Abstract
OBJECTIVES
The authors assessed the use of dual antiplatelet therapy (DAPT) and outcomes in patients undergoing percutaneous coronary intervention (PCI) during the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation).
BACKGROUND
The frequency, patterns, and outcomes when adding DAPT to non-vitamin K antagonist oral anticoagulants in the setting of PCI in patients with AF are largely unknown.
METHODS
The study population included all patients in the treatment group of the ROCKET AF trial divided by the receipt of PCI during follow-up. Clinical characteristics, PCI frequency, and rates of DAPT were reported. Clinical outcomes were adjudicated independently as part of the trial.
RESULTS
Among 14,171 patients, 153 (1.1%) underwent PCI during a median 806 days of follow-up. Patients treated with rivaroxaban were significantly less likely to undergo PCI compared with warfarin-treated patients (61 vs. 92; p ¼ 0.01). Study drug was continued during PCI in 81% of patients. Long-term DAPT ($30 days) was used in 37% and single antiplatelet therapy in 34%. A small number switched from DAPT to monotherapy within 30 days of PCI (n ¼ 19 [12.3%]) and 15% of patients received no antiplatelet therapy after PCI. Rates of stroke/systemic embolism and major bleeding events were high in post-PCI patients (4.5/100 patient-years and 10.2/100 patient-years) in both treatment groups.
CONCLUSIONS
In patients with AF at moderate to high risk for stroke, PCI occurred in <1% per year. DAPT was used in a variable manner, with the majority of patients remaining on study drug after PCI. Rates of both thrombotic and bleeding events were high after PCI, highlighting the need for studies to determine the optimal antithrombotic therapy.
Keywords: anticoagulants, coronary disease, direct-acting oral anticoagulants, fibrillation, percutaneous coronary intervention
In patients undergoing percutaneous coronary intervention (PCI), the prevalence of atrial fibrillation (AF) is substantial and may be increasing (1). An indication for oral anticoagulation, such as AF, in patients who require antiplatelet therapy for PCI can make therapeutic choices difficult (2). Previous investigation has shown that the combination of oral anticoagulation and antiplatelet therapy increases the risk of bleeding (3–6). However, there are few data on the combination of direct oral anticoagulants with dual antiplatelet therapy (DAPT) in the post-PCI setting. In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) (7), patients taking DAPT were excluded from enrollment, but participants who underwent PCI during the conduct of the trial were allowed to continue study drug and the use of antiplatelet therapy, as well as duration, was left to the investigators’ discretion.
To understand more about the combination of rivaroxaban and antiplatelet therapy in the post-PCI setting, we analyzed patients in the ROCKET AF trial who underwent PCI during the study. We aimed first to describe the incidence of PCI and characteristics of the population that required PCI during the ROCKET AF trial. We then aimed to describe the patterns of antiplatelet therapy in the post-PCI setting, and finally the clinical outcomes of patients undergoing PCI and on combinations of oral anticoagulation and antiplatelet therapy.
Methods
STUDY POPULATION.
The ROCKET AF trial design, methods, and primary results have been published (7,8). Briefly, ROCKET AF was a randomized, double-blind, double-dummy international noninferiority trial that compared fixed-dose rivaroxaban (20 mg/d or 15 mg/d in participants with a creatinine clearance of 30–49 ml/min) versus doseadjusted warfarin (maintaining an international normalized ratio in the therapeutic range [2.0–3.0]) for the prevention of stroke in nonvalvular AF. Patients enrolled had to have prior stroke or transient ischemic attack or $2 risk factors for stroke. Patients with only 2 risk factors were capped at 10% of the overall trial population. The primary endpoint was stroke or non–central nervous system embolism. For these analyses, we included all patients in the ROCKET AF trial who received $1 dose of study drug; the intention-to-treat population was used for efficacy outcomes and the safety population for bleeding outcomes.
OUTCOMES.
The outcomes evaluated in our study included the composite (and component events) of all stroke and non–central nervous system (or systemic) embolism, vascular death, and myocardial infarction (MI). Stroke was defined as a sudden, focal neurologic deficit resulting from a presumed cerebrovascular cause that is not reversible within 24 hours and not due to a readily identifiable cause, such as a tumor or seizure. Non–central nervous system systemic embolism was defined as abrupt vascular insufficiency associated with clinical or radiologic evidence of arterial occlusion in the absence of other likely mechanisms (e.g., trauma, atherosclerosis, or instrumentation). MI was defined by clinical symptoms consistent with MI and cardiac biomarker elevation (troponin I or T, creatine kinase myocardial band) greater than the upper limit of normal, the development of new pathological Q waves in $2 contiguous electrocardiography leads, or confirmed by autopsy. Vascular death was defined as having been caused by vascular events such as stroke, embolism, or acute MI. The safety outcomes evaluated were International Society on Thrombosis and Haemostasis major bleeding, defined as clinically overt bleeding associated with any of the following: fatal outcome, involving a critical site (i.e., intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal), or clinically overt bleeding associated with a fall in hemoglobin concentration of $2 g/dl or leading to transfusion of $2 U of packed red blood cells or whole blood, and clinically relevant nonmajor bleeding defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact with a physician (visit or telephone call), temporary (i.e., by delaying the next study drug administration) cessation of study drug, pain, or impairment of daily activities. All events in ROCKET AF were adjudicated independently by a blinded, multispecialty adjudication committee.
STATISTICAL METHODS.
Baseline characteristics, including patient demographics, risk factors, medical history, and baseline medications were calculated with cohorts divided by PCI status and randomized treatment group. Continuous variables are presented as medians (25th, 75th percentiles) and categorical variables are presented as counts (percentages). Within the PCI study population, the time to first PCI was described in both treatment groups as well as baseline characteristics of these patients, the patterns of antiplatelet use with mean duration of use, and the clinical outcomes stratified by treatment group and analyzed as unadjusted event rate per 100 patientyears. Type of procedure and stents placed were also described. All statistical analyses of the aggregate, de-identified data were performed by the Duke Clinical Research Institute (Durham, North Carolina) using SAS software (version 9.2, SAS Institute, Cary, North Carolina).
RESULTS
There were 153 patients (1.1%) who underwent PCI in ROCKET AF, 61 in the rivaroxaban treatment arm and 92 in the warfarin treatment arm during a median 806 days of follow-up. Median length of follow-up was statistically similar between treatment groups (841 days in rivaroxaban-treated patients vs. 791 days in warfarin-treated patients; p ¼ 0.29). Patients undergoing PCI were similar in age, but less often female, more likely to have been on prior vitamin K antagonist agents, and more likely to have had a prior MI or diabetes compared with patients who did not undergo PCI (Table 1). They had similar stroke risk, as measured by CHADS2 (Congestive Heart Failure, Hypertension, Age $75 Years, Diabetes Mellitus [1 point for presence of each], and Stroke/ TIA [2 points]; scores range from 0 to 6) scores, and similar creatinine clearance. Among the PCI cohort, the clinical characteristics of those receiving rivaroxaban were similar to those receiving warfarin (Online Table 1).
TABLE 1.
Baseline Characteristics
| Overall (N ¼ 14,171) |
Patients With PCI (n ¼ 153) |
Patients Without PCI (n = 14,018) |
p Value | |
|---|---|---|---|---|
| Age, yrs | 73 (65, 78) | 73 (67, 79) | 73 (65, 78) | 0.305 |
| Female | 5,605 (39.6) | 27 (17.6) | 5,578 (39.8) | <0.001 |
| BMI, kg/m2 | 28.2 (25.1, 32.0) | 28.9 (26.0, 32.4) | 28.2 (25.1, 32.00 | 0.105 |
| SBP, mm Hg | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 0.039 |
| DBP, mm Hg | 80 (70, 85) | 80 (70, 82) | 80 (70, 85) | 0.024 |
| Type of AF | 0.247 | |||
| Persistent | 11,485 (81.1) | 116 (75.8) | 11,369 (81.1) | |
| Paroxysmal | 2,490 (17.6) | 34 (22.2) | 2,456 (17.5) | |
| Newly diagnosed | 196 (1.4) | 3 (2.0) | 193 (1.4) | |
| Previous medication use | ||||
| Aspirin | 5,184 (36.6) | 65 (42.5) | 5,119 (36.5) | 0.128 |
| VKA | 8,853 (62.3) | 113 (73.9) | 8,740 (62.3) | 0.004 |
| CHADS2 score | 3.5 _ 0.9 | 3.5 _ 1.0 | 3.5 _ 0.9 | 0.928 |
| CHADS2 score | ||||
| 1 | 3 (0.0) | 0 (0.0) | 3 (0.0) | |
| 2 | 1,857 (13.1) | 25 (16.3) | 1,832 (13.1) | |
| 3 | 6,169 (43.5) | 60 (39.2) | 6,109 (43.6) | |
| 4 | 4,067 (28.7) | 40 (26.1) | 4,027 (28.7) | |
| 5 | 1,797 (12.7) | 26 (17.0) | 1,771 (12.6) | |
| 6 | 278 (2.0) | 2 (1.3) | 276 (2.0) | |
| Coexisting condition | ||||
| Prior stroke/TIA/non-CNS embolism |
7,767 (54.8) | 86 (56.2) | 7,681 (54.8) | 0.726 |
| Congestive HF | 8,851 (62.5) | 82 (53.6) | 8,769 (62.6) | 0.023 |
| Hypertension | 12,824 (90.1) | 132 (86.3) | 12,692 (90.5) | 0.074 |
| Diabetes | 5,647 (39.9) | 75 (49.0) | 5,572 (39.7) | 0.020 |
| Prior MI | 2,446 (17.3) | 50 (32.7) | 2,396 (17.1) | <.001 |
| PAD | 832 (5.9) | 18 (11.8) | 814 (5.8) | 0.002 |
| COPD | 1,481 (10.5) | 17 (11.1) | 1,464 (10.4) | 0.790 |
| CrCl, ml/min* | 67 (52, 87) | 71 (53, 88) | 67 (52, 87) | 0.483 |
Values are median (25th, 75th percentile), n (%), or mean _ SD.
Creatinine clearance was calculated using the Cockcroft-Gault equation.
AF ¼ atrial fibrillation; BMI ¼ body mass index; CHADS2 ¼ Congestive Heart Failure, Hypertension, Age $75 Years, Diabetes Mellitus [1 point for presence of each], and Stroke/TIA [2 points]; scores range from 0 to 6; CNS ¼ central nervous system; COPD ¼ chronic obstructive pulmonary disease; CrCl ¼ creatinine clearance; DBP ¼ diastolic blood pressure; HF ¼ heart failure; MI ¼ myocardial infarction; PAD ¼ peripheral artery disease; PCI ¼ percutaneous coronary intervention; SBP ¼ systolic blood pressure; SD ¼ standard deviation; TIA ¼ transient ischemic attack; VKA ¼ vitamin K antagonist.
FREQUENCY OF PCI AND PROCEDURAL CHARACTERISTICS.
There was a consistently small but increasing incidence of PCI over time (Figure 1). Warfarin-treated patients had a significantly shorter time (273 vs. 370 days; p ¼ 0.011) to first PCI compared with the rivaroxaban-treated patients, and warfarin-treated patients were more likely to undergo PCI during the conduct of the trial. For procedural characteristics, the use of bare metal stents was slightly higher than the use of drug-eluting stents, and a minority of patients received balloon angioplasty alone (Table 2). Across treatment groups, the use of stents/balloon angioplasty was similar.
FIGURE 1. Time to First PCI Event for Patients on Rivaroxban Versus Warfarin.
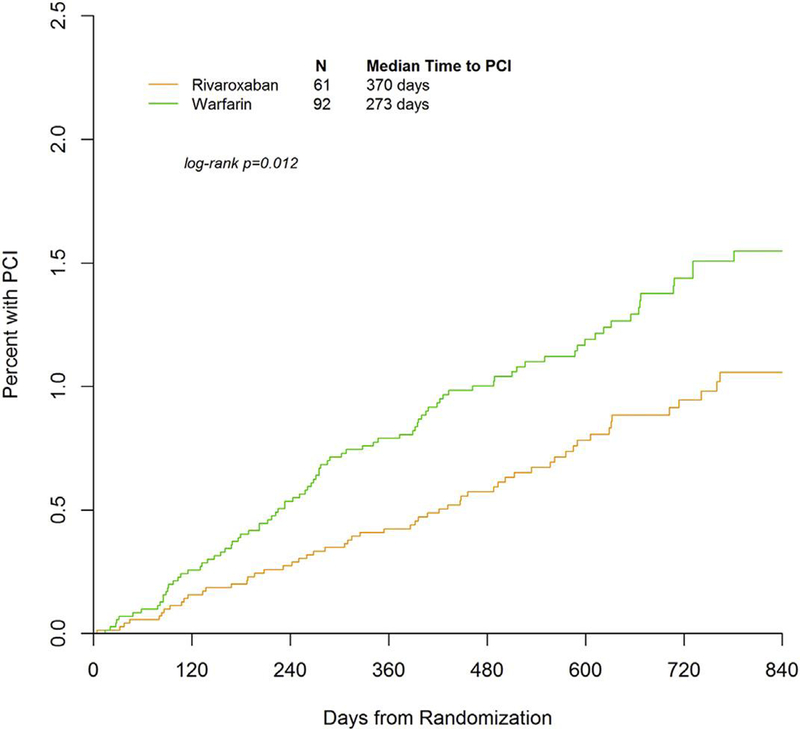
Warfarin-treated patients had significantly shorter time to first percutaneous cardiac intervention (PCI) compared with rivaroxaban-treated patients.
TABLE 2.
Procedure Characteristics
| All PCIs (n ¼ 153) | Rivaroxaban (n ¼ 61) | Warfarin (n ¼ 92) | |
|---|---|---|---|
| BMS | 68 (44) | 25 (41) | 43 (47) |
| DES | 53 (35) | 21 (34) | 32 (35) |
| Ballon angioplasty | 25 (16) | 14 (23) | 11 (12) |
| Missing | 7 (5) | 1 (2) | 6 (7) |
Values are n (%).
BMS ¼ bare-metal stent(s); DES ¼ drug-eluting stent(s); other abbreviation as in Table 1.
USE OF STUDY AND ANTIPLATELET THERAPY DURING PCI.
Patterns of antiplatelet therapy use after PCI were variable; trial protocol recommended timing of study drug cessation (Figure 2) but left study drug resumption and antiplatelet medications to the investigators’ discretion. Nearly 81% of all patients who underwent PCI remained on study drug for $30 days after the procedure. After PCI, 37% of patients received DAPT (clopidogrel and aspirin) for $30 days, and approximately 34% of patients were given monotherapy with clopidogrel or aspirin. There was a small group of patients (15%) who received study drug without antiplatelet therapy, and a 12% rate of switching at approximately 30 days from DAPT to single antiplatelet therapy (Figure 3).
FIGURE 2. Periprocedural Strategy in ROCKET AF.
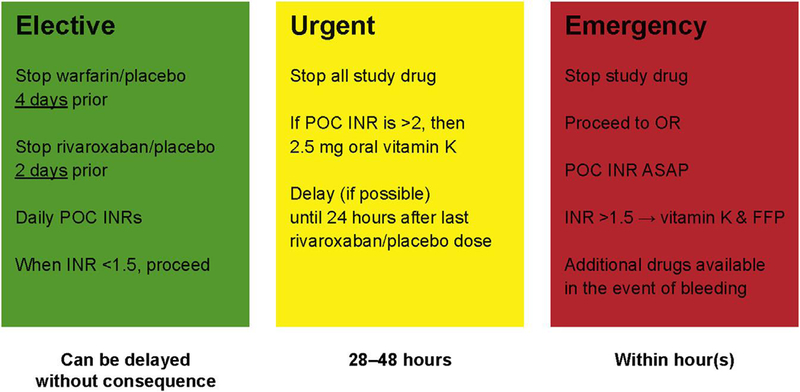
Investigators were given discretion over how to manage study drug in the event of an invasive procedure, but were provided protocol guidance in the setting of elective versus urgent versus emergent procedure for study drug management. For urgent and emergent procedures, immediate cessation of study drug was necessary with monitoring and administration of therapeutic agents if the situation warranted.
FIGURE 3. Frequency of Concomitant Antiplatelet Therapy Use.
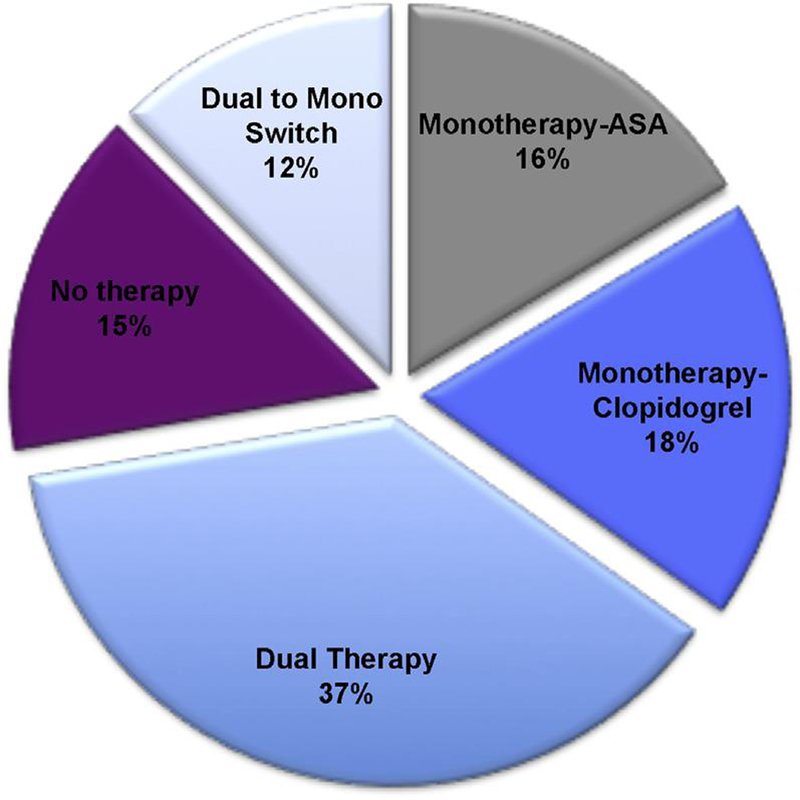
At 30 days after PCI in all patients undergoing PCI in ROCKET AF, the majority of patients (80.5%) remained on study drug after PCI therapy. Abbreviations as in Figures 1 and 2.
OUTCOMES.
In the 153 patients who underwent PCI, rates of thrombotic and bleeding outcomes were high. Rates of stroke or systemic embolism were 4.5 per 100 patient-years, whereas rates of major bleeding were 10.5 per 100 patient-years. Comparing patients with and without PCI, all ischemic and thrombotic outcome event rates were higher in post-PCI patients compared with patients who did not undergo PCI (Table 3). Similar patterns were seen for bleeding outcomes; patients with PCI were at substantially greater risk for bleeding events compared with those who did not undergo PCI (Table 3). On comparison of the treatment groups, there was a numerically higher rate of stroke and vascular death events in the warfarin group compared with the rivaroxaban group (Table 4). The risk of the composite outcome of stroke and systemic embolism was similar between treatment groups as was the risk of MI. For bleeding events, there was a numerically increased rate of both major bleeding and the composite of major and clinically relevant nonmajor bleeding in those treated with rivaroxaban compared with those treated with warfarin (Table 4). When patients were divided by use of antiplatelet therapy at 30 days after PCI, the rates of major bleeding were high and similar across treatment groups for all patients (Online Table 2) and for those patients who remained on study drug (81%) (Online Table 3). When event frequency was plotted at follow-up time intervals, there was a clustering of thrombotic and bleeding events (Figure 4). Stroke, MI, systemic embolic events, and major bleeding events predominantly occurred within the first 6 months after PCI. Vascular deaths slowly accrued across the follow-up time intervals.
TABLE 3.
Event Rates for Efficacy and Safety Endpoints
| Unadjusted Event Rate/100 Patient-Years (No. of Events) | ||
|---|---|---|
| Endpoint | No PCI (n ¼ 14,018) | After PCI (n ¼ 153) |
| Stroke or systemic embolism | 2.3 (568) | 4.5 (7) |
| Stroke | 2.1 (529) | 3.1 (5) |
| MI | 1.0 (253) | 6.2 (7) |
| Vascular death | 3.0 (763) | 8.0 (13) |
| Major or NMCR bleeding | 14.7 (2886) | 31.7 (27) |
| Major bleeding | 3.5 (766) | 10.5 (12) |
NMCR ¼ nonmajor clinically relevant; other abbreviation as in Table 1.
TABLE 4.
Event Rates for Efficacy and Safety Endpoints According to Study Treatment Among PCI Patients
| Unadjusted Event Rate/100 Patient-Years (No. of Events) | ||
|---|---|---|
| Endpoint | Rivaroxaban (n ¼ 61) | Warfarin (n ¼ 92) |
| Stroke or systemic embolism | 5.0 (3) | 4.1 (4) |
| Stroke | 1.6 (1) | 4.1 (4) |
| MI | 6.8 (3) | 5.9 (4) |
| Vascular death | 3.1 (2) | 11.1 (11) |
| Major or NMCR bleeding | 57.7 (15) | 20.3 (12) |
| Major bleeding | 15.0 (6) | 8.1 (6) |
Figure 4. Proportion of Adverse Events After PCI.
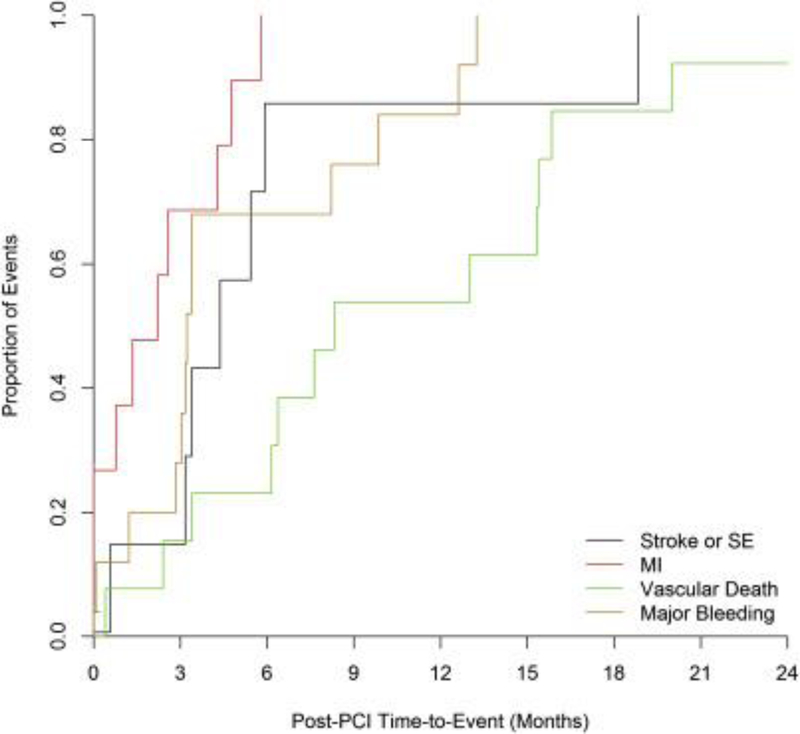
There is a clustering of events in the 6-month time window. Abbreviation as in Figure 1.
DISCUSSION
In a large, international cohort of patients with nonvalvular AF at increased risk for ischemic stroke, PCI was rare, occurring in only 1% of the patients during follow-up. The majority of these patients remained on anticoagulant study drug in the post-PCI period, although the use of antiplatelet therapy was variable. The rates of thrombotic and bleeding outcomes were substantially elevated in patients undergoing PCI and this risk persisted for 6 months after the procedure. Although comparisons between warfarin and rivaroxaban cannot be made with these data, our results highlight the importance of caution in these patients, and the need for further research on the combination of non-vitamin K antagonist oral anticoagulants (direct-acting oral anticoagulants [DOACs]) and antiplatelet therapy in the setting of PCI.
RATE OF PCI IN ROCKET AF.
Previous investigations estimate that approximately 1% to 2% of adults have AF and, over time, approximately 20% to 30% of these adults will undergo PCI (9,10). Recently published registry data indicate that rates of MI are higher in patients with AF versus those without AF. Soliman et al. (11) explored the Atherosclerosis Risk in Communities (ARIC) database and found that rates of MI were higher in patients with AF, particularly in women and black patients. The unadjusted rates were approximately 1% per year in patients with AF compared with 0.5% per year in others. Soliman et al. (12) found similar results in a post hoc analysis of the REGARDS (REasons for Geographic and Racial Differences in Stroke) study. Unadjusted rates of MI were significantly higher in patients with preexisting AF (approximately 1% per year) than in those without. When the population from the ROCKET AF trial was examined, there were few patients (<1% per year) who underwent PCI during the Median 2.5 year follow-up. Although this rate may seem low compared with observational estimates, recent data from large clinical trials provide context. In the ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation), ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis In Myocardial Infarction 48) and RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trials, rates of MI during follow-up were #1% per year (13–15). Thus, the experience in the ROCKET AF trial seems similar to other large international clinical trial cohorts.
Despite the double-blind, double-dummy design of the ROCKET AF trial, there was a significant difference in rates of PCI between treatment arms. Patients treated with rivaroxaban were significantly less likely to undergo PCI compared with those on warfarin. Potential explanations for this phenomenon include the play of chance, a protective effect seen with rivaroxaban above that seen with warfarin, or “pseudo-unblinding.” There are 2 prospective randomized clinical studies with rivaroxaban in the prevention of acute coronary events. The ATLAS TIMI 46 (Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Patients With Acute Coronary Syndromes-Thrombolysis In Myocardial Infarction 46) (16) and ATLAS 2 TIMI-51 (17) trials showed that in the post-ACS setting, rivaroxaban at low doses (5 and 2.5 mg) was effective in reducing rates of cardiovascular death, stroke, and MI, while also increasing rates of bleeding. However, the patient population and concomitant medications were very different in the ATLAS trials compared with ROCKET AF, as were the dosing strategies. Thus, the comparison may not be valid. Other factor Xa inhibitors have been tested in a similar manner (18), without signals for substantial efficacy in the secondary prevention of major adverse cardiovascular events, but with increased risk for major bleeding events. More detailed investigation on the effect of rivaroxaban versus warfarin on further ischemic events in patients with and without prior MI has been published showing a nonsignificant 14% reduction in the hazard (hazard ratio: 0.86; 95% confidence interval: 0.73 to 1.00; p ¼ 0.0509) for Cardiovascular death, MI, or unstable angina in patients assigned to rivaroxaban compared with patients assigned to warfarin (19). Pseudo-unblinding (in this context) is the phenomenon by which investigators may have obtained open laboratory values in the process of clinical care (e.g., treatment for acute coronary syndrome) that would indicate the treatment group assignment for their patient. This phenomenon may have led to a differential practice pattern with respect to performance of PCI. At the time of the ROCKET AF trial, there were few data available on performance of procedures like PCI while undergoing treatment with a DOAC like rivaroxaban. Thus there may have been hesitation to perform PCI in rivaroxaban-treated patients. Although this explanation is plausible, there is no way to validate it.
Patients with an indication for chronic anticoagulation undergoing PCI still present a unique challenge to health care providers. The optimal medical therapy to reduce the risk of subsequent thrombotic events while balancing the risk of bleeding is currently unknown. This lack was evident in our population, with variable use of antiplatelet therapy after PCI. This could also have been due to the clinical trial setting, in which clinical providers in the ROCKET AF trial may have been less comfortable in the long-term prescription of antiplatelet therapy, because the type of oral anticoagulation remained blinded. Based on previous studies (20), the scope of this problem remains large (>50,000 patients annually in the United States alone) and would seem to only increase in the coming years, with the increasing elderly population in Western countries, and the more frequent use of DOACs. Our results highlight the pressing need for further investigation of optimal therapeutic combinations in these patients to understand both pharmacodynamic as well as therapeutic effects of these drugs.
TRIPLE THERAPY VERSUS ALTERNATIVES AND COMBINATION OF DOAC WITH ANTIPLATELET THERAPY.
DAPT is effective in reducing stent thrombosis and further adverse thrombotic events in patients with acute coronary syndrome (21–23). Oral anticoagulation is an effective therapy for reduction of stroke risk in patients with AF at moderate to high risk for stroke (7,14,24,25). Previous investigation shows that any combination of oral anticoagulants and antiplatelet therapy substantially increases the risk of bleeding compared with either alone (2–4,6). Much of the data are observational, but are convincing given their consistent message. A current European consensus statement suggests a risk-based approach dependent on thrombotic risk (as estimated by the CHA2DS2-VASc score) and clinical situation (stable coronary artery disease vs. acute coronary syndrome) (26). There have been few randomized clinical trials on this important question. The WOEST (What is the Optimal antiplatelet and anticoagulant therapy in patients with oral anticoagulation and coronary Stenting) trial was an open-label, randomized clinical trial that examined the use of oral anticoagulation (warfarin) and clopidogrel with or without additional aspirin (9). The WOEST trial of 573 patients randomized to warfarin and clopidogrel, with or without concomitant aspirin, demonstrated that additional aspirin was not associated with added thrombotic protection, but significantly increased bleeding risk (9). Data from the WOEST trial are compelling, and have prompted revisions to the aforementioned European consensus document. The ISAR-TRIPLE (Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation) study evaluated a different strategy for patients on oral anticoagulation undergoing PCI (27). In a randomized, open-label trial of 614 patients receiving concomitant aspirin and oral anticoagulation, subjects were randomized to receive either 6 weeks of clopidogrel therapy or 6 months of clopidogrel therapy. Over a follow-up of $9 months, there was no difference in the primary endpoint (composite of death, MI, stent thrombosis, or TIMI major bleeding) between treatment arms. The authors concluded that further studies evaluating the duration of potential therapy should be pursued. Despite these encouraging findings, these results cannot be extrapolated and applied to the use of DOACs with clopidogrel or aspirin.
There are limited data available on the combination of aspirin or P2Y12 antagonists and DOACs. Dans et al. (28) studied the combination of antiplatelet agents with dabigatran versus warfarin in a secondary analysis of the RE-LY trial. They found that concomitant antiplatelet therapy significantly increased rates of major and minor bleeding for patients on either dabigatran (150 and 110 mg) or warfarin, but did not affect the beneficial reduction in stroke or systemic embolism seen with dabigatran. In further analyses, DAPT further increased the risk of major and minor bleeding compared with antiplatelet monotherapy in combination with oral anticoagulation. Alexander et al. (29) showed similar results in their secondary analysis of the ARISTOTLE trial. Concomitant aspirin use was associated with an increased risk of major and nonmajor clinically relevant bleeding, but did not affect the beneficial reduction in thrombotic events seen with apixaban. Our results also show an high risk for bleeding events when combining antiplatelet therapy with oral anticoagulation with either warfarin or rivaroxaban, though the post hoc, nonrandomized, subgroup analysis nature of our study limits further interpretation.In addition, despite the frequent use of concomitant antiplatelet therapy and anticoagulation, the rates of thrombotic events were also high in the post-PCI setting. These findings highlight the challenges of treating patients with AF undergoing PCI. These patients remain at very high risk for bleeding events, but need potent therapies to address their high thrombotic event risk (30,31). Although our study population was small, limiting the depth of analyses and comparisons that we could perform, these data are among the very limited published experience of patients with AF specifically undergoing PCI on DOAC therapy.
FUTURE DIRECTIONS.
These data continue to raise questions about how to handle these new drugs, both in the setting of PCI, and in concert with antiplatelet agents. Currently, the PIONEER AF-PCI (A Study Exploring Two Strategies of Rivaroxaban [JNJ39039039; BAY-59–7939] and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention) trial (NCT01830543) (32) has been initiated to evaluate this specific question for rivaroxaban. Trials evaluating the combination of antiplatelet drugs with dabigatran and apixaban in the PCI setting have also been initiated (33). There are several challenges to investigators, such as the choice of drug-eluting versus bare metal stents, the duration of potential triple therapy, and the use of newer more potent antiplatelet agents in patients at high risk for bleeding. Although these are complex issues, trials such as PIONEER AF-PCI, REDUAL PCI (Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting), and AUGUSTUS (An Open-label, 2 × 2 Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome or Percutaneous Coronary Intervention) are necessary to determine the optimal medical therapy for patients who require chronic oral anticoagulation and undergo PCI.
STUDY LIMITATIONS.
First, it was a post hoc subgroup analysis of the ROCKET AF trial and thus is subject to selection bias, and both measured and unmeasured confounding. There were few PCIs and clinical events after PCI, which limits the power and types of comparisons that could be made. The timing of medications and urgency of indications were not available for all PCIs, again limiting the analyses we could perform. Indications for PCI were not available for all patients, but of those patients (86 [56%]) with clearly referenced indications, 42 (49%) had PCI for an indication of acute coronary syndrome, 20 (23%) were preplanned elective cases, and the remainder had PCI for other adverse events (atypical chest pain, etc.).
CONCLUSIONS
In the ROCKET AF trial, a large international population of patients with AF at increased risk for stroke, PCI occurred infrequently, in 1.1% of patients during follow-up. DAPT was used in a variable manner, but the majority of patients remained on the study drug after PCI. Rates of both thrombotic and bleeding events were high in the post-PCI period and most events occurred within 6 months. These results highlight the need for caution in these patients and should drive further research on the combination of DOACs and antiplatelet therapy in the setting of PCI.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
In patients taking oral anticoagulation, the addition of dual antiplatelet therapy significantly increases bleeding risk with little proven benefit. Although the combination of vitamin K–based agents and P2Y12 inhibitors has been studied, there is very little published on the combination of direct-acting oral anticoagulants and dual antiplatelet therapy, specifically in the setting of PCI.
WHAT IS NEW?
We studied the antithrombotic therapy practice patterns and outcomes in patients undergoing PCI during the ROCKET AF trial. In patients with AF at moderate-to-high risk for stroke, PCI occurred in <1% per year. Dual antiplatelet therapy was used in a variable manner, with the majority of patients remaining on the study drug after PCI. Rates of both thrombotic and bleeding events were high after PCI, highlighting the need for studies to determine the optimal antithrombotic therapy for patients requiring OAC who undergo PCI.
WHAT IS NEXT?
Several ongoing studies are investigating the optimal combination of DOACs and antiplatelet therapy.
Acknowledgments
The ROCKET AF trial was supported by Johnson & Johnson Pharmaceutical Research & Development (Raritan, New Jersey) and Bayer HealthCare AG (Leverkusen, Germany).
Funding/Disclosures
Dr. Sherwood was funded by NIH T-32 training grant #5 T32 HL 7101–37. Dr. Jones received research grants from the American Heart Association, AstraZeneca, Boston Scientific Corporation, and Daiichi-Sankyo.
Dr. Becker is a consultant/advisory board member for Janssen Research & Development, Portola, Cook, and Boehringer Ingelheim.
Dr. Berkowitz is an employee of Bayer HealthCare Pharmaceuticals. Dr. Breithardt has received consulting fees from Bayer HealthCare, Johnson & Johnson, Boehringer Ingelheim, Sanofi, Merck Sharp & Dohme, and 3M.
Dr. Fox has received research funding from Bayer, Janssen, and AstraZeneca; honoraria from Bayer, AstraZeneca, GlaxoSmithKline, Janssen, and Sanofi; consulting fees from Bayer, Lilly, AstraZeneca, and Sanofi.
Dr. Halperin has received consulting fees from Bayer AG HealthCare, Boehringer Ingelheim, Daiichi-Sankyo, Johnson & Johnson, Ortho-McNeil-Janssen Pharmaceuticals, Pfizer, and Sanofi-Aventis.
Dr. Hankey has received consulting fees from Bayer and Sanofi. Dr. Inger has received research funding from Johnson & Johnson, Bristol-Myers Squibb, Boehringer Ingelheim, and Medtronic; consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, CVS Health, Johnson & Johnson, Merck, Pfizer, and St. Jude Medical.
Dr. Piccini has received research grants from ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, ResMed, and St Jude Medical; and consulting fees from GlaxoSmithKline, Johnson & Johnson, Laguna Pharmaceuticals, Medtronic, and Spectranetics.
Dr. Nessel is an employee of Janssen Research and Development.
Dr. Mahaffey has provided full disclosures prior to August 1, 2013, available at www.dcri.org. Disclosures after August 1, 2013 available at ss://med.stanford.
edu/profiles/47970?tab¼research-and-scholarship.
Dr. Patel has received research funding from Johnson & Johnson, AstraZeneca;
advisory board fees from Bayer, Janssen, AstraZeneca, and Genzyme.
ABBREVIATI ONS AND ACRONYMS
- AF
atrial fibrillation
- DAPT
dual antiplatelet therapy
- DOAC
direct-acting oral anticoagulants
- MI
myocardial infarction
- PCI
percutaneous coronary
References
- 1.Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011;4:522–34. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Nodar JM, Marin F, Roldan V, et al. Should we recommend oral anticoagulation therapy in patients with atrial fibrillation undergoing coronary artery stenting with a high HAS-BLED bleeding risk score? Circ Cardiovasc Interv 2012; 5:459–66. [DOI] [PubMed] [Google Scholar]
- 3.Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010;170:1433–41. [DOI] [PubMed] [Google Scholar]
- 4.Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013;62:981–9. [DOI] [PubMed] [Google Scholar]
- 5.Paikin JS, Wright DS, Crowther MA, Mehta SR, Eikelboom JW. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation 2010;121:2067–70. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation 2013;128: 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 8.Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 2010;159:340–7. [DOI] [PubMed] [Google Scholar]
- 9.Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an openlabel, randomised, controlled trial. Lancet 2013;381:1107–15. [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Huber K, Andreotti F, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary–a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2010;31:1311–8. [DOI] [PubMed] [Google Scholar]
- 11.Soliman EZ, Lopez F, O’Neal WT, et al. Atrial Fibrillation and risk of ST-segment-elevation versus non-ST-segment-elevation myocardial infarction: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2015;131:1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahit MC, Lopes RD, Wojdyla DM, et al. Apixaban in patients with atrial fibrillation and prior coronary artery disease: insights from the ARISTOTLE trial. Int J Cardiol 2013;170: 215–20. [DOI] [PubMed] [Google Scholar]
- 14.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 16.Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 2009;374:29–38. [DOI] [PubMed] [Google Scholar]
- 17.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 18.Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011;365:699–708. [DOI] [PubMed] [Google Scholar]
- 19.Mahaffey KW, Stevens SR, White HD, et al. Ischaemic cardiac outcomes in patients with atrial fibrillation treated with vitamin K antagonism or factor Xa inhibition: results from the ROCKET AF trial. Eur Heart J 2014;35:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142–7. [DOI] [PubMed] [Google Scholar]
- 21.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001;358:527–33. [DOI] [PubMed] [Google Scholar]
- 22.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 23.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 24.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343:687–91. [PubMed] [Google Scholar]
- 25.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Windecker S, Huber K, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J 2014;35:3155–79. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler KA, Maeng M, Mehilli J, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: The ISAR-TRIPLE trial. J Am Coll Cardiol 2015;65:1619–29. [DOI] [PubMed] [Google Scholar]
- 28.Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RELY) trial. Circulation 2013;127:634–40. [DOI] [PubMed] [Google Scholar]
- 29.Alexander JH, Lopes RD, Thomas L, et al. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2014;35:224–32. [DOI] [PubMed] [Google Scholar]
- 30.Mooe T, Eriksson P, Stegmayr B. Ischemic stroke after acute myocardial infarction. A population-based study. Stroke 1997;28:762–7. [DOI] [PubMed] [Google Scholar]
- 31.Capodanno D, Angiolillo DJ. Management of antiplatelet and anticoagulant therapy in patients with atrial fibrillation in the setting of acute coronary syndromes or percutaneous coronary interventions. Circ Cardiovasc Interv 2014;7:113–24. [DOI] [PubMed] [Google Scholar]
- 32.Gibson CM, Mehran R, Bode C, et al. An openlabel, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin K antagonist treatment strategy in subjects with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). Am Heart J 2015;169:472–8. [DOI] [PubMed] [Google Scholar]
- 33.Capodanno D, Lip GY, Windecker S, et al. Triple antithrombotic therapy in atrial fibrillation patients with coronary syndromes or undergoing percutaneous coronary intervention or transcatheter aortic valve replacement. Euro-Intervention 2015;10:1015–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


