Abstract
Sediments play an essential role in the functioning of aquatic ecosystems but simultaneously retain harmful compounds. However, sediment quality assessment methods that consider the risks caused by the combined action of all sediment-associated contaminants to benthic biota are still underrepresented in water quality assessment strategies. Significant advancements have been made in the application of effect-based methods, but methodological improvements can still advance sediment risk assessment. The present study aimed to explore such improvements by integrating effect-monitoring and chemical profiling of sediment contamination. To this end, 28 day life cycle bioassays with Chironomus riparius using intact whole sediment cores from contaminated sites were performed in tandem with explorative chemical profiling of bioavailable concentrations of groups of legacy and emerging sediment contaminants to investigate ecotoxicological risks to benthic biota. All contaminated sediments caused effects on the resilient midge C. riparius, stressing that sediment contamination is ubiquitous and potentially harmful to aquatic ecosystems. However, bioassay responses were not in line with any of the calculated toxicity indices, suggesting that toxicity was caused by unmeasured compounds. Hence, this study underlines the relevance of effect-based sediment quality assessment and provides smarter ways to do so.
Introduction
Sediments play an indispensable role in the functioning of aquatic ecosystems because benthic organisms drive ecosystem processes supporting biogeochemical cycling and therewith the entire aquatic food web.1 Simultaneously, sediments are also the largest chemical repositories on earth, where harmful, often persistent hydrophobic compounds accumulate and are retained long after the pollution of the overlying water has decreased.2 The vast variety of these sediment-associated contaminants may exert harmful effects on the benthic community and can thereby impair ecosystem functioning.2,3 Despite this apparent threat to aquatic ecosystems, the European Union Water Framework Directive (EU-WFD)4 mentions water on 373 occasions, but sediment only seven times, and does not require the member states to monitor sediment quality.5 When performed at all, water authorities most often monitor sediment quality by means of chemical target analysis, focusing on only a limited set of specific compounds, potentially overlooking ecotoxicological risks caused by the myriad of (un)known mixtures of sediment-associated compounds.6 Therefore, there is a need for integrated sediment quality assessment methods that consider the risks caused by the combined action of all sediment-associated contaminants to benthic biota.
In recent years, significant advancements have been made in the application of effect-based methods and subsequent risk assessment in environmental quality monitoring.7−10 For sediments, particular attention has been given to the integration of toxicity identification evaluation (TIE) and effect-directed analysis (EDA).11 The integration of TIE and EDA presents promising initial steps in toxicant identification in the quality assessment of contaminated sediments.12,13 Nonetheless, further developments of the methods that are currently used offer opportunities for improved understanding of sediment contamination and the accompanying risks to benthic biota.
The importance of bioavailability-based toxicity assessment was recently highlighted, as it allows a much more relevant representation of the exposure of benthic invertebrates to contaminants.12,14 Moreover, the concentrations obtained this way allow for subsequent comparison with environmental quality standards or effect concentrations for the water phase, which are much more readily available for water than for sediments.15 A variety of methods for the determination of bioavailable contaminant concentrations in sediment has been described;14,16−19 however, the manipulation of sediments (e.g., sieving and homogenization) that is required for application in toxicity tests and especially for bioavailability-based extractions leads to altered sediment characteristics, affecting layering and pore water concentrations of contaminants,20 which can lead to over- or underestimation of sediment toxicity.21−24 Hence, the use of undisturbed sediments in laboratory toxicity testing mimics the natural situation most closely, increasing the realism of the sediment quality assessment. Similarly, the use of chronic life cycle bioassays mimics the exposure of organisms on relevant time scales, representing ecologically relevant endpoints, and should allow for a more realistic interpretation of sediment toxicity to benthic biota.
The present study aimed to advance sediment quality assessment by combining invertebrate life cycle effect-monitoring and chemical profiling of sediment contamination to gain insight into the drivers of sediment toxicity to benthic biota. To this end, bioassays with intact whole sediment cores from contaminated sites were performed in tandem with explorative bioavailability-based chemical profiling of groups of legacy and emerging sediment contaminants, followed by the calculation of the potential toxicity of the detected contaminants. Based on the results, considerations and recommendations on the integration of effect-monitoring and chemical profiling in future sediment quality assessment are given.
Materials and Methods
Outline of the Study
Sampling locations were selected in collaboration with the Dutch Water Authorities and subdivided into four categories according to the predominant surrounding land use or pollution source as follows: urban, waste water treatment plant (WWTP) effluent, agriculture, and reference. Urban sites were located in the city of Amsterdam and were identified as “chemical hotspots” requiring mitigation measures by the local water authority, based on high levels of polycyclic aromatic hydrocarbons (PAHs) and metals in the sediment (Table S1). WWTP sites received effluent from treatment plants from the cities Eindhoven, Hilversum, and Utrecht with a capacity of 120.000–750.000 inhabitant equivalents per day. Agricultural sites were located in areas with predominantly agricultural land use where a wide array of herbicides, insecticides, and fungicides is applied (Table S2). A site on the University of Amsterdam Science Park campus served as the reference location.
To assess the toxicity of the sediments, 28 day life cycle whole sediment bioassays with the nonbiting midge Chironomus riparius were performed on the intact whole sediment cores. Survival, emergence after 28 d, and mean emergence time (EmT50) were monitored as endpoints.25,26 Standard 48 h Daphnia magna immobilization tests indicated no significant effects for simultaneous grab samples of the overlying water for all sampled locations (data not shown).
To elucidate the drivers of effects observed in the sediment bioassays, sediments were investigated for physical characteristics and chemical contamination [see Table S4 for compound properties and limits of quantification (LOQs)]. To this end, a selection of legacy and emerging sediment contaminants was made based on expected or indicative land use specific compounds.
Sediment pollution at the urban locations was expected to arise from metals and PAHs.27 Therefore, Al, As, Ag, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Se, and Zn were selected representing the metals, while phenanthrene and pyrene were selected as model PAHs because they are representative of the presence and toxicity of complex PAH mixtures and because their pore water dissolved concentrations can be accurately quantified by passive samplers (see below).28
WWTP effluents typically contain large numbers of contaminants of emerging concern (CECs),29 including pharmaceuticals, illicit drugs, and personal care products and their metabolites.30 Five WWTP effluent marker compounds were selected, representing CECs that accumulate in the sediment: the synthetic polycyclic musk fragrance HHCB (galaxolide, 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-g-2-benzopyran), the nonionic surfactant precursor and metabolite nonylphenol, the antimicrobial agent triclosan and its metabolite methyl-triclosan, and the plastic precursor bisphenol A (BPA). These compounds were selected based on their common occurrence in WWTP effluents, high production volumes, persistence, and their tendencies to sorb to the sediment.31−34
Agricultural locations were expected to suffer most from pollution by pesticides used on the surrounding fields.35 Therefore, the sediments were subjected to chemical screening for 150 commonly used pesticides (Table S4).
Metal concentrations were determined in total sediment extracts, after which freely dissolved pore water concentrations were calculated. Passive sampling with solid-phase microextraction (SPME) fibers was applied to determine pore water-equilibrated concentrations of the selected organic compounds. The obtained compound concentrations were thus representative of the bioavailable concentrations in the sediment14,17 and thereby directly relatable to the toxicity observed in the bioassays.36
To identify the compound-based ecotoxicological risks of the contaminated sediments, the potential toxicity of the detected sediment-pore water contaminant concentrations was calculated for each location using three well-established toxicity indices:
-
i.
The exposure-activity ratio (EAR), based on bioactivity data for a wide variety of contaminants generated by the U.S. Environmental Protection Agency (USEPA) ToxCast program.37
-
ii.
The multisubstance potentially affected fraction (msPAF), based on species sensitivity distributions (SSDs) for multiple species and contaminant combinations.38
-
iii.
The toxic unit (TU),39 based on reported effect concentrations of the detected contaminants for a relevant species and endpoint.
Sampling Locations and Sample Collection
Sediment samples were collected at urban (n = 5), WWTP (n = 3), agriculture (n = 3), and reference (n = 1) locations in The Netherlands during March and April 2017 (Supporting Information 2). Each location was sampled on a single occasion, and intact whole sediment cores for the bioassays (n = 5 for contaminated sites, n = 10 for the reference site) and for the physical and chemical analyses (n = 5 for all sites) were collected using a sediment core sampler (UWITEC, Mondsee, Austria) loaded with acrylic tubes (l: 60 cm, d: 6 cm). Cores were collected over a 20 m transect with at least 0.5 m distance between each core, which, given the sediment homogeneity of the heavily modified water bodies that were selected for this study, resulted in a representative sampling of each location. In the laboratory, the top 5.5 cm was transferred to small acrylic tubes (l: 15 cm, d: 6 cm) using a sediment core cutter (UWITEC). The top 5.5 cm was used to ensure sediment stability and a water-sediment ratio that allows sufficient volume for quality measurements in the overlying water. To eliminate indigenous fauna, cores were stored at −20 °C for at least 48 h before the start of the experiments. Sediment cores were selected at random for subsequent analyses. For chemical profiling, the top 2 cm of the sediments was analyzed, as it is the zone that is inhabited by chironomids40 and thus representative of organism exposure to contaminants.
Physical Chemical Characterization of the Sediment
Particle size distribution, C/N ratio, and total organic carbon (TOC) content were determined for all sediments (see Supporting Information 4 for experimental details).
Whole Sediment Bioassays
Test Organism and Culturing Conditions
C. riparius larvae originated from the University of Amsterdam in-house laboratory culture, which was kept at 20 ± 1 °C and a 16:8 h light/dark photoperiod. The culture was maintained in aquaria containing quartz sand overlaid with Dutch standard water (DSW).41 The culture was fed a mixture of Trouvit (Trouw, Fontaine-les-Vervins, France) and Tetraphyll (Tetrawerke, Melle, Germany) in a ratio 20:1. This mixture was also used as food for the whole sediment bioassays.
Sediment Preparation
Sediment cores were topped off with 125 mL of DSW and thawed for 24 h. In addition to testing a natural reference sediment (SP), a negative laboratory control was performed using artificial sediment according to OECD guideline 21842 with slight modifications41 containing 140 mg of food, representing 0.5 mg/larva/day food mixture for the entire duration of the experiment. The artificial sediment was sterilized by autoclaving and homogenized in glass bottles on a roller bank at 20 rpm for >24 h. Per acrylic tube, 260 g of artificial sediment was added, topped off with 125 mL of DSW, and left to settle for 24 h. All sediment cores were aerated throughout the experiment using glass Pasteur pipettes and compressed air. Aeration was turned on 24 h prior to the start of the experiment.
C. riparius 28 Day Life Cycle Whole Sediment Bioassays
The C. riparius 28 d life cycle whole sediment bioassays were performed with first instar larvae (<24 h) based on OECD guideline 218.42 There were five replicates for each contaminated site and 10 replicates for the reference site and the negative control to ensure sufficient quality control of the experiment. One core from the reference site was lost during the transfer from the tube to the small acrylic core. Ten larvae per replicate sediment core were added at the start of the experiment. On day 7 and 14, 17.5 mg of additional food was added, corresponding with 0.25 mg food/larva/day for a period of 7 days. Demineralized water was added to compensate for water loss by evaporation. From day 14 onward, the sediment cores were covered with fine mesh gauze and checked daily for emerging midges, which were sexed and removed. At the end of the 28 d experiment, the sediments were sieved (350 μm) and the surviving larvae were counted. Dissolved oxygen concentration, conductivity, and pH were measured in the overlying water of each core on day 1, 14, and 28 using a benchtop multimeter (HACH, Tiel, The Netherlands). The ammonium concentration was determined by analyzing 1 mL of filtered (0.2 μm pore size) overlying water of each core on an Autoanalyzer (San++, Skalar, Breda, The Netherlands). The number of surviving adults and larvae, the number of emerged adults, and the emergence time of the adults were monitored as endpoints.
Chemical Profiling
Metal Concentrations in Sediments and Pore Water
To determine the total metal concentrations in the sediments, ground and homogenized sediments were digested in a microwave with HNO3/HCl.43 From two replicate cores per location, 250 mg sample from the top 2 cm of the cores was used to extract metals (Supporting Information 3). Metal concentrations, as total concentration per sediment dry weight, were determined using an inductively coupled plasma mass spectrometer (Optima 8300; PerkinElmer). Freely dissolved metal concentrations were subsequently calculated using the SEDIAS tool,44 which considers the TOC content of the sediments to determine sediment-pore water partitioning coefficients for metals. This was possible for Cd, Cr, Cu, Ni, Pb, and Zn.
Passive Sampling with SPME Fibers
SPME fibers (Polymicro Technologies, Phoenix, AZ, USA) consisted of 200 m length glass fibers with an internal diameter of 108 μm and a 34.5 μm polyacrylate coating (coating volume 15.4 μL/m). SPME fibers were wrapped in aluminum foil and cut in 4 cm pieces, sequentially cleaned in three solvents (acetonitrile, methanol, and a 1:1 ultrapure water/methanol mixture; J.T. Baker, Deventer, The Netherlands) for 30 min each and stored in ultrapure water until application. From one frozen sediment core per site, the top 2 cm of each core was removed after 24 h of thawing and homogenized. Three replicate 10 mL vials per core were filled with ∼5 g of wet sediment, three SPME fibers, and 5 mL of demineralized water. Three vials were filled with only ultrapure water and three SPME fibers as negative controls. The sediment slurries with SPME fibers were agitated for 28 d (pesticides and WWTP marker compounds) and 56 d (PAHs) on a roller mixer (20 rpm, Stuart SRT9; Cole-Parmer, Stone, UK) at 20 °C to ensure equilibrium partitioning with the sediment pore water.45 Next, fibers were cleaned using a wet tissue, cut into 1 cm pieces, and placed in 0.2 mL inserts in 1.5 mL high-performance liquid chromatography (HPLC) vials. Organic compounds were subsequently extracted from the fibers by the addition of solvents and agitation on a roller mixer for 1–3 h and stored at −20 °C until analysis. LC-grade acetonitrile (J.T. Baker) (200 μL) was used as the solvent for PAH, pesticide, and BPA analyses, and n-hexane (J.T. Baker) (150 μL) was used as the solvent for the other WWTP marker compounds. Chromatographic details for the analyses of organic contaminants are provided in Supporting Information 3 (Tables S5–S10).
Polycyclic Aromatic Hydrocarbons
Phenanthrene and pyrene detection in acetonitrile SPME extracts was performed on a LC system with a fluorescence detector (Shimadzu, Kyoto, Japan).
WWTP Markers
For detection of HHCB, nonylphenol (mixture of isomers), triclosan, and methyl-triclosan, the hexane SPME extracts were analyzed by gas chromatography coupled to mass spectrometry (GC–MS) using a Finnigan Trace MS quadrupole MS (Thermo Fisher Scientific) set to selected ion monitoring mode.
For detection of BPA, acetonitrile SPME extracts were analyzed by LC coupled to tandem MS (LC–MS/MS) using electrospray ionization operating in negative mode coupled to a QTRAP 4000 MS system (AB Sciex, MA, USA).
Pesticides
Acetonitrile SPME extracts were subjected to chemical screening for 150 commonly used pesticides at the laboratory of the water authority of Fryslân using LC–MS/MS and GC–MS, as previously described by de Baat et al. (2018).46
Deriving Concentrations of Organic Contaminants in the Sediment Pore Water
Quantified concentrations in the (diluted) SPME extracts were converted to concentrations in the SPME polymer phase (0.616 μL per 4 cm fiber). The fiber-water partition coefficient (Kfw) was used to calculate the sediment-pore water concentrations of the target compounds. The log Kfw values for phenanthrene and pyrene were 4.29 and 4.99, respectively.47 No Kfw values were available for the pesticides and the WWTP markers. Therefore, Kfw values for these compounds were estimated using their octanol–water partition coefficients (Kow) and eq 1,48 which describes the relationship between Kow and Kfw for the polyacrylate fibers used in the present study.
| 1 |
Finally, the freely dissolved pore water concentrations were calculated assuming chemical equilibrium between the sediment solids, the slurry water, and the SPME polymer (eq 2).
| 2 |
where Caq,free is the pore water concentration and CSPME,equilibrium is the concentration in the fiber.
Data Analyses
A detailed description of the performed data analyses is provided in Supporting Information 5. In short, bioassay responses were compared between contaminated sites and the reference site (SP). Midge survival and emergence were tested for statistical differences using a Mann–Whitney U test. The mean emergence time (EmT50), that is, the day at which 50% emergence occurred, was calculated separately for males and females according to a previously described protocol,49 and significant differences were checked using a one-way ANOVA with Dunnett’s multiple comparison post hoc test (p < 0.05).
Three previously described toxicity indices were calculated to determine the potential toxicity of the contaminant concentrations in the sediments. A cumulative EAR of the mixture of detected compounds (EARmixture) was calculated for each location by summing the EAR profiles of each of the compounds using the R package toxEval.8,50 For metals, no toxicity data were available within the USEPA ToxCast database51 at the time of writing, and these were hence excluded from EARmixture calculations. Toxic pressures of individual chemicals, expressed as PAFs, were derived using previously reported chronic NOEC SSDs.52 Subsequently, mixture toxic pressures, expressed as msPAF-NOEC, were derived assuming mixture toxicity according to the “mixed model” by De Zwart and Posthuma (2005).38 Cumulative TUs were calculated per location assuming response additivity, in which TU was defined as the ratio of the measured concentration of a given compound to its EC50 for D. magna.39 Previously reported threshold values that indicate risks of chemical contamination in surface waters were applied to interpret the calculated compound-based toxicity indices (EARmixture = 1; msPAF = 5%; TU = 0.1).8,53,54 Bioassay responses were summarized in a toxicity index in which each location was attributed a point for the occurrence of lethal and sublethal effects, respectively.
Results
Physical Chemical Sediment Characteristics
Sediment characteristics varied among the different sediments (Table S12), but no land userelated pattern in particle size distribution, TOC content, and C/N ratio became apparent (Supporting Information 4).
Sediment Toxicity
Quality Criteria
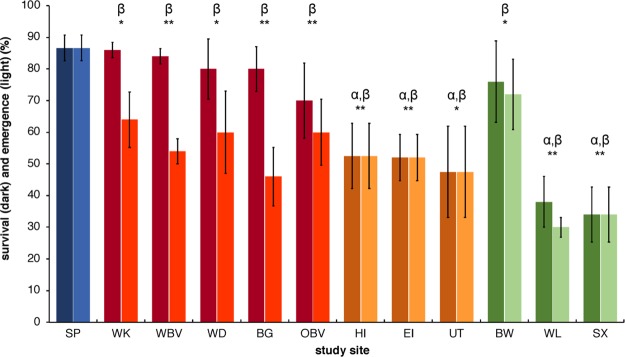
All quality parameters were in accordance with OECD guideline 218, except for the oxygen concentrations in two cores which were excluded from subsequent analyses (Table S15). The results of the 28 d life cycle whole sediment bioassays with C. riparius are depicted in Figure 1. Survival and emergence in the negative control were 93 and 92%, respectively. There was no significant difference in survival and emergence between the negative control and the field reference site (SP).
Figure 1.
Survival (dark bars) and emergence (light bars) (mean ± SE; % of initial individuals) of C. riparius after 28 d exposure to whole sediment cores from reference (blue), urban (red), WWTP (orange), and agricultural (green) sites. Significant differences between mean values of the reference (SP) vs contaminated sites are shown, where α indicates a difference in survival and β indicates a difference in emergence, and significance levels for α and β are indicated as * = p < 0.05 and ** = p < 0.01.
Survival
For all urban sites, survival was slightly but not significantly (p > 0.05) lower (70–86%) than that for the reference site (SP). Survival on the WWTP sediments (47.5–52.5%) was significantly (p < 0.05) lower than that on the reference sediment (SP). The agricultural sediment from BW did not significantly (p > 0.05) impact midge survival (76%). In contrast, the sediments from agricultural sites WL and SX impacted midge survival significantly (p < 0.05) and most strongly, with only 38 and 34% survival, respectively.
Emergence
In contrast to survival, adult emergence for urban sites (46–64%) was significantly (p < 0.05) lower than that for the reference site (SP), indicating a reduced larval development rate. Emergence for WWTP sites was significantly (p < 0.05) lower (48–53%) than that for the reference site (SP), yet this was attributable to the low survival. Emergence for the agricultural site BW (72%) was significantly (p < 0.05) lower than that for the reference site (SP) but higher than on all other contaminated sediments. Almost all surviving midges on the WL and SX agricultural sediments emerged (30 and 34%, respectively).
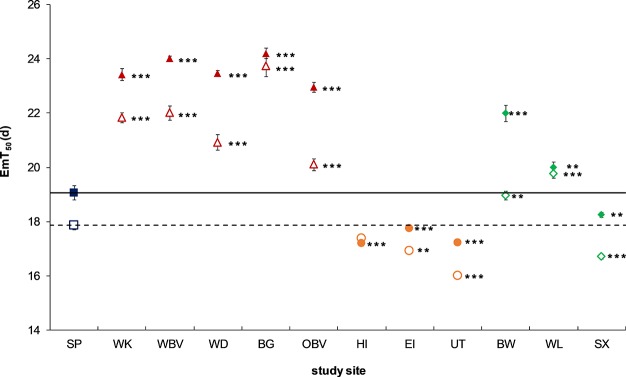
Emergence Time
As an example of cumulative emergence data, EmT50 curves for both genders on the reference sediment are shown in Figure S2. The EmT50 values on the artificial and the reference sediment (SP) were 17.2 and 19.1 days, respectively, for female midges and 16.4 and 17.9 days, respectively, for male midges (Figure 2). For both genders, all EmT50 values differed significantly from the reference site (SP), except for males for WWTP location HI. The EmT50 values for the urban sites were higher than those for the reference site (SP), indicating delayed midge emergence on urban sediments. In contrast, EmT50 values on the WWTP sediments were lower than those on the reference sediment (SP), indicating accelerated emergence. The EmT50 values for the WWTP location HI were the lowest (17.2 days for females and 16.0 days for males) of all field sediments and were nearly identical to the EmT50 values of the artificial sediment. Agricultural sediments affected EmT50 values differentially. The sediment from BW and WL caused delayed emergence (22.0 and 20.0 days, respectively, for females and 19.0 and 19.8 days, respectively, for males), and the sediment from location SX caused accelerated emergence (18.3 days for females and 16.7 days for males).
Figure 2.
Mean (±SE) 50% emergence time in days (EmT50) of C. riparius females (solid icons) and males (open icons) after 28 d exposure to whole sediment cores from reference (blue squares), urban (red pyramids), WWTP (orange circles), and agricultural (green diamonds) sites. The horizontal line represents the EmT50 value of the reference sediment for females (solid) and males (dashed), to which all locations were compared. Significance is indicated as **p < 0.01 and ***p < 0.001.
Chemical Profiling
Quality Criteria
The LOQs (analytical and corresponding dissolved field concentration) of the compounds targeted in the chemical profiling are reported in Table S4. SPME measurement reliability and reproducibility were deemed sufficient for chemical profiling purposes (Supporting Information 3). Blank SPME signals of the target analytes were below LOQs, and the artificial sediment showed no signal except for low freely dissolved concentrations of the organic contaminants BPA and nonylphenol and the metals Cr, Cu, Pb, and Zn (Table S11).
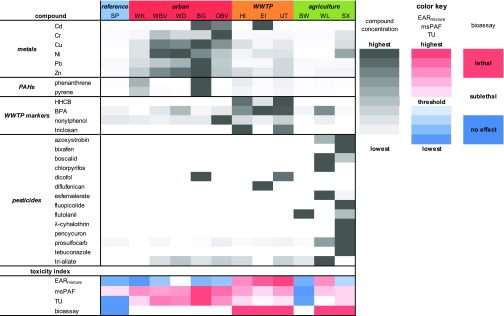
Detected Contaminants
An overview of the detected freely dissolved contaminant concentrations is depicted as a heat map in Figure 3. As, Ag, and Se were not detected in any of the sediments. Freely dissolved sediment concentrations of Cd, Cr, Cu, Ni, Pb, and Zn differed between locations. Phenanthrene and pyrene, four of the WWTP markers, and 14 of the target pesticides were detected in the sediment from one or more locations. Metals were detected most frequently and at the highest concentrations for urban locations. PAHs were present in all land use types, but the highest pore water dissolved concentrations were detected for the urban locations WK (phenanthrene 22.2 μg/L, pyrene 2.4 μg/L) and BG (phenanthrene 42.4 μg/L, pyrene 6.3 μg/L). WWTP markers were detected for all sampling sites but more frequently and at higher freely dissolved concentrations for WWTP sites. HHCB was detected for all WWTP locations (HI 40 ng/L; EI 10 ng/L; UT 50 ng/L). Triclosan was detected for two of the three WWTP locations (HI 2.9 μg/L; UT 2.4 μg/L). HHCB and triclosan were not detected in sediments from other land uses. BPA and nonylphenol were present at most of the investigated locations but at higher concentrations in WWTP sediments (HI 0.7 and 0.3 μg/L; EI 0.9 and 0.2 μg/L; and UT 0.9 and 0.4 μg/L, respectively), except for nonylphenol for the urban location OBV (1.1 μg/L). Traces of the herbicides prosulfocarb and triallate were detected in nearly all field sediments, but a higher diversity and higher freely dissolved concentrations of pesticides were detected for the agricultural sites. For WL, the fungicide boscalid (2.6 μg/L) and the pesticide esfenvalerate (2.9 ng/L) were detected. For SX, the fungicides azoxystrobin (4.8 μg/L), boscalid (1.1 μg/L), and fluopicolide (1.1 μg/L) and the insecticide λ-cyhalothrin (0.04 ng/L) were detected. For the third agricultural location BW, only the systemic fungicide flutolanil was found at a relatively high concentration of 0.3 μg/L.
Figure 3.
Heat map depicting freely dissolved contaminant concentrations and toxicity indices for sediments from sites with different land uses.
Toxicity Indices
A wide range of values was calculated for the EAR (0.06–2.3), msPAF (0.7–74.5%), and TU (0.05–2.7) toxicity indices (Figure 3 and Table S11). The top contributing contaminants differed between the toxicity indices yet were very similar for the different locations within an index. The EAR was dominated by toxicity of BPA, msPAF by nonylphenol, and TU by Cu. A detailed overview per location and index is given in Table S14. The highest EARmixture value was found for the WWTP location UT (2.3) and the lowest was found for the urban location WK (0.06). The EARmixture threshold value (≥1) was met or exceeded by sediments from the urban location WD; WWTP locations HI, EI, and UT; and the agricultural location WL. The highest msPAF value was found for the urban location BG (74.5%) and the lowest was found for the agricultural location BW (0.7%). The msPAF threshold value (5%) was exceeded by all but the agricultural location BW. The highest TU value was found for the urban location BG (2.7) and the lowest was found for the reference location SP (0.05). For TU, the threshold value (0.1) was exceeded by all locations except the agricultural location BW and the reference location SP. The bioassay-based toxicity index, as it followed directly from the (sub)lethal bioassay responses, was the highest for all WWTP locations and the agricultural locations WL and SX and the lowest for the reference location SP. Thus, the toxicity indices produced a divergent outcome for potential toxicity based on the detected compound concentrations, which in turn was different from the outcome of the bioassays.
Discussion
All contaminated sediments caused lethal and/or sublethal effects on the midge C. riparius, a species that is relatively resilient to sediment contamination.55,56 Contrastingly, acute bioassays with the sensitive invertebrate D. magna elucidated no toxicity in the overlying water at the same locations. This illustrates the severity of sediment contamination, present at a variety of locations with different pollution sources, on benthic invertebrates. No relationship between TOC, C/N ratio, or particle size distribution and bioassay responses was observed, and the addition of food ensured that differences in the nutritional quality of the sediments did not affect bioassay responses. Hence, the clear differences in toxic effects could be attributed to the chemical profiles of the investigated sediments.
Intact Sediment Cores Can Simulate Realistic Contaminant Exposure
The need to improve the accuracy of ecological risk assessment, especially with regard to sediment manipulation before use in toxicity testing, was recently highlighted.24 In the present study, the traditionally performed sediment manipulation was minimized because the use of intact whole sediment cores maintained natural layering and thereby contaminant availability in the sediments. As the upper centimeters of freshwater sediments are occupied by benthic organisms that live on top of and in the layered sediment,1,57 a realistic exposure of the test species to sediment contamination was achieved in the present study. The presently described methods thus introduce increased realism into toxicity assessment, while they do not present increased infrastructural demands compared to the use of manipulated sediments.
Life Cycle Bioassays Can Accommodate Sensitive Quality Assessment
The use of the life cycle bioassays allowed the measurement of lethal and sublethal endpoints in a chronic exposure scenario and differences in effects between the different land uses became more distinct with increasing endpoint sensitivity (survival < emergence < EmT50). This illustrates the benefit of the inclusion of sensitive sublethal endpoints in effect-based sediment quality assessment, especially when the endpoints are indicative of stress responses that directly relate to the population level.58 Responses to toxic compounds can, however, vary greatly between benthic species59,60 and the risk that contaminated sediments pose to benthic communities can be over- or underestimated if only one test organism is used. For example, chironomids are relatively resilient to chemical contamination,61 and sediment bioassays with more sensitive species may elucidate toxicity at lower compound concentrations or for other toxic modes of action. Therefore, in line with Tuikka et al. (2011),60 the use of additional test organisms is recommended. In an ideal situation, a suite of life cycle bioassays would be used as a powerful tool in the interpretation of a wide variety of ecologically relevant effects of sediment contamination. However, the high resolution of life cycle bioassays comes at a significant cost of time and labor intensity, making their regular implication in monitoring strategies less likely. Therefore, the development of simplified shorter bioassays could represent a valuable advancement in effect-based sediment quality assessment, provided that they will allow the determination of equally sensitive or more sensitive endpoints and maintain realistic exposure to the full pollution spectrum. Candidate endpoints include biomarkers for specific oxidative, neuronal, and energy metabolism stress that were shown to sensitively elucidate responses in C. riparius after 48 h at lower effect concentrations than responses in larval development and emergence after 28 d.62 Additionally, molecular endpoints such as stress-related gene expression can be used to demonstrate responses of chironomids at the cellular level,63 which can be observed on time scales from hours to days. These cellular or molecular responses may provide test setups that can more readily be implemented in sediment quality assessment because of their lower infrastructural demands, but they are more difficult to extrapolate to population-level effects and still come at possibly high operational costs. Hence, given the more realistic interpretation of sediment toxicity to benthic biota that life cycle bioassays offer, their value in regular sediment quality assessment, especially compared to the traditional approaches based only on compound concentrations, should not be underestimated despite their high infrastructural demands.
Bioavailable Contaminant Concentrations Support Realistic Risk Interpretation
The use of total sediment concentrations can lead to misinterpretation of the contaminant exposure that aquatic invertebrates actually experience, as the organic carbon content of the sediment can influence the bioavailability of sediment-associated contaminants.12,14,18 Therefore, freely dissolved concentrations for metals were estimated based on the TOC content of the sediments. Although this approach presented an estimation of the bioavailable metal concentrations, ideally these should be determined directly by bioaccessibility-based extraction methods.19 For metals, this can, for example, be achieved by means of diffusive gradient in thin films,64 which is hence recommended for future sediment quality assessment. For organic compounds, passive sampling with SPME fibers was applied to measure freely dissolved concentrations of organic contaminants in the investigated sediments. The use of SPME in sediment chemical profiling had several advantages: (i) SPME material availability and cost, (ii) ease of use in terms of method simplicity and scale, (iii) the measurement consistency and reliability, and (iv) the availability of a validated method to calculate freely dissolved concentrations for a broad diversity of chemicals from concentrations in the SPME polymer phase.48 The most prominent disadvantage of SPME in sediment passive sampling applications is the limited sorption capacity of the polymer phase. This limits the contaminant concentrations that can be obtained in SPME extracts, resulting in analytical detection limits that may exceed (sublethal) effect concentrations of highly toxic compounds (Table S4). Moreover, the small extract volumes that are obtained with SPME limit subsequent application in explorative analytical methods such as EDA,65 or the recently proposed integration of TIE and EDA, that can greatly aid in diagnosing drivers of toxicity in sediments.12 Alternatively, polymeric materials with a higher sorption capacity for organic compounds, such as XAD resin12 or sheets made of silicone rubber, polyoxymethylene or polyethylene,14 allow for large-volume bioaccessibility-based extraction of sediment-related contaminants. Additionally, because of the frequently acidic or basic nature of pharmaceuticals and pesticides, the application of ion-exchange polymers alongside polymers that target neutral organic compounds can improve the passive sampling of strongly sorbing but still predominantly charged compounds from sediments. An additional advantage of the use of polymers is their potential application in passive-dosing setups66 that can aid the integration of EDA and TIE for sediment quality assessment by allowing for high throughput determination of bioavailability-based in vivo and in vitro endpoints.67
The determination of the bioavailable toxicant concentrations in the present study allowed a more accurate representation of the exposure of benthic invertebrates to organic contaminants.14,36 Because toxic effect concentrations are much more readily available for water than for sediments,15 the concentrations obtained this way allowed for subsequent calculation of toxicity indices originally designed for the water phase.
Toxicity Indices Fail to Predict Sediment Toxicity
EAR, msPAF, and TU approaches were used to determine the potential toxicity of the detected contaminant concentrations in the sediments. Low toxicity index scores coincided with low bioassay responses for the reference location SP (except msPAF) and the agricultural location BW, but for all other locations, such convergent outcomes of the compound- and effect-based approaches were not observed. The relatively clean chemical profile and corresponding low bioassay response for the agriculture location BW can be explained by its position in front of a pumping station where the water from the entire agricultural area is collected, which leads to a more diluted pesticide loading to the sediment. The msPAF and TU indices responded most strongly for the urban locations, driven by the detected legacy contaminants, despite low bioassay responses. Contrastingly, the EAR responded most strongly for WWTP locations, strongly driven by WWTP markers, which is partly in line with the observed bioassay responses. It must be noted, however, that the lack of toxicity data for metals in the ToxCast database may have contributed to the relatively low EARmixture scores for the urban locations. Moreover, the TU calculations were based on toxicity data for D. magna, and species-specific sensitivities of C. riparius to the detected contaminants may have been over- or underestimated. This is also the case for the other toxicity indices, which are based on responses of a wide variety of either in vitro endpoints (EARmixture) or organisms (msPAF). As such, none of the used toxicity indices take species-specific sensitivity of C. riparius and the toxic mode of action of the detected contaminants into consideration, even though these very likely impacted bioassay responses.
Interestingly, all three contaminant concentration-based toxicity indices underestimated the toxicity for the agricultural locations WL and SX that showed the highest toxicity in the bioassays. Apparently, the bioassay responses were caused by contaminants that did not contribute strongly to the toxicity indices, or, more likely, were caused by unmeasured compounds. This illustrates that toxicity indices are strongly dependent on a priori selected compound lists, underlining the importance of careful selection of target compounds in chemical profiling.
Target Compound Lists Inevitably Lead to Misinterpretation of Ecotoxicological Risks
The present selection of target compounds was based on the expected pollutants at the sampling locations, originating from their main pollution sources. Land use-specific chemical profiles became apparent, with metals and PAHs predominantly present at urban locations, WWTP markers at WWTP locations, and pesticides at agricultural locations. In turn, land use-specific bioassay responses were observed, suggesting a correlation between the detected compounds and the toxic effects. However, toxicity indices only partly explained the observed bioassay responses, suggesting that a broader selection of target compounds may have better explained the observed toxicity. This was previously shown to improve the explanatory power of toxicity indices,68 and the selection of target compounds for future chemical profiling can be customized to suit any type of sediment, pollution source, or compound (group) of interest. However, toxicity indices will always depend on target compound lists and will consequently overlook the risks of unmeasured or unknown contaminants. Hence, the use of only compound-based toxicity indices can result in misinterpretation of risks in sediment quality assessment.
Sediment: An Environmental Compartment of Concern
In spite of more strict water quality regulations coming into place and generally decreasing dissolved contaminant concentrations, this study underlines the continued and increasing relevance of sediment contamination to aquatic ecosystem health. Midge survival was less impacted for the urban sites, which contained the highest legacy contaminant concentrations, than for WWTP-impacted and agricultural sites, which contained relatively low legacy contaminant concentrations. This illustrates that sediment-associated pesticides and emerging contaminants related to sewage effluent pose an even more severe risk to benthic invertebrates than legacy contaminants. Sediment is not only a reservoir for poorly degradable legacy contaminants but also a sink for other strongly binding, poorly degradable pesticides and emerging contaminants. As long as the prioritization of hazardously contaminated sediments remains based only on legacy contaminants, many sediments that pose an even greater environmental risk will not be identified. As sediments can act as a source of contamination to the relatively clean overlying water,15 this underlines the importance of sediment as a vital environmental compartment in aquatic ecosystem health assessment.
Acknowledgments
The authors thank Frenk Selhorst, Rick Helmus, Samira Absalah, Rutger van Hall, Leo Posthuma, Brett Blackwell, Piet Verdonschot, Pim de Voogt, John Parsons, and three anonymous reviewers for their valuable contribution. This work is a part of the research program “Promotiebeurs voor leraren” with the project number 023.008.029, financed by the Dutch Research Council (NWO).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b02732.
Additional information on the sampling locations; chemical target analyses; physical chemical sediment characteristics; and data analyses and bioassay results (PDF)
Author Contributions
§ M.L.d.B. and N.W. have equally contributed to this study.
The authors declare no competing financial interest.
Supplementary Material
References
- Covich A. P.; Palmer M. A.; Crowl T. A. The Role of Benthic Invertebrate Species in Freshwater Ecosystems. Bioscience 1999, 49, 119. 10.2307/1313537. [DOI] [Google Scholar]
- Burton G. A. Assessing Sediment Toxicity: Past, Present, and Future. Environ. Toxicol. Chem. 2013, 32, 1438–1440. 10.1002/etc.2250. [DOI] [PubMed] [Google Scholar]
- Massei R.; Hollert H.; Krauss M.; von Tümpling W.; Weidauer C.; Haglund P.; Küster E.; Gallampois C.; Tysklind M.; Brack W. Toxicity and Neurotoxicity Profiling of Contaminated Sediments from Gulf of Bothnia (Sweden): A Multi-Endpoint Assay with Zebrafish Embryos. Environ. Sci. Eur. 2019, 31, 8. 10.1186/s12302-019-0188-y. [DOI] [Google Scholar]
- European Commission . Directives of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy, 2013; Vol. 2013. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32013L0039.
- Borja A.; Valencia V.; Franco J.; Muxika I.; Bald J.; Belzunce M. J.; Solaun O. The Water Framework Directive: Water Alone, or in Association with Sediment and Biota, in Determining Quality Standards?. Mar. Pollut. Bull. 2004, 49, 8–11. 10.1016/j.marpolbul.2004.04.008. [DOI] [PubMed] [Google Scholar]
- den Besten P. J.; de Deckere E.; Babut M. P.; Power B.; DelValls T. A.; Zago C.; Oen A. M. P.; Heise S. Biological Effects-Based Sediment Quality in Ecological Risk Assessment for European Waters. J. Soils Sediments 2003, 3, 144–162. 10.1065/jss2003.08.084. [DOI] [Google Scholar]
- Altenburger R.; Brack W.; Burgess R. M.; Busch W.; Escher B. I.; Focks A.; Mark Hewitt L.; Jacobsen B. N.; de Alda M. L.; Ait-Aissa S.; Backhaus T.; Ginebreda A.; Hilscherová K.; Hollender J.; Hollert H.; Neale P. A.; Schulze T.; Schymanski E. L.; Teodorovic I.; Tindall A. J.; de Aragão Umbuzeiro G.; Vrana B.; Zonja B.; Krauss M. Future Water Quality Monitoring: Improving the Balance between Exposure and Toxicity Assessments of Real-World Pollutant Mixtures. Environ. Sci. Eur. 2019, 31, 12. 10.1186/s12302-019-0193-1. [DOI] [Google Scholar]
- Blackwell B. R.; Ankley G. T.; Bradley P. M.; Houck K. A.; Makarov S. S.; Medvedev A. V.; Swintek J.; Villeneuve D. L. Potential Toxicity of Complex Mixtures in Surface Waters from a Nationwide Survey of United States Streams: Identifying in Vitro Bioactivities and Causative Chemicals. Environ. Sci. Technol. 2019, 53, 973–983. 10.1021/acs.est.8b05304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baat M. L.; Kraak M. H. S.; Van der Oost R.; De Voogt P.; Verdonschot P. F. M. Effect-Based Nationwide Surface Water Quality Assessment to Identify Ecotoxicological Risks. Water Res. 2019, 159, 434–443. 10.1016/j.watres.2019.05.040. [DOI] [PubMed] [Google Scholar]
- Doyle E.; Biales A.; Focazio M.; Griffin D.; Loftin K.; Wilson V. Effect-Based Screening Methods for Water Quality Characterization Will Augment Conventional Analyte-by-Analyte Chemical Methods in Research As Well As Regulatory Monitoring. Environ. Sci. Technol. 2015, 49, 13906–13907. 10.1021/es5053254. [DOI] [PubMed] [Google Scholar]
- Li H.; Zhang J.; You J. Diagnosis of Complex Mixture Toxicity in Sediments: Application of Toxicity Identification Evaluation (TIE) and Effect-Directed Analysis (EDA). Environ. Pollut. 2018, 237, 944–954. 10.1016/J.ENVPOL.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Li H.; Yi X.; Cheng F.; Tong Y.; Mehler W. T.; You J. Identifying Organic Toxicants in Sediment Using Effect-Directed Analysis: A Combination of Bioaccessibility-Based Extraction and High-Throughput Midge Toxicity Testing. Environ. Sci. Technol. 2019, 53, 996–1003. 10.1021/acs.est.8b05633. [DOI] [PubMed] [Google Scholar]
- Burgess R. M.; Ho K. T.; Brack W.; Lamoree M. Effects-Directed Analysis (EDA) and Toxicity Identification Evaluation (TIE): Complementary but Different Approaches for Diagnosing Causes of Environmental Toxicity. Environ. Toxicol. Chem. 2013, 32, 1935–1945. 10.1002/etc.2299. [DOI] [PubMed] [Google Scholar]
- Jonker M. T. O.; Van Der Heijden S. A.; Adelman D.; Apell J. N.; Burgess R. M.; Choi Y.; Fernandez L. A.; Flavetta G. M.; Ghosh U.; Gschwend P. M.; Hale S. E.; Jalalizadeh M.; Khairy M.; Lampi M. A.; Lao W.; Lohmann R.; Lydy M. J.; Maruya K. A.; Nutile S. A.; Oen A. M. P.; Rakowska M. I.; Reible D.; Rusina T. P.; Smedes F.; Wu Y. Advancing the Use of Passive Sampling in Risk Assessment and Management of Sediments Contaminated with Hydrophobic Organic Chemicals: Results of an International Ex Situ Passive Sampling Interlaboratory Comparison. Environ. Sci. Technol. 2018, 52, 3574–3582. 10.1021/acs.est.7b05752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deckere E.; de Cooman W.; Leloup V.; Meire P.; Schmitt C.; von der Ohe P. C. Development of Sediment Quality Guidelines for Freshwater Ecosystems. J. Soils Sediments 2011, 11, 504–517. 10.1007/s11368-010-0328-x. [DOI] [Google Scholar]
- Li Y.; Chen C.-E. L.; Chen W.; Chen J.; Cai X.; Jones K. C.; Zhang H. Development of a Passive Sampling Technique for Measuring Pesticides in Waters and Soils. J. Agric. Food Chem. 2019, 67, 6397–6406. 10.1021/acs.jafc.9b00040. [DOI] [PubMed] [Google Scholar]
- Mayer P.; Parkerton T. F.; Adams R. G.; Cargill J. G.; Gan J.; Gouin T.; Gschwend P. M.; Hawthorne S. B.; Helm P.; Witt G.; You J.; Escher B. I. Passive Sampling Methods for Contaminated Sediments: Scientific Rationale Supporting Use of Freely Dissolved Concentrations. Integr. Environ. Assess. Manage. 2014, 10, 197–209. 10.1002/ieam.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge S. T. J.; Postma J. F.; Hermens J. L. M. Sediment Toxicity of a Rapidly Biodegrading Nonionic Surfactant: Comparing the Equilibrium Partitioning Approach with Measurements in Pore Water. Environ. Sci. Technol. 2008, 42, 4215–4221. 10.1021/es702802p. [DOI] [PubMed] [Google Scholar]
- Peijnenburg W. J.; Teasdale P. R.; Reible D.; Mondon J.; Bennett W. W.; Campbell P. G. Passive Sampling Methods for Contaminated Sediments: State of the Science for Metals. Integr. Environ. Assess. Manage. 2014, 10, 179–196. 10.1002/ieam.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. L.; Batley G. E. Disturbances to Metal Partitioning during Toxicity Testing of Iron(II)-Rich Estuarine Pore Waters and Whole Sediments. Environ. Toxicol. Chem. 2003, 22, 424–432. . [DOI] [PubMed] [Google Scholar]
- Anderson B. S.; Hunt J. W.; Phillips B. M.; Fairey R.; Puckett H. M.; Stephenson M.; Taberski K.; Newman J.; Tjeerdema R. S. Influence of Sample Manipulation on Contaminant Flux and Toxicity at the Sediment-Water Interface. Mar. Environ. Res. 2001, 51, 191–211. 10.1016/S0141-1136(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Castro B. B.; Guilhermino L.; Ribeiro R. In Situ Bioassay Chambers and Procedures for Assessment of Sediment Toxicity with Chironomus Riparius. Environ. Pollut. 2003, 125, 325–335. 10.1016/S0269-7491(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Eriksson Wiklund A.-K.; Dag Broman B. S. Toxicity Evaluation by Using Intact Sediments and Sediment Extracts. Mar. Pollut. Bull. 2005, 50, 660–667. 10.1016/j.marpolbul.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Costello D. M.; Harrison A. M.; Hammerschmidt C. R.; Mendonca R. M.; Burton G. A. Hitting Reset on Sediment Toxicity: Sediment Homogenization Alters the Toxicity of Metal-amended sediments. Environ. Toxicol. Chem. 2019, 38, 1995–2007. 10.1002/etc.4512. [DOI] [PubMed] [Google Scholar]
- Hruska K. A.; Dubé M. G. Using Artificial Streams to Assess the Effects of Metal-Mining Effluent on the Life Cycle of the Freshwater Midge (Chironomus Tentans) in Situ. Environ. Toxicol. Chem. 2004, 23, 2709–2718. 10.1897/03-508. [DOI] [PubMed] [Google Scholar]
- Paumen M. L.; Borgman E.; Kraak M. H. S.; van Gestel C. A. M.; Admiraal W. Life Cycle Responses of the Midge Chironomus Riparius to Polycyclic Aromatic Compound Exposure. Environ. Pollut. 2008, 152, 225–232. 10.1016/j.envpol.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Beasley G.; Kneale P. Reviewing the Impact of Metals and PAHs on Macroinvertebrates in Urban Watercourses. Prog. Phys. Geogr. 2002, 26, 236–270. 10.1191/0309133302pp334ra. [DOI] [Google Scholar]
- Arp H. P. H.; Azzolina N. A.; Cornelissen G.; Hawthorne S. B. Predicting Pore Water EPA-34 PAH Concentrations and Toxicity in Pyrogenic-Impacted Sediments Using Pyrene Content. Environ. Sci. Technol. 2011, 45, 5139–5146. 10.1021/es2007935. [DOI] [PubMed] [Google Scholar]
- Loos R.; Carvalho R.; António D. C.; Comero S.; Locoro G.; Tavazzi S.; Paracchini B.; Ghiani M.; Lettieri T.; Blaha L.; Jarosova B.; Voorspoels S.; Servaes K.; Haglund P.; Fick J.; Lindberg R. H.; Schwesig D.; Gawlik B. M. EU-Wide Monitoring Survey on Emerging Polar Organic Contaminants in Wastewater Treatment Plant Effluents. Water Res. 2013, 47, 6475–6487. 10.1016/j.watres.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Petrie B.; Barden R.; Kasprzyk-Hordern B. A Review on Emerging Contaminants in Wastewaters and the Environment: Current Knowledge, Understudied Areas and Recommendations for Future Monitoring. Water Res. 2015, 72, 3–27. 10.1016/j.watres.2014.08.053. [DOI] [PubMed] [Google Scholar]
- Götz C. W.; Stamm C.; Fenner K.; Singer H.; Schärer M.; Hollender J. Targeting Aquatic Microcontaminants for Monitoring: Exposure Categorization and Application to the Swiss Situation. Environ. Sci. Pollut. Res. 2010, 17, 341–354. 10.1007/s11356-009-0167-8. [DOI] [PubMed] [Google Scholar]
- Venkatesan A. K.; Halden R. U. Wastewater Treatment Plants as Chemical Observatories to Forecast Ecological and Human Health Risks of Manmade Chemicals. Sci. Rep. 2015, 4, 3731. 10.1038/srep03731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwientek M.; Guillet G.; Rügner H.; Kuch B.; Grathwohl P. A High-Precision Sampling Scheme to Assess Persistence and Transport Characteristics of Micropollutants in Rivers. Sci. Total Environ. 2016, 540, 444–454. 10.1016/J.SCITOTENV.2015.07.135. [DOI] [PubMed] [Google Scholar]
- Yuan J.; Van Dyke M. I.; Huck P. M. Identification of Critical Contaminants in Wastewater Effluent for Managed Aquifer Recharge. Chemosphere 2017, 172, 294–301. 10.1016/j.chemosphere.2016.12.120. [DOI] [PubMed] [Google Scholar]
- Schreiner V. C.; Szöcs E.; Bhowmik A. K.; Vijver M. G.; Schäfer R. B. Pesticide Mixtures in Streams of Several European Countries and the USA. Sci. Total Environ. 2016, 573, 680–689. 10.1016/j.scitotenv.2016.08.163. [DOI] [PubMed] [Google Scholar]
- Leslie H. A.; ter Laak T. L.; Busser F. J. M.; Kraak M. H. S.; Hermens J. L. M. Bioconcentration of Organic Chemicals: Is a Solid-Phase Microextraction Fiber a Good Surrogate for Biota?. Environ. Sci. Technol. 2002, 36, 5399–5404. 10.1021/es0257016. [DOI] [PubMed] [Google Scholar]
- Blackwell B. R.; Ankley G. T.; Corsi S. R.; Decicco L. A.; Houck K. A.; Judson R. S.; Li S.; Martin M. T.; Murphy E.; Schroeder A. L.; Smith E. R.; Swintek J.; Villeneuve D. L. An “EAR” on Environmental Surveillance and Monitoring: A Case Study on the Use of Exposure-Activity Ratios (EARs) to Prioritize Sites, Chemicals, and Bioactivities of Concern in Great Lakes Waters. Environ. Sci. Technol. 2017, 51, 8713–8724. 10.1021/acs.est.7b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zwart D.; Posthuma L. Complex Mixture Toxicity for Single and Multiple Species: Proposed Methodologies. Environ. Toxicol. Chem. 2005, 24, 2665–2676. 10.1897/04-639R.1. [DOI] [PubMed] [Google Scholar]
- Sprague J. B. Measurement of Pollutant Toxicity to Fish-III. Sublethal Effects and “Safe” Concentrations. Water Res. 1971, 5, 245–266. 10.1016/0043-1354(71)90171-0. [DOI] [Google Scholar]
- Chaloner D. T.; Wotton R. S. Substratum Preferences by Larvae of Three Species of Midge (Diptera: Chironomidae). Hydrobiologia 1996, 339, 93–99. 10.1007/BF00008917. [DOI] [Google Scholar]
- Marinković M.; Verweij R. A.; Nummerdor G. A.; Jonker M. J.; Kraak M. H. S.; Admiraal W. Life Cycle Responses of the Midge Chironomus Riparius to Compounds with Different Modes of Action. Environ. Sci. Technol. 2011, 45, 1645–1651. 10.1021/es102904y. [DOI] [PubMed] [Google Scholar]
- OECD . OECD Guideline 218 (Sediment-Water Chironomid Toxicity Test Using Spiked Sediment); OECD, 2004. 10.1787/9789264070264-en. [Google Scholar]
- Bettinelli M.; Baroni U.; Pastorelli N. Microwave Oven Sample Dissolution for the Analysis of Environmental and Biological Materials. Anal. Chim. Acta 1989, 225, 159–174. 10.1016/S0003-2670(00)84604-8. [DOI] [Google Scholar]
- SEDIAS (SEDIment ASsistent) . https://www.helpdeskwater.nl/secundaire-navigatie/english/sediment/guidance-document/ (accessed Aug 26, 2019).
- Heijden S. A. v. d.; Jonker M. T. O. PAH Bioavailability in Field Sediments: Comparing Different Methods for Predicting in Situ Bioaccumulation. Environ. Sci. Technol. 2009, 43, 3757–3763. 10.1021/es803329p. [DOI] [PubMed] [Google Scholar]
- de Baat M. L.; Bas D. A.; van Beusekom S. A. M.; Droge S. T. J.; van der Meer F.; de Vries M.; Verdonschot P. F. M.; Kraak M. H. S. Nationwide Screening of Surface Water Toxicity to Algae. Sci. Total Environ. 2018, 645, 780–787. 10.1016/j.scitotenv.2018.07.214. [DOI] [PubMed] [Google Scholar]
- Paschke A.; Popp P. Solid-Phase Microextraction Fibre-Water Distribution Constants of More Hydrophobic Organic Compounds and Their Correlations with Octanol-Water Partition Coefficients. J. Chromatogr. A 2003, 999, 35–42. 10.1016/S0021-9673(03)00538-7. [DOI] [PubMed] [Google Scholar]
- Endo S.; Droge S. T. J.; Goss K.-U. Polyparameter Linear Free Energy Models for Polyacrylate Fiber-Water Partition Coefficients to Evaluate the Efficiency of Solid-Phase Microextraction. Anal. Chem. 2011, 83, 1394–1400. 10.1021/ac102868e. [DOI] [PubMed] [Google Scholar]
- Vogt C.; Nowak C.; Diogo J. B.; Oetken M.; Schwenk K.; Oehlmann J. Multi-Generation Studies with Chironomus Riparius - Effects of Low Tributyltin Concentrations on Life History Parameters and Genetic Diversity. Chemosphere 2007, 67, 2192–2200. 10.1016/j.chemosphere.2006.12.025. [DOI] [PubMed] [Google Scholar]
- De Cicco L. A.; Corsi S. R.; Villeneuve D. L.; Blackwell B.; Ankley G. T.. ToxEval: Evaluation of Measured Concentration Data Using the ToxCast High-Throughput Screening Database or a User-Defined Set of Concentration Benchmarks, R Package, version 1.0.0; U.S. Geological Survey, 2018. http://usgs-r.github.io/toxEval/index.html (accessed Sep 3, 2019).
- U.S. EPA . iCSS ToxCast Dashboard. https://comptox.epa.gov/dashboard/ (accessed Sep 3, 2019).
- Posthuma L.; van Gils J.; Zijp M. C.; van de Meent D.; de Zwart D. Species Sensitivity Distributions for Use in Environmental Protection, Assessment, and Management of Aquatic Ecosystems for 12 386 Chemicals. Environ. Toxicol. Chem. 2019, 38, 905–917. 10.1002/etc.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindim C.; de Zwart D.; Cousins I. T.; Kutsarova S.; Kühne R.; Schüürmann G. Exposure and Ecotoxicological Risk Assessment of Mixtures of Top Prescribed Pharmaceuticals in Swedish Freshwaters. Chemosphere 2019, 220, 344–352. 10.1016/j.chemosphere.2018.12.118. [DOI] [PubMed] [Google Scholar]
- Bundschuh M.; Goedkoop W.; Kreuger J. Evaluation of Pesticide Monitoring Strategies in Agricultural Streams Based on the Toxic-Unit Concept - Experiences from Long-Term Measurements. Sci. Total Environ. 2014, 484, 84–91. 10.1016/j.scitotenv.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Wilson R. S. Monitoring Organic Enrichment of Rivers Using Chironomid Pupal Exuvial Assemblages. Neth. J. Aquat. Ecol. 1992, 26, 521–525. 10.1007/BF02255285. [DOI] [Google Scholar]
- Calle-Martínez D.; Casas J. J. Chironomid Species, Stream Classification, and Water-Quality Assessment: The Case of 2 Iberian Mediterranean Mountain Regions. J. North Am. Benthol. Soc. 2006, 25, 465–476. 10.1899/0887-3593(2006)25[465:csscaw]2.0.co;2. [DOI] [Google Scholar]
- Simpson S.; Batley G.. Sediment Quality Assessment: A Practical Guide; CSIRO Publishing, 2016. [Google Scholar]
- Sibley P. K.; Ankley G. T.; Benoit D. A. Factors Affecting Reproduction And The Importance Of Adult Size On Reproductive Output Of The Midge Chironomus Tentans. Environ. Toxicol. Chem. 2001, 20, 1296. 10.1002/etc.5620200618. [DOI] [PubMed] [Google Scholar]
- De Lange H. J.; De Haas E. M.; Maas H.; Peeters E. T. H. M. Contaminated Sediments and Bioassay Responses of Three Macroinvertebrates, the Midge Larva Chironomus Riparius, the Water Louse Asellus Aquaticus and the Mayfly Nymph Ephoron Virgo. Chemosphere 2005, 61, 1700–1709. 10.1016/j.chemosphere.2005.03.083. [DOI] [PubMed] [Google Scholar]
- Tuikka A. I.; Schmitt C.; Höss S.; Bandow N.; von der Ohe P. C.; de Zwart D.; de Deckere E.; Streck G.; Mothes S.; van Hattum B.; Kocan A.; Brix R.; Brack W.; Barceló D.; Sormunen A. J.; Kukkonen J. V. K. Toxicity Assessment of Sediments from Three European River Basins Using a Sediment Contact Test Battery. Ecotoxicol. Environ. Saf. 2011, 74, 123–131. 10.1016/j.ecoenv.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Van den Berg S. J. P.; Baveco H.; Butler E.; De Laender F.; Focks A.; Franco A.; Rendal C.; Van Den Brink P. J. Modeling the Sensitivity of Aquatic Macroinvertebrates to Chemicals Using Traits. Environ. Sci. Technol. 2019, 53, 6025–6034. 10.1021/acs.est.9b00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro H. R.; Pestana J. L. T.; Novais S. C.; Soares A. M. V. M.; Lemos M. F. L. Toxicity of the Insecticides Spinosad and Indoxacarb to the Non-Target Aquatic Midge Chironomus Riparius. Sci. Total Environ. 2019, 666, 1283–1291. 10.1016/j.scitotenv.2019.02.303. [DOI] [PubMed] [Google Scholar]
- Planelló R.; Servia M. J.; Gómez-Sande P.; Herrero Ó.; Cobo F.; Morcillo G. Transcriptional Responses, Metabolic Activity and Mouthpart Deformities in Natural Populations of Chironomus Riparius Larvae Exposed to Environmental Pollutants. Environ. Toxicol. 2015, 30, 383–395. 10.1002/tox.21893. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Davison W. Use of Diffusive Gradients in Thin-Films for Studies of Chemical Speciation and Bioavailability. Environ. Chem. 2015, 12, 85. 10.1071/en14105. [DOI] [Google Scholar]
- Brack W.; Ait-Aissa S.; Burgess R. M.; Busch W.; Creusot N.; Di Paolo C.; Escher B. I.; Mark Hewitt L.; Hilscherova K.; Hollender J.; Hollert H.; Jonker W.; Kool J.; Lamoree M.; Muschket M.; Neumann S.; Rostkowski P.; Ruttkies C.; Schollee J.; Schymanski E. L.; Schulze T.; Seiler T.-B.; Tindall A. J.; De Aragão Umbuzeiro G.; Vrana B.; Krauss M. Effect-Directed Analysis Supporting Monitoring of Aquatic Environments—An in-Depth Overview. Sci. Total Environ. 2016, 544, 1073–1118. 10.1016/j.scitotenv.2015.11.102. [DOI] [PubMed] [Google Scholar]
- Birch H.; Gouliarmou V.; Holten Lützhøft H.-C.; Mikkelsen P. S.; Mayer P. Passive Dosing to Determine the Speciation of Hydrophobic Organic Chemicals in Aqueous Samples. Anal. Chem. 2010, 82, 1142–1146. 10.1021/ac902378w. [DOI] [PubMed] [Google Scholar]
- Jahnke A.; Mayer P.; Schäfer S.; Witt G.; Haase N.; Escher B. I. Strategies for Transferring Mixtures of Organic Contaminants from Aquatic Environments into Bioassays. Environ. Sci. Technol. 2016, 50, 5424–5431. 10.1021/acs.est.5b04687. [DOI] [PubMed] [Google Scholar]
- Moschet C.; Wittmer I.; Simovic J.; Junghans M.; Piazzoli A.; Singer H.; Stamm C.; Leu C.; Hollender J. How a Complete Pesticide Screening Changes the Assessment of Surface Water Quality. Environ. Sci. Technol. 2014, 48, 5423–5432. 10.1021/es500371t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.