Abstract
The electrochemical production of hydrogen peroxide (H2O2) by 2-electron oxygen reduction reaction (ORR) is an attractive alternative to the present complex anthraquinone process. The objective of this paper is to provide a state-of-the-arts review of the most important aspects of this process. First, recent advances in H2O2 production are reviewed and the advantages of H2O2 electrogeneration via 2-electron ORR are highlighted. Second, the selectivity of the ORR pathway towards H2O2 formation as well as the development process of H2O2 production are presented. The cathode characteristics are the decisive factors of H2O2 production. Thus the focus is shifted to the introduction of commonly used carbon cathodes and their modification methods, including the introduction of other active carbon materials, hetero-atoms doping (i.e., O, N, F, B, and P) and decoration with metal oxides. Cathode stability is evaluated due to its significance for long-term application. Effects of various operational parameters, such as electrode potential/current density, supporting electrolyte, electrolyte pH, temperature, dissolved oxygen, and current mode on H2O2 production are then discussed. Additionally, the environmental application of electrogenerated H2O2 on aqueous and gaseous contaminants removal, including dyes, pesticides, herbicides, phenolic compounds, drugs, VOCs, SO2, NO, and Hg0, are described. Finally, a brief conclusion about the recent progress achieved in H2O2 electrogeneration via 2-electron ORR and an outlook on future research challenges are proposed.
Keywords: Hydrogen peroxide, oxygen reduction reaction, electro-Fenton, carbon materials, environmental remediation
Graphical Abstract
1. Introduction
1.1. H2O2 production methods
Hydrogen peroxide (H2O2), one of the 100 most important chemicals, is a versatile and environmentally friendly oxidant over the full pH range with high oxidation potential (E0=1.763 V at pH 0, E0=0.878 V at pH 14) (Campos-Martin et al., 2006). It leaves no hazardous residuals but only water and oxygen. H2O2 plays a significant role in a wide range of applications, including synthesis of organic compounds, pulp and bleaching, treatment of wastewater, groundwater remediation, disinfection, semiconductor cleaning, detergent, and waste gas treatment (Qiang et al., 2002; Yang et al., 2018). The industrial demand, especially the increased demand for water treatment and environmental remediation are driving an increase in H2O2 demand. The annual production of H2O2 reached 5.5 million t in 2015 (Ciriminna et al., 2016).
Currently, the majority of global H2O2 is manufactured by the anthraquinone oxidation process (AO, also known as the auto-oxidation process), it is the most widely used process and accounts for more than 95% of total H2O2 production each year (Yi et al., 2016). The AO process involves the hydrogenation and subsequent oxidation of an alkylanthraquinone precursor dissolved in a mixture of organic solvents followed by liquid-liquid extraction to recover H2O2 (Campos-Martin et al., 2006). However, this process cannot be recognized as a green process. The major drawbacks of the multi-step AO process are that it requires massive infrastructure and significant energy input (Campos-Martin et al., 2006). The centralized production requires additional transport, storage, and handling of high-concentration H2O2, which involves hazards and escalating costs (Campos-Martin et al., 2006; Yang et al., 2018).
There are various applications where dilute H2O2 solutions are sufficient, which makes many other H2O2 synthesis methods possible candidates for replacing the AO process. For example, chemical synthesis, medical disinfection, pulp bleaching, and cosmetic uses only require H2O2 with a concentration lower than 9 wt% (Russo et al., 2013; Edwards et al., 2015). For wastewater treatment, only <0.1 wt% is necessary (Young et al., 2016). The decentralized requirements of users and drawbacks of the AO process stimulate the industry and academic community to develop other H2O2 synthesis methods.
Table SM-1 summarizes H2O2 synthesis approaches reported in the literature. These methods can be divided into three categories, that is direct H2O2 synthesis from H2 and O2, photo-catalysis, and O2 electroreduction. The direct H2O2 synthesis route is achieved by the principles of noble metal catalysis (García et al., 2015; Freakley et al., 2016), fuel-cell method (Yamanaka, 2014; Yi et al., 2016), and plasma methods (Yi et al., 2016). Noble metal catalysis was first reported by Henkel et al. (Yang et al., 2018) in 1914. In this process, H2 and O2 mixture feed gas (O2 excess, H2 concentration less than 4 mol%) are introduced to a liquid medium in the presence of noble metal catalysts. The reaction system of the H2/O2 fuel cell method includes an anode, an electrolyte membrane, and a cathode. H2 dissociates into H+ on the anode, passes through the membrane and reacts with the adsorbed O2 on the cathode to form H2O2 (Yi et al., 2016). Yamanaka and co-workers developed this method by using proton exchange membrane (PEM) electrolytes, where O2 evolution is used to supply protons (Yamanaka, 2018). Photo-catalysis is another promising method to generate H2O2. Photocatalysts such as TiO2 (Shiraishi et al., 2003), g-C3N4 (He et al., 2018; Hu et al., 2018; Zhu et al., 2018) are capable of generating dilute H2O2 from O2-saturated water under UV light irradiation. The photo-generated electron (e−) induces the 2-electron O2 reduction and generates H2O2 (Eq. (S6)), whereas the photo-excited hole (h+) oxidizes the water. Additionally, H2O2 can theoretically be generated by the 2-electron oxidation of water on a photoanode (Eq. (S7)). Photoanodes such as BiVO4 and WO3/BiVO4 have been reported to produce H2O2 (Fuku et al., 2016; Fuku et al., 2017). However, H2O2 produced by this pathway has inherent drawbacks since O2 evolution occurs more easily than H2O2 production due to its more positive potentials, and H2O2 is easily oxidized to O2 on the anode (Eq. (S8)).
Among these methods, H2O2 production via 2-electron O2 electroreduction pathway is one of the most attractive alternative because it enables the in situ H2O2 production at moderate temperature and atmospheric pressure. The electrocatalysts cover a wide range from noble metals, and metal alloys to carbon-based materials (Chen et al., 2018). The electrocatalysts, especially the earth-abundant carbon-based materials, can be tuned to achieve efficient, selective and stable electrocatalysts towards 2-electron O2 reduction.
Berl et al. first reported H2O2 production via O2 electrochemical reduction by using activated carbon cathode (Berl, 1939). Based on this method, in the 1980s, the Huron-Dow process was developed by Dow and Huron Technologies, Inc. to achieve on-site dilute alkaline H2O2 production (Yang et al., 2018). The dilute alkaline H2O2 can be used for the pulp and paper bleaching process. This process was commercialized in 1991. However, the highly alkaline working solution limit its further development. Since the 2000s, the Electro-Fenton (EF) process, a process based on O2 electroreduction to produce H2O2, was developed by Brilla’s group and Oturan’s group (Brillas et al., 1996; Oturan et al., 2000). In this process, H2O2 is continuously generated in situ via 2-electron O2 reduction on the cathode (Eq. (1)), Fe2+ was added externally or regenerated on the cathode (Eq. (2)) (Sirés et al., 2014). ·OH (oxidation potential of 2.80 V vs. NHE) are continuously generated by the reaction between H2O2 and Fe2+ for organic contaminants degradation. The H2O2 concentration produced by the EF process range from 10 mg L−1 to 2% (Brillas et al., 2009).
| (1) |
| (2) |
In this review, we highlight our current understanding of factors that control the activity, selectivity, and stability of cathode materials that control the H2O2 production. Various cathode modification methods are systematically introduced and compared. We also review the influence of several operational parameters (potential/current, electrolyte, pH, temperature, oxygen, power mode) on H2O2 productivity. The reactors for H2O2 production are summarized. Finally, the application in water treatment and decontamination of aqueous and gaseous pollutants based on electrogenerated H2O2 are reviewed.
1.2. Mechanism of H2O2 generation from O2 electroreduction
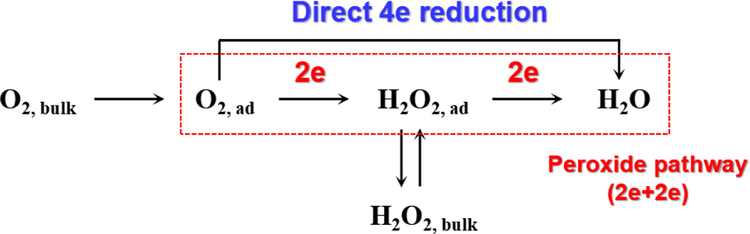
The ORR is a multi-electron reaction, which involves several elementary steps and reaction intermediates (Chai et al., 2017). Several schemes have been proposed to describe the reaction pathways. Among them, the one suggested by Wroblowa et al. (Wroblowa et al., 1976) is the mostly commonly used. As shown in Fig. 1, after adsorption at the cathode, the oxygen may be reduced via the direct 4-electron pathway or the series 2-electron pathway (peroxide pathway) (Katsounaros et al., 2012):
Fig. 1.
Pathway of oxygen reduction reaction.
The direct 4-electron pathway, where H2O is formed without formation of other intermediates:
| (3) |
The series 2-electron pathway, where H2O2 is formed as an intermediate (Eq. (1)).
H2O2 could undergo several reactions after its formation on the cathode. First, H2O2 could be further electroreduced to H2O via Eq. (4) on the cathode (Sánchez-Sánchez and Bard, 2009; Sirés et al., 2014), especially on porous cathodes (Zhou et al., 2018b). Second, H2O2 could be oxidized to O2 at the anode via HO2· as an intermediate (Eq. (5)) (Hickling and Wilson, 1951; Sirés et al., 2014). Moreover, H2O2 may disproportionate to O2 and H2O in a non-electrochemical bimolecular reaction (Eq. (6)) (Vasudevan and Oturan, 2014).
| (4) |
| (5-1) |
| (5-2) |
| (6) |
The difference between the 2-electron and 4-electron pathway is based on whether H2O2 can be detected as an intermediate or not. The 4-electron pathway is desirable in the field of fuel cells, metal-air batteries (Raj et al., 2016). Meanwhile, the 2-electron pathway to H2O2 is of particular interest for decentralized applications such as wastewater treatment (Sirés et al., 2014) and groundwater remediation (Zhang et al., 2017). Thus, significant efforts have focused on developing efficient electrocatalysts with high selectivity for the reduction of O2 to H2O2.
Currently, noble metals and their alloys (such as Pd (Fortunato et al., 2018), Pd-Au (Jirkovský et al., 2011; Freakley et al., 2015; Menegazzo et al., 2015; Pizzutilo et al., 2017), Pt-Hg (Siahrostami et al., 2013), Pd-Hg (Verdaguer-Casadevall et al., 2014), and Pt-Ni (Yang et al., 2004)) are the most efficient electrocatalysts. They exhibit small overpotential for O2 reduction as well as high H2O2 selectivity (up to ~98%) (Lu et al., 2018). However, their large-scale application could be restricted due to the scarcity of noble metals. Carbon materials, which is a key focus of this review, are earth-abundant, highly tunable, environmentally friendly, and electrochemically stable. They exhibit great promise as an alternative for H2O2 electrosynthesis. In recent years, research in the field of H2O2 electrogeneration by carbon materials covers a wide range of topics aimed at improving the activity, selectivity, and stability of catalysts, including heteroatom doping (O (Zhou et al., 2013b; Miao et al., 2014; Lu et al., 2018), N (Fellinger et al., 2012; Lee et al., 2012; Zhong et al., 2013; Hasché et al., 2016; Sun et al., 2018), F (Zhao et al., 2018a; Zhao et al., 2018b), B (Chen et al., 2018), S (Perazzolo et al., 2015; Jiang et al., 2016; Roldán et al., 2018), and P (Li et al., 2018)), material structure regulation (Park et al., 2014; Liu et al., 2015a; Liu et al., 2015b; Zhao et al., 2018b), optimization of reaction conditions (Qiang et al., 2002; Yang et al., 2018).
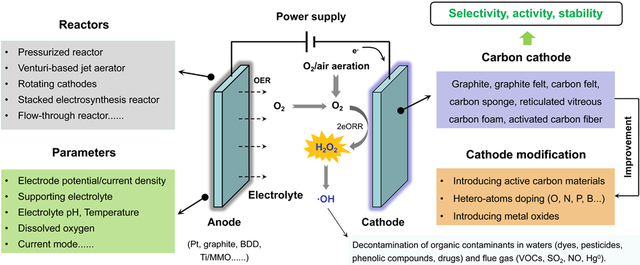
2. Cathodes and its modification
Cathode is the determining factor of H2O2 electrosynthesis. This section will specifically focus on carbon cathodes and their various modification methods. The modification methods are divided into the following (Fig. 2): (1) introducing other active carbon materials to carbon support, such as carbon nanotubes (CNTs) (Zarei et al., 2009; Khataee et al., 2011; Babaei-Sati and Basiri Parsa, 2017a; Flores et al., 2017), carbon black (Darvishi et al., 2013; Soltani et al., 2013; Yu et al., 2015), acetylene black (Sheng et al., 2011; Sheng et al., 2014), and graphene (Le et al., 2015a; Le et al., 2015b; Yang et al., 2017; Divyapriya et al., 2018; Kim et al., 2018); (2) hetero-atoms doping, such as O (Zhou et al., 2013b; Miao et al., 2014; Lu et al., 2018), N (Fellinger et al., 2012; Lee et al., 2012; Zhong et al., 2013; Hasché et al., 2016; Sun et al., 2018), F (Zhao et al., 2018a; Zhao et al., 2018b), P (Li et al., 2018), B (Chen et al., 2018), and S (Perazzolo et al., 2015; Jiang et al., 2016; Roldán et al., 2018); (3) depositing metal oxides to carbon support (Bonakdarpour et al., 2011; Carneiro et al., 2015; Aveiro et al., 2018; Pinheiro et al., 2018). Additionally, gas diffusion electrodes (GDEs) are widely recognized as high-performance cathode configurations for various gas (O2 (Luo et al., 2015), CO2 (Li et al., 2018), NH3 (Chen et al., 2017)) electroreduction, thus its utilization for O2 electroreduction will be reviewed.
Fig. 2.
The classification of modification approaches of carbon materials.
2.1. Commonly used carbon cathodes
The most frequently used carbon cathodes are commercially available materials, such as graphite (Da Pozzo et al., 2005), graphite felt (GF) (Zhou et al., 2013b; Zhou et al., 2014; Wang et al., 2015; Castañeda et al., 2017), carbon felt (CF) (Isarain-Chávez et al., 2010; Pérez et al., 2016; Pérez et al., 2018), reticulated vitreous carbon (RVC) foam (Li et al., 2014; Zhou et al., 2018b; Zhou et al., 2018d), and activated carbon fiber (ACF) (Wang et al., 2005; Wang et al., 2008; Lei et al., 2010; Wang et al., 2010).
Graphite has excellent electrical conductivity and chemical stability, it is relatively cheap and easy to get. However, its surface area is difficult to increase (Liu et al., 2005), and its activity for H2O2 electrogeneration is not satisfactory. Thus research efforts aimed to modify graphite electrodes (Khataee et al., 2017) or use them to support achieving higher H2O2 productivity (Khataee et al., 2011; Babaei-Sati and Basiri Parsa, 2017a).
As a typical three-dimensional (3-D) electrodes, GF has a high volumetric surface area (22,100–22,700 m2 m−3), an electrical conductivity (370.37 S m−1) (Chaudhuri and Lovley, 2003) close to that of metals, good chemical resistance and stability, good mechanical integrity, it is easy to manufacture and scale-up, and its cost is reasonable (Kim et al., 2007; Logan et al., 2007). In recent years, GF has been extensively used to electrogenerate H2O2 in the field of electrochemical advanced oxidation process (EAOP) for wastewater treatment and drinking water disinfection (Brillas et al., 2009). Additionally, GF is recognized as promising electrode materials in applications such as all-vanadium redox flow batteries (Gao et al., 2013; Zhang et al., 2013), fuel cells (Castañeda et al., 2017), microbial fuel cells (MFCs) (Kim et al., 2007).
Activated carbon fiber (ACF) is a potential carbon material with characteristics of adsorption, conductivity, and catalysis. It has been used for H2O2 electrogeneration in some reports (Wang et al., 2005; Wang et al., 2008). Moreover, in the application of wastewater treatment, electrosorption processes can be coupled with H2O2 electrogeneration and H2O2 activation for various organic contaminants removal in waters via adsorption and electrooxidation. Compared with the above materials, RVC foam is less frequently used for H2O2 electrosynthesis due to its large resistance and fragile nature (Zhou et al., 2011). However, due to its 3D structure and macro-pores, RVC foam has advantages over others especially in flow-through reactors, where low resistance of water flow is required (Drogui et al., 2001; Li et al., 2014; Zhou et al., 2018d).
2.2. Modification of carbon cathodes
2.2.1. Introduction of other active carbon materials
Recently, different carbon materials (in the form of powder or thin film) are introduced to assist the O2 2-electron electroreduction reaction, where commercially available carbon materials are usually used as support. These “foreign aid” materials include carbon black (Assumpção et al., 2011; Soltani et al., 2013; Yu et al., 2015a; Yu et al., 2015), acetylene black (Sheng et al., 2011; Sheng et al., 2014), graphene (Le et al., 2015a; Le et al., 2015b; Liu et al., 2016; Yang et al., 2017; Garcia-Rodriguez et al., 2018), carbon nanotubes (CNTs) (Zarei et al., 2009; Khataee et al., 2011; Babaei-Sati and Basiri Parsa, 2017a), and activated carbon (Zarei et al., 2009; Khataee et al., 2011).
Carbon black has been introduced to conventional carbon support to improve its performance on H2O2 production significantly. Using GF as support, Yu et al. (Yu et al., 2015) introduced carbon black and polytetrafluoroethylene (PTFE) mixture to GF, improving the H2O2 production by about 10.7 times at the optimum carbon black to PTFE mass ratio of 1:5. Linear sweeping voltammetry (LSV) results show that the presence of carbon black increases the catalytic activity towards O2 reduction and also higher conductivity. The BET surface area and pore volume increased from 1.565 m2 g−1 and 0.004 cm3 g−1 to 5.320 m2 g−1 and 0.087 cm3 g−1, respectively, a significant enhancement of H2O2 production. In another study (Yu et al., 2015a), carbon black and PTFE mixtures were deposited on carbon fiber, by using a novel dual gas diffusion electrodes, the accumulation of H2O2 reached 566 mg L−1 (production rate: 22.3 mg cm−2 h−1) in 0.05 M Na2SO4 electrolyte at a current density of 7.1 mA cm−2 and air flow rate of 500 mL min−1. Yang et al. and co-workers (Xu et al., 2017) fabricated gas diffusion electrodes using stainless wire substrate, graphite gas diffusion layer and carbon black (Vulcan X-72R) catalysis layer. The electrodes achieved a H2O2 production rate as high as 8.86 mg cm−2 h−1 with a low air flow rate of 50 mL min−1. Different from the work of Yu et al., carbon black is the only catalyst that is responsible for the high-performance H2O2 production, showing its great promise as a “foreign aid” material. The high H2O2 yield also originates from the formation of the 3-phase interface by using gas diffusion electrodes, which facilitate the O2 mass transfer.
As an active carbon material, acetylene black also has been used to modify the carbon material support. Sheng et al. (Sheng et al., 2014) fabricated a composite acetylene black-PTFE electrode by using a sheet active core and a damp proof coating, which sets up a hydrophile-hydrophobe balance on the electrode surface. Different than most other work whose primary goal is to increase the surface hydrophilicity and enhance the mass transfer between electrolyte and electrode surface, this work balanced the hydrophilicity and hydrophobicity. This work achieved an accumulation of H2O2 concentration of 677.5 mg L−1 with a H2O2 production rate of 54.2 mg cm−2 h−1, which is among the highest H2O2 production rates.
Graphene, a 2-dimensional (2D) one-atom-thick sheet composed of sp2 carbon atoms arranged in a honeycomb structure, has received extensive attention due to its remarkable electrical, optical, physical, thermal, high specific surface area and mechanical properties (Le et al., 2015b; Yang et al., 2017).
Recently, many authors are exploiting the potential use of graphene decorated materials for H2O2 electrogeneration (Le et al., 2015b; Divyapriya et al., 2017; Yang et al., 2017; Divyapriya et al., 2018; Garcia-Rodriguez et al., 2018). Yang et al. (Yang et al., 2017) developed a GF cathode coated with electrochemically exfoliated graphene and carbon black. Resulted from the accelerated electron transfer rate, the O2 electroreduction was facilitated, and the electrode presented a high H2O2 generation rate of 7.7 mg cm−2 h−1. Using CF as a substrate, Le et al. (Le et al., 2015b) set up a new cathode by electrochemically depositing reduced graphene oxide (rGO) on the surface of CF. The authors found the rGO modified cathode exhibited improved electrochemical properties such as an increase of the redox current and a decrease of the charge transfer resistance compared with the raw CF. Most recently, Divyapriya et al. (Divyapriya et al., 2018) employed disposed liquid crystal display (LCD) glass as a substrate, deposited thin graphene on it by reducing graphene oxide (GO) electrochemically without binder/linker. A H2O2 production rate of 0.23 mg cm−2 h−1 under air aeration, pH 3.5, potential of −1.5 V (vs. Ag/AgCl) was obtained. Liu et al. fabricated a N-doped graphene@carbon nanotube composite material (N-G@CNT) and found that its ORR activity towards H2O2 production was significantly improved (Liu et al., 2016). What is worth mentioning is that graphene is often used in the gas diffusion electrode. Zhang et al. prepared a graphene@graphite-based gas diffusion electrode (G-GDE) with high conductivity and remarkable activity, it shows excellent corrosion resistance and reusability when used in EF process for organic pollutants degradation (Zhang et al., 2018).
Carbon nanotubes (CNTs) is another active material that receives special attention. Khataee et al. and co-workers (Khataee et al., 2011) compared the activity of three cathode materials (i.e., bare graphite, activated carbon/graphite, and CNTs/graphite) on H2O2 production. The amount of H2O2 using CNTs/graphite fed with air was nearly 3 times higher than that of AC/graphite and 7 times higher than that of bare graphite. Zarei et al. (Zarei et al., 2009) fabricated an activated carbon/PTFE/carbon paper and CNTs/PTFE/carbon paper electrodes and obtained an H2O2 production rate of 2.00 mg cm−2 h−1 and 4.85 mg cm−2 h−1, respectively. Babaei-Sati et al. (Babaei-Sati and Basiri Parsa, 2017a) deposited polypyrrole/multi-walled carbon nanotube (MWCNT) onto graphite and investigated the maximum addition of MWCNT for H2O2 production. Results showed that 2.5% w/w MWCNT had the highest electrocatalytic activity. Activated carbon was also used as active carbon materials. However, its performance is inferior to other active materials discussed above.
2.2.2. Doping with hetero-atoms
One of the merits of carbon materials is that its structure is highly tunable by doping with various heteroatoms, such as O, N, F, B, and P. It is always an active topic in the field of carbon materials for various applications (i.e., fuel cells (Qu et al., 2010; Zhong et al., 2013), microbial fuel cells (Feng et al., 2011), Li-ion batteries (Qie et al., 2012), super-capacitors (Si et al., 2013), pollutants adsorption (Shin et al., 2011), capacitive deionization (Liu et al., 2015)). As catalysts in 2-electron O2 electroreduction for H2O2 production, carbon materials have been doped with various heteroatoms aimed at improving the activity and selectivity on H2O2 production. Generally, the incorporation of heteroatom into carbon framework changes the electronic structure of carbon materials. The electrocatalytic activity in the doped carbon materials originates from the electronic modulation of the prevalent conjugated sp2-sp2 linkages or the delocalization of π-orbital electrons in their graphitic domains due to the presence of heteroatoms (Aveiro et al., 2018). The heteroatom dopants change the localized electronic structure of the carbon lattice and generate partial positively and negatively charged groups without obviously influencing the conductive properties of materials (Daems et al., 2014; Asefa, 2016). Here, carbon materials doped with various heteroatoms for efficient and selective H2O2 production via 2-electron O2 electroreduction are reviewed.
O doping, which forms various oxygen-containing functional groups (OGs) on carbon surface, is the most facile and low-cost approach to achieve higher electrical conductivity and electrocatalytic activity (Kim et al., 2018; Lu et al., 2018; Pan et al., 2018). Methods that introduce OGs to carbon surface can be generally divided into 3 types, that is oxidation by (1) concentrated strong oxidants (H2O2 (He et al., 2017), HNO3 (Lu et al., 2018), H2SO4 (Miao et al., 2014)), (2) hydroxyl radicals (Zhang et al., 2009; Gao et al., 2013)), and (3) electrochemical method (Barton et al., 1997; Zhou et al., 2013b; Miao et al., 2014). All these methods are effective to introduce OGs to carbon surface while the electrochemical oxidation is recognized as the most promising method since it is practical, controllable and environmentally friendly.
Lu et al. (Lu et al., 2018) demonstrated that oxidation could be a facile and general approach for carbon catalysts to significantly improve both the selectivity and activity for H2O2 production from O2 electroreduction. The HNO3-oxidized CNT catalysts can efficiently produce around 1975 mg L−1 H2O2 within 30 min with a high selectivity of >90%. Suggested by density functional theory calculations, the carbon atoms adjacent to OGs such as −COOH and C-O-C are the active sites for the 2-electron pathway. In some authors’ work, GF was electrochemically modified for enhanced H2O2 production. Zhou et al. (Zhou et al., 2013b) anodically oxidized the GF electrodes and found the modified electrodes exhibited much higher electrocatalytic activity towards O2 electroreduction reaction than the unmodified one. The surface oxygen in the form of acidic groups (i.e., −COOH, −COH, −COO-, R-OH, >C=O) can improve the hydrophilicity of electrode surface and also behave as surface-active sites for 2-electron O2 electroreduction, thus resulting in a H2O2 production 2.7 times higher than the pristine one. In Miao et al.’s work (Miao et al., 2014), GF was electrochemically modified by cyclical polarization in different concentration of H2SO4 solution in the range of 0.0 V to +2.0 V (vs. SCE) at a rate of 10 mV s−1. A significant increase of carboxyl groups on the GF surface was observed and the H2O2 production significantly increased by 6 times in a static cell compared with the original electrode. Due to its simplicity, some authors are continuously investigating the applicability of O-doping on other carbon materials (Berenguer et al., 2009; Zhang et al., 2009; Tang et al., 2011; Yoon et al., 2011; Thostenson et al., 2017) and the performance optimization (Zhang et al., 2013; Tabti et al., 2014). However, what should be noted is that under negative polarization (i.e., used as a cathode), the OGs could be partially electroreduced, resulting in a less amount of active sites for 2-electron O2 electroreduction (Cheng and Teng, 2003). In some works, the stability of oxidized electrodes for H2O2 production was tested (Zhou et al., 2013b; Lu et al., 2018). However, the cycles or operation time are not long enough. Thus, investigation on the stability and possible strategies to improve the long-term stability of O-doped carbon electrodes is necessary.
Compared with O-doping, N-doped carbon materials receives even more attention aimed at 4-electron O2 electroreduction pathway for H2O generation (Qu et al., 2010; Geng et al., 2011; Zhao et al., 2015; Guo et al., 2016; Liu et al., 2016) and most recently, 2-electron O2 electroreduction pathway for H2O2 production (Fellinger et al., 2012; Yang et al., 2017; Iglesias et al., 2018). As Fellinger et al. (Fellinger et al., 2012) suggested, the number of transferred electrons during ORR was not always four. Thus it is possible to find a selective and efficient catalyst for a pure 2-electron process towards H2O2 production on N-containing conductive carbons. The authors synthesized N-doped carbon using ionic liquid N-butyl-3-methylpyridinium dicyanamide (BMP-dca) as a direct precursor. The catalysts achieved a H2O2 production rate of 0.17 g (g[cat] h)−1 with a faradaic current efficiency of 65.15% under a working voltage of 0.1 V (vs. RHE), O2 flow rate of 226 mL min−1, catalyst loading of ~325 μg cmgeo−2 in 0.1 M HClO4 medium. Suggested by the authors, the pyrrolic nitrogen sites within the carbon, might contribute to the activity of the 2-electron pathway (Fellinger et al., 2012). Zhu et al. (Zhu et al., 2018) fabricated a highly porous nitro-enriched graphitic carbon (NGC) from melamine. The NGC cathode achieved a H2O2 production rate of 0.07 mg cm−2 h−1. Characterized by several techniques, the authors proposed that the pyrrolic N structure, together with the improved electro-conductivity contributed to the H2O2 production. Most recently, Sun et al. (Sun et al., 2018) explored a number of nitrogen-doped mesoporous carbon catalysts on H2O2 production. They found nitrogen doping could sharply boost the activity and selectivity of catalysts, obtained a previously unachieved H2O2 selectivity of ~95–98% in acidic solution. In alkaline solution, the authors got a H2O2 production rate as high as 561.7 mM (gcat h)−1 with faradaic selectivity above 70%.
F doping, another method to modify the electronic structure of carbon materials by breaking the electroneutrality of carbon lattice and induce charge redistribution, draws particular attention recently. Pioneering work was done by Zhao et al. and co-workers (Zhao et al., 2018a; Zhao et al., 2018b). The F-doped hierarchically porous carbon was synthesized by using aluminum-based MOF as a precursor. It exhibited H2O2 selectivity of 97.5–83.0% and the H2O2 production rate of 112.6–792.6 mM (gcat h)−1 in the potential range of −0.1 to −0.6 V (vs. RHE) at pH 1, the corresponding current efficiency is as high as 93.6–81.6%. The high yield and selectivity resulted from the introduction of CF2,3 atom into the carbon lattice, which facilitates the adsorption of O2 and the subsequent desorption of OOH intermediates (Zhao et al., 2018b). In another work (Zhao et al., 2018a), the author proposed that F-doped carbon materials could induce charges polarization and change Fermi level, thus tune the electron transfer between electrode and species in the electrolyte. This is an interesting design that by using the F-doped porous carbon, both H2O2 production and Fe2+ electro-regeneration were achieved, resulting in a high-performance Electro-Fenton process for various organic contaminants degradation.
There are limited reports on other heteroatom-doped carbon materials for H2O2 production, in which some are focused on co-doping by two or more heteroatoms (Li et al., 2018). For example, Li et al. (Li et al., 2018) synthesized pure N mono- and N/P dual-doped cotton-stalk-derived activated carbon fibers and used as cathode in Electro-Fenton process. Results show the N/P dual-doped catalyst demonstrate the highest electrochemical activity on H2O2 generation and activation, which are in agreement with the enhancement effect of other heteroatoms discussed above. Most recently, Chen et al. (Chen et al., 2018) produced several boron-carbon-nitrogen (BCN) materials and studied their electrochemical performance. They revealed that the existence of h-BN domains in the graphitic structures yield higher activity and selectivity towards 2-electron O2 electroreduction than structures with individual N or B doping.
2.2.3. Introducing metal oxides
Another strategy is based on the utilization of non-noble metals catalysts, including metal oxides, metal-doped conductive polymers (Asefa, 2016; Nie et al., 2015; Jaouen et al., 2011), and metal-phthalocyanines (Jaouen et al., 2011; Nie et al., 2015; Asefa, 2016;) either as such or supported. Among these, metal oxides especially those with high abundance, low cost, low environmental impact, and good electrocatalytic activities, receive special attention. Additionally, such materials decorated carbon materials can be easily converted into gas diffusion electrodes (GDEs), which can generate H2O2 at a higher rate than plate electrodes, and are very attractive for industrial and practical applications (Antonin et al., 2013; Antonin et al., 2017).
Aveiro et al. (Aveiro et al., 2018) synthesized hybrid materials composed of MnO2 nanoflowers supported on Vulcan XC-72 carbon with controlled number of oxygen vacancies. The authors studied the relationship between the activity of MnO2/C on H2O2 production and the MnO2 loading. Using MnO2-/C 3% w/w as an electrocatalyst, up to 391 mg L−1 H2O2 was obtained at the potential of −1.1 V (vs. Ag/AgCl), 5-fold increase than the Vulcan XC-72 carbon itself. Carneiro et al. (Carneiro et al., 2015) prepared Nb2O5 decorated reduced graphene oxide (rGO) nanocomposites for H2O2 electrogeneration. Compared with unmodified rGO, the Nb2O5-rGO electrode gave higher H2O2 yields either in acidic (70.5% vs. 85.3%) or alkaline (63.4% vs. 74.9%) solutions. In another work, the authors investigated the Ta2O5 nanoparticles modified carbon black (Printex 6L carbon) as electrocatalysts for H2O2 electrogeneration (Carneiro et al., 2016a). Results show a higher H2O2 yield and current efficiency was obtained by Ta2O5/C catalyst than carbon black. Most recently, more research are emerging using non-noble metal oxides decorated carbon materials for H2O2 electrogeneration, such as CeO2 (Pinheiro et al., 2018), WO2.72 (Paz et al., 2018), and Fe3O4 (Barros et al., 2015), cobalt oxides (Bonakdarpour et al., 2011; Campos et al., 2013), and vanadium oxides (Moraes et al., 2014). A schematic illustration of the aforementioned modification methods of carbon cathodes and the corresponding mechanism is shown in Fig. SM-1.
2.3. Gas diffusion electrodes (GDEs)
Gas diffusion electrodes (GDEs) are one cathode configuration that deserves a special introduction. It has been widely applied to enhance gas transport to the electrochemical interface, such as that in fuel cells, CO2 electrocatalysis (Li et al., 2018), and N2 electrocatalysis (S. Chen et al., 2017). Actually, some of the works we reviewed before used GDEs as their cathode configuration to achieve high H2O2 production rate.
Conventionally, cathodes are submerged in the electrolyte. Thus, the process efficiency is controlled by the solubility of the molecular O2 in the electrolyte, which is only ~1 mM at 1 atmosphere. For GDEs, a completely different mechanism through which O2 reaches the electrode surface is applied. GDEs are composed of a gas diffusion layer and a catalysis layer. During the process of H2O2 production, the gas diffusion layer faces to air or oxygen while the catalysis layer faces to electrolyte (Sheng et al., 2011; Sheng et al., 2014). The gas diffuses through micropores of the diffusion layer to the catalysis layer and reacts with H+ from electrolyte and electron from catalysis layer to form H2O2 molecules. The H2O2 molecules are then transported to the bulk electrolyte. The key advantages of GDEs is that a three-phase interface could be generated thus the low solubility was no longer a problem for the continuous O2 supply. Fig. 3 is a schematic explanation of the difference between GDEs and conventionally submerged cathodes. Considering the high performance on H2O2 production with high current efficiency, as shown in Table 1, more and more works are using GDEs with various electrocatalysts for H2O2 production. In Table 1, H2O2 production rate, corresponding current efficiency by various cathodes are also compared.
Fig. 3.
Different mechanism on H2O2 production via O2 2-electron electroreduction on gas diffusion electrodes (GDEs) and conventionally submerged electrodes
Table 1.
H2O2 production rate and current efficiency in literature.
| Cathode | pH | Current density (A m−2) | Time (min) | O2 flow rate (L min−1) | [H2O2] (mg cm−2 h−1) | Current efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Commonly used carbon materials | |||||||
| Graphite | 3 | 600 | 150 | Air aeration | 0.0087 | - | (Khataee et al., 2017) |
| Graphite | Na2SO4/NaHSO4 buffer | −0.9 V (vs. SCE) | 500 | 0.13 | 0.24 | 41–62 | (Da Pozzo et al., 2005) |
| Graphite felt | 3 | 132 | 300 | 0.14 | 0.11 | - | (Zarei et al., 2009) |
| Graphite felt | 3 | 50 | 60 | 0 | 0.58 | 33–56 | (Yu et al., 2014) |
| Carbon felt | 3 | 161 | 180 | 0.1 | 0.62 | - | (Özcan et al., 2008b) |
| RVC foam | 7 | 125 | 40 | 0 | 0.67 | 7 | (Zhou et al., 2018b) |
| RVC foam | 2 | 167 | 70 | 0 | 0.25 | 4 | (Zhou et al., 2018d) |
| Activated carbon fiber | 3 | 180 | 180 | 0.1 | 0.55 | - | (Wang et al., 2005) |
| Introduction of other active carbon materials | |||||||
| Graphene/Carbon black/PTFE/GF | 7 | −0.9 V (vs. SCE) | 120 | 0.7 (air aeration) | 7.7 | 42–92 | (Yang et al., 2017) |
| Activated carbon/PTFE/GF | 3 | 200 | 300 | 0.14 | 2.00 | 16–45 | (Zarei et al., 2009) |
| CNTs/PTFE/GF | 3 | 200 | 300 | 0.14 | 4.85 | 40–61 | (Zarei et al., 2009) |
| CNTs/PTFE/Graphite | 3 | 25 | 180 | 2.5 (air aeration) | 0.04 | - | (Khataee et al., 2011) |
| Carbon black/PTFE/GF | 7 | 50 | 60 | 0 | 22.3 | 68–98 | (Yu et al., 2015) |
| Acetylene black/PTFE | 3 | 200 | 150 | 2.0 | 6.0 | 92.7 | (Sheng et al., 2011) |
| Hydrazine hydrate modified GF | 6.4 | −0.65 V (vs. SCE) | 120 | 0.4 | 0.96 | 70–85 | (Zhou et al., 2013a) |
| Polypyrrole/MWCNT/Graphite a | 3 | −0.55 V (vs. SCE) | 10 | 0.3 (air aeration) | 0.34 | - | (Babaei-Sati and Basiri Parsa, 2017a) |
| Polyaniline/MWCNT/SS | 2 | −0.6 V (vs. SCE) | 60 | 0.3 (air aeration) | 0.19 | 41–85 | (Babaei-Sati and Basiri Parsa, 2017b) |
| Doping with hetero-atoms | |||||||
| Electrochemically oxidized GF | 7 | −0.4 V (vs. SCE) | 80 | 0.04 | 0.22 | - | (Miao et al., 2014) |
| Electrochemically oxidized GF | 6.4 | −0.65 V (vs. SCE) | 120 | 0.4 | 1.56 | 65–90 | (Zhou et al., 2013b) |
| Electrochemically oxidized RVC foam | 7 | 125 | 50 | 0 | 0.33 | - | (Zhou et al., 2018a) |
| Biomass derived N/C catalyst | 13 | −0.6746 V (vs. RHE) | 90 | Pre-saturated with O2 | 1.69 g (g[cat] h)−1 | 40 | (Yang et al., 2017) |
| N-doped meso-porous carbon | 0.1 M HClO4 | −0.1 V (vs. RHE) | 344 (5.74 h) | 0.226 | 0.17 g (g[cat] h)−1 | 65 | (Fellinger et al., 2012) |
| N-enriched graphitic carbon | 6 | 10 | 60 | - | 0.07 | - | (Zhu et al., 2018) |
| N-functionalized CNT cathode | 3 | −0.85 V (vs. RHE) | 90 | 0.4 | 2.12 | 63 | (Zhang et al., 2008) |
| N/P dual-doped activated carbon fiber | 3 | 15 V cell voltage | 150 | 0.08 (air aeration) | 0.41 | - | (Li et al., 2018) |
| F-doped hierarchically porous carbon | 1 | −0.5 V (vs. SCE) | 180 | O2 saturated | 34.9 | 82–94 | (Zhao et al., 2018b) |
| Introducing metal oxides | |||||||
| V2O5/Carbon | 0.1 M NaOH | −1.5 V (vs. Ag/AgCl) | 120 | O2 pressure: 0.2 Bar | 620 mg L−1 b | - | (Moraes et al., 2014) |
| MnO2/Carbon | 0.1 M H2SO4 | −1.1 V (vs. Ag/AgCl) | 120 | O2 pressure: 0.2 Bar | 32.58 | - | (Aveiro et al., 2018) |
| WO2.72/Carbon | 0.1 M H2SO4 | −0.7 V (vs. Ag/AgCl) | 120 | O2 pressure: 0.2 Bar | 27.94 | 41.9 | (Paz et al., 2018) |
| CeO2/Carbon | 0.1 M NaOH | −2.3 V (vs. Hg/HgO) | 120 | O2 pressure: 0.2 Bar | 871 mg L−1 c | - | (Assumpão et al., 2012) |
| Gas diffusion electrodes | |||||||
| Activated carbon/PTFE/Carbon paper based GDEs | 3 | 204 | 300 | 0.14 | 1.94 | - | (Salari et al., 2009) |
| Carbon/PTFE based GDEs | 3 | 300 | 60 | 1.2 (air aeration) | 12.85 | 50 | (Panizza and Cerisola, 2008) |
| Tert-butyl-anthraquinone modified carbon black based GDEs | 0.1 M H2SO4 | −1.0 V (vs. SCE) | 90 | O2 pressure: 0.2 Bar | 7.08 | - | (Valim et al., 2013) |
| Graphene/PTFE/Carbon cloth based GDEs | 3 | 400 | 180 | 0.2 | 9.43 | 35 | (Garcia-Rodriguez et al., 2018) |
| Carbon black/PTFE/Carbon fiber based GDEs | 7 | 357 | 180 | 0.1 (air aeration) | 11.2 | 45–84 | (Yu et al., 2015a) |
| Carbon black/PTFE/Carbon fiber based GDEs | 7 | 357 | 180 | 0.5 (air aeration) | 12.2 | 51–88 | (Yu et al., 2015a) |
| Ta2O5/Carbon black based GDEs | 2 | −1.0 V (vs. Ag/AgCl) | 120 | O2 pressure: 0.2 Bar | 1.64 | 83.2 | (Carneiro et al., 2016b) |
| N-doped grapheme@CNT based GDEs | 3 | −0.5 V (vs. SCE) | 120 | 0.45 | 0.26 | - | (Liu et al., 2016) |
| CoS2 based GDEs | 3 | 1000 | 60 | 0.5 | 51.0 | 82 | (Ridruejo et al., 2018) |
| Graphene@Graphite based GDEs | 3 | 200 | 60 | 0.06 | 2.0 | - | (Zhang et al., 2018) |
| PTFE/Graphite based GDEs | 3 | −0.55 V (vs. SCE) | 30 | 0.4 | 4.5 | 70–78 | (Zhou et al., 2007) |
| Mesoporous carbon based GDEs | 3 | 1500 | 240 | Air aeration (1 psi) | 14.2 | 15–35 | (Garza-Campos et al., 2018) |
| Carbon black/MWCNT/PTFE based GDEs | 7 | 35 | 180 | 0.09 | 0.98 | 65.1 | (Chen et al., 2017) |
| Activated carbon based GDEs | 3 | 300 mA, 1 g AC | 360 | Air aeration | 0.009 g (g[cat] h)−1 | - | (Bañuelos et al., 2014) |
| Carbon black/PTFE based GDEs | 1 M KOH | −1.1 V (vs. Ag/AgCl) | 90 | O2 pressure: 0.2 Bar | 44.93 | 33–61 | (Willyam R. P. Barros et al., 2015) |
| Carbon black/Graphite/PTFE/stainless wire based GDEs | 7 | Cell voltage: 4 V | 120 | 0.05 (air aeration) | 8.86 | 84 | (Xu et al., 2017) |
| Others | |||||||
| Polypyrrole/anthraquinonedisulph onate film modified graphite | 3 | −0.65 V (vs. SCE) | 120 | 0.33 | 1.00 | 64–73 | (Zhang et al., 2008) |
| Plasma-treated graphite | 3 | 600 | 150 | Air aeration | 0.032 | - | (Khataee et al., 2017) |
| Lignin/polypyrrole cathode | 3 | −0.5 V (vs. SCE) | 360 | 0.25 | 1.67 | 96 (after 180 min operation) | (Huang et al., 2018) |
| Graphene film/Conductive LCD matrix | 3.5 | −1.5 V (vs. Ag/AgCl) | 180 | 1 (air aeration) | 0.23 | 59 | (Divyapriya et al., 2018) |
| Quinone functionalized grapheme/Fe3O4 cathode | 3 | −1.2 V (vs. Ag/AgCl) | 180 | O2 saturated | 0.30 | - | (Divyapriya et al., 2017) |
Note: a-MWCNT means multi-wall carbon nanotubes, b and c-the area of electrodes and volume of electrolytes are not provided in the literature.
However, GDEs has disadvantages such as hydrophobicity decrease after extended use (Li et al., 2018), costly commercial cathode materials (Pérez et al., 2018), and modest mechanical resistance (Pérez et al., 2018). These problems should be addressed in future research before its commercialization.
2.4. A summary
3. Stability of electrodes
Considerable success has been achieved in enhancing the activity and selectivity of different carbon-based cathodes on H2O2 production via 2-electron ORR. However, considering the fact that systems that based on H2O2 production (such as EF process) are developed for long-term and large-scale application, the cycling stability plays an equal or even more significant role in determining the performance of cathodes on H2O2 production. The decay of the performance on H2O2 production would result in a loss of the precious active materials and an increase in the capital cost for maintenance (Jiang et al., 2018). Thus, the long-term stability of electrodes deserves special attention.
Previous works usually include stability test of cathode materials. For example, Lu et al. (Lu et al., 2018) achieved high activity and selectivity upon H2O2 production by introducing oxygen functional groups to carbon nanotubes (CNTs). They tested the stability of oxidized CNTs by chronoamperometric technique and observed negligible changes in current response after 10 h. Zhou et al. (Zhou et al., 2013b) evaluated the stability of anodically modified GF electrode for p-nitrophenol degradation. The TOC removal efficiency decreased within 15% over 10-times continuous runs. Zhao et al. (Zhao et al., 2018b) prepared fluorine-doped hierarchically porous carbon, which achieved good H2O2 selectivity of 97.5–83.0% and the H2O2 production rate could reach 112.6–792.6 mM (gcat h)−1 over the potential range of 0.2 V to −0.3 V vs. RHE. The durability of catalyst through 8 successive cycles (each lasting for 3 h) for H2O2 electrosynthesis was tested, the H2O2 concentration was almost unchanged compared with the fresh catalyst. Chen et al. (S. Chen et al., 2018) designed boron and nitrogen co-doped carbon materials (BCN). The synthesized structures yield high activity and selectivity toward 2-electron ORR to H2O2. More importantly, the BCN materials exhibited stable stability over 50 h. In the work of Sun et al. (Sun et al., 2018), nitrogen-doped mesoporous carbon could achieve ~95–98% on H2O2 production in acidic solution. The catalyst exhibits excellent stability during the continuous electrochemical H2O2 production within 6 h in acid solution. However, the operation time is not enough for practical applications.
From our perspectives, 5–10 cycles or duration less than 10 h operated in many research are not convincing to draw the conclusion that the cathode materials are stable upon H2O2 electrosynthesis. The stability of carbon-based catalysts is determined by the stability of active sites on 2-electron ORR. Various sites on carbon matrix are active on 2-electron ORR, such as oxygen functional groups (especially −COOH and C-O-C (Lu et al., 2018)), nitrogen-containing sites (especially pyrrolic N (Fellinger et al., 2012; Yang et al., 2017)), boron-containing sites (Chen et al., 2018), and fluorine-containing sites (Zhao et al., 2018b). Based on the literature, N,B,F-containing sites are relatively stable during the electrochemical process. However, oxygen functional groups are not stable during prolonged operation. Cheng and co-workers (Cheng and Teng, 2003) observed the reduction of carboxyl groups upon negative polarization, which suggests that O-doped carbon materials could exhibit gradually decreased activity towards H2O2 production. Recently, the results were also confirmed by Wang et al., where the authors found the obvious relationship between decreased H2O2 production and decreased carboxyl groups on graphite felt cathode (Wang et al., 2019).
4. Reactors for H2O2 synthesis
The performance of cathode is one of the key factors affecting the H2O2 production. Thus many studies focused on the design, modification, and optimization of the cathode. In comparison, there are not so many reports on reactor design, which is important for the scale-up and application of H2O2 electrosynthesis. In this part, we summarize some recent works that focus on the design of reactors. These reactors are designed for high-performance H2O2 production with lower energy consumption, or aimed at a specific application.
Mass transport of oxygen to the cathode vicinity is one crucial factor to consider when design reactors. Thus, some reactors such as pressurized (Pérez et al., 2018), venturi-based jet aerator ones (Pérez et al., 2016), reactor equipped with rotating cathodes (Yu et al., 2014), and stacked electrosynthesis reactor (Lu et al., 2017) were developed. Perez et al. (Pérez et al., 2018) studied the effect of air pressure on the EF process and found that the removal of maleic acid was dramatically accelerated by using pressurized air. An innovative venturi-based jet aerator was also developed by Perez and co-workers (Fig. 4a) (Pérez et al., 2016). The aerator stands as an effective and promising oxygen supply to CF cathode for H2O2 production. To improve the oxygen delivery to the cathode, Yu et al. (Yu et al., 2014) developed a rotating disks reactor (Fig. 4b) using two round GF disks cathodes arranged in parallel. The design resulted in the efficient H2O2 production and methyl orange degradation without external oxygen aeration. As Fig. 4c shows, to enhance the H2O2 generation rate and reduce energy consumption, Lu et al. (Lu et al., 2017) developed a novel stacked electrosynthesis reactor (SER). The SER was composed with different electrode pairs in various spacing distances as gas diffusion cathode, and Ti plate coated with IrO2 and Ta2O5 as anode. The maximum H2O2 production rate in the SER could reach 1929 ± 51 mg L−1 min−1. Most recently, Perez et al. (Pérez et al., 2018) invented a filter-press flow cell which uses GF as a gas-diffusion cathode. In this design, cheap and unmodified GF was positioned on top of carbon cloth as an air-diffusion cathode and was fitted into an undivided filter-press cell. Optimal conditions achieved at E=−0.3 V (vs. SHE) yielded 100.4 mg L−1 H2O2 with efficiency close to 100% and low energy consumption.
Fig. 4.
Various reactors reported in literature for H2O2 production: (a) Venturi-based jet aerator ones (Adapted with permission (Pérez et al., 2016), Copyright 2016, Elsevier), (b) reactor equipped with rotating cathodes (Adapted with permission (Yu et al., 2014), Copyright 2014, American Chemical Society), (c) stacked electrosynthesis reactor (Adapted with permission (Lu et al., 2017), Copyright 2017, Elsevier).
In our previous work, a 3-electrode flow-through electrochemical cell was designed to develop localized acidic conditions (Zhou et al., 2018d), coupled with simultaneous formation and utilization of O2 to enhance H2O2 formation. As shown in Fig. 5a, the O2 generated on anode could be transferred to cathode vicinity, and a localized pH of 2.75 ± 0.25 is developed to support effective O2 reduction.
Fig. 5.
Various reactors reported in literature for H2O2 production and contaminants degradation:(a) Carbon nanotube membrane stack filter (Adapted with permission (Gao et al., 2015). Copyright 2015, American Chemical Society), (b) 3-electrode flow-through reactor (Adapted with permission (Zhou et al., 2018d). Copyright 2018, Elsevier), and (c) vertical-flow reactor (Adapted with permission (Ren et al., 2016). Copyright 2016, Elsevier).
Some other works also consider the reactor design for pollutants degradation. Gao et al. (Gao et al., 2015) designed a carbon nanotube (CNT) membrane stack for the flow-through sequential regenerative Electro-Fenton process (Fig. 5b). The CNT membrane stack consisted of 4 layers, where the CNT network cathode for O2 reduction to H2O2, a CNT-COOFe2+ cathode to activate H2O2 to ·OH and regenerate Fe2+ in situ, a porous PVDF/PTFE insulating separator, and a CNT filter anode for remaining oxidation intermediates. Ren et al. (Ren et al., 2016) suggested that most Electro-Fenton reactors were either in intermittent flow in a single cell or a continuous parallel-flow mode, these operations cause a low space-time treatment efficiency and mass transfer limitation. Thus, they designed a vertical-flow reactor to accelerate the reaction rate and enhance the treatment efficiency (Fig. 5c).
5. Parameters affecting the H2O2 yield
5.1. Effect of electrode potential or current intensity
Electrosynthesis of H2O2 can be operated at potentiostatic condition where the electrode is kept at a constant potential or galvanostatic conditions where the current is kept constant. Most of the experimental works in laboratory use potentiostatic condition, while the galvanostatic condition is more suitable for applicative purposes since various reference electrodes are often avoided in commercial reactors to decrease the running costs (Ma et al., 2018). Electrode potential or current can be easily controlled. Thus their influence on H2O2 yield was extensively investigated.
The dissolved oxygen is electroreduced to H2O2 at the cathode via Eq. (1). However, two parasitic reactions occur simultaneously at the cathode. (1) the electroreduction of H2O2 to H2O at the cathode-electrolyte interface, especially at porous cathodes (Zhou et al., 2018b), and (2) the H2 evolution reaction (HER) via Eq. (7) (Qiang et al., 2003).
| (7) |
Qiang et al. investigated the cathodic potential (Ec) on H2O2 production in the range from 0.2–0.9 V (vs. SCE). Results indicated that in the range of 0.2–0.5 V, the H2O2 concentration increases linearly with reaction time. At −Ec > 0.5V, the H2O2 concentration decrease notably with reaction time. It implies that high −Ec stresses the side reactions (i.e., H2O2 electroreduction, HER) (Qiang et al., 2002). In Ma and co-authors’ work, the curve H2O2 concentration vs. current density shows a maximum for 5.75 mA cm−2, which corresponds to a H2O2 yield of 4.2 mM and to a working potential of about −0.9 V (vs. SCE). Under lower values of current density (i.e., less negative values of the working cathodic potential), the O2 reduction occurs under the kinetic control of the charge transfer or a mixed kinetic regime. Thus the increase of current density induce an increase on H2O2 yield. However, under higher current density (and of the working potential), the O2 reduction does not benefit from the larger amount of charge passed because the process is kinetically limited by the mass transfer of oxygen to the cathode surface (Xia et al., 2017; Ma et al., 2018). Moreover, the higher potential favors the H2O2 electroreduction to H2O (Eq. (4)). Khataee et al. studied the H2O2 production with respect to the applied current in the range of 100~400 mA. They found 300 mA induced the maximum H2O2 production and higher current of 400 mA decelerated the process. The authors explained that it was caused by the further electroreduction of H2O2, acceleration of HER, as well as anodic decomposition of H2O2 (Khataee et al., 2017).
5.2. Effect of the surface area of cathode
H2O2 is electrogenerated on the cathode surface, thus increasing the cathode surface area is a convenient way to raise the limiting current, and consequently, the H2O2 production. Usually, authors use cathodes with a constant area to test the electrocatalytic activity of cathode materials or effect of various parameters on H2O2 production and current efficiency. According to Ma et al. (Ma et al., 2018), higher H2O2 concentrations could be obtained using a high ratio between the cathode area and the anode one. It is because higher cathode surfaces favor the O2 cathodic reduction while low surfaces of the anode reduce the extent of H2O2 anodic oxidation.
5.3. Effect of supporting electrolyte
In an electrochemical process, the conductivity of aqueous solution affects the voltage, current efficiency, and the consumption of electrical energy (Daghrir et al., 2012). From the aspect of electrochemical reaction, the conductivity of electrolyte should be high to ensure good ion transfer in the aqueous medium. If the conductivity is too low, the resistance could be high, and consequently, a higher applied cell voltage is required. However, from the aspect of the application, low concentration of salts is favorable because it is a source of secondary pollution (Martínez-Huitle et al., 2015).
Both the type of electrolytes and its concentration affect the 2-electron ORR for H2O2 production. In lab-scale studies, sodium chloride (NaCl) and sodium sulfate (Na2SO4) are the two electrolytes most often used (Qiang et al., 2003; Zhou et al., 2013b; Sirés et al., 2014; Ma et al., 2016). According to Khataee et al.’s results (Khataee et al., 2017), increasing the concentration of Na2SO4 form 0.01 M to 0.025 M and 0.05 M could increase significantly the H2O2 production. However, a further increase to 0.1 M doesn’t show an obvious increase in H2O2 production. This could be explained that under low concentration of Na2SO4, there is a need for high voltages to overcome the decrease in electrical conductivity. Thus, as mentioned before, side reactions (i.e., H2 evolution, H2O2 cathodic reduction, and H2O2 anodic oxidation) result in the decreased H2O2 yield. However, Na2SO4 concentration as high as 0.1 M is not realistic. Additionally, the containing of chloride and bromide in the electrolyte was reported to promote the H2O2 generation (Qiang et al., 2002).
It is worth highlighting that from the aspect of H2O2 utilization in the oxidation process, such as Electro-Fenton process, the type of electrolytes could significantly impact the process due to the ions-induced active species and the interaction of ions with hydroxyl radicals. Such as when chlorides are used, the Cl-mediated oxidation will be favored (Martínez-Huitle and Brillas, 2008; Anglada et al., 2009; Sirés et al., 2014). When sulfates are used, persulfates can be electrogenerated at nonactive anodes (Brillas and Martínez-Huitle, 2011; Martínez-Huitle et al., 2012; Sirés et al., 2014), while sulfate radicals can be generated at active anodes (Farhat et al., 2015; Chen et al., 2018). Additionally, the existence of ions in an aqueous matrix such as HCO3− and Cl− could scavenge hydroxyl radicals (Pignatello et al., 2006).
5.4. Effect of electrolyte pH
The electrolyte pH plays an essential role in H2O2 electrosynthesis and H2O2-based applications such as Electro-Fenton process. From Eq. (1), it seems that a low pH is favorable for H2O2 electrogeneration since its synthesis consumes protons. However, a high concentration of protons may accelerate the H2 evolution reaction, which is a primary competitive reaction with 2-electron ORR for H2O2 generation on the cathode, thus reducing the H2O2 production and current efficiency. Additionally, higher pH in the basic range could promote the decomposition of H2O2. above pH 9, H2O2 decomposes markedly with increasing pH, temperature and reaction time (Qiang et al., 2002), which could be attributed to the role of HO2− in the base H2O2 solution (Eq. (8)) (Qiang et al., 2002).
| (8) |
Many works have reported the influence of electrolyte pH on H2O2 production, most of which found an optimal pH and explained in the same way as we discussed above. Results in Qiang et al.’s work indicate that pH 2 is the optimal condition. Above pH 2, the yield decreases due to the insufficient H+, while below pH 2, the yield also decreases due to the enhanced H2 evolution (Qiang et al., 2002). Khataee et al. investigated the H2O2 yield under pH values of 3, 5, 7, and 9. The acidic condition of pH 3 was found to be the most effective condition for H2O2 production, wherein, the production decrease reduced with the increase of solution pH due to the lack of H+. At more acidic pH, side reactions including H2O2 reduction to H2O and H2 evolution concurrently happen on the cathode and decrease the H2O2 yield (Khataee et al., 2017).
5.5. Effect of temperature
In contrast to other parameters, the effect of temperature on H2O2 electrosynthesis has not been widely studied (Daghrir et al., 2012; Martínez-Huitle et al., 2015). Usually, the process is operated at room temperature. Some authors have suggested that temperature exerts conflicting effects on H2O2 generation. The O2 diffusion coefficient will increase as temperature rises, which facilitates the O2 reduction. However, on the other hand, increasing temperature will decrease the O2 solubility in the electrolyte and increase the H2O2 decomposition rate (Qiang et al., 2002).
5.6. Effect of oxygen
Pure oxygen gas or air are usually used as the source of oxygen. Typically, O2 is supplied to the cathode surface by aeration or by an external pure oxygen source. Both the oxygen purity and mass flow rate affect the H2O2 production. It has been well recognized that higher oxygen purity and flow rate facilitates H2O2 production (Qiang et al., 2002; Martínez-Huitle and Brillas, 2009; Yu et al., 2014). However, the oxygen utilization efficiency (OUE) by these methods is extremely low (<0.1%), a significant energy loss and operation complexity occur especially when up-scaling the process (Yu et al., 2015b; Pérez et al., 2016). Thus, various attempts were conducted to achieve higher OUE as well as high current efficiency for H2O2 production.
The oxidation of water to O2 (O2 evolution reaction, OER) is typically considered a reaction that is not relevant with 2-electron ORR occurs on the cathode. However, some authors employ this pathway as the source of DO for H2O2 production. In Zhou et al.’s work, Ti/mixed metal oxides (Ti/MMO) anode was used to in situ supply O2 for GF cathode, and an OUE of 9.8% was obtained (Zhou et al., 2018c). Zhang et al. used the same method to supply O2 for the cathode in a three-electrode system, and OUE of 20.2% was achieved (Zhang et al., 2017). Some other strategies concerning the O2 supply and mass transport to cathode surface include pressurized reactors (Scialdone et al., 2015; Pérez et al., 2018), Venturi-based jet aerators (Pérez et al., 2016), reactors equipped with rotating cathodes (Yu et al., 2014), gas diffusion electrodes (GDEs), and stacked electrosynthesis reactors (Lu et al., 2017).
5.7. Effect of mixing
As previously discussed, the performance of H2O2 electrosynthesis process is likely to be affected by the mass transfer of DO to the cathode surface and of H2O2 to the anode (in undivided reactors). On the one hand, mixing facilitates the mass transfer of DO to the cathode surface and thus enhances the H2O2 production. On the other hand, mixing also facilitates the mass transfer of H2O2 to the anode, which induces invalid anodic oxidation of H2O2 (Eq. (5)). Reported in several works (Ma et al., 2018; Zhou et al., 2018d), the higher is the mixing rate, the higher is the H2O2 yield.
5.8. Effect of pulsed current electrolysis
Little attention was paid to the decomposition pathways of H2O2 in an electrochemical system, especially methods to decrease the extent of invalid pathways. Some authors reported that H2O2 molecules might undergo several pathways after its formation on the cathode surface, such as further electroreduction to H2O (Sánchez-Sánchez and Bard, 2009; Coria et al., 2015), diffusion to the bulk electrolyte, disproportion in bulk electrolyte to H2O and O2 (Brillas et al., 2009; Sirés et al., 2014), and anodic oxidation to O2 (Vasudevan and Oturan, 2014). In our previous work (Zhou et al., 2018b; Zhou et al., 2018c), these pathways are systematically considered. The disproportion of H2O2 cannot be inhibited due to the intrinsic nature of H2O2. The anodic oxidation of H2O2 could be avoided when divided cells are used (Riccobono et al., 2017), however, for undivided cells, it can not be avoided. The key feature of H2O2 electroreduction pathway is that it occurs during the diffusion of H2O2 molecule from cathode inside and surface to bulk electrolyte. Thus we propose that pulsed current assisted electrolysis can decrease the extent of H2O2 electroreduction and thus increase the H2O2 concentration in bulk electrolyte (mechanism shown in Fig. SM-2). Results show pulsed current parameters significantly influence the H2O2 yield. Under optimal conditions, the H2O2 yield was 61.6% higher than under constant currents. This could be an attractive method due to its simple operation without any change on cathode and cells.
6. Application in decontamination of aqueous and gaseous contaminants
In situ and de-centralized H2O2 production by 2-electron O2 reduction solves the drawbacks of H2O2-based advanced oxidation processes (AOPs), such as the hazards of storage, transportation, and handling of high-concentration H2O2. Thus, the in situ supply of H2O2 via O2 electroreduction is of great importance for the H2O2-based AOPs for environmental applications. Principally, for the existing H2O2-based AOPs, such as Fenton (Fe2+/H2O2) (Zhou et al., 2017), UV/Fenton (Shemer et al., 2006), UV/H2O2 (Liu et al., 2017), and ultrasound/H2O2 (Vončina and Majcen-Le-Marechal, 2003), the H2O2 can be provided via O2 electroreduction. Coupled with various H2O2 activation techniques, highly oxidative hydroxyl radicals (·OH) can be generated and transforms organic contaminants into salts, CO2, and H2O. Fig. 6 shows the possible procedure of electrosynthesized H2O2 on various environmental applications. In terms of H2O2 activation, despite the conventional activation methods such as Fe2+ (Pignatello et al., 2006), non-iron metal ions (Bokare and Choi, 2014), UV light (Liu et al., 2017), ultrasound (Pignatello et al., 2006; Wang and Wang, 2018), and activated carbon (Khalil et al., 2001; Ribeiro et al., 2013; Anfruns et al., 2014), some new catalysts towards highly selective H2O2 activation are continuously emerging, includes iron oxychloride (FeOCl) (Yang et al., 2016; Zhang et al., 2016; Sun et al., 2018), Cu-doped γ-Al2O3 (Lyu et al., 2015; Yun et al., 2016), MoS2 (Xing et al., 2018), WS2 (Dong et al., 2018), g-C3N4 (Lyu et al., 2017), and various carbon-based catalysts (Fang et al., 2014; Fang et al., 2015a; Fang et al., 2015b; Duan et al., 2018).
Fig. 6.
Schematic procedures of H2O2 electrogeneration via 2-electron O2 reduction, H2O2 activation, and its utilization in decontamination of aqueous and gaseous contaminants
Currently, some review papers have been published to summarize the H2O2-based AOPs for environmental remediation (Neyens and Baeyens, 2003; Pignatello et al., 2006; Brillas et al., 2009; Wang and Xu, 2012; Oturan and Aaron, 2014; Cheng et al., 2016). One of the key factors that determine the decontamination efficiency is the dosage of H2O2. Thus, in the following part, to highlight the important role of H2O2 production, the decontamination performance will be analyzed based on the H2O2 production.
6.1. Decontamination of aqueous organic contaminants
The most popular system for organic contaminants degradation based on in situ H2O2 electrosynthesis is the well-known Electro-Fenton (EF) process. Developed by Brilla’s group and Oturan’s group (Brillas et al., 1996; Oturan et al., 2000), the process couples H2O2 electrogeneration and Fe2+ electro-regeneration and continuously generates ·OH via the reaction between H2O2 and Fe2+ for organic contaminants degradation. The process efficiency is highly dependent on the H2O2 productivity. Thus in the following parts, the decontamination performance was analyzed based on the H2O2 productivity.
Till now, the EF process has been widely used to treat non-biodegradable or refractory organic contaminants, such as dyes (Wang et al., 2005; Özcan et al., 2008b; Yu et al., 2014; Yang et al., 2017; Zhou et al., 2018a), pesticides and herbicides (Özcan et al., 2008a; Oturan et al., 2012; Zhao et al., 2012; Zhao et al., 2018a), phenolic compounds (Oturan et al., 2000; Yuan et al., 2013; Zhou et al., 2013b; Divyapriya et al., 2017; Yang et al., 2017), and drugs (Skoumal et al., 2009; Divyapriya et al., 2018; Garza-Campos et al., 2018; Zhou et al., 2018b). As summarized in Table SM-2, the performance of the EF process enabled by different cathodes on organic contaminants degradation was systematically summarized, with a special focus on H2O2 production.
The dyes are usually generated from the textile dyeing and printing industries (Nidheesh and Gandhimathi, 2012). Their presence in water is of particular environmental concern because they give undesirable color to water sources and can be toxic and carcinogenic (Eren and Acar, 2006). In some cases, the dyes can generate dangerous byproducts via oxidation, hydrolysis, or other chemical reactions (Wang et al., 2005). Many papers have reported the degradation and mineralization of different dyes (such as Methyl Orange (Yu et al., 2014), Basic Blue 3 (Özcan et al., 2008b), Acid Red 14 (Wang et al., 2005), Methylene Blue (Li et al., 2018), Orange II (Paz et al., 2018), Tartrazine (Yu et al., 2015b; Ren et al., 2016), and Rhodamine B (Zhang et al., 2018)) in EF process by various cathodes materials. Wang et al. (Wang et al., 2005) compared the performance of the EF process using activated carbon fiber (ACF) felt and graphite as cathode for Acid Red 14 removal. They found the decolorization rate of both cathodes was almost the same. However, TOC could be reduced by 70% by ACF felt cathode, while it was only 40% by graphite cathode after 6 h. The difference was majorly due to the different H2O2 production rate, where it was 0.55 mg cm−2 h−1, and 0.05 mg cm−2 h−1 for ACF felt and graphite cathode, respectively. Li et al. (Li et al., 2018) also observed that higher H2O2 production rate could induce higher removal efficiency of dyes. In their work, pure N mono- and N/P dual-doped cotton-stalk-derived ACF were prepared and used as the cathode in EF process for methylene blue (MB) degradation. Results showed that N/P dual-doped ACF and the original ACF exhibited H2O2 production rate of 0.41 mg cm−2 h−1 and 0.25 mg cm−2 h−1, respectively, resulting in a 24.1% higher MB removal efficiency by N/P dual-doped ACF than the original ACF. Thus, we can draw a conclusion that higher H2O2 production is the determining factor of EF performance.
Pesticides and herbicides have been widely and extensively used in agricultural activities all over the world in the past century (Özcan et al., 2008a; Nidheesh and Gandhimathi, 2012). Currently, it has been widely detected in surface water, groundwater, and wastewater effluents. These chemicals usually have direct adverse effects on the living organisms and they are generally toxic and carcinogenic even in low concentrations (Nidheesh and Gandhimathi, 2012). The EF process has been proved to be a powerful method to degrade various hazardous pesticides and herbicides. F-doped porous carbon was employed by Zhao et al. (Zhao et al., 2018a) to degrade atrazine by EF process. As an important operational parameter, cathode potential influences the H2O2 production and thus, the degradation performance of atrazine. At −0.6 V (vs. SCE) and −0.2 V (vs. SCE), H2O2 production rate of 8.77 mg cm−2 h−1 and 3.05 mg cm−2 h−1 were obtained, resulting in a different degradation kinetic constant of 0.31 min−1 and 0.10 min−1, respectively. Zhao et al. (Zhao et al., 2012) constructed an EF system with the Fe3O4@Fe2O3/activated carbon aerogel as a cathode and achieved effective imidacloprid degradation over a wide pH range from 3 to 9, which also based on in situ H2O2 production but activated with different mechanism under different pHs. 43 μM and 20 μM ·OH was produced at pH 3 and pH 9, respectively.
Phenolic compounds are a family of organic contaminants that are released to the surface water from a wide number of industries such as pharmaceutical plants, coke plants, food-processing industries, gas and oil refineries, and other chemical plants (Nidheesh and Gandhimathi, 2012). Phenolic compounds are very toxic to human health and aquatic life. Some of these that have been studied by EF process includes p-nitrophenol (p-Np) (Zhou et al., 2014), phenol (Yang et al., 2017), bisphenol A (Yuan et al., 2013; Divyapriya et al., 2017), and triclosan (Yuan et al., 2013). Hydrazine hydrate modified GF was produced by Zhou et al. (Zhou et al., 2014). At pH 3, p-Np with an initial concentration of 50 mg L−1 was completely removed after 20 min by the modified electrode, and the TOC removal was 73.7% after 2 h, which was 21.5% higher than the unmodified GF electrode. The result could be ascribed to the higher H2O2 production by modified GF electrode (0.96 mg cm−2 h−1 and 0.37 mg cm−2 h−1, respectively). Electrochemically exfoliated graphene modified GF electrode was synthesized by Yang et al. (Yang et al., 2017). The authors proved that the TOC removal efficiency of phenol was about 42% by modified electrode at 1 h, while it was only about 25% by the unmodified electrode, which is also in accordance with the H2O2 production rates (7.7 mg cm−2 h−1 and 4.6 mg cm−2 h−1, respectively).
Various pharmaceutical drugs have been detected in different water sources in recent years, such as ibuprofen (Skoumal et al., 2009; Zhou et al., 2018b), sulfadiazine (Yang et al., 2017), tetracaine (Ridruejo et al., 2018), amoxicillin (Elmolla and Chaudhuri, 2010; Garza-Campos et al., 2018), and ciprofloxacin (Divyapriya et al., 2018). Some of the drugs are mutagenic and carcinogenic. Thus EF process was examined to remove drugs and their metabolites from water sources to avoid the hazardous effects of human and animals. The same with other types of organic contaminants, the degradation performance mainly depends on the cathode and H2O2 production. In our previous work (Zhou et al., 2018b), the pulsed current was confirmed to promote the H2O2 production from 0.25 mg cm−2 h−1 to 0.67 mg cm−2 h−1, thus inducing a significantly higher ibuprofen removal. At 10 min, the ibuprofen removal efficiency under constant and pulsed current is 34.1% and 75.0%, respectively. The time required for total removal is 60 min and 100 min for pulsed current and constant current, respectively. Reported by Yang et al. and co-workers (Yang et al., 2017), electrochemically exfoliated graphene modified GF electrode was synthesized and used as a cathode in the EF process for sulfadiazine degradation. The modified electrode increase the H2O2 production from 4.6 mg cm−2 h−1 to 7.7 mg cm−2 h−1. Thus, the TOC removal efficiency of sulfadiazine was about 100% by modified electrode at 1 h, while it was only 69% by the unmodified electrode.
Recently, some new AOPs based on H2O2 electrochemical production have been developed, such as electro-peroxone (Yuan et al., 2013), photoelectro-peroxone (Frangos et al., 2016b), electrochemically driven UV/H2O2 processes (Barazesh et al., 2015; Frangos et al., 2016a). Various studies have shown that these new EAOPs are effective for wastewater treatment (Bakheet et al., 2013), micropollutant abatement (Yao et al., 2016; Yao et al., 2017; Yao et al., 2018), disinfection/oxidation by-product control (Li et al., 2015; Hou et al., 2016; Mao et al., 2018), and carbon based adsorbents regeneration (Zhan et al., 2016a; Zhan et al., 2016b).
Apart from the studies on the synthetic solutions, there are some studies dealing with real wastewater (Wang et al., 2008; Priambodo et al., 2011), which are important for real applications. Moreover, the evolution and nature of toxicity during the transformation of contaminants is another important factor to consider. The incomplete mineralization of contaminants can generate by-products that exhibit toxicity comparable to or greater than that of the original contaminants. Thus, the change of toxicity during treatment process could be conducted.
6.2. Decontamination of gaseous contaminants
The H2O2 based AOPs have been widely used for organic contaminants degradation in the aqueous phase. As discussed in section 6.1, various organic contaminants can be effectively removed by ·OH in a homogeneous manner. However, limited work was reported on heterogeneous decontamination of gaseous contaminants by H2O2-based AOPs, especially where the H2O2 was in situ supplied from O2 electroreduction. This process can solve the problem of H2O2 transport and handling (Qiang et al., 2002), thus has advantages over the traditional H2O2-based AOPs. Due to the few reports on decontamination of gaseous contaminants by AOPs based on electrogenerated H2O2, here, the applications of H2O2-based AOPs for various gaseous contaminants removal are briefly reviewed.
Some studies based on H2O2-based AOPs have attempted to oxidize or destruct various contaminants in gas phase, such as nitric oxide (NO) (Liu et al., 2011; Liu and Zhang, 2011; Ding et al., 2014; Liu et al., 2014a; Hao and Zhao, 2016), elemental mercury (Hg0) (Martinez and Deshpande, 2007; Liu et al., 2014b; Zhou et al., 2015; Xu et al., 2017), sulfur dioxide (SO2) (Chen et al., 2017), toluene (Handa et al., 2013; Liu et al., 2017), and dichloromethane (DCM) (Feitz et al., 2002). Cooper et al. (Collins et al., 2001; Limvoranusorn et al., 2005) firstly used H2O2-based AOP to oxidize and remove NO from simulated flue gas by activating H2O2 with UV and high-temperature flue gas. Later, Liu et al. and co-workers systematically investigated NO oxidation and removal by UV/H2O2 process which includes the mechanism study (Liu et al., 2014a), parameters optimization (Liu and Zhang, 2011; Liu et al., 2014a), and development of kinetic models (Liu et al., 2011). Recently, Hao et al. (Hao and Zhao, 2016) also used UV light to activate vaporized H2O2, which achieved efficient NO oxidation. Besides the above activation methods, some work also reported other catalysts such as hematite (Ding et al., 2014), which exhibited a lower running cost than UV and heat.
The trace elemental mercury (Hg0) is majorly from the coal-fired power plants. It has gradually become a major concern due to its volatility, high toxicity, and bioaccumulation (Zhou et al., 2015). H2O2-based AOPs have been examined as practical approach to remove Hg0 from flue gas. Zhou et al. (Zhou et al., 2015) synthesized magnetically separable Fe2.45Ti0.55O4 catalystand used it as H2O2 activator for Hg0 oxidation. 96% of Hg0 was removed with 0.5 M H2O2 and 0.6 g L−1 catalyst at weak acid medium. Most recently, Xu et al. (Xu et al., 2017) designed a new diffusion electrochemical reactor to remove Hg0. The reactor used self-made gas diffusion electrode (GDE) as cathode and Ti/IrO2 as anode. A high H2O2 production rate of 8.86 mg cm−2 h−1 was obtained at pH 7. The high H2O2 production rate resulted in a 90% Hg0 removal efficiency under 70 °C after 40 min electrolysis. This result highlights the critical role of H2O2 concentration on Hg0 removal by H2O2-based AOPs.
Sulfur dioxide (SO2) in the flue gas is recognized as one of the main initiators for haze formation which severely influence the air quality in many developing countries (Zhao et al., 2011; Chen et al., 2017). It also leads to acid rain and photochemical smog. Thus, many technologies are developed to achieve clean, efficient and energy-saving flue gas desulfurization (FGD). Even the wet flue gas desulfurization (WFGD) has been well developed and widely used, the alkaline adsorbents or organic solvents used in the process inevitably lead to secondary pollution (Chen et al., 2017). Fortunately, electrochemical approach based on H2O2 electrogeneration offers an alternative due to its environmental friendliness and superior desulfurization efficiency. One typical work was reported by Chen et al. recently (Chen et al., 2017). The authors used carbon black-based GDEs to produce H2O2 for in situ oxidation of SO2 to SO42− in an electrolysis system. Up to 1002.4 mg L−1 H2O2 (H2O2 production rate: 0.98 mg cm−2 h−1) and SO2 removal efficiency of 98% were obtained.
Volatile organic compounds (VOCs) are another type of gaseous contaminants that can be removed by H2O2-based AOPs. Various methods have been developed for the removal of VOCs (especially VOCs emitted from industrial plants with high concentration), such as catalytic combustion (Li et al., 2009), adsorption (Poddar and Sirkar, 1997) and biological treatment (Handa et al., 2013). However, low-concentration VOCs are hardly removed by a single aforementioned method. Thus, some authors proposed that H2O2-based AOPs could be a facile process towards complete removal of VOCs with low concentration. Feitz et al. (Feitz et al., 2002) used the photo-Fenton process and successfully achieved the degradation of gaseous DCM. Compared with the Fenton-based process, Liu et al. (Liu et al., 2017) reported that UV/H2O2 process could moderately generate ·OH and yielded removal efficiency of toluene over 80% without any loss, while it declined to 32% and 45% after 2 h in the Fenton and UV/Fenton process, respectively. The result obtained by Liu et al. clearly demonstrated that a continuous in situ supply of H2O2 could possibly guarantee a stable VOCs removal within the whole process, which can be realized by H2O2 electrogeneration system.
From the above analysis, it can be concluded that compared with aqueous contaminants, limited work was reported on decontamination of gaseous contaminants by H2O2-based AOPs, especially where the H2O2 was in situ supplied from O2 electroreduction. However, many studies draw the same conclusion that the electrode materials that could produce a higher concentration of H2O2 result in a higher removal efficiency of contaminants. Moreover, removal of gaseous contaminants is a heterogeneous process, which is different from homogeneous decontamination of aqueous contaminants. Thus, in terms of reactor design, the effective mass transfer could be an important factor to consider.
7. Concluding remarks
A review of the recent literature on H2O2 production from O2 electroreduction and its environmental applications is presented. The success of H2O2 electrochemical production from O2 reduction covers several aspects, including cathode materials, operational parameters and reactor configurations. Cathode materials are the fundamental factor that directly determines the selectivity, activity and stability of cathodes towards 2-electron ORR. Operational parameters, another essential aspect that has been explored in most of the recent literature, not only impact the ORR pathway but also impact the mass transfer of ORR species (O2, H+, H2O). For large-scale applications, reactor configurations are especially essential to guarantee the performance as well as economic soundness.
Recently, the field has witnessed a growing interest and understanding in electrochemical H2O2 production from O2 reduction. However, these studies are still far from sufficient, especially for practical applications. From our perspectives, there are still some challenges in this fascinating field.
(1) Establishment of cost-effective and active carbon based cathodes. Recently, not only scientists from environmental field but also from the field of chemistry and materials begin to focus on H2O2 production via 2-electron ORR, where their previous interests only lie in 4-electron ORR aimed at applications such as fuel cells and metal-air batteries. Thus, it can be anticipated that new and advanced carbon materials could be continuously developed for electrochemical H2O2 production. Theoretical studies and simulation could help to predict the activity trends of carbon based materials.
(2) Long-term stability and failure mechanism of cathode materials. Stability of electrodes are especially significant for practical applications. However, the operation time in many previous works are far from enough. Thus, longer testing time are suggested to evaluate the stability of cathode materials.
(3) GDEs are recommended as various catalysts can be used by this cathode structure to obtain high H2O2 production due to the formation of three-phase interface. However, more research is needed to reveal the flooding mechanism over extended use and thus, guarantee the long-term stability of GDEs.
(4) More realistic conditions are suggested in future work, such as low concentration of supporting electrolyte (e.g. Na2SO4, NaNO3) and air aeration as a replacement of pure O2 injection. More realistic conditions are expected to offer more direct guidance to practical applications.
(5) Only limited setups at device level have been proposed for H2O2 electrogeneration and water treatment, with a low efficiency and relatively complicated configurations. Thus, more engineering work are suggested to develop better reactor configurations in the future.
Supplementary Material
Highlights:
Recent advances in H2O2 production by various methods are reviewed.
H2O2 electrogeneration by various carbon-based materials are summarized.
Effects of operation parameters and reactors on H2O2 production are reviewed.
The applications of electrogenerated H2O2 on organic contaminants degradation are presented.
Future perspectives on O2 electroreduction for H2O2 production are suggested.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51776055), Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. QA201815), and the US National Institute of Environmental Health Sciences (NIEHS, Grant No. P42ES017198). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, the National Institutes of Health and the National Natural Science Foundation of China. We also thank China Scholarship Council for the financial support to Wei Zhou.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anfruns A, García-Suárez EJ, Montes-Morán MA, Gonzalez-Olmos R, Martin MJ, 2014. New insights into the influence of activated carbon surface oxygen groups on H2O2 decomposition and oxidation of pre-adsorbed volatile organic compounds. Carbon 77, 89–98. [Google Scholar]
- Anglada Angela, Urtiaga A, Ortiz I, 2009. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biot 84, 1747–1755. [Google Scholar]
- Antonin VS, Assumpcao MHMT, Silva JCM, Parreira LS, Lanza MRV, Santos MC, 2013. Synthesis and characterization of nanostructured electrocatalystsbased on nickel and tin for hydrogen peroxide electrogeneration. Electrochim. Acta 109, 245–251. [Google Scholar]
- Antonin VS, Parreira LS, Aveiro LR, Silva FL, Valim RB, Hammer P, Lanza MRV, Santos MC, 2017. W@Au nanostructures modifying carbon as materials for hydrogen peroxide electrogeneration. Electrochim. Acta 231, 713–720. [Google Scholar]
- Asefa T, 2016. Metal-free and noble metal-free heteroatom-doped nanostructured carbons as prospective sustainable electrocatalysts. Accounts of Chem. Res 49, 1873–1883. [DOI] [PubMed] [Google Scholar]
- Assumpão MHMT, Moraes A, De Souza RFB, Gaubeur I, Oliveira RTS, Antonin VS, Malpass GRP, Rocha RS, Calegaro ML, Lanza MRV, Santos MC, 2012. Low content cerium oxide nanoparticles on carbon for hydrogen peroxide electrosynthesis. Appl. Catal. A-Gen 411–412, 1–6. [Google Scholar]
- Assumpção MHMT, De Souza RFB, Rascio DC, Silva JCM, Calegaro ML, Gaubeur I, Paixão TRLC, Hammer P, Lanza MRV, Santos MC, 2011. A comparative study of the electrogeneration of hydrogen peroxide using Vulcan and Printex carbon supports. Carbon 49, 2842–2851. [Google Scholar]
- Aveiro LR, da Silva AGM, Antonin VS, Candido EG, Parreira LS, Geonmonond RS, de Freitas IC, Lanza MRV, Camargo PHC, Santos MC, 2018. Carbon-supported MnO2 nanoflowers: Introducing oxygen vacancies for optimized volcano-type electrocatalytic activities towards H2O2 generation. Electrochim. Acta 268, 101–110. [Google Scholar]
- Babaei-Sati R, Basiri Parsa J, 2017a. Electrogeneration of H2O2 using graphite cathode modified with electrochemically synthesized polypyrrole/MWCNT nanocomposite for electro-Fenton process. J. Ind. Eng. Chem 52, 270–276. [Google Scholar]
- Babaei-Sati R, Basiri Parsa J, 2017b. Electrodeposition of PANI/MWCNT nanocomposite on stainless steel with enhanced electrocatalytic activity for oxygen reduction reaction and electro-Fenton process. New J. Chem 41, 5995–6003. [Google Scholar]
- Bakheet B, Yuan S, Li Z, Wang H, Zuo J, Komarneni S, Wang Y, 2013. Electro-peroxone treatment of Orange II dye wastewater. Water Res 47, 6234–6243. [DOI] [PubMed] [Google Scholar]
- Bañuelos JA, El-Ghenymy A, Rodríguez FJ, Manríquez J, Bustos E, Rodríguez A, Brillas E, Godínez LA, 2014. Study of an air diffusion activated carbon packed electrode for an electro-fenton wastewater treatment. Electrochim. Acta 140, 412–418. [Google Scholar]
- Barazesh JM, Hennebel T, Jasper JT, Sedlak DL, 2015. Modular advanced oxidation process enabled by cathodic hydrogen peroxide production. Environ. Sci. Technol 49, 7391–7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros WRP, Ereno T, Tavares AC, Lanza MRV, 2015. In situ electrochemical generation of hydrogen peroxide in alkaline aqueous solution by using an unmodified gas diffusion electrode. ChemElectroChem 2, 714–719. [Google Scholar]
- Barros WRP, Wei Q, Zhang G, Sun S, Lanza MRV, Tavares AC, 2015. Oxygen reduction to hydrogen peroxide on Fe3O4 nanoparticles supported on Printex carbon and Graphene. Electrochim. Acta 162, 263–270. [Google Scholar]
- Barton SS, Evans MJB, Halliop E, MacDonald JAF, 1997. Anodic oxidation of porous carbon. Langmuir 13, 1332–1336. [Google Scholar]
- Berenguer R, Marco-Lozar JP, Quijada C, Cazorla-Amorós D, Morallón E, 2009. Effect of electrochemical treatments on the surface chemistry of activated carbon. Carbon 47, 1018–1027. [Google Scholar]
- Berl E, 1939. A new cathodic process for the production of H2O2. Trans. Electrochem. Soc 76, 359–369. [Google Scholar]
- Bokare AD, Choi W, 2014. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater 275, 121–135. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour A, Esau D, Cheng H, Wang A, Gyenge E, Wilkinson DP, 2011. Preparation and electrochemical studies of metal-carbon composite catalysts for small-scale electrosynthesis of H2O2. Electrochim. Acta 56, 9074–9081. [Google Scholar]
- Brillas E, Martínez-Huitle CA, 2011. Synthetic Diamond Films: Preparation, Electrochemistry, Characterization, and Applications
- Brillas E, Mur E, Casado J, 1996. Iron(II) Catalysis of the Mineralization of Aniline Using a Carbon-PTFE O2 - Fed Cathode. J. Electrochem. Soc 143, L49–L53. [Google Scholar]
- Brillas E, Sirés I, Oturan MA, 2009. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev 109, 6570–6631. [DOI] [PubMed] [Google Scholar]
- Campos-Martin JM, Blanco-Brieva G, Fierro JLG, 2006. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Edit 45, 6962–6984. [DOI] [PubMed] [Google Scholar]
- Campos M, Siriwatcharapiboon W, Potter RJ, Horswell SL, 2013. Selectivity of cobalt-based catalysts towards hydrogen peroxide formation during the reduction of oxygen. Catal. Today 202, 135–143. [Google Scholar]
- Carneiro JF, Paulo MJ, Siaj M, Tavares AC, Lanza MRV, 2015. Nb2O5 nanoparticles supported on reduced graphene oxide sheets as electrocatalyst for the H2O2 electrogeneration. J. Catal 332, 51–61. [Google Scholar]
- Carneiro JF, Rocha RS, Hammer P, Bertazzoli R, Lanza MRV, 2016a. Hydrogen peroxide electrogeneration in gas diffusion electrode nanostructured with Ta2O5. Appl. Catal. A-Gen 517, 161–167. [Google Scholar]
- Carneiro JF, Rocha RS, Hammer P, Bertazzoli R, Lanza MRV, 2016b. Hydrogen peroxide electrogeneration in gas diffusion electrode nanostructured with Ta2O5. Appl. Catal. A-Gen 517, 161–167. [Google Scholar]
- Castañeda LF, Walsh FC, Nava JL, Ponce de León C, 2017. Graphite felt as a versatile electrode material: Properties, reaction environment, performance and applications. Electrochim. Acta 258, 1115–1139. [Google Scholar]
- Chai GL, Hou Z, Ikeda T, Terakura K, 2017. Two-electron oxygen reduction on carbon materials catalysts: mechanisms and active sites. Journal of Physical Chemistry C 121, 14524–14533. [Google Scholar]
- Chaudhuri SK, Lovley DR, 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol 21, 1229–1232. [DOI] [PubMed] [Google Scholar]
- Chen L, Lei C, Li Z, Yang B, Zhang X, Lei L, 2018. Electrochemical activation of sulfate by BDD anode in basic medium for efficient removal of organic pollutants. Chemosphere 210, 516–523. [DOI] [PubMed] [Google Scholar]
- Chen S, Chen Z, Siahrostami S, Higgins D, Nordlund D, Sokaras D, Kim TR, Liu Y, Yan X, Nilsson E, Sinclair R, Norskov JK, Jaramillo TF, Bao Z, 2018. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide. J. Am. Chem. Soc 140, 7851–7859. [DOI] [PubMed] [Google Scholar]
- Chen S, Perathoner S, Ampelli C, Mebrahtu C, Su D, Centi G, 2017. Room-temperature electrocatalytic synthesis of NH3 from H2O and N2 in a gas-liquid-solid three-phase reactor. ACS Sustain. Chem. Eng 5, 7393–7400. [Google Scholar]
- Chen Z, Dong H, Yu H, Yu H, 2017. In-situ electrochemical flue gas desulfurization via carbon black-based gas diffusion electrodes: Performance, kinetics and mechanism. Chem. Eng. J 307, 553–561. [Google Scholar]
- Cheng M, Zeng G, Huang D, Lai C, Xu P, Zhang C, Liu Y, 2016. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J 284, 582–598. [Google Scholar]
- Cheng PZ, Teng H, 2003. Electrochemical responses from surface oxides present on HNO3-treated carbons. Carbon 41, 2057–2063. [Google Scholar]
- Ciriminna R, Albanese L, Meneguzzo F, Pagliaro M, 2016. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 9, 3374–3381. [DOI] [PubMed] [Google Scholar]
- Collins MM, Cooper CD, Dietz JD, C. CA Iii, Tazi LM, 2001. Pilot-scale evaluation of H2O2 injection to control NOx emissions. J. Environ. Eng 127, 329–336. [Google Scholar]
- Coria G, Pérez T, Sirés I, Nava JL, 2015. Mass transport studies during dissolved oxygen reduction to hydrogen peroxide in a filter-press electrolyzer using graphite felt, reticulated vitreous carbon and boron-doped diamond as cathodes. J. Electroanal. Chem 757, 225–229. [Google Scholar]
- Da Pozzo A, Di Palma L, Merli C, Petrucci E, 2005. An experimental comparison of a graphite electrode and a gas diffusion electrode for the cathodic production of hydrogen peroxide. J. Appl. Electrochem 35, 413–419. [Google Scholar]
- Daems N, Sheng X, Vankelecom IFJ, Pescarmona PP, 2014. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2, 4085–4110. [Google Scholar]
- Daghrir R, Drogui P, Robert D, 2012. Journal of Photochemistry and Photobiology A : Chemistry Photoelectrocatalytic technologies for environmental applications. J. Photochem. Photobio. A- Chem 238, 41–52. [Google Scholar]
- Darvishi R, Soltani C, Rezaee A, Khataee A, 2013. Combination of carbon black- ZnO/UV process with an electrochemical process equipped with a carbon black −PTFE-coated gas-diffusion cathode for removal of a textile dye. Ind. Eng. Chem. Res 52, 14133–14142. [Google Scholar]
- Ding J, Zhong Q, Zhang S, Song F, Bu Y, 2014. Simultaneous removal of NOx and SO2 from coal-fired flue gas by catalytic oxidation-removal process with H2O2. Chem. Eng. J 243, 176–182. [Google Scholar]
- Divyapriya G, Nambi IM, Senthilnathan J, 2017. An innate quinone functionalized electrochemically exfoliated graphene/Fe3O4 composite electrode for the continuous generation of reactive oxygen species. Chem. Eng. J 316, 964–977. [Google Scholar]
- Divyapriya G, Thangadurai P, Nambi I, 2018. Green Approach To Produce a Graphene thin film on a conductive LCD matrix for the oxidative transformation of ciprofloxacin. ACS Sustainable Chem. Eng 6, 3453–3462. [Google Scholar]
- Dong C, Ji J, Shen B, Xing M, Zhang J, 2018. Enhancement of H2O2 decomposition by the cocatalytic effect of WS2 on the Fenton reaction for the synchronous reduction of Cr(VI) and remediation of phenol. Environ. Sci. Technol [DOI] [PubMed] [Google Scholar]
- Drogui P, Elmaleh S, Rumeau M, Bernard C, Rambaud A, 2001. Oxidising and disinfecting by hydrogen peroxide produced in a two-electrode cell. Water Res 35, 3235–3241. [DOI] [PubMed] [Google Scholar]
- Duan X, Sun H, Wang S, 2018. Metal-Free Carbocatalysis in advanced oxidation reactions. Accounts Chem. Res 51, 678–687. [DOI] [PubMed] [Google Scholar]
- Edwards JK, Freakley SJ, Lewis RJ, Pritchard JC, Hutchings GJ, 2015. Advances in the direct synthesis of hydrogen peroxide from hydrogen and oxygen. Catal. Today 248, 3–9. [Google Scholar]
- Elmolla ES, Chaudhuri M, 2010. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 252, 46–52. [DOI] [PubMed] [Google Scholar]
- Eren Z, Acar FN, 2006. Adsorption of Reactive Black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination 194, 1–10. [Google Scholar]
- Fang G, Gao J, Liu C, Dionysiou DD, Wang Y, Zhou D, 2014. Key role of persistent free radicals in hydrogen peroxide activation by biochar: Implications to organic contaminant degradation. Environ. Sci. Technol 48, 1902–1910. [DOI] [PubMed] [Google Scholar]
- Fang G, Liu C, Gao J, Dionysiou DD, Zhou D, 2015a. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ. Sci. Technol 49, 5645–5653. [DOI] [PubMed] [Google Scholar]
- Fang G, Zhu C, Dionysiou DD, Gao J, Zhou D, 2015b. Mechanism of hydroxyl radical generation from biochar suspensions: Implications to diethyl phthalate degradation. Bioresource Technol 176, 210–217. [DOI] [PubMed] [Google Scholar]
- Farhat A, Keller J, Tait S, Radjenovic J, 2015. Removal of persistent organic contaminants by electrochemically activated sulfate. Environ. Sci. Technol 49, 14326–14333. [DOI] [PubMed] [Google Scholar]
- Feitz AJ, Guan J, Chattopadhyay G, David Waite T, 2002. Photo-Fenton degradation of dichloromethane for gas phase treatment. Chemosphere 48, 401–406. [DOI] [PubMed] [Google Scholar]
- Fellinger TP, Hasché F, Strasser P, Antonietti M, 2012. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. Journal of the American Chemical Society 134, 4072–4075. [DOI] [PubMed] [Google Scholar]
- Feng L, Yan Y, Chen Y, Wang L, 2011. Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ. Sci 4, 1892–1899. [Google Scholar]
- Flores N, Thiam A, Rodríguez RM, Centellas F, Cabot PL, Garrido JA, Brillas E, Sirés I, 2017. Electrochemical destruction of trans-cinnamic acid by advanced oxidation processes: kinetics, mineralization, and degradation route. Environ. Sci. Pollut. Res 24, 6071–6082. [DOI] [PubMed] [Google Scholar]
- Fortunato GV, Pizzutilo E, Mingers AM, Kasian O, Cherevko S, Cardoso ESF, Mayrhofer KJJ, Maia G, Ledendecker M, 2018. The impact of palladium loading and interparticle distance on the selectivity for the oxygen reduction reaction towards hydrogen peroxide. J. Phys. Chem C 122, 15878–15885. [Google Scholar]
- Frangos P, Shen W, Wang H, Li X, Yu G, Deng S, Huang J, Wang B, Wang Y, 2016a. Improvement of the degradation of pesticide deethylatrazine by combining UV photolysis with electrochemical generation of hydrogen peroxide. Chem. Eng. J 291, 215–224. [Google Scholar]
- Frangos P, Wang H, Shen W, Yu G, Deng S, Huang J, Wang B, Wang Y, 2016b. A novel photoelectro-peroxone process for the degradation and mineralization of substituted benzenes in water 286, 239–248. [Google Scholar]
- Freakley SJ, He Q, Harrhy JH, Lu L, Crole DA, Morgan DJ, Ntainjua EN, Edwards JK, Carley AF, Borisevich AY, Kiely CJ, Hutchings GJ, 2016. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 351, 965–968. [DOI] [PubMed] [Google Scholar]
- Freakley SJ, Lewis RJ, Morgan DJ, Edwards JK, Hutchings GJ, 2015. Direct synthesis of hydrogen peroxide using Au-Pd supported and ion-exchanged heteropolyacids precipitated with various metal ions. Catal. Today 248, 10–17. [Google Scholar]
- Fuku K, Miyase Y, Miseki Y, Funaki T, Gunji T, Sayama K, 2017. Photoelectrochemical hydrogen peroxide production from water on a WO3/BiVO4 photoanode and from O2 on an Au cathode without external bias. Chem.-Asian J 12, 1111–1119. [DOI] [PubMed] [Google Scholar]
- Fuku K, Miyase Y, Miseki Y, Gunji T, Sayama K, 2016. Enhanced oxidative hydrogen peroxide production on conducting glass anodes modified with metal oxides. ChemistrySelect 1, 5721–5726. [Google Scholar]
- Gao C, Wang N, Peng S, Liu S, Lei Y, Liang X, Zeng S, Zi H, 2013. Influence of Fenton’s reagent treatment on electrochemical properties of graphite felt for all vanadium redox flow battery. Electrochim. Acta 88, 193–202. [Google Scholar]
- Gao G, Zhang Q, Hao Z, Vecitis CD, 2015. Carbon nanotube membrane stack for flow-through sequential regenerative electro-Fenton. Environ. Sci. Technol 49, 2375–2383. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez O, Lee YY, Olvera-Vargas H, Deng F, Wang Z, Lefebvre O, 2018. Mineralization of electronic wastewater by electro-Fenton with an enhanced graphene-based gas diffusion cathode. Electrochim. Acta 276, 12–20. [Google Scholar]
- García T, Agouram S, Dejoz A, Sánchez-Royo JF, Torrente-Murciano L, Solsona B, 2015. Enhanced H2O2 production over Au-rich bimetallic Au-Pd nanoparticles on ordered mesoporous carbons. Catal. Today 248, 48–57. [Google Scholar]
- Garza-Campos B, Morales-Acosta D, Hernández-Ramírez A, Guzmán-Mar JL, Hinojosa-Reyes L, Manríquez J, Ruiz-Ruiz EJ, 2018. Air diffusion electrodes based on synthetized mesoporous carbon for application in amoxicillin degradation by electro-Fenton and solar photo electro-Fenton. Electrochim. Acta 269, 232–240. [Google Scholar]
- Geng D, Chen Y, Chen Y, Li Y, Li R, Sun X, Ye S, Knights S, 2011. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy and Environ. Sci 4, 760–764. [Google Scholar]
- Guo D, Shibuya R, Akiba C, Saji S, Kondo T, Nakamura J, 2016. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351, 361–365. [DOI] [PubMed] [Google Scholar]
- Jiang HR, Shyy W, Zeng L, Zhang RH, Z. TS, 2018. Highly efficient and ultra-stable boron-doped graphite felt electrodes for vanadium redox flow batteries. J. Mater. Chem. A 1–10. [Google Scholar]
- Handa M, Lee Y, Shibusawa M, Tokumura M, Kawase Y, 2013. Removal of VOCs in waste gas by the photo-Fenton reaction: Effects of dosage of Fenton reagents on degradation of toluene gas in a bubble column. J. Chem. Technol. Biot 88, 88–97. [Google Scholar]
- Hao R, Zhao Y, 2016. Macrokinetics of NO oxidation by vaporized H2O2 association with ultraviolet light. Energy Fuel 30, 2365–2372. [Google Scholar]
- Hasché F, Oezaslan M, Strasser P, Fellinger TP, 2016. Electrocatalytic hydrogen peroxide formation on mesoporous non-metal nitrogen-doped carbon catalyst. J. Energy Chem 25, 251–257. [Google Scholar]
- He Z, Chen J, Chen Y, Makwarimba CP, Huang X, Zhang S, Chen J, Song S, 2018. An activated carbon fiber-supported graphite carbon nitride for effective electro-Fenton process. Electrochim. Acta 276, 377–388. [Google Scholar]
- He Z, Jiang Y, Meng W, Jiang F, Zhou H, Li Y, Zhu J, Wang L, Dai L, 2017. HF/H2O2 treated graphite felt as the positive electrode for vanadium redox flow battery. Appl. Surf. Sci 423, 111–118. [Google Scholar]
- Hickling A, Wilson WH, 1951. The anodic decomposition of hydrogen peroxide. J. Electrochem. Soc 98, 425–433. [Google Scholar]
- Hou M, Chu Y, Li X, Wang H, Yao W, Yu G, Murayama S, Wang Y, 2016. Electro-peroxone degradation of diethyl phthalate: Cathode selection, operational parameters, and degradation mechanisms. J. Hazard. Mater 319, 61–68. [DOI] [PubMed] [Google Scholar]
- Hu S, Qu X, Li P, Wang F, Li Q, Song L, Zhao Y, Kang X, 2018. Photocatalytic oxygen reduction to hydrogen peroxide over copper doped graphitic carbon nitride hollow microsphere: The effect of Cu(I)-N active sites. Chem. Eng. J 334, 410–418. [Google Scholar]
- Huang H, Han C, Wang G, Feng C, 2018. Lignin combined with polypyrrole as a renewable cathode material for H2O2 generation and its application in the electro-Fenton process for azo dye removal. Electrochim. Acta 259, 637–646. [Google Scholar]
- Iglesias D, Giuliani A, Melchionna M, Marchesan S, Criado A, Nasi L, Bevilacqua M, Tavagnacco C, Vizza F, Prato M, Fornasiero P, 2018. N-doped graphitized carbon nanohorns as a forefront electrocatalyst in highly selective O2 reduction to H2O2. Chem 4, 106–123. [Google Scholar]
- Isarain-Chávez E, Arias C, Cabot PL, Centellas F, Rodríguez RM, Garrido JA, Brillas E, 2010. Mineralization of the drug β-blocker atenolol by electro-Fenton and photoelectro-Fenton using an air-diffusion cathode for H2O2 electrogeneration combined with a carbon-felt cathode for Fe2+ regeneration. Appl. Catal. B-Environ 96, 361–369. [Google Scholar]
- Jaouen F, Proietti E, Lefèvre M, Chenitz R, Dodelet J-P, Wu G, Chung HT, Johnston CM, Zelenay P, 2011. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuelcells. Energy Environ. Sci 4, 114–130. [Google Scholar]
- Jiang T, Wang Y, Wang K, Liang Y, Wu D, Tsiakaras P, Song S, 2016. A novel sulfur-nitrogen dual doped ordered mesoporous carbon electrocatalyst for efficient oxygen reduction reaction. Appl. Catal. B-Environ 189, 1–11. [Google Scholar]
- Jirkovský JS, Panas I, Ahlberg E, Halasa M, Romani S, Schiffrin DJ, 2011. Single atom hot-spots at Au-Pd nanoalloys for electrocatalytic H2O2 production. J. Am. Chem. Soc 133, 19432–19441. [DOI] [PubMed] [Google Scholar]
- Katsounaros I, Schneider WB, Meier JC, Benedikt U, Biedermann PU, Auer AA, Mayrhofer KJJ, 2012. Hydrogen peroxide electrochemistry on platinum: Towards understanding the oxygen reduction reaction mechanism. Phys. Chem. Chem. Phys 14, 7384–7391. [DOI] [PubMed] [Google Scholar]
- Khalil LB, Girgis BS, Tawfik TAM, 2001. Decomposition of H2O2 on activated carbon obtained from olive stones. J. Chem. Technol. Biot 76, 1132–1140. [Google Scholar]
- Khataee A, Sajjadi S, Pouran SR, Hasanzadeh A, Joo SW, 2017. A comparative study on electrogeneration of hydrogen peroxide through oxygen reduction over various plasma-treated graphite electrodes. Electrochim. Acta 244, 38–46. [Google Scholar]
- Khataee AR, Safarpour M, Zarei M, Aber S, 2011. Electrochemical generation of H2O2 using immobilized carbon nanotubes on graphite electrode fed with air: Investigation of operational parameters. J. Electroanal. Chem 659, 63–68. [Google Scholar]
- Kim HW, Ross MB, Kornienko N, Zhang L, Guo J, Yang P, McCloskey BD, 2018. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nature Catalysis 1, 282–290. [Google Scholar]
- Kim JR, Jung SH, Regan JM, Logan BE, 2007. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresource Technol 98, 2568–2577. [DOI] [PubMed] [Google Scholar]
- Le TXH, Bechelany M, Champavert J, Cretin M, 2015a. A highly active based graphene cathode for the electro-fenton reaction. RSC Adv 5, 42536–42539. [Google Scholar]
- Le TXH, Bechelany M, Lacour S, Oturan N, Oturan MA, Cretin M, 2015b. High removal efficiency of dye pollutants by electron-Fenton process using a graphene based cathode. Carbon 94, 1003–1011. [Google Scholar]
- Lee YH, Li F, Chang KH, Hu CC, Ohsaka T, 2012. Novel synthesis of N-doped porous carbons from collagen for electrocatalytic production of H2O2. Appl. Catal. B-Environ 126, 208–214. [Google Scholar]
- Lei H, Li H, Li Z, Li Z, Chen K, Zhang X, Wang H, 2010. Electro-Fenton degradation of cationic red X-GRL using an activated carbon fiber cathode. Process Saf. Environ 88, 431–438. [Google Scholar]
- Li J, Chen G, Zhu Y, Liang Z, Pei A, Wu C-L, Wang H, Lee HR, Liu K, Chu S, Cui Y, 2018. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nature Catalysis 1, 592–600. [Google Scholar]
- Li K, Liu J, Li J, Wan Z, 2018. Effects of N mono- and N/P dual-doping on H2O2, OH generation, and MB electrochemical degradation efficiency of activated carbon fiber electrodes. Chemosphere 193, 800–810. [DOI] [PubMed] [Google Scholar]
- Li Q, Batchelor-Mcauley C, Lawrence NS, Hartshorne RS, Jones CJV, Compton RG, 2014. A flow system for hydrogen peroxide production at reticulated vitreous carbon via electroreduction of oxygen. J. Solid State Electr 18, 1215–1221. [Google Scholar]
- Li WB, Wang JX, Gong H, 2009. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 148, 81–87. [Google Scholar]
- Li Y, Shen W, Fu S, Yang H, Yu G, Wang Y, 2015. Inhibition of bromate formation during drinking water treatment by adapting ozonation to electro-peroxone process. Chem. Eng. J 264, 322–328. [Google Scholar]
- Limvoranusorn P, Cooper CD, Dietz JD, Clausen C. a., Pettey L, Collins MM, 2005. Kinetic modeling of the gas-phase oxidation of nitric oxide using hydrogen peroxide. J. Environ. Eng 131, 518–525. [Google Scholar]
- Liu G, Ji J, Huang H, Xie R, Feng Q, Shu Y, Zhan Y, Fang R, He M, Liu S, Ye X, Leung DYC, 2017. UV/H2O2: An efficient aqueous advanced oxidation process for VOCs removal. Chem. Eng. J 324, 44–50. [Google Scholar]
- Liu H, Cheng S, Logan BE, 2005. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol 39, 5488–5493. [DOI] [PubMed] [Google Scholar]
- Liu J, Song P, Ruan M, Xu W, 2016. Catalytic properties of graphitic and pyridinic nitrogen doped on carbon black for oxygen reduction reaction. Chinese J. Catal 37, 1119–1126. [Google Scholar]
- Liu T, Wang K, Song S, Brouzgou A, Tsiakaras P, Wang Y, 2016. New Electro-Fenton gas diffusion cathode based on nitrogen-doped graphene@carbon nanotube composite materials. Electrochim. Acta 194, 228–238. [Google Scholar]
- Liu Y, Chen S, Quan X, Yu H, Zhao H, Zhang Y, 2015a. Efficient mineralization of perfluorooctanoate by Electro-Fenton with H2O2 electro-generated on hierarchically porous carbon. Environ. Sci. Technol 49, 13528–13533. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nie C, Liu X, Xu X, Sun Z, Pan L, 2015. Review on carbon-based composite materials for capacitive deionization. RSC Adv 5, 15205–15225. [Google Scholar]
- Liu Y, Quan X, Fan X, Wang H, Chen S, 2015b. High-yield electrosynthesis of hydrogen peroxide from oxygen reduction by hierarchically porous carbon. Angew. Chem. Int. Edit 54, 6837–6841. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Q, Yin Y, Pan J, Zhang J, 2014a. Advanced oxidation removal of NO and SO2 from flue gas by using ultraviolet/H2O2/NaOH process. Chem. Eng. Res. Des 92, 1907–1914. [Google Scholar]
- Liu Y, Zhang J, Pan J, 2014b. Photochemical oxidation removal of Hg0 from flue gas containing SO2/NO by an ultraviolet irradiation/hydrogen peroxide (UV/H2O2) process. Energy Fuel 28, 2135–2143. [Google Scholar]
- Liu Y, Zhang J, Sheng C, 2011. Kinetic model of NO removal from SO2-containing simulated flue gas by wet UV/H2O2 advanced oxidation process. Chem. Eng. J 168, 183–189. [Google Scholar]
- Liu YX, Zhang J, 2011. Photochemical oxidation removal of NO and SO2 from simulated flue gas of coal-fired power plants by wet scrubbing using UV/H2O2 advanced oxidation process. Ind. Eng. Chem. Res 50, 3836–3841. [Google Scholar]
- Logan B, Cheng S, Watson V, Estadt G, 2007. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol 41, 3341–3346. [DOI] [PubMed] [Google Scholar]
- Lu Y, Liu G, Luo H, Zhang R, 2017. Efficient in-situ production of hydrogen peroxide using a novel stacked electrosynthesis reactor. Electrochim. Acta 248, 29–36. [Google Scholar]
- Lu Z, Chen G, Siahrostami S, Chen Z, Liu K, Xie J, Liao L, Wu T, Lin D, Liu Y, Jaramillo TF, Nørskov JK, Cui Y, 2018. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nature Catalysis 1, 156–162. [Google Scholar]
- Luo H, Li C, Wu C, Zheng W, Dong X, 2015. Electrochemical degradation of phenol by in situ electro-generated and electro-activated hydrogen peroxide using an improved gas diffusion cathode. Electrochim. Acta 186, 486–493. [Google Scholar]
- Lyu L, Zhang L, He G, He H, Hu C, 2017. Selective H2O2 conversion to hydroxyl radicals in the electron-rich area of hydroxylated C-g-C3N4/CuCo-Al2O3. J. Mater. Chem. A 5, 7153–7164. [Google Scholar]
- Lyu L, Zhang L, Wang Q, Nie Y, Hu C, 2015. Enhanced Fenton catalytic efficiency of γ-Cu-Al2O3 by σ-Cu2+-ligand complexes from aromatic pollutant degradation. Environ. Sci. Technol 49, 8639–8647. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhou M, Ren G, Yang W, Liang L, 2016. A highly energy-efficient flow-through electro-Fenton process for organic pollutants degradation. Electrochim. Acta 200, 222–230. [Google Scholar]
- Ma P, Ma H, Galia A, Sabatino S, Scialdone O, 2018. Reduction of oxygen to H2O2 at carbon felt cathode in undivided cells. Effect of the ratio between the anode and the cathode surfaces and of other operative parameters. Sep. Purif. Technol 208, 116–122. [Google Scholar]
- Mao Y, Guo D, Yao W, Wang X, Yang H, Xie YF, Komarneni S, Yu G, Wang Y, 2018. Effects of conventional ozonation and electro-peroxone pretreatment of surface water on disinfection by-product formation during subsequent chlorination. Water Res 130, 322–332. [DOI] [PubMed] [Google Scholar]
- Martínez-Huitle CA, Brillas E, 2009. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B-Environ 87, 3–4. [Google Scholar]
- Martínez-Huitle CA, Brillas E, 2008. Electrochemical alternatives for drinking water disinfection. Angew. Chem. Int. Edit 47, 1998–2005. [DOI] [PubMed] [Google Scholar]
- Martínez-Huitle CA, Dos Santos EV, De Araújo DM, Panizza M, 2012. Applicability of diamond electrode/anode to the electrochemical treatment of a real textile effluent. J. Electroanal. Chem 674, 103–107. [Google Scholar]
- Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O, 2015. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev 115, 13362–13407. [DOI] [PubMed] [Google Scholar]
- Martinez AI, Deshpande BK, 2007. Kinetic modeling of H2O2-enhanced oxidation of flue gas elemental mercury. Fuel Process. Technol 88, 982–987. [Google Scholar]
- Menegazzo F, Manzoli M, Signoretto M, Pinna F, Strukul G, 2015. H2O2 direct synthesis under mild conditions on Pd-Au samples: Effect of the morphology and of the composition of the metallic phase. Catal. Today 248, 18–27. [Google Scholar]
- Miao J, Zhu H, Tang Y, Chen Y, Wan P, 2014. Graphite felt electrochemically modified in H2SO4 solution used as a cathode to produce H2O2 for pre-oxidation of drinking water. Chem. Eng. J 250, 312–318. [Google Scholar]
- Moraes A, Assumpção MHMT, Papai R, Gaubeur I, Rocha RS, Reis RM, Calegaro ML, Lanza MRV, Santos MC, 2014. Use of a vanadium nanostructured material for hydrogen peroxide electrogeneration. J. Electroanal. Chem 719, 127–132. [Google Scholar]
- Neyens E, Baeyens J, 2003. A Reivew of Classic Fenton’s Peroxidaion as an Advanced Technique. J. Hazard. Mater B98, 33–50. [DOI] [PubMed] [Google Scholar]
- Nidheesh PV, Gandhimathi R, 2012. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 45, 2611–2692. [Google Scholar]
- Nie Y, Li L, Wei Z, 2015. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev 44, 2168–2201. [DOI] [PubMed] [Google Scholar]
- Oturan MA, Aaron JJ, 2014. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Env. Sci. Tec 44, 2577–2641. [Google Scholar]
- Oturan MA, Peiroten J, Chartrin P, Acher AJ, 2000. Complete destruction of p-Nitrophenol in aqueous medium by electro-fenton method. Environ. Sci. Technol 34, 3474–3479. [Google Scholar]
- Oturan N, Brillas E, Oturan MA, 2012. Unprecedented total mineralization of atrazine and cyanuric acid by anodic oxidation and electro-Fenton with a boron-doped diamond anode. Environ. Chem. Lett 10, 165–170. [Google Scholar]
- Özcan A, Şahin Y, Koparal AS, Oturan MA, 2008a. Degradation of picloram by the electro-Fenton process. J. Hazard. Mater 153, 718–727. [DOI] [PubMed] [Google Scholar]
- Özcan A, Şahin Y, Savaş Koparal A, Oturan MA, 2008b. Carbon sponge as a new cathode material for the electro-Fenton process: Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J. Electroanal. Chem 616, 71–78. [Google Scholar]
- Pan Z, Wang K, Wang Y, Tsiakaras P, Song S, 2018. In-situ electrosynthesis of hydrogen peroxide and wastewater treatment application: A novel strategy for graphite felt activation. Appl. Catal. B-Environ 237, 392–400. [Google Scholar]
- Panizza M, Cerisola G, 2008. Electrochemical generation of H2O2 in low ionic strength media on gas diffusion cathode fed with air. Electrochim. Acta 54, 876–878. [Google Scholar]
- Park J, Nabae Y, Hayakawa T, Kakimoto MA, 2014. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon. ACS Catal 4, 3749–3754. [Google Scholar]
- Paz EC, Aveiro LR, Pinheiro VS, Souza FM, Lima VB, Silva FL, Hammer P, Lanza MRV, Santos MC, 2018. Evaluation of H2O2 electrogeneration and decolorization of Orange II azo dye using tungsten oxide nanoparticle-modified carbon. Appl. Catal. B-Environ 232, 436–445. [Google Scholar]
- Perazzolo V, Durante C, Pilot R, Paduano A, Zheng J, Rizzi GA, Martucci A, Granozzi G, Gennaro A, 2015. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 95, 949–963. [Google Scholar]
- Pérez JF, Llanos J, Sáez C, López C, Cañizares P, Rodrigo MA, 2016. Electrochemical jet-cell for the in-situ generation of hydrogen peroxide. Electrochem. Commun 71, 65–68. [Google Scholar]
- Pérez JF, Sabatino S, Galia A, Rodrigo MA, Llanos J, Sáez C, Scialdone O, 2018. Effect of air pressure on the electro-Fenton process at carbon felt electrodes. Electrochim. Acta 273, 447–453. [Google Scholar]
- Pérez T, Coria G, Sirés I, Nava JL, Uribe AR, 2018. Electrosynthesis of hydrogen peroxide in a filter-press flow cell using graphite felt as air-diffusion cathode. J. Electroanal. Chem 812, 54–58. [Google Scholar]
- Pignatello JJ, Oliveros E, MacKay A, 2006. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Env. Sci. Tec 36, 1–84. [Google Scholar]
- Pinheiro VS, Paz EC, Aveiro LR, Parreira LS, Souza FM, Camargo PHC, Santos MC, 2018. Ceria high aspect ratio nanostructures supported on carbon for hydrogen peroxide electrogeneration. Electrochim. Acta 259, 865–872. [Google Scholar]
- Pizzutilo E, Kasian O, Choi CH, Cherevko S, Hutchings GJ, Mayrhofer KJJ, Freakley SJ, 2017. Electrocatalytic synthesis of hydrogen peroxide on Au-Pd nanoparticles: From fundamentals to continuous production. Chem. Phys. Lett 683, 436–442. [Google Scholar]
- Poddar TK, Sirkar KK, 1997. A hybrid of vapor permeation and membrane-based absorption-stripping for VOC removal and recovery from gaseous emissions. J. Membrane Sci 132, 229–233. [Google Scholar]
- Priambodo R, Shih Y, Huang Y, Huang Y, 2011. Treatment of real wastewater using semi-batch (Photo)-Electro-Fenton method. Sustain. Environ. Res 21, 389–393. [Google Scholar]
- Qiang Z, Chang JH, Huang CP, 2003. Electrochemical regeneration of Fe2+ in Fenton oxidation processes. Water Res 37, 1308–1319. [DOI] [PubMed] [Google Scholar]
- Qiang ZM, Chang JH, Huang CP, 2002. Electrochemical generation of hydrogen peroxide from dissolved oxygen in acidic solutions. Water Res 36, 85–94. [DOI] [PubMed] [Google Scholar]
- Qie L, Chen WM, Wang ZH, Shao QG, Li X, Yuan LX, Hu XL, Zhang WX, Huang YH, 2012. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater 24, 2047–2050. [DOI] [PubMed] [Google Scholar]
- Qu L, Liu Y, Baek J-B, Dai L, 2010. Nitrogen-Doped Graphene as Efficient Metal-Free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4, 1321–1326. [DOI] [PubMed] [Google Scholar]
- Raj CR, Samanta A, Noh SH, Mondal S, Okajima T, Ohsaka T, 2016. Emerging new generation electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 4, 11156–11178. [Google Scholar]
- Ren G, Zhou M, Liu M, Ma L, Yang H, 2016. A novel vertical-flow electro-Fenton reactor for organic wastewater treatment. Chem. Eng. J 298, 55–67. [Google Scholar]
- Ribeiro RS, Silva AMT, Figueiredo JL, Faria JL, Gomes HT, 2013. The influence of structure and surface chemistry of carbon materials on the decomposition of hydrogen peroxide. Carbon 62, 97–108. [Google Scholar]
- Riccobono G, Pastorella G, Vicari F, D’Angelo A, Galia A, Quatrini P, Scialdone O, 2017. Abatement of AO7 in a divided microbial fuel cells by sequential cathodic and anodic treatment powered by different microorganisms. J. Electroanal. Chem 799, 293–298. [Google Scholar]
- Ridruejo C, Alcaide F, Álvarez G, Brillas E, Sirés I, 2018. On-site H2O2 electrogeneration at a CoS2-based air-diffusion cathode for the electrochemical degradation of organic pollutants. J. Electroanal. Chem 808, 364–371. [Google Scholar]
- Roldán L, Truong-Phuoc L, Ansón-Casaos A, Pham-Huu C, García-Bordejé E, 2018. Mesoporous carbon doped with N,S heteroatoms prepared by one-pot auto-assembly of molecular precursor for electrocatalytic hydrogen peroxide synthesis. Catal. Today 301, 2–10. [Google Scholar]
- Russo V, Tesser R, Santacesaria E, Di Serio M, 2013. Chemical and technical aspects of propene oxide production via hydrogen peroxide (HPPO process). Ind. Eng. Chem. Res 52, 1168–1178. [Google Scholar]
- Salari D, Niaei A, Khataee A, Zarei M, 2009. Electrochemical treatment of dye solution containing C.I. Basic Yellow 2 by the peroxi-coagulation method and modeling of experimental results by artificial neural networks. J. Electroanal. Chem 629, 117–125. [Google Scholar]
- Sánchez-Sánchez CM, Bard AJ, 2009. Hydrogen peroxide production in the oxygen reduction reaction at different electrocatalysts as quantified by scanning electrochemical microscopy. Anal. Chem 81, 8094–8100. [DOI] [PubMed] [Google Scholar]
- Scialdone O, Galia A, Gattuso C, Sabatino S, Schiavo B, 2015. Effect of air pressure on the electro-generation of H2O2 and the abatement of organic pollutants in water by electro-Fenton process. Electrochim. Acta 182, 775–780. [Google Scholar]
- Shemer H, Kunukcu YK, Linden KG, 2006. Degradation of the pharmaceutical Metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 63, 269–276. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Song S, Wang X, Song L, Wang C, Sun H, Niu X, 2011. Electrogeneration of hydrogen peroxide on a novel highly effective acetylene black-PTFE cathode with PTFE film. Electrochim. Acta 56, 8651–8656. [Google Scholar]
- Sheng Y, Zhao Y, Wang X, Wang R, Tang T, 2014. Electrogeneration of H2O2 on a composite acetylene black-PTFE cathode consisting of a sheet active core and a dampproof coating. Electrochim. Acta 133, 414–421. [Google Scholar]
- Shin KY, Hong JY, Jang J, 2011. Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: Isotherms and kinetic study. J. Hazard. Mater 190, 36–44. [DOI] [PubMed] [Google Scholar]
- Shiraishi F, Nakasako T, Hua Z, 2003. Formation of hydrogen peroxide in photocatalytic reactions. J. Phys. Chem. A 107, 11072–11081. [Google Scholar]
- Si W, Zhou J, Zhang S, Li S, Xing W, Zhuo S, 2013. Tunable N-doped or dual N, S-doped activated hydrothermal carbons derived from human hair and glucose for supercapacitor applications. Electrochim. Acta 107, 397–405. [Google Scholar]
- Siahrostami S, Verdaguer-Casadevall A, Karamad M, Deiana D, Malacrida P, Wickman B, Escudero-Escribano M, Paoli EA, Frydendal R, Hansen TW, Chorkendorff I, Stephens IEL, Rossmeisl J, 2013. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater 12, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M, 2014. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res 21, 8336–8367. [DOI] [PubMed] [Google Scholar]
- Skoumal M, Rodríguez RM, Cabot PL, Centellas F, Garrido JA, Arias C, Brillas E, 2009. Electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton degradation of the drug ibuprofen in acid aqueous medium using platinum and boron-doped diamond anodes. Electrochim. Acta 54, 2077–2085. [Google Scholar]
- Soltani RDC, Rezaee A, Khataee AR, Godini H, 2013. Electrochemical generation of hydrogen peroxide using carbon black-, carbon nanotube-, and carbon black/ carbon nanotube-coated gas-diffusion cathodes: effect of operational parameters and decolorization study 39, 4277–4286. [Google Scholar]
- Sun M, Chu C, Geng F, Lu X, Qu J, Crittenden J, Elimelech M, Kim JH, 2018. Reinventing Fenton chemistry: Iron oxychloride nanosheet for pH-insensitive H2O2 activation. Environ. Sci. Tech. Lett 5, 186–191. [Google Scholar]
- Sun Y, Sinev I, Ju W, Bergmann A, Dresp S, Kühl S, Spöri C, Schmies H, Wang H, Bernsmeier D, Paul B, Schmack R, Kraehnert R, Roldan Cuenya B, Strasser P, 2018. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts. ACS Catal 8, 2844–2856. [Google Scholar]
- Tabti Z, Ruiz-Rosas R, Quijada C, Cazorla-Amorós D, Morallón E, 2014. Tailoring the surface chemistry of activated carbon cloth by electrochemical methods. ACS Appl. Mater. Inter 6, 11682–11691. [DOI] [PubMed] [Google Scholar]
- Tang X, Guo K, Li H, Du Z, Tian J, 2011. Electrochemical treatment of graphite to enhance electron transfer from bacteria to electrodes. Bioresource Technol 102, 3558–3560. [DOI] [PubMed] [Google Scholar]
- Thostenson JO, Ngaboyamahina E, Sellgren KL, Hawkins BT, Piascik JR, Klem EJD, Parker CB, Deshusses MA, Stoner BR, Glass JT, 2017. Enhanced H2O2 Production at Reductive Potentials from Oxidized Boron-Doped Ultrananocrystalline Diamond Electrodes. ACS Appl. Mater. Inter 9, 16610–16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valim RB, Reis RM, Castro PS, Lima AS, Rocha RS, Bertotti M, Lanza MRV, 2013. Electrogeneration of hydrogen peroxide in gas diffusion electrodes modified with tert-butyl-anthraquinone on carbon black support. Carbon 61, 236–244. [Google Scholar]
- Vasudevan S, Oturan MA, 2014. Electrochemistry: As cause and cure in water pollution-an overview. Environ. Chem. Lett 12, 97–108. [Google Scholar]
- Verdaguer-Casadevall A, Deiana D, Karamad M, Siahrostami S, Malacrida P, Hansen TW, Rossmeisl J, Chorkendorff I, Stephens IEL, 2014. Trends in the electrochemical synthesis of H2O2: Enhancing activity and selectivity by electrocatalytic site engineering. Nano Lett 14, 1603–1608. [DOI] [PubMed] [Google Scholar]
- Vončina DB, Majcen-Le-Marechal A, 2003. Reactive dye decolorization using combined ultrasound/H2O2. Dyes Pigments 59, 173–179. [Google Scholar]
- Wang A, Qu J, Liu H, Ru J, 2008. Mineralization of an azo dye Acid Red 14 by photoelectro-Fenton process using an activated carbon fiber cathode. Appl. Catal. B-Environ 84, 393–399. [Google Scholar]
- Wang A, Qu J, Ru J, Liu H, Ge J, 2005. Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dyes Pigments 65, 227–233. [Google Scholar]
- Wang CT, Chou WL, Chung MH, Kuo YM, 2010. COD removal from real dyeing wastewater by electro-Fenton technology using an activated carbon fiber cathode. Desalination 253, 129–134. [Google Scholar]
- Wang CT, Hu JL, Chou WL, Kuo YM, 2008. Removal of color from real dyeing wastewater by Electro-Fenton technology using a three-dimensional graphite cathode. J. Hazard. Mater 152, 601–606. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang S, 2018. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J 334, 1502–1517. [Google Scholar]
- Wang JL, Xu LJ, 2012. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Env. Sci. Tec 42, 251–325. [Google Scholar]
- Wang Y, Liu Y, Wang K, Song S, Tsiakaras P, Liu H, 2015. Preparation and characterization of a novel KOH activated graphite felt cathode for the electro-Fenton process. Appl. Catal. B-Environ 165, 360–368. [Google Scholar]
- Wang Y, Zhou W, Gao J, Ding Y, Kou K, 2019. Oxidative modification of graphite felts for efficient H2O2 electrogeneration : Enhancement mechanism and long-term stability. J. Electroanal. Chem 833, 258–268. [Google Scholar]
- Wroblowa HS, Pan Yen-Chi, Razumney G, 1976. Electroreduction of oxygen. J. Electroanal. Chem. Interfacial Electrochem 69, 195–201. [Google Scholar]
- Xia G, Wang Y, Wang B, Huang J, Deng S, Yu G, 2017. The competition between cathodic oxygen and ozone reduction and its role in dictating the reaction mechanisms of an electro-peroxone process. Water Res 118, 26–38. [DOI] [PubMed] [Google Scholar]
- Xing M, Xu W, Dong C, Bai Y, Zeng J, Zhou Y, Zhang J, Yin Y, 2018. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 4, 1359–1372. [Google Scholar]
- Xu Y, Cao L, Sun W, Yang J, 2017. In-situ catalytic oxidation of Hg0 via a gas diffusion electrode. Chem. Eng. J 310, 170–178. [Google Scholar]
- Yamanaka I, 2018. Direct synthesis of pure H2O2 qqueous solution by CoTPP/Ketjen-black electrocatalyst and the fuel cell reactor. Electrocatalysis 9, 236–242. [Google Scholar]
- Yamanaka I, 2014. Direct and safe synthesis of H2O2 from O2 and H2 using fuel cell reactors. J. Jap. Petrol. Inst 57, 237–250. [Google Scholar]
- Yang H, Vogel W, Lamy C, Alonso-Vante N, 2004. Structure and electrocatalytic activity of carbon-supported Pt-Ni alloy nanoparticles toward the oxygen reduction reaction. J. Phys. Chem. B 108, 11024–11034. [Google Scholar]
- Yang S, Verdaguer-Casadevall A, Arnarson L, Silvioli L, Čolić V, Frydendal R, Rossmeisl J, Chorkendorff I, Stephens IEL, 2018. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis. ACS Catal 8, 4064–4081. [Google Scholar]
- Yang W, Zhou M, Cai J, Liang L, Ren G, Jiang L, 2017. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. J. Mater. Chem. A 5, 8070–8080. [Google Scholar]
- Yang X. jing, Xu X. meng, Xu X. chao, Xu J., Wang, lin H, Semiat R, Han Y. fan, 2016. Modeling and kinetics study of Bisphenol A (BPA) degradation over an FeOCl/SiO2 Fenton-like catalyst. Catal. Today 276, 85–96. [Google Scholar]
- Yang Y, He F, Shen Y, Chen X, Mei H, Liu S, Zhang Y, 2017. A biomass derived N/C-catalyst for the electrochemical production of hydrogen peroxide. Chem. Commun 53, 9994–9997. [DOI] [PubMed] [Google Scholar]
- Yao W, Qu Q, Von Gunten U, Chen C, Yu G, Wang Y, 2017. Comparison of methylisoborneol and geosmin abatement in surface water by conventional ozonation and an electro-peroxone process. Water Res 108, 373–382. [DOI] [PubMed] [Google Scholar]
- Yao W, Wang X, Yang H, Yu G, Deng S, Huang J, Wang B, Wang Y, 2016. Removal of pharmaceuticals from secondary effluents by an electro-peroxone process. Water Res 88, 826–835. [DOI] [PubMed] [Google Scholar]
- Yao W, Waqi S, Rehman U, Wang H, Yang H, Yu G, Wang Y, 2018. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res 138, 106–117. [DOI] [PubMed] [Google Scholar]
- Yi Y, Wang L, Li G, Guo H, 2016. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: Noble-metal catalytic method, fuel-cell method and plasma method. Catal. Sci. Technol 6, 1593–1610. [Google Scholar]
- Yoon C-M, Long D, Jang S-M, Qiao W, Ling L, Miyawaki J, Rhee C-K, Mochida I, Yoon S-H, 2011. Electrochemical surface oxidation of carbon nanofibers. Carbon 49, 96–105. [Google Scholar]
- Young MN, Links MJ, Popat SC, Rittmann BE, Torres CI, 2016. Tailoring microbial electrochemical cells for production of hydrogen peroxide at high concentrations and efficiencies. ChemSusChem 9, 3345–3352. [DOI] [PubMed] [Google Scholar]
- Yu F, Zhou M, Yu X, 2015. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim. Acta 163, 182–189. [Google Scholar]
- Yu F, Zhou M, Zhou L, Peng R, 2014. A Novel Electro-Fenton Process with H2O2 Generation in a Rotating Disk Reactor for Organic Pollutant Degradation. Environ. Sci. Tech. Lett 1, 320–324. [Google Scholar]
- Yu X, Zhou M, Hu Y, Groenen Serrano K, Yu F, 2014. Recent updates on electrochemical degradation of bio-refractory organic pollutants using BDD anode: A mini review. Environ. Sci. Pollut. Res 21, 8417–8431. [DOI] [PubMed] [Google Scholar]
- Yu X, Zhou M, Ren G, Ma L, 2015a. A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-Fenton. Chem. Eng. J 263, 92–100. [Google Scholar]
- Yu X, Zhou M, Ren G, Ma L, 2015b. A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-Fenton. Chem. Eng. J 263, 92–100. [Google Scholar]
- Yuan S, Gou N, Alshawabkeh AN, Gu AZ, 2013. Efficient degradation of contaminants of emerging concerns by a new electro-Fenton process with Ti/MMO cathode. Chemosphere 93, 2796–2804. [DOI] [PubMed] [Google Scholar]
- Yuan S, Li Z, Wang Y, 2013. Effective degradation of methylene blue by a novel electrochemically driven process. Electrochem. Comm 29, 48–51. [Google Scholar]
- Yun Y, Li Z, Chen Y-H, Saino M, Cheng S, Zheng L, 2016. Catalytic reduction of nitrate in secondary effluent of wastewater treatment plants by Fe0 and Pd-Cu/ -Al2O3. Water Sci. Technol 73, 2697–2703. [DOI] [PubMed] [Google Scholar]
- Zarei M, Salari D, Niaei A, Khataee A, 2009. Peroxi-coagulation degradation of C.I. Basic Yellow 2 based on carbon-PTFE and carbon nanotube-PTFE electrodes as cathode. Electrochim. Acta 54, 6651–6660. [Google Scholar]
- Zhan J, Wang H, Pan X, Wang J, Yu G, Deng S, Huang J, Wang B, Wang Y, 2016a. Simultaneous regeneration of p-nitrophenol-saturated activated carbon fiber and mineralization of desorbed pollutants by electro-peroxone process. Carbon 101, 399–408. [Google Scholar]
- Zhan J, Wang Y, Wang H, Shen W, Pan X, Wang J, Yu G, 2016b. Electro-peroxone regeneration of phenol-saturated activated carbon fiber : The effects of irreversible adsorption and operational parameters. Carbon 109, 321–330. [Google Scholar]
- Zhang G, Yang F, Gao M, Fang X, Liu L, 2008. Electro-Fenton degradation of azo dye using polypyrrole/anthraquinonedisulphonate composite film modified graphite cathode in acidic aqueous solutions. Electrochim. Acta 53, 5155–5161. [Google Scholar]
- Zhang H, Wan X, Li G, Zhang F, 2017. A three-electrode electro-Fenton system supplied by self-generated oxygen with automatic pH-regulation for groundwater remediation. Electrochim. Acta 250, 42–48. [Google Scholar]
- Zhang J, Jiao XL, Xia YG, Liu FF, Pang YP, Zhao XF, Chen DR, 2016. Enhanced catalytic activity in liquid-exfoliated FeOCl nanosheets as a Fenton-like catalyst. Chem.-Eur. J 22, 9321–9329. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xi J, Li Z, Zhou H, Liu L, Wu Z, Qiu X, 2013. Electrochemical activation of graphite felt electrode for VO2+/VO2+ redox couple application. Electrochim. Acta 89, 429–435. [Google Scholar]
- Zhang X, Fu J, Zhang Y, Lei L, 2008. A nitrogen functionalized carbon nanotube cathode for highly efficient electrocatalytic generation of H2O2 in Electro-Fenton system. Sep. Purif. Technol 64, 116–123. [Google Scholar]
- Zhang X, Lei L, Xia B, Zhang Y, Fu J, 2009. Oxidization of carbon nanotubes through hydroxyl radical induced by pulsed O2 plasma and its application for O2 reduction in electro-Fenton. Electrochim. Acta 54, 2810–2817. [Google Scholar]
- Zhang Z, Meng H, Wang Y, Shi L, Wang X, Chai S, 2018. Fabrication of graphene@graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process. Electrochim. Acta 260, 112–120. [Google Scholar]
- Zhao H, Sun C, Jin Z, Wang D-W, Yan X, Chen Z, Zhu G, Yao X, 2015. Carbon for the oxygen reduction reaction: a defect mechanism. J. Mater. Chem. A 3, 11736–11739. [Google Scholar]
- Zhao H, Wang Y, Wang Y, Cao T, Zhao G, 2012. Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism. Appl. Catal. B-Environ 125, 120–127. [Google Scholar]
- Zhao K, Quan X, Chen S, Yu H, Zhang Y, Zhao H, 2018a. Enhanced electro-Fenton performance by fluorine-doped porous carbon for removal of organic pollutants in wastewater. Chem. Eng. J 354, 606–615. [Google Scholar]
- Zhao K, Su Y, Quan X, Liu Y, Chen S, Yu H, 2018b. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon. J. Catal 357, 118–126. [Google Scholar]
- Zhao L, Li X, Quan X, Chen G, 2011. Effects of surface features on sulfur dioxide adsorption on calcined NiAl hydrotalcite-like compounds. Environ. Sci. Technol 45, 5373–5379. [DOI] [PubMed] [Google Scholar]
- Zhong R-S, Qin Y-H, Niu D-F, Tian J-W, Zhang X-S, Zhou X-G, Sun S-G, Yuan W-K, 2013. Effect of carbon nanofiber surface functional groups on oxygen reduction in alkaline solution. J. Power Sources 225, 192–199. [Google Scholar]
- Zhou C, Sun L, Zhang A, Ma C, Wang B, Yu J, Su S, Hu S, Xiang J, 2015. Elemental mercury (Hg0) removal from containing SO2/NO flue gas by magnetically separable Fe2.45Ti0.55O4/H2O2 advanced oxidation processes. Chem. Eng. J 273, 381–389. [Google Scholar]
- Zhou L, Hu Z, Zhang C, Bi Z, Jin T, Zhou M, 2013a. Electrogeneration of hydrogen peroxide for electro-Fenton system by oxygen reduction using chemically modified graphite felt cathode. Sep. Purif. Technol 111, 131–136. [Google Scholar]
- Zhou L, Zhou M, Hu Z, Bi Z, Serrano KG, 2014. Chemically modified graphite felt as an efficient cathode in electro-Fenton for p-nitrophenol degradation. Electrochim. Acta 140, 376–383. [Google Scholar]
- Zhou L, Zhou M, Zhang C, Jiang Y, Bi Z, Yang J, 2013b. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts. Chem. Eng. J 233, 185–192. [Google Scholar]
- Zhou M, Chi M, Luo J, He H, Jin T, 2011. An overview of electrode materials in microbial fuel cells. J. Power Sources 196, 4427–4435. [Google Scholar]
- Zhou M, Yu Q, Lei L, Barton G, 2007. Electro-Fenton method for the removal of methyl red in an efficient electrochemical system. Sep. Purif. Technol 57, 380–387. [Google Scholar]
- Zhou W, Ding Y, Gao J, Kou K, Wang Y, Meng X, Wu S, Qin Y, 2018a. Green electrochemical modification of RVC foam electrode and improved H2O2 electrogeneration by applying pulsed current for pollutant removal. Environ. Sci. Pollut. Res 6015–6025. [DOI] [PubMed] [Google Scholar]
- Zhou W, Gao J, Ding Y, Zhao H, Meng X, Wang Y, Kou K, Xu Y, Wu S, Qin Y, 2018b. Drastic enhancement of H2O2 electro-generation by pulsed current for ibuprofen degradation: Strategy based on decoupling study on H2O2 decomposition pathways. Chem. Eng. J 338, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Gao J, Kou K, Meng X, Wang Y, Ding Y, Xu Y, Zhao H, Wu S, Qin Y, 2018c. Highly efficient H2O2 electrogeneration from O2 reduction by pulsed current: Facilitated release of H2O2 from porous cathode to bulk. J. Taiwan Inst. Chem. Eng 83, 59–63. [Google Scholar]
- Zhou W, Gao J, Zhao H, Meng X, Wu S, 2017. The role of quinone cycle in Fe2+–H2O2 system in the regeneration of Fe2+. Environ. Technol 38, 1887–1896. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rajic L, Zhao Y, Gao J, Qin Y, Alshawabkeh AN, 2018d. Rates of H2O2 electrogeneration by reduction of anodic O2 at RVC foam cathodes in batch and flow-through cells. Electrochim. Acta 277, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qiu S, Ma F, Li G, Deng F, Zheng Y, 2018. Melamine-derived carbon electrode for efficient H2O2 electro-generation. Electrochim. Acta 261, 375–383. [Google Scholar]
- Zhu Z, Pan H, Murugananthan M, Gong J, Zhang Y, 2018. Visible light-driven photocatalytically active g-C3N4 material for enhanced generation of H2O2. Appl. Catal. B-Environ 232, 19–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.