared to DI water, the magnitudes of intensities of free radicals decreased by 29.02% (p = 0.0007) and 12.80% (p = 0.0199), respectively, with as-prepared and 7 day aged PAW. The magnitude of activity of PAW on scavenging free radicals decreased by 56% after aging for 7 d. Similarly, the ability of as-prepared and 7 day aged PAW to scavenge radicals also demonstrated a positive effect on decreasing the corresponding ESR intensities of 2,2-diphenyl-1-picrylhydrazyl (DPPH) stable free radicals, as shown in Figure S2. Compared to DI water, the magnitudes of intensities of free radicals decreased by 28.42% (p = 0.0001) and 15.10% (p = 0.0060) , respectively, with as-prepared and 7 day aged PAW. Also, the magnitude of activity of PAW on scavenging DPPH free radicals decreased by 47% after aging for 7 d. These results indicated that the activity of the metastable liquid PAW would be decayed with time when it was saved in ambient laboratory air. Thus, developing an innovative strategy on persisting in distinct activity of PAW is necessary for its extensive applications.
In biotechnology, low-temperature systems using liquid nitrogen are popularly employed to save cells and tissues for persisting their activities in future using. Moreover, effects of different freezing rates and thawing temperatures on the post-thaw qualities of sperm36 and camel spermatozoa37 were evaluated in the literature. These ideas encourage us to utilize the facile liquid nitrogen system to maintain the activity of the created liquid PAW for further application in the future. Also, the effects of the freezing rates and the corresponding melting rates on the persisting in distinct activity of treated PAW are investigated. Figure 1A shows the corresponding statistically significant results of scavenging abilities of as-prepared and 1 day aged PAW, compared to DI water, on active hydroxyl radicals. Compared to DI water, the magnitudes of intensities of free radicals decreased by 30.68% (p = 0.0096) and 21.09% (p = 0.0019), respectively, with as-prepared PAW and 1 day aged PAW-4-1. Maintaining the activity of PAW on scavenging hydroxyl radicals by quickly freezing at −80 °C and quickly melting in a warm-water bath seems to be the optimum condition, as compared to the as-prepared PAW. In this experimental condition, the activity of the treated PAW on scavenging free radicals decreased by a magnitude of 31% after aging for 1 day. Basically, quickly freezing at −80 °C is better than slowly freezing at −20 °C, and quickly melting in a warm-water bath is better than slowly melting in the ambient laboratory air for persisting the activity of PAW, as shown in Figure 1A. Similarly, compared to DI water, the magnitudes of intensity of DPPH free radicals decreased by 27.81% (p = 0.0202) and 22.06% (p = 0.0412), respectively, with as-prepared PAW and 1 day aged PAW-4-1, as shown in Figure 1B. In the optimal experimental condition (quickly freezing/quickly melting) for maintaining the activity of treated PAW on scavenging DPPH radicals decreased by a magnitude of 21% after aging for 1 day, as compared to as-prepared PAW. Figure S3 exhibits the corresponding experimental results after aging for 30 days. Maintaining the activity of PAW on scavenging hydroxyl and DPPH radicals by quickly freezing at −80 °C and quickly melting in a warm-water bath still is the optimal experimental condition. The magnitudes of activities of treated PAW-4-30 on scavenging hydroxyl and DPPH radicals just decreased by 30 and 13% , respectively, after aging for 30 days, as compared to as-prepared PAW. Interestingly, the decreases in activities on scavenging free radicals of hydroxyl and DPPH are slightly different between the 1 day aged treated PAW and 30 day aged treated PAW based on optimum conditions. For scavenging hydroxyl radicals, the decreased magnitudes are 31 and 30% after aging for 1 and 30 days, respectively, as compared to as-prepared PAW. For scavenging DPPH radicals, the decreased magnitudes are 21 and 13% after aging for 1 and 30 days, respectively, as compared to as-prepared PAW. These results indicate that the storage time has little influence on the activity of the frozen PAW, making the activity available after long-term storage of PAW in a solid state.
Figure 1.
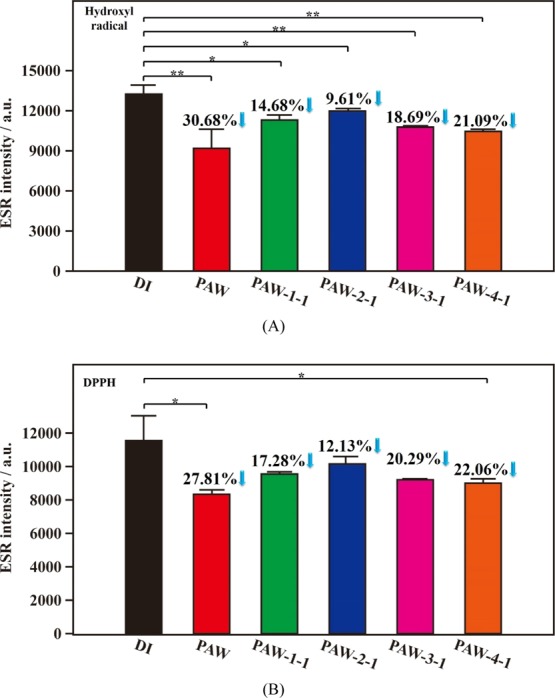
Statistical results of ESR spectra of hydroxyl and DPPH free radicals based on as-prepared PAW (red), various 1 day aged PAW, and DI water (black) for reference. (A) Hydroxyl free radicals. (B) DPPH free radicals. Green: slowly frozen/slowly melted PAW-1-1; blue: slowly frozen/quickly melted PAW-2-1; pink: quickly frozen/slowly melted PAW-3-1; orange: quickly frozen/quickly melted PAW-4-1. *, p < 0.05; **, p < 0.01.
Moreover, the liquid nitrogen system was used to quickly freeze PAW (called super-quickly frozen PAW) for persisting its distinct property. Figure 2A shows the corresponding statistically significant results of scavenging abilities of as-prepared and 1 day aged PAW, compared to DI water, on active hydroxyl radicals. Compared to DI water, the magnitudes of intensity of free radicals decreased by 29.26% (p = 0.0063) and 21.39% (p = 0.0006), respectively, with as-prepared PAW and 1 day aged PAW-6-1. Similarly, as compared to DI water, the magnitudes of intensity of DPPH free radicals decreased by 23.32% (p = 0.0006) and 19.60% (p = 0.0001), respectively, with as-prepared PAW and 1 day aged PAW-6-1, as shown in Figure 2B. In this experimental set, the sample of PAW-6-1 based on the most quickly freezing (using liquid nitrogen) and melting process demonstrates the best effect on maintaining the activities on scavenging free radicals. Moreover, as exhibited in Figure 2, the effect of the freezing/melting process on the corresponding activity of treated PAW is basically similar to that shown in Figure 1. A quick freezing and melting process takes advantage on persisting the distinct activity of prepared PAW. For activities of PAW-6-1 on scavenging hydroxyl and DPPH radicals, the decreased magnitudes are just 27 and 16%, respectively, after aging for one day, as compared to as-prepared PAW. Meanwhile, similar experiments were performed for 30 days to investigate the effect of storage time on the corresponding activity of treated PAW, as demonstrated in Figure 3. Compared to DI water, the magnitudes of intensity of hydroxyl free radicals decreased by 30.80% (p = 0.0078) and 22.31% (p = 0.0174), respectively, with as-prepared PAW and 30 day aged PAW-6-30, as shown in Figure 3A. Compared to DI water, the magnitudes of intensity of DPPH free radicals decreased by 25.07% (p = 0.0007) and 19.82% (p = 0.0004), respectively, with as-prepared PAW and 30 day aged PAW-6-30, as shown in Figure 3B. Also, the sample of PAW-6-1 based on the most quickly freezing and melting process demonstrates the best effect on maintaining the activities on scavenging free radicals after the aging experiment was performed for 30 days. For activities of PAW-6-1 on scavenging hydroxyl and DPPH radicals, the decreased magnitudes are just 28 and 21%, respectively, as compared to as-prepared PAW. Encouragingly, the decreases in activities on scavenging free radicals are very close to each other between 1 day aged PAW-6-1 and 30 day aged PAW-6-30, as comparing Figures 2 with 3. These similar results are observed on PAW-6-1 and PAW-6-30 suggesting that the storage time has less influence on the corresponding activity of the frozen PAW, promising the activity available after long-term storage of PAW in a stable solid state. Moreover, as discussed in Figures 1–3, S1, and S2, the obtained various decreased magnitudes in ESR intensities of free radicals based on similar experiments performed in as-prepared PAW, as compared to those performed in DI water, are examined. The intensities of the hydroxyl free radicals decreased by 29.02, 30.68, 29.26, and 30.80%. These decreased magnitudes for as-prepared PAW compared to DI water are very consistent, promising good experimental reproducibility. Also, the intensities of the DPPH free radicals decreased by 28.42, 27.81, 23.32, and 25.07%. The experimental reproducibility is satisfactory.
Figure 2.
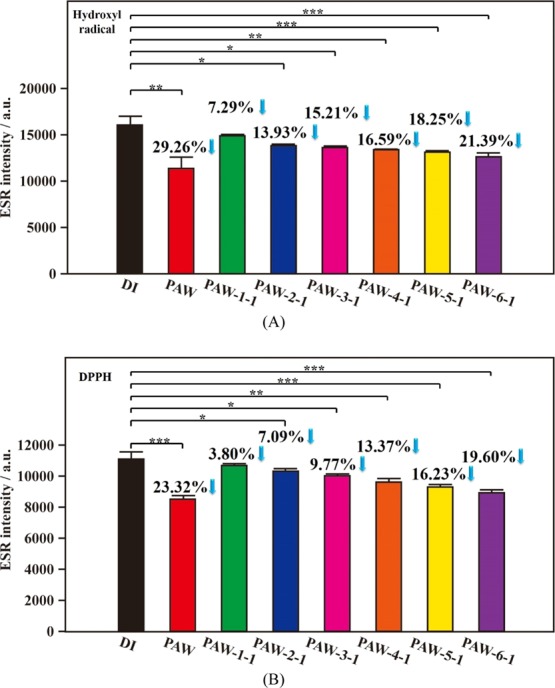
Statistical results of ESR spectra of hydroxyl and DPPH free radicals based on as-prepared PAW (red), various 1 day aged PAW and DI water (black) for reference. (A) Hydroxyl free radicals. (B) DPPH free radicals. Green: slowly frozen/slowly melted PAW-1-1; blue: slowly frozen/quickly melted PAW-2-1; pink: quickly frozen/slowly melted PAW-3-1; orange: quickly frozen/quickly melted PAW-4-1; yellow: super-quickly frozen/slowly melted PAW-5-1; purple: super-quickly frozen/quickly melted PAW-6-1. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Figure 3.
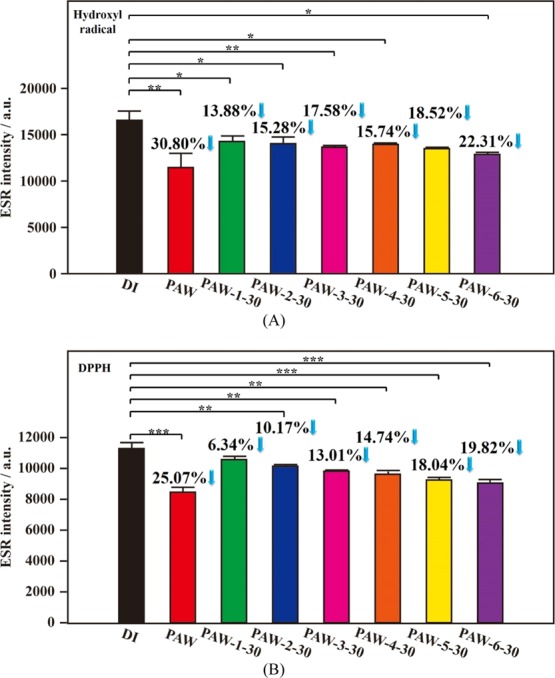
Statistical results of ESR spectra of hydroxyl and DPPH free radicals based on as-prepared PAW (red), various 30 day aged PAW and DI water (black) for reference. (A) Hydroxyl free radicals. (B) DPPH free radicals. Green: slowly frozen/slowly melted PAW-1-30; blue: slowly frozen/quickly melted PAW-2-30; pink: quickly frozen/slowly melted PAW-3-30; orange: quickly frozen/quickly melted PAW-4-30; yellow: super-quickly frozen/slowly melted PAW-5-30; purple: super-quickly frozen/quickly melted PAW-6-30. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Persisting in the Ability on Higher Evaporation Rates of PAW
Compared to DI water, the prepared PAW possesses a reduced HB structure. This distinct property is responsible for a higher evaporation rate in ambient laboratory air, as reported before.23,29 Water’s energy is associated with the bounded state of water molecules. It was reported that the osmotic effects,38 involving HBs, capillary forces, and requirement to restore water’s free state, can reduce the potential (or activity) of water. This discourse is based on the assumption of “free water”, in which HBs do not exist. So far, changes in water’s activity in different states have not been revealed for pure water. Generally, a higher vapor pressure of pure water is responsible for its higher potential (chemical activity),39 making a higher evaporation rate at room temperature. Also, as shown in our previous report,25 PAW possesses higher activity, which is in accordance with the increase in activity observed for confined water.40 As shown in Figure S4, the magnitude of the evaporation rate of as-prepared PAW is markedly higher by 41.22%, compared to that of DI water, after experiments for a half hour at 24.3 °C with 57.5 relative humidity (RH) %. Basically, quickly freezing at −80 °C and quickly melting in a warm-water bath still is the optimum condition for maintaining the activity on a higher evaporation rate. In this experimental condition, the magnitude of activity of 1 day aged PAW-4-1 on a higher evaporation rate (the magnitude of the evaporation rate is higher by 35.81%, compared to that of DI water) decreased by 13% after aging for 1 day, as compared to as-prepared PAW.
Compared to instrumental ESR experiments, the experimental results of the evaporation rate are easily influenced by many factors, like duration in the experiment, temperature, and RH. Thus, two more batch experiments were performed to examine the freezing/melting process on the corresponding activity of the aged PAW. Figure 4A shows the evaporated quantities with time of DI water and as-prepared and various 30 day aged PAW. In the first half hour, compared to DI water, the magnitudes of evaporation rates increased by 44.09 and 39.23%, respectively, with as-prepared PAW and 30 day aged PAW-4-30 (the optimum sample for maintaining this activity based on as-prepared PAW). In this experimental condition, the magnitude of activity of 30 day aged PAW-4-30 on a higher evaporation rate just decreased by 11% after aging for 30 days, as compared to as-prepared PAW. These results are consistent with those observed in Figure S4. After evaporation for 1 h, the quickly frozen and melted PAW-4-30 is still the optimally treated PAW. Compared to DI water, the magnitudes of evaporation rates increased by 24.62 and 22.21%, respectively, with those of as-prepared PAW and 30 day aged PAW-4-30. In this optimally experimental condition, the magnitude of activity of the higher evaporation rate decreased by 10% after aging for 30 days, as compared to as-prepared PAW. After evaporations for 2 and 3 h, as shown in Figure 4B, the quickly frozen and melted PAW-4-30 is still the optimally treated PAW, as compared to other treating processes, although some fluctuations are observed because of the competing advantage between quickly freezing and quickly melting in a longer duration. After evaporations for 2 h, compared to DI water, the magnitudes of evaporation rates increased by 16.23% (16.56% for 3 h) and 13.20% (9.97% for 3 h), respectively, with as-prepared PAW and 30 day aged PAW-4-30. In this optimally experimental condition, the magnitude of activity on the higher evaporation rate decreased by 19% (40% for 3 h) after aging for 30 days, as compared to as-prepared PAW. Figure S5A demonstrates similar experimental results based on another batch experiment performed at 24.0 °C with 58.5 RH %. After aging for 30 days, in the optimal experimental condition, the magnitudes of activities on higher evaporation rates of PAW-4-30 (quickly frozen and quickly melted PAW), compared to as-prepared PAW, decreased by 14 and 27% after evaporations for 0.5 and 1 h, respectively. Interestingly, compared to DI water, the higher evaporation rates of as-prepared PAW and PAW-4-30 are very close after evaporations for 2 and 3 h, as shown in Figure S5B. Meanwhile, as discussed in Figures 4, S4, and S5, the magnitudes of higher evaporation rates decreased by 10, 13, and 14%, respectively, compared to as-prepared PAW after the evaporation experiments for 0.5 h. These close decreased values based on similar batch experiments indicate that the experimental reproducibility is satisfactory.
Figure 4.
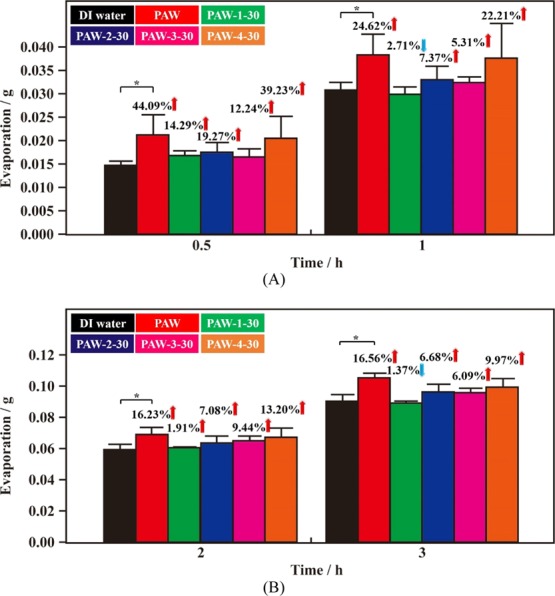
Evaporation quantities (g) with time of as-prepared PAW (red), various 30 day aged PAW and DI water (black) for reference. (A) 0.5 and 1 h after experiments. (B) 2 and 3 h after experiments. Green: slowly frozen/slowly melted PAW-1-30; blue: slowly frozen/quickly melted PAW-2-30; pink: quickly frozen/slowly melted PAW-3-30; orange: quickly frozen/quickly melted PAW-4-30. *, p < 0.05. The evaporation experiments were performed at 1 atm and 24.2 °C with 57.5 RH %.
Persisting in the Distinct Property of Negatively Charged PAW and in Activity on Effective Oxygen Evolution Reactions Utilizing PAW
As reported in our previous study on the mechanism of creation of PAW,25 the resulted PAW is slightly negatively charged because the water clusters are surrounded by hot electrons. The persistence of metastable PAW is time-dependent and the prepared PAW loses its activity with time because of the destruction of the metastable conformation. Figure 5 exhibits the zeta potentials of as-prepared PAW, various 30 day aged PAW, and DI water for reference. As shown in Figure 5A, the zeta potentials of as-prepared PAW and PAW-6-30 are significantly negative, indicating they are electron-doping states; while DI water is close to electronically neutral. Figure 5B shows the statistical results of zeta potentials of DI water, as-prepared and various 30 day aged PAW. Compared to DI water at −0.61 ± 0.04 mV, the zeta potentials are −27.93 ± 0.65, −23.87 ± 1.32, and −23.77 ± 0.64 mV for as-prepared PAW, 30 day aged PAW-5-30 and PAW-6-30 (the optimum samples for maintaining this distinct property based on as-prepared PAW), respectively. In this experiment, super-quickly freezing PAW utilizing liquid nitrogen can maintain the distinct property of PAW with a negatively charged state; while the quickly or slowly melting process is less important. Compared to as-prepared PAW, the distinct property of the negatively charged state of PAW-5-30 or PAW-6-30 just decreased by 15% of magnitude after aging for 30 days. In other experimental conditions, this distinct property of the negatively charged state of PAW can be maintained at around 50% magnitude, ignoring the speed of freezing and melting, after aging for 30 days.
Figure 5.
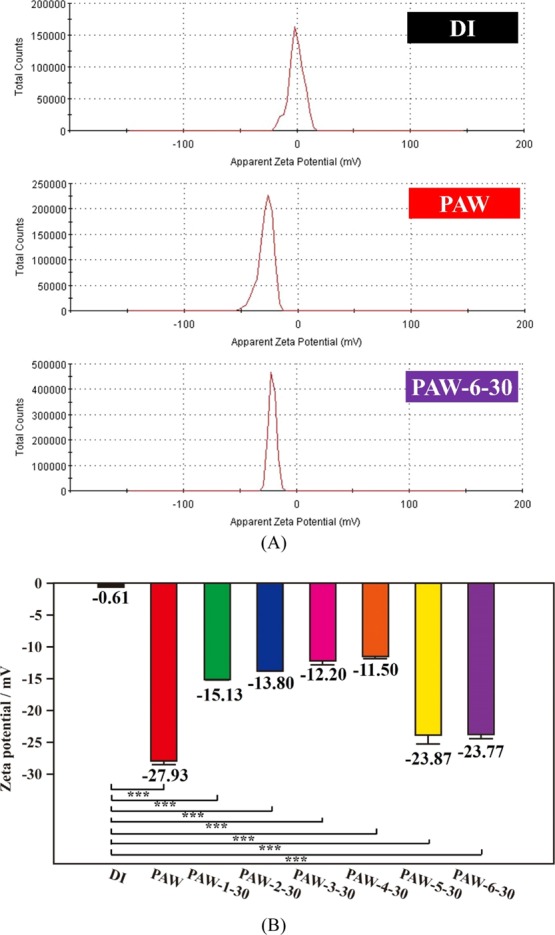
Zeta potential of as-prepared PAW (red), various 30 day aged PAW and DI water (black) for reference. (A) Zeta potentials of as-prepared PAW, 30 day aged PAW-6-30 and DI water. (B) Statistical results of zeta potentials of various samples. Green: slowly frozen/slowly melted PAW-1-30; blue: slowly frozen/quickly melted PAW-2-30; pink: quickly frozen/slowly melted PAW-3-30; orange: quickly frozen/quickly melted PAW-4-30; yellow: super-quickly frozen/slowly melted PAW-5-30; purple: super-quickly frozen/quickly melted PAW-6-30. ***, p < 0.001.
As per the study on triggering comprehensive enhancement in oxygen evolution reactions (OERs) by using PAW, we reported that the created PAW has an advantage in OERs because the corresponding activation energy can be effectively reduced by itself.31Figure 6A shows the linear scan voltammograms (LSVs) of OERs performed in 0.1 N KCl based on as-prepared PAW, 10 day aged PAW-4-10, and DI water. Clearly, the recorded currents are higher for as-prepared PAW and PAW-4-10, compared to DI water. Figure 6B shows the corresponding statistical results of recorded currents at 1.5 V versus Ag/AgCl in OERs using different water samples. Compared to DI water, the magnitudes of currents increased by 39.52 and 32.55%, respectively, with as-prepared PAW and 10 day aged PAW-4-10 (the optimum sample for maintaining the activity on effective OERs based on as-prepared PAW). Obviously, quickly freezing in storage has the advantage of maintaining PAW’s activity on effective OERs. Certainly, quickly freezing plus quickly melting assembles the best process. Compared to as-prepared PAW, the magnitude of distinct activity of PAW-4-10 in OERs just decreased by 18% after aging for 10 days.
Figure 6.
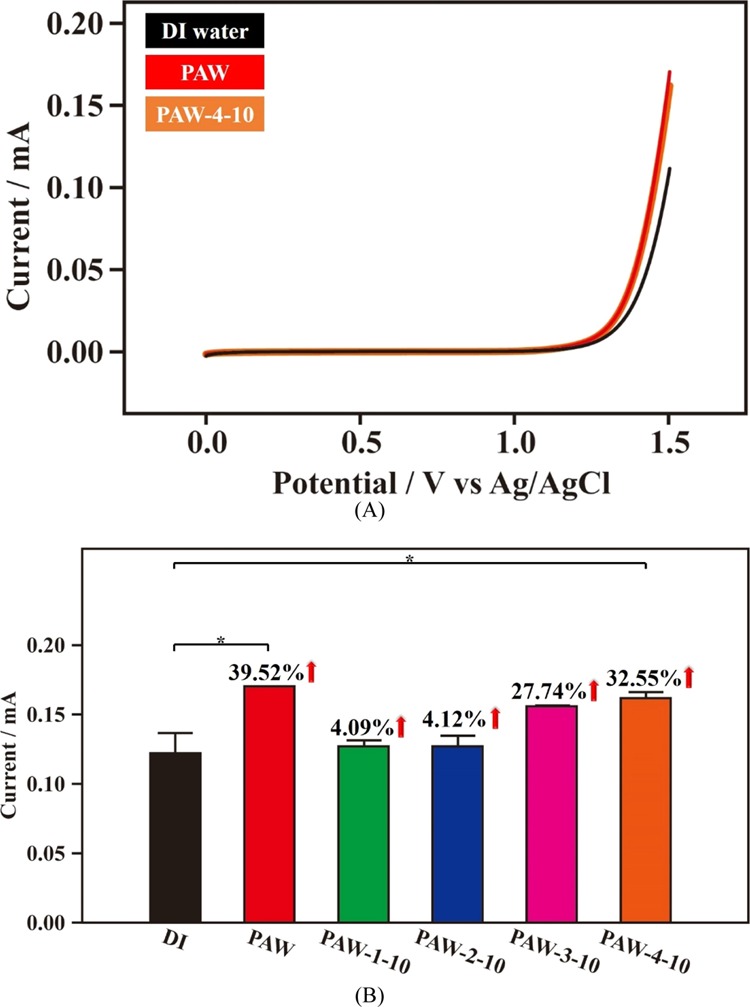
OERs (in 0.1 N KCl) of as-prepared PAW (red), various 10 day aged PAW and DI water (black) for reference. (A) LSV of as-prepared PAW, 10 day aged PAW-4-10 and DI water. (B) Statistical results of currents at 1.5 V vs Ag/AgCl of various samples. Blue: slowly frozen/quickly melted PAW-2-10; pink: quickly frozen/slowly melted PAW-3-10; orange: quickly frozen/quickly melted PAW-4-10. *, p < 0.05.
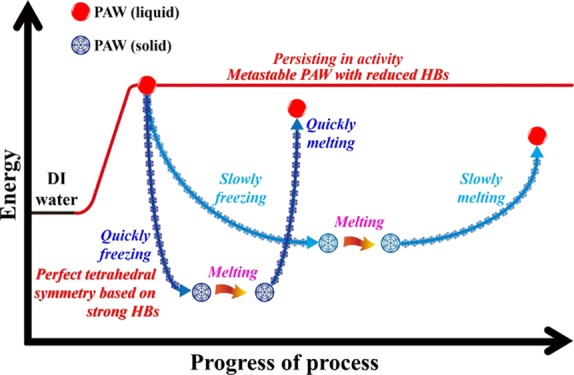
In biochemistry, alive cell cryopreservation based on freezing–thawing technology has broad application in protecting cell activity for long-term storage. Studies on complicated parametric optimization in cryopreservation are warranted to improve the quality of frozen–thawed cells, which is dependent on cell viability or motility.36,37 The freezing rate is one of the most important factor controlling the life or death of the cell during freezing. The thawing rate is also critical in preserving the viability of the cell. As shown in the literature,36 a rapid freezing rate appeared to be beneficial for kinematics and a higher thawing temperature resulted in better kinematics. In physical chemistry, the freezing process results in chemical processes that are deemed to be slow at room temperature becoming promoted by freezing; it can also produce unexpected chemical products.41 The cooling of a solution under laboratory conditions can lead to temperature variations within the sample. The effect can result in differing thermodynamics and kinetics of the reaction.41 Compared to the dynamic equilibrium of HBs in liquid water, the detailed HB network of ice with a nearly perfect tetrahedral symmetry is more difficult to be instrumentally certified. In this work, the freezing rate was controlled by using liquid nitrogen at ca. −200, −20 °C-controlled, and −80 °C-controlled refrigerators. The melting rate was controlled by using a warm-water bath at 40 °C and an ambient laboratory air at room temperature. As shown in our previous studies,23,25 the created PAW from HET is electron-doping with reduced HBs. The doped hot electron may promote some physical and/or chemical processes in the freezing process of PAW. In other words, adequate energies are necessary during the freezing processes for ordering and attracting. Hence, providing additional energy is favorable for freezing water.25 It was previously stated that PAW with fewer HBs possesses a higher energy potential. Stored energy is available for water molecules with extra kinetic energy when the ambient temperature rapidly decreases, namely, the non-HB structure in a high-energy state can transform into an HB structure in a low energy state, accompanied by a release of available energy.25 We believe that the more quickly freezing process in treating PAW is beneficial for ordering and attracting to form ice with a more perfect tetrahedral symmetry around each water molecule. More melting heat is necessary for melting the more quickly frozen PAW to be present in a more energetic liquid state. Certainly, the more quickly melting process results in liquid PAW maintaining most of its original distinct properties and activities. Figure 7 demonstrates a proposed mechanism for the developed strategy on persisting in distinct activity of PAW based on the experimental results. These interesting findings and the corresponding detailed mechanisms are worthy of further study in the future.
Figure 7.
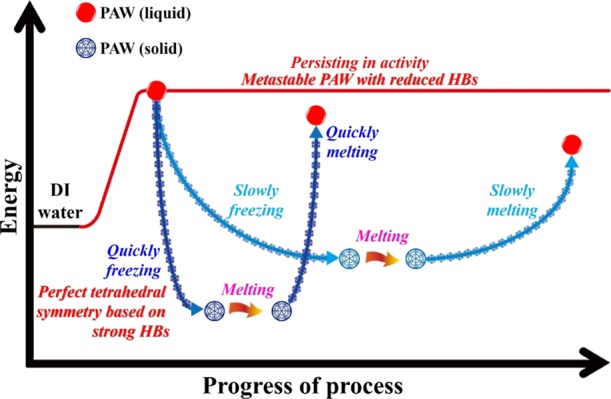
Schematic description for the persistence of liquid PAW under freezing and melting processes.
Conclusions
We have successfully utilized the quickly freezing/melting process to persist the distinct properties and the corresponding activity of innovative liquid PAW. After aging for one month, the distinct property of the negatively charged state of PAW can be maintained on a level above 80% of magnitude, compared to as-prepared PAW. Also, the distinct activities on a higher evaporation rate, scavenging free radicals and effective OERs can be maintained on a level above 70% of magnitudes, compared to as-prepared PAW. The experimental results indicate that quickly freezing (using liquid nitrogen or atmosphere at −80 °C) is more advantageous than quickly melting for persisting in the distinct activities of PAW. This innovative utilization of cryopreservation on persisting in the distinct properties and activities of PAW is the first to be virtually shown in the literature. These findings promise PAW conveniently applicable in water-related fields to investigate innovative aspects of effects from liquid PAW.
Materials and Methods
Chemicals and Materials
Electrolyte of KCl and reagents of 5,5-dimethyl-1-pyrroline N-oxide and DPPH were purchased from Sigma-Aldrich Organics. Reagents of H2O2 and iron(II) chloride tetrahydrate were purchased from Acros Organics. Reagent of phosphate-buffered saline was purchased from Bioman Organics. Reagent of ethylenediaminetetraacetic acid was purchased from Bioshop Organics. All of the reagents were used as received without further purification. All of the solutions were prepared using DI 18.2 MΩ cm water provided from a Milli-Q system. All of the experiments were performed in an air-conditioned room at ca. 24 °C. The water temperature is ca. 24.3 °C.
Acknowledgments
The authors thank the Ministry of Science and Technology (MOST) of ROC and Taipei Medical University for their financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02627.
Experimental methods, ESR spectra of hydroxyl free radicals and DPPH free radicals, statistical results of ESR spectra of hydroxyl and DPPH free radicals, and evaporation quantities (g) after 0.5 h of as-prepared plasmon-activated water, various 1-day and 30-day aged PAW and DI water for reference (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wang D.; Li Q.; Han C.; Xing Z.; Yang X. When NiO@Ni Meets WS2 Nanosheet Array: A Highly Efficient and Ultrastable Electrocatalyst for Overall Water Splitting. ACS Cent. Sci. 2018, 4, 112–119. 10.1021/acscentsci.7b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl P.; Barth F. G. Biomaterial Systems for Mechanosensing and Actuation. Nature 2009, 462, 442–448. 10.1038/nature08603. [DOI] [PubMed] [Google Scholar]
- Ma M.; Guo L.; Anderson D. G.; Langer R. Bio-Inspired Polymer Composite Actuator and Generator Driven by Water Gradients. Science 2013, 339, 186–189. 10.1126/science.1230262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K.; Mitsuoka K.; Hirai T.; Walz T.; Agre P.; Heymann J. B.; Engel A.; Fujiyoshi Y. Structural Determinants of Water Permeation through Aquaporin-1. Nature 2000, 407, 599–605. 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Jin J.; Goddard W. A. III. Mechanisms Underlying The Mpemba Effect in Water from Molecular Dynamics Simulations. J. Phys. Chem. C 2015, 119, 2622–2629. 10.1021/jp511752n. [DOI] [Google Scholar]
- Shultz M. J.; Vu T. H.; Meyer B.; Bisson P. Water: A Responsive Small Molecule. Acc. Chem. Res. 2012, 45, 15–22. 10.1021/ar200064z. [DOI] [PubMed] [Google Scholar]
- Davis J. G.; Gierszal K. P.; Wang P.; Ben-Amotz D. Water Structural Transformation at Molecular Hydrophobic Interfaces. Nature 2012, 491, 582–585. 10.1038/nature11570. [DOI] [PubMed] [Google Scholar]
- Scatena L. F.; Brown M. G.; Richmond G. L. Water at Hydrophobic Surfaces: Weak Hydrogen Bonding and Strong Orientation Effects. Science 2001, 292, 908–912. 10.1126/science.1059514. [DOI] [PubMed] [Google Scholar]
- Schulz R.; Von Hansen Y.; Daldrop J. O.; Kappler J.; Noé F.; Netz R. R. Collective hydrogen-bond rearrangement dynamics in liquid water. J. Chem. Phys. 2018, 149, 244504. 10.1063/1.5054267. [DOI] [PubMed] [Google Scholar]
- Chaban V. V.; Prezhdo V. V.; Prezhdo O. V. Confinement by Carbon Nanotubes Drastically Alters The Boiling and Critical Behavior of Water Droplets. ACS Nano 2012, 6, 2766–2773. 10.1021/nn3002533. [DOI] [PubMed] [Google Scholar]
- Tunuguntla R. H.; Henley R. Y.; Yao Y.-C.; Pham T. A.; Wanunu M.; Noy A. Enhanced Water Permeability and Tunable Ion Selectivity in Subnanometer Carbon Nanotube Porins. Science 2017, 357, 792–796. 10.1126/science.aan2438. [DOI] [PubMed] [Google Scholar]
- Pestana L. R.; Felberg L. E.; Head-Gordon T. Coexistence of Multilayered Phases of Confined Water: The Importance of Flexible Confining Surfaces. ACS Nano 2018, 12, 448–454. 10.1021/acsnano.7b06805. [DOI] [PubMed] [Google Scholar]
- Davis J. G.; Rankin B. M.; Gierszal K. P.; Ben-Amotz D. On The Cooperative Formation of Non-Hydrogen-Bonded Water at Molecular Hydrophobic Interfaces. Nat. Chem. 2013, 5, 796–802. 10.1038/nchem.1716. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ángeles F.; Firoozabadi A. Hydrophobic Hydration and The Effect of NaCl Salt in The Adsorption of Hydrocarbons and Surfactants on Clathrate Hydrates. ACS Cent. Sci. 2018, 4, 820–831. 10.1021/acscentsci.8b00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X.; Toubin C.; Habartova A.; Pluharova E.; Roeselova M.; Pettersson J. B. C. Rapid Water Transport through Organic Layers on Ice. J. Phys. Chem. A 2018, 122, 4861–4868. 10.1021/acs.jpca.8b01951. [DOI] [PubMed] [Google Scholar]
- Pyrgiotakis G.; Vasanthakumar A.; Gao Y.; Eleftheriadou M.; Toledo E.; DeAraujo A.; McDevitt J.; Han T.; Mainelis G.; Mitchell R.; et al. Inactivation of Foodborne Microorganisms Using Engineered Water Nanostructures (EWNS). Environ. Sci. Technol. 2015, 49, 3737–3745. 10.1021/es505868a. [DOI] [PubMed] [Google Scholar]
- Pyrgiotakis G.; Vedantam P.; Cirenza C.; McDevitt J.; Eleftheriadou M.; Leonard S. S.; Demokritou P. Optimization of A Nanotechnology Based Antimicrobial Platform for Food Safety Applications Using Engineered Water Nanostructures (EWNS). Sci. Rep. 2016, 6, 21073. 10.1038/srep21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I.; Ishikawa M.; Takahashi K.; Watanabe M.; Nishimaki K.; Yamagata K.; Katsura K.-i.; Katayama Y.; Asoh S.; Ohta S. Hydrogen Acts as A Therapeutic Antioxidant by Selectively Reducing Cytotoxic Oxygen Radicals. Nat. Med. 2007, 13, 688–694. 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Liao W.-T.; Huang T.-S.; Chiu C.-C.; Pan J.-L.; Liang S.-S.; Chen B.-H.; Chen S.-H.; Liu P.-L.; Wang H.-C.; Wen Z.-H.; Wang H.-M.; Hsiao S.-W. Biological Properties of Acidic Cosmetic Water from Seawater. Int. J. Mol. Sci. 2012, 13, 5952–5971. 10.3390/ijms13055952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S.; Benvenuti F.; Nappi G.; Fortunati N. A.; Marino L.; Aureli T.; De Luca S.; Pagliarani S.; Canestrari F. Antioxidative Effects of Sulfurous Mineral Water: Protection Against Lipid and Protein Oxidation. Eur. J. Clin. Nutr. 2009, 63, 106–112. 10.1038/sj.ejcn.1602892. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Du J.; Luo R.; Wang Z.; Wang Z.; Han J.; Liu P.; Fujita T.; Xue Q.; Chen M. 3D Bicontinuous Nanoporous Plasmonic Heterostructure For Enhanced Hydrogen Evolution Reaction Under Visible Light. Nano Energy 2019, 58, 552–559. 10.1016/j.nanoen.2019.01.073. [DOI] [Google Scholar]
- Zhang L.; Ding N.; Lou L.; Iwasaki K.; Wu H.; Luo Y.; Li D.; Nakata K.; Fujishima A.; Meng Q. Localized Surface Plasmon Resonance Enhanced Photocatalytic Hydrogen Evolution via Pt@Au NRs/C3N4 Nanotubes under Visible-Light Irradiation. Adv. Funct. Mater. 2019, 29, 1806774. 10.1002/adfm.201806774. [DOI] [Google Scholar]
- Chen H.-C.; Hwang B.-J.; Mai F.-D.; Liu Y.-C.; Lin C.-M.; Kuo H.-S.; Chou D.-S.; Lee M.-J.; Yang K.-H.; Yu C.-C.; et al. Active and Stable Liquid Water Innovatively Prepared Using Resonantly Illuminated Gold Nanoparticles. ACS Nano 2014, 8, 2704–2713. 10.1021/nn406403c. [DOI] [PubMed] [Google Scholar]
- Chen H.-C.; Mai F.-D.; Yang K.-H.; Chen L.-Y.; Yang C.-P.; Liu Y.-C. Quantitative Evaluation on Activated Property-Tunable Bulk Liquid Water with Reduced Hydrogen Bonds Using Deconvoluted Raman Spectroscopy. Anal. Chem. 2015, 87, 808–815. 10.1021/ac5039434. [DOI] [PubMed] [Google Scholar]
- Chen H.-C.; Mai F. D.; Hwang B. J.; Lee M. J.; Chen C. H.; Wang S. H.; Tsai H. Y.; Yang C. P.; Liu Y. C. Creation of Electron-Doping Liquid Water with Reduced Hydrogen Bonds. Sci. Rep. 2016, 6, 22166. 10.1038/srep22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-C.; Lin H. C.; Chen H. H.; Mai F. D.; Liu Y. C.; Lin C. M.; Chang C. C.; Tsai H. Y.; Yang C. P. Innovative Strategy with Potential to Increase Hemodialysis Efficiency and Safety. Sci. Rep. 2014, 4, 4425. 10.1038/srep04425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-C.; Mai F.-D.; Yang K.-H.; Chen L.-Y.; Yang C.-P.; Liu Y.-C. Quantitative Evaluation on Activated Property-Tunable Bulk Liquid Water with Reduced Hydrogen Bonds Using Deconvoluted Raman Spectroscopy. Anal. Chem. 2015, 87, 808–815. 10.1021/ac5039434. [DOI] [PubMed] [Google Scholar]
- Hwang B.-J.; Chen H. C.; Mai F. D.; Tsai H. Y.; Yang C. P.; Rick J.; Liu Y. C. Innovative Strategy on Hydrogen Evolution Reaction Utilizing Activated Liquid Water. Sci. Rep. 2015, 5, 16263. 10.1038/srep16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-P.; Chen H. C.; Wang C. C.; Tsai P. W.; Ho C. W.; Liu Y. C. Effective Energy Transfer via Plasmon-Activated High-Energy Water Promotes Its Fundamental Activities of Solubility, Ionic Conductivity, and Extraction at Room Temperature. Sci. Rep. 2015, 5, 18152. 10.1038/srep18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-C.; Mai F.-D.; Yang K.-H.; Tsai H.-Y.; Yang C.-P.; Chen C.-C.; Chen C.-H.; Liu Y.-C. Environmentally Friendly Etching Agent: Vapor from Hot Electron-Activated Liquid Water. Green Chem. 2016, 18, 3098–3105. 10.1039/c6gc00353b. [DOI] [Google Scholar]
- Chen H.-C.; Mai F. D.; Yang K. H.; Chen L. Y.; Yang C. P.; Liu Y. C. Triggering Comprehensive Enhancement in Oxygen Evolution Reaction by Using Newly Created Solvent. Sci. Rep. 2016, 6, 28456. 10.1038/srep28456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-C.; Cheng C.-Y.; Lin H.-C.; Chen H.-H.; Chen C.-H.; Yang C.-P.; Yang K.-H.; Lin C.-M.; Lin T.-Y.; Shih C.-M.; et al. Multifunctions of Excited Gold Nanoparticles Decorated Artificial Kidney with Efficient Hemodialysis and Therapeutic Potential. ACS Appl. Mater. Interfaces 2016, 8, 19691–19700. 10.1021/acsami.6b05905. [DOI] [PubMed] [Google Scholar]
- Chen H.-C.; Chen Y.-R.; Yang K.-H.; Yang C.-P.; Tung K.-L.; Lee M.-J.; Shih J.-H.; Liu Y.-C. Effective reduction of water molecules’ interaction for efficient water evaporation in desalination. Desalination 2018, 436, 91–97. 10.1016/j.desal.2018.02.013. [DOI] [Google Scholar]
- Chen H.-C.; Cheng C.-Y.; Chen L.-Y.; Chang C.-C.; Yang C.-P.; Mai F.-D.; Liao W.-C.; Chang H.-M.; Liu Y.-C. Plasmon-Activated Water Effectively Relieves Hepatic Oxidative Damage Resulted from Chronic Sleep Deprivation. RSC Adv. 2018, 8, 9618–9626. 10.1039/c7ra13559a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-K.; Chen H. C.; Fang S. U.; Ho C. W.; Tai C. J.; Yang C. P.; Liu Y. C. Innovatively Therapeutic Strategy on Lung Cancer by Daily Drinking Aantioxidative Plasmon-Induced Activated Water. Sci. Rep. 2018, 8, 6316. 10.1038/s41598-018-24752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C.; Han Y.-L.; Shi Y.-X.; Zhu J.-Q. Cryopreservation of Plagiognathops Microlepis Sperm. Cryobiology 2018, 85, 105–112. 10.1016/j.cryobiol.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Malo C.; Elwing B.; Soederstroem L.; Lundeheim N.; Morrell J. M.; Skidmore J. A. Effect of Different Freezing Rates and Thawing Temperatures on Cryosurvival of Dromedary Camel Spermatozoa. Theriogenology 2019, 125, 43–48. 10.1016/j.theriogenology.2018.07.037. [DOI] [PubMed] [Google Scholar]
- Uzzan M.; Nechrebeki J.; Zhou P.; Labuza T. P. Effect of Water Activity and Temperature on the Stability of Creatine During Storage. Drug Dev. Ind. Pharm. 2009, 35, 1003–1008. 10.1080/03639040902755197. [DOI] [PubMed] [Google Scholar]
- van den Berg C.; Bruin S.. Water Activity and Its Estimation in Food Systems: Theoretical Aspects. In Water Activity: Influences on Food Quality; Rockland L. B., Stewart G. F., Eds.; Academic Press: New York, 1981. [Google Scholar]
- Zhou H.-X. Helix Formation Inside a Nanotube: Possible Influence of Backbone-Water Hydrogen Bonding by the Confining Surface Through Modulation of Water Activity. J. Chem. Phys. 2007, 127, 245101–245104. 10.1063/1.2812282. [DOI] [PubMed] [Google Scholar]
- O’Concubhair R.; Sodeau J. R. The Effect of Freezing on Reactions with Environmental Impact. Acc. Chem. Res. 2013, 46, 2716–2724. 10.1021/ar400114e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


