Abstract
Background
The aim of this study was to observe the concentration of serum anti-PLA2R antibody in idiopathic membranous nephropathy (IMN) patients and analyze its relationship with clinical and laboratory parameters.
Material/Methods
We treated 72 patients with idiopathic membranous nephropathy diagnosed by renal biopsy; all these patients who presented nephrotic syndrome were enrolled for investigation, and then underwent combination therapy with prednisone and cyclosporine A for 6 months. We collected data on 24-h total proteinuria (TUpro), creatinine clearance rate (Ccr), and serum albumin (Alb) levels before and after immunosuppressive treatment. Serum anti-PLA2R antibody was measured by enzyme-linked immunosorbent assay (ELISA).
Results
Fifty-six out of 72 IMN patients presented positive serum anti-PLA2R antibody. The titer of anti-PLA2R antibody was significantly correlated with both TUpro and serum Alb levels of pre- and post-therapeutic values in IMN (P<0.05), but did not have a relationship with Ccr (P>0.05). In comparison with the anti-PLA2R antibody-negative group, there were significantly higher TUpro and lower Alb levels in the anti-PLA2R antibody-positive group (P<0.05). However, Ccr was comparatively lower in the anti-PLA2R antibody-positive group, but the difference was not statistically significant (P>0.05). There were 24 patients with negative anti-PLA2R antibody and 14 patients had complete remission in the positive anti-PLA2R antibody group, while anti-PLA2R antibody of all 14 patients became negative. Eight out of 16 patients without anti-PLA2R antibody went into complete remission.
Conclusions
Serum anti-PLA2R antibody, as determined by non-invasive technique, is a specific biomarker for diagnosis of IMN. Our results suggest that serum anti-PLA2R antibody has great potential to guide clinical diagnosis and treatment, as well as prognosis determination, in IMN patients.
MeSH Keywords: Glomerulonephritis, Membranous; Nephrotic Syndrome; Receptors, Phospholipase A2
Background
Idiopathic membranous nephropathy (IMN) is one of the most common pathological lesions in primary nephrotic syndrome, and is also a common cause of refractory nephrotic syndrome in elderly patients [1]. Previously, diagnosis of IMN was based on renal biopsy; however, many patients are reluctant to undergo renal biopsy, which limits the ability yo determine the exact type of pathology. Since the discovery of MN half a century ago [2], physicians have continued exploring the growing number of diagnostic and therapeutic strategies available. Beck et al. [3] found anti-PLA2R antibody in 70% IMN patients and reported this important discovery in the New England Journal of Medicine in 2009, and PLA2R antibody is now widely used for diagnosis of IMN. Many clinical studies were carried out worldwide, showing that 52–78% of IMN patients have positive anti-PLA2R antibody [4]. Research in China also indicated high specificity of anti-PLA2R antibody for diagnosis of IMN and recent research has mainly focused on the observation of clinical indicators [5]. The Nephrology Department of the Nantong Affiliated Hospital routinely assays the serum anti-PLA2R antibody in proteinuria patients to rule out potential IMN. The present study specifically focused on IMN patients with nephrotic syndrome diagnosed by renal biopsy, who received combination therapy with prednisone and cyclosporine A for 6 months. We then analyzed the relationship between anti-PLA2R antibody and clinical parameters in IMN based on our single-center data. As there are few similar studies in Chinese populations, our results are of great clinical significance.
Material and Methods
Subjects
From April 2017 through July 2018, we enrolled 72 patients with nephrotic syndrome diagnosed by renal biopsy as having IMN. Diseases such as hepatitis, malignant tumors, lupus nephritis, and other common etiologies of secondary MN were excluded initially. None of the patients had previously received immunosuppressive treatment. This study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University. Signed written informed consent was obtained from all participants before the study began.
Research methods
The titer of serum anti-PLA2R antibody was assayed by enzyme-linked immunosorbent assay (ELISA), TUpro, Alb, and Ccr, as well as by anti-PLA2R antibody collected soon after admission. Patients received basic treatments with hypotensive agents, hypoglycemic agents, statins, anticoagulants, diuretics, and immunosuppressive treatment including corticosteroids (initial dose of prednisone 1mg/kg of body weight) and cyclosporine A (the initial dose was 3 mg/kg of body weight and the serum drug concentration was kept at 50–150 μg/L). Based on the 2012 KDIGO guideline [6], complete remission was defined as urinary protein excretion less than 300 mg/mM creatinine with normal serum albumin, and partial remission was defined as total urinary protein less than 3.5 g/d with at least 50% reduction versus baseline, accompanied by an increase or a return to normal of the serum albumin concentration and stable serum creatinine. Non-remission was defined as none of the above. After 6 months of treatment, TUpro, Alb, Ccr, and anti-PLA2R antibody were collected again, and data were processed for statistical analysis.
ELISA was used for serum anti-PLA2R antibody assay
All serum samples were prepared for PLA2R-Ab assay using the ELISA test commercialized by EUROImmune AG (Luebeck, Germany). Briefly, serum samples were diluted 1: 100 and then incubated with PLA2R pre-coated ELISA plates. The plates were incubated with anti-human IgG-horseradish peroxidase conjugate, and then were read at 450 nm. Concentrations of samples were calculated by the calibration curve extinction values. ELISA cutoff values were established according to the manufacturer’s protocol.
Statistics analysis
Statistical Product and Service Solutions (SPSS) 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Variables with symmetric distribution are reported as mean±standard deviation (SD). The comparison of clinical parameters during the follow-up period was made using the chi-square test for count variables and the t test for continuous variables. Spearman rank correlation was used to assess relationships between 2 variables with asymmetric distribution.
Results
General information
In this study, the serum samples of 122 patients with idiopathic membranous nephropathy, 118 patients with other kidney diseases, and 87 healthy subjects were detected by renal biopsies in our hospital. According to the cutoff value 20 RU/mL provided in the user’s manual, the sensitivity and specificity of the kit in the diagnosis of idiopathic membranous nephropathy were 97.5% and 100%, respectively. It was preliminarily accepted that this value met the experimental requirements. The OD value and the negative and positive results were imported into SPSS software, and the ROC curve was obtained as shown in Figure 1. The results show that when the cutoff value was 20 RU/mL, the diagnostic efficiency was greater.
Figure 1.
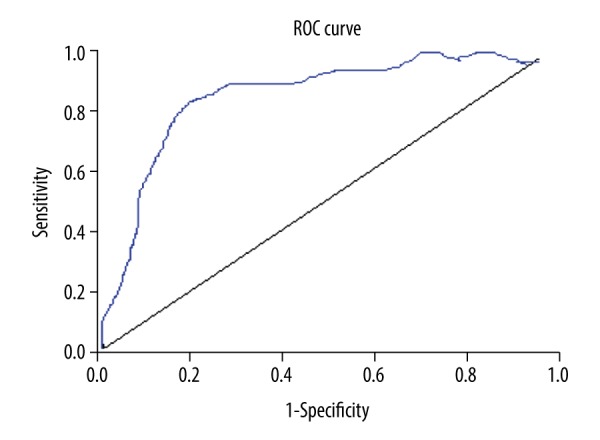
Sensitivity and specificity of ROC curve analysis about cutoff value. AUC=0.937, standard error=0.03, P≤0.000, 95% CI (0.83–0.95).
Seventy-two patients with nephrotic syndrome were diagnosed as having IMN (48 males, 24 females, average age 51±20.6 years), 48 patients also had hypertension (HTN), and 12 patients had type 2 diabetes mellitus (T2DM). Among them, 56 patients had positive anti-PLA2R antibody (average age 51±15.2 years), which accounted for 77.7% (38 of them were male). HTN accounted for 67.8% and T2DM accounted for 17.8%. Another 16 patients (10 males, average age 50±10.6 years) had negative anti-PLA2R antibody, which accounted for 22.3%. There were 10 cases of HTN and 2 case of T2DM, which accounted for 62.5% and 12.5%, respectively.
Observation parameters analysis
There were no significant differences in age, sex, HTN, or T2DM between anti-PLA2R antibody-positive and −negative groups (P>0.05) (Table 1). TUpro was significantly higher and Alb was significantly lower in the positive anti-PLA2R antibody group than in the negative group (P<0.05). However, Ccr was not significantly different between the 2 groups (P>0.05) (Table 2). In the anti-PLA2R antibody-positive group, the PLA2R antibody titer and total proteinuria were clearly decreased (P<0.05), and serum albumin was remarkably increased after treatment (P<0.05), but there was no significant difference in creatinine clearance rates before and after treatment (P>0.05) (Table 3). Among the 56 anti-PLA2R antibody-positive patients, 14 entered into complete remission, 24 entered into partial remission, and the remaining patients had no remission. The anti-PLA2R antibody in 24 patients became negative, accounting for 42.8%, and 14 out of these 24 patients entered into complete remission. In the complete remission group, there were significant differences in TUpro, Alb, and anti-PLA2R antibody levels between pre- and post-treatment (P<0.05). In the partial remission group, anti-PLA2R antibody in 10 cases became negative, and there were significant differences in TUpro, Alb, and anti-PLA2R antibody levels between pre- and post-treatment (P<0.05). In the non-remission group, there were no significant differences in TUpro and Alb between pre- and post-treatment (P>0.05), but anti-PLA2R antibody levels decreased significantly after treatment (P<0.05). There were no significant differences in Ccr level among the 3 groups (Table 4). The prevalence of remission in anti-PLA2R antibody-negative group was comparatively higher, but no statistical difference was found (P>0.05), (see Table 5).
Table 1.
Comparison of age, sex, HTN, and T2DM between anti-PLA2R antibody-positive and −negative groups.
| Anti-PLA2R positive (56 cases) | Anti-PLA2R negative (16 cases) | P | |
|---|---|---|---|
| Age (years) | 51±15.2 | 50±10.6 | 0.136 |
| Sex (male) | 38 (67.8%) | 10 (62.5%) | 0.78 |
| HTN | 38 (67.8%) | 10 (62.5%) | 0.78 |
| T2DM | 10 (17.8%) | 2 (12.5%) | 0.724 |
Table 2.
Comparison of TUpro, Alb, and Ccr levels between anti-PLA2R antibody-positive and −negative groups before treatment.
| Anti-PLA2R positive (56 cases) | Anti-PLA2R negative (16 cases) | P | |
|---|---|---|---|
| TUpro | 6.9±1.3 | 4.8±0.83 | 0.016 |
| Alb | 22.6±3.4 | 27.3±2.05 | 0.032 |
| Ccr | 78.3±28.1 | 84.7±30.16 | 0.283 |
Table 3.
Relationship between anti-PLA2R antibody and TUpro, Alb, and Ccr levels before and after treatment (56 cases).
| Pre-treatment | Post-treatment | P | |
|---|---|---|---|
| UTpro | 6.9±1.3 | 2.5±0.94* | 0.006 |
| Alb | 24.6±3.4 | 32.7±5.28* | 0.012 |
| Ccr | 78.3±28.1 | 80.5±23.7 | 0.654 |
| Anti-PLA2R | 194.2±84.7 | 64.9±37.6* | 0.015 |
Pre-treatment, post-treatment;
P<0.05 compared with before treatment.
Table 4.
Comparison of UTpro, Alb, Ccr, and anti-PLA2R antibody levels between pre- and post-treatment among complete, partial, and non-remission groups.
| Complete remission (14 cases) | Partial remission (24 cases) | Non-remission (18 cases) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | Percent changes | Pre− | Post− | Reduction/rise rate | Pre− | Post− | Percent changes | |
| UTpro | 5.14±1.69 | 0.2±0.06* | 90.2±5.9%* | 7.53±2.16 | 2.74±1.52* | 63.6±8.8%* | 7.7±3.52 | 5.4±2.13 | 30.2±13.6%* |
| Alb | 26.1±2.52 | 37.48±1.69* | 30.5±3.3%* | 23.5±2.09 | 32.±2.41* | 25.1±2.5%* | 20.3±5.75 | 24.1±4.85 | 15.8±5.7%* |
| Ccr | 82.5±20.3 | 83.9±22.7 | 2.0±1.1% | 82.04±25.8 | 82.4±23.5 | 1.9±1.2% | 80.2±27.4 | 80.5±24.9 | 2.2±1.9% |
| Anti-PLA2R | 66.3±20.4 | 8.3±5.17* | 88.2±3.6%* | 132.6±39.2 | 47.2±17.4* | 63.9±15.8%* | 620.4±205.93 | 350.2±135.7* | 33.5±10.9%* |
Pre – before treatment, post – after treatment;
P<0.05 compared with pre-treatment.
Table 5.
Comparison of remission rate between anti-PLA2R antibody-positive and −negative groups after treatment.
| Anti-PLA2R positive (56 cases) | Anti-PLA2R negative (16 cases) | P | |
|---|---|---|---|
| Complete remission | 14 (25%) | 8 (50%) | 0.182 |
| Partial remission | 24 (42.8%) | 4 (25%) | 0.368 |
| Non-remission | 18 (32.1%) | 4 (25%) | 0.703 |
Discussion
The mechanism for IMN is mainly induced by immunocomplex deposition on subepithelial cells and complement activation, which results in the alteration of basement membrane structure and injury of glomerular filtration barrier, eventually leading to proteinuria [7], and this disease is ascribed to podocytopathy. The vast majority of IMN patients are anti-PLA2R antibody-positive. The reason why IMN patients have high prevalence of anti-PLA2R antibody expression is that PLA2R is expressed by podocytes, and a process of subepithelial immune complex deposition in situ is the mechanism involved [8]. The incidence rate of anti-PLA2R antibody in IMN patients was previously reported to be 52–78% worldwide [4], but a higher incidence rate of 81.7–96.4% has been reported in China [5,9,10]; this discrepancy may be due to differences in genetic backgrounds. We found that 77.7% of IMN patients were anti-PLA2R antibody-positive, which is slightly lower than in previous Chinese studies. This may be because we did not routinely conduct IgG subtype staining, so there might have been secondary membranous nephropathy patients intermingled into our observation group.
Our study showed that there were no significant differences in age, sex, HTN, or T2DM between anti-PLA2R antibody-positive and −negative groups, but UTpro was significantly higher and Alb was significantly lower in the antibody-positive group (P<0.05). We also found that TUpro and Alb levels were correlated with anti-PLA2R antibody levels, in agreement with previous reports [11]. Complete and total remission (25% and 67.8%, respectively) in the antibody-positive group were lower than those matched values of 50% and 75% in the antibody-negative group, but no statistically significant difference was observed(P>0.05) (Table 5). The higher remission rates in the antibody-negative group might be due to the comparatively mild immuno-activity of the disease, and previous research [5] found that serum anti-PLA2R antibody levels were correlated with disease activity. The disease activity was comparatively low in patients with lower titer of antibody or without antibody when serum samples were extracted. We found higher remission rates in the antibody-negative group, but there was no statistically significant difference between the 2 groups, and this might be due in part to our small sample sizes.
Ruggenenti et al. [12] found that initial low titer of antibody and transferred negative antibody after 6 months of treatment were the best predictors for disease remission, as a higher initial serum antibody titer is associated with a lower rate of disease remission. Meanwhile, detection of dynamic changes in antibody titer in patients with complete and partial remissions can predict relapse, and an increase in titer of antibody precedes the proteinuria rise [13]. Zhen Qu et al. found that higher levels of anti-PLA2R antibody may predict risks of non-remission [14]. Xueping Wu et al. showed that changes in serum anti-PLA2R antibody level are closely related to the status of IMN patients [15]. Previous research also showed that patients with significantly elevated initial antibody titer were more vulnerable to kidney dysfunction, and even had doubled serum creatinine levels a few years later [16]. Although there was no statistically significant difference in Ccr levels between the 2 groups in this study, Ccr was obviously lower in the antibody-positive group. This might be related with the short duration of follow-up, and a significant discrepancy might appear after a longer follow-up.
There are some limitations in this study. On the one hand, the sample sizes are smaller in a single-center study, and the duration of observation is comparatively short; on the other hand, in addition to routine exclusion from common etiology for secondary MN, such as SLE, hepatitis virus, and malignant diseases, there was no staining for immunoglobulin IgG subtypes in renal biopsy specimens. The IgG4 dominating immune complex deposition on the subepithelia cell was uncertain, which might affect pathological diagnosis of IMN to a certain extent. In this regard, more samples and longer follow-up are needed, and IgG4 staining should be carried out in future research.
Conclusions
Serum anti-PLA2R antibody is a highly specific biomarker for IMN; it can be utilized for IMN diagnosis and for evaluation of treatment and prognosis. Detection of dynamic changes in antibody titer in IMN patients can help assess remission and relapse of the disease.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Austin HR, Antonovych TT, MacKay K, et al. NIH conference. Membranous nephropathy. Ann Intern Med. 1992;116:672–82. doi: 10.7326/0003-4819-116-8-672. [DOI] [PubMed] [Google Scholar]
- 2.Jones DB. Nephrotic glomerulonephritis. Am J Pathol. 1957;33:313–29. [PMC free article] [PubMed] [Google Scholar]
- 3.Beck LJ, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoxha E, Harendza S, Zahner G, et al. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–32. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 5.Qin W, Beck LJ, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–43. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck L, Bomback AS, Choi MJ, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62:403–41. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Salant DJ, Belok S, Madaio MP, Couser WG. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980;66:1339–50. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck LJ, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong HR, Wang YY, Cheng XH, et al. Retrospective study of phospholipase A2 receptor and IgG subclasses in glomerular deposits in Chinese patients with membranous nephropathy. PLoS One. 2016;11:e156263. doi: 10.1371/journal.pone.0156263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Wang L, Zhang Y, et al. A novel time-resolved fluoroimmunoassay for the quantitative detection of antibodies against the phospholipase A2 receptor. Sci Rep. 2017;7:46096. doi: 10.1038/srep46096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoxha E, Kneissler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–58. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahan K, Debiec H, Plaisier E, et al. Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–58. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu Z, Zhang MF, Cui Z, et al. Antibodies against M-type phospholipase A2 receptor may predict treatment response and outcome in membranous nephropathy. Am J Nephrol. 2018;48:438–46. doi: 10.1159/000494662. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Liu L, Guo Y, Yang L. Clinical value of a serum anti-PLA2R antibody in the diagnosis and monitoring of primary membranous nephropathy in adults. Int J Nephrol Renovasc Dis. 2018;11:241–47. doi: 10.2147/IJNRD.S176665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–48. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]


