Short abstract
Background
Vagus nerve stimulation (VNS) paired with a motor task improves motor outcome in rat stroke models. It is hypothesised that VNS delivered during rehabilitation will improve upper limb function compared to control rehabilitation therapy. Two pilot clinical studies demonstrated acceptable safety and feasibility of VNS paired with rehabilitation for improved upper limb function after stroke. Participants who received rehabilitation paired with VNS demonstrated clinically meaningful improvements in motor function that exceed gains seen among controls who received similar rehabilitation without VNS. These preliminary data support a larger pivotal trial.
Methods
VNS-REHAB (VNS-Rehabilitation) is a pivotal, multi-site, double-blinded, randomised trial designed to evaluate safety and efficacy of VNS paired with upper limb rehabilitation after ischaemic stroke. The study will include up to 120 participants with upper limb weakness due to stroke nine months to 10 years prior. All participants will be implanted with a VNS device and randomised to receive either Active (0.8 mA) or Control VNS (0.0 mA) paired with upper limb rehabilitation. All participants receive 18 sessions of in-clinic therapy for six weeks, followed by a home-based therapy for three months. The rehabilitation therapy involves progressive, functionally based and intensive practice of hand and arm tasks. VNS is delivered during each movement repetition. After blinded follow-up is completed, the Active vagus nerve stimulation group continues with home-based Active VNS and the Control group receive six weeks of in-clinic therapy with Active VNS followed by home-based Active VNS. The primary efficacy endpoint will be the difference in Fugl-Meyer assessment-upper extremity scores between the Active VNS and Control VNS groups at the end of six weeks of in-clinic therapy. Additional secondary endpoints will also be measured. Safety will be assessed with analysis of adverse events and device complications during study participation.
Discussion
This pivotal trial will determine whether VNS paired with rehabilitation is a safe and effective treatment for improving arm function after stroke.
Trial Registration: ClinicalTrials.gov, NCT03131960. Registered on 27 April 2017.
Keywords: Vagus nerve, stroke, rehabilitation, neuromodulation, upper limb, plasticity
Background
Stroke is the leading cause of long-term disability worldwide.1 A major contribution to this disability is upper limb weakness which severely affects quality of life.2 Rehabilitation improves upper limb function but the improvement is often incomplete and typically plateaus after 4–6 months.3 Combining rehabilitation with therapies that aim at augmenting neuroplasticity may help further improve upper limb function after stroke.
Animal studies have demonstrated that vagus nerve stimulation (VNS) during motor training improves forelimb recovery in rat models of ischaemic stroke.4–6 Repeatedly pairing brief bursts of VNS with movement modifies motor synaptic connectivity, reorganises motor cortex and facilitates recovery of the forelimb after ischaemic stroke.5,6 The mechanisms for VNS-driven improvements likely involves VNS activation of the cholinergic nucleus basalis and the noradrenergic locus coeruleus that engages the sensory-motor network during task-specific learning.7,8
In a recent multi-site, double-blinded randomised controlled pilot trial,9 all participants were implanted with a VNS device and received six weeks of in-clinic rehabilitation followed by a home exercise programme. Participants were randomised to Active VNS (n = 8) or Control VNS (n = 9) paired with rehabilitation. The average Fugl-Myer assessment-upper extremity (FMA-UE) scores increased 7.6 points after Active VNS compared to 5.3 points in Control VNS after six weeks in-clinic therapy (difference = 2.3 points, confidence interval (CI): –1.8 to 6.4, p = 0.20). Three months after the in-clinic therapy, FMA-UE scores increased by 9.5 points from baseline after Active VNS and 3.8 points from baseline after Control VNS (difference = 5.7 points; CI: –1.4 to 11.5, p = 0.055). Response rates, defined as FMA-UE change of six points or greater,10 were 88% with Active VNS and 33% with Control VNS (p < 0.05). No serious adverse events related to stimulation or therapy were reported. The study provided evidence of feasibility and safety of VNS paired with upper limb rehabilitation after stroke and, therefore, supported a pivotal trial for efficacy of the intervention. A first clinical feasibility study found similar results at the end of six weeks of in-clinic VNS (long-term home-based VNS was not a part of the feasibility study).11
We report the protocol for the pivotal (VNS-REHAB) trial of VNS paired with upper limb rehabilitation after ischaemic stroke. The primary objective of this trial is to provide evidence of efficacy and safety of VNS paired with rehabilitation. All participants will be implanted with the VNS device and then randomised to either Active VNS or Control VNS paired with upper limb rehabilitation. It is hypothesised that the Active VNS group will demonstrate greater improvements in their upper limb compared to the Control VNS group. Additionally, we expect the safety profile for VNS therapy for stroke rehabilitation to be comparable to VNS for epilepsy and depression.12
It is the intent that these data support a premarket approval application to Food and Drug Administration (FDA) for market clearance.
Methods
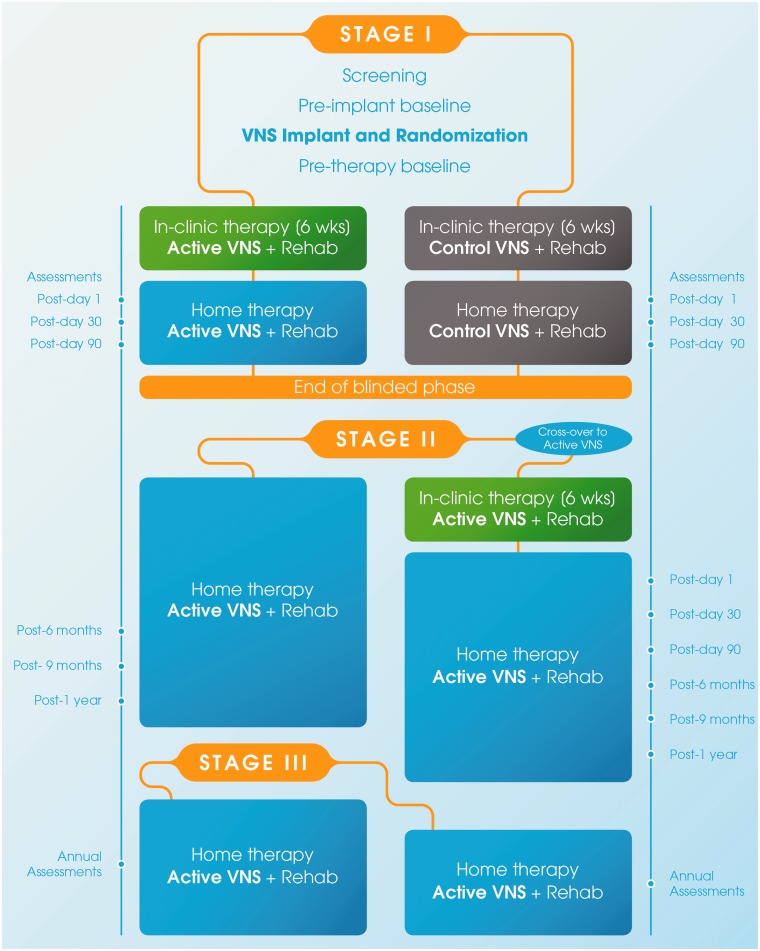
This pivotal (VNS-REHAB) study is a multi-site, double-blinded and randomised clinical trial of VNS paired with rehabilitation compared to Control VNS with rehabilitation in people with upper limb weakness after ischaemic stroke. The trial includes an initial blinded period (Stage I) during which participants receive in-clinic rehabilitation and a home exercise programme with or without VNS, dependent on randomisation allocation. The primary outcome is assessed during this period which lasts approximately six months. This is then followed by an open treatment phase where all participants receive Active VNS for one year (Stage II). After this, participants continue to receive home-based Active VNS and are assessed annually until commercial approval of the therapy (Stage III) (Figures 1 to 3).
Figure 1.
Study flow for all stages of the VNS-REHAB trial. During the blinded phase (Stage I), after initial screening and baseline measures, all participants will be implanted with the VNS device and randomised to receive either Active or Control VNS paired with upper limb rehabilitation. Both groups will receive in-clinic therapy for six weeks, followed by home-based therapy of three months. During Stage II (unblinded), the Active VNS group will continue with home-based Active VNS until the end of the year, while the Control group will cross-over to receive the Active VNS therapy. During Stage III, participants will continue to receive home-based active VNS therapy. Assessment timepoints for the Active VNS and Control VNS groups are shown on the left and right, respectively. VNS: vagus nerve stimulation; wks: weeks; green: in-clinic active VNS; grey: in-clinic and home-based control VNS; blue: home-based Active VNS.
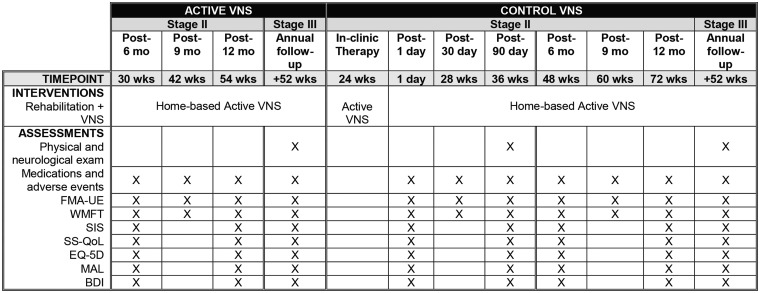
Figure 3.
Assessments and interventions. BDI: beck depression inventory; FMA-UE: Fugl-Meyer assessment-upper extremity; mo: months; MAL: motor activity log; SIS: stroke impact scale; SS-QOL: stroke specific quality of life; VNS: vagus nerve stimulation; wks: weeks; WMFT: Wolf motor function test. Note: Stages II and III: Stage II starts with a quarterly assessment for the Active VNS group three months after the end of Stage I (or 30 weeks after the start of the blinded in-clinic therapy). In contrast, the Control group starts the crossover therapy in Stage II and the therapy period will end 24 weeks after the blinded in-clinic therapy.
Trial status
The study is registered on ClinicalTrials.gov (NCT03131960, 27 April 2017). Participant recruitment started in 2017 (protocol version 1.1, 23 March 2017). Recruitment is still ongoing and, so far, more than 100 participants have been enrolled and 84 implanted. Enrolment is expected to close in Summer 2019.
Ethical/regulatory approval
Participants will be enrolled at centres in the United States (US) and United Kingdom (UK) and recruited from hospitals, clinics, and rehabilitation centres in proximity to study sites. The study is approved by an institutional review board (IRB) at each institution (US) or ethics committee (UK), and subject to appropriate regulatory approvals (US FDA Investigational Device Exemption (IDE, #G170031) and UK Medicines and Healthcare products Regulatory Agency (MHRA) No. #CI/2015/0011). The IRBs and principal investigators governing each site are listed in the Declarations section. All participants will provide/have provided written informed consent to designated staff at each site prior to undergoing any study procedures, in compliance with the requirements set forth in US FDA (Code of Federal Regulations Title 21). All relevant parties (investigators, IRB's and participants) will be notified of important protocol modifications. The study will be conducted according to the Declaration of Helsinki.
Recruitment and eligibility criteria
Participants aged between 22 and 80 years with a history of supratentorial ischaemic stroke that occurred at least nine months but not more than 10 years prior to enrolment will be included in the study. Potential participants are identified by clinical care teams and through use of advertisements. Once identified, site personnel discuss study requirements with potential participants including time commitments and entry criteria. Potential participants who are eligible for the study, meet with the site investigator and consent to the study. Study assessments are then performed to verify entry criteria. The study will enroll participants with moderate to severe upper limb weakness with an FMA-UE of 20–50 points. A full list of inclusion and exclusion criteria is provided in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
| 1. History of unilateral supratentorial ischaemic stroke that occurred at least nine months but not more than 10 years prior to enrolment.2. Age >22 years and <80 years. 3. FMA-UE score of 20 to 50 (inclusive of 20 and 50).4. Ability to communicate, understand and give appropriate consent. Participants should be able to follow two-step commands. 5. Right- or left-sided weakness of upper limb. 6. Active wrist flexion/extension; active abduction/extension of thumb and at least two additional digits. |
| Exclusion criteria |
| 1. History of haemorrhagic stroke. 2. Presence of ongoing dysphagia or aspiration difficulties. 3. Medication that may significantly interfere with the actions of VNS on neurotransmitter systems at study entry. 4. Prior injury to vagus nerve, either bilateral or unilateral. 5. Severe or worse depression (beck depression inventory >29). 6. Unfavourable candidacy for device implant surgery. 7. Current use of any other stimulation device, such as a pacemaker or other neurostimulator; current use of any other investigational device or drug. 8. Medical or mental instability. 9. Pregnancy or plans to become pregnant or to breastfeed during the study period. 10. Requirement of diathermy during the study duration. 11. Active rehabilitation within four weeks prior to consent. 12. Botulinum toxin injections or other non-study active rehabilitation of the upper limb within four weeks prior to therapy through the post-90 day visit after active VNS. 13. Severe spasticity of the upper limb (modified Ashworth ≥3). 14. Significant sensory loss. Sensory loss will be measured using the upper extremity sensory section of the Fugl-Meyer assessment of physical performance. The assessment addresses light touch (two items) and proprioception (four items). The highest point attained is 12; subjects with scores less than 6 will be excluded from the study. |
FMA-UE: Fugl-Meyer assessment-upper extremity; VNS: vagus nerve stimulation.
Screening and baseline assessments
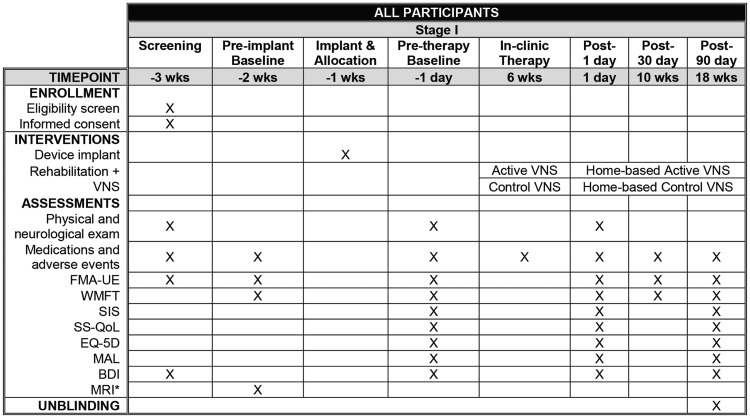
Participants will undergo a screening visit, a pre-implantation assessment and a post-implantation baseline assessment. These include the FMA-UE, Beck Depression Inventory (BDI), physical and neurological exams, and a brain magnetic resonance imaging (MRI) scan (Figure 2). The post-implantation visit just prior to the start of study therapy will be used in study analyses.
Figure 2.
Stage I: Enrolment, assessments, and interventions. *Optional. BDI: beck depression inventory; FMA-UE: Fugl-Meyer assessment-upper extremity; mo: months; MAL: motor activity log; MRI: magnetic resonance imaging; SIS: stroke impact scale; SS-QOL: stroke specific quality of life; VNS: vagus nerve stimulation; wks: weeks; WMFT: Wolf motor function test.
Randomisation (allocation to groups)
Subjects are randomised at implant surgery to either the investigational treatment (Active VNS) or Control VNS on a 1:1 basis. The Contract Research Organization (CRO) performing data analysis/collection will supply the randomisation sequence via email to an unblinded clinical engineer testing the device during implantation. The device is set to allow correct group settings such that site treatment personnel remain blinded. Randomisation is stratified by FMA-UE score and age to provide relatively equal distributions of upper limb impairment and age between groups. Personnel and patient blinding are described below under Interventions.
Device and implantation
The implantable and external devices for this study are identical in function to those used in the feasibility and pilot studies.11,13 Briefly, the device includes an implantable system (Vivistim System®) consisting of an implantable neurostimulator (implantable pulse generator or IPG, Model 1001, MicroTransponder Inc., Austin, TX) and an implantable lead and electrode (Model 3000, MicroTransponder Inc., Austin, TX). An external system consisting of a controller (Model 2000 Wireless Transmitter, MicroTransponder Inc., Austin, TX) and a software system (Stroke Application Programming Software, SAPS®) will allow control of settings for the IPG. Clinicians and therapists are trained in proper use of the system to provide VNS treatment during rehabilitation sessions. No changes to the device system are expected during the trial.
Device implantation takes place after the screening and pre-implant visits (Figures 1 and 2) and will follow similar guidelines as the pilot studies in humans.11,13 All participants implanted are considered fully enrolled into the study. Details of the surgical procedure have been published previously.11,13 Participants will be implanted under general anaesthesia. Operative times for primary VNS implantation vary, but are typically expected to take between 1 and 2 h. The participant will return home following an expected recovery period of 1 to 24 hours (same day surgery). Participants recover for approximately three to seven days before testing begins, depending on the investigator’s medical opinion and scheduling. If a subject discontinues the study or refuses further treatment after the end of the randomised phase, the IPG and the portion of the lead coiled in the chest may be removed. The electrode can be removed or left on the nerve. The surgeon will examine each subject after lead and IPG removal to verify appropriate recovery. No further follow-up will occur after device removal, and study staff will note subject participation status as completely discontinued at that time.
In-clinic rehabilitation therapy
In-clinic therapy will occur approximately three days a week for six weeks, for a total of 18 sessions. Participants will be considered compliant with treatment if they complete at least 12 sessions of in-clinic rehabilitation.
Stimulation parameters and therapist blinding
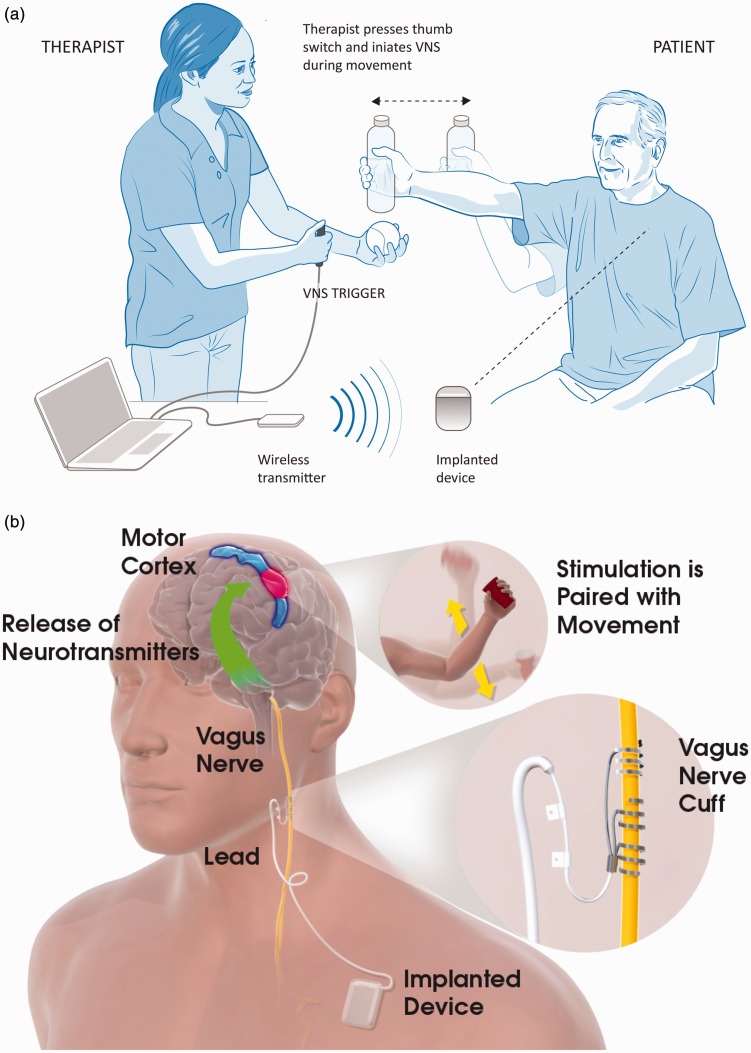
Stimulation parameters were selected based on preclinical studies and the two pilot human studies.11,13–15 An unblinded programmer at each site will set the device parameters prior to the first therapy session using password-protected functions in the SAPS® system. The programmer will not be involved in therapy or assessments to help maintain the blind. During each session, the therapist will use the SAPS® and a push-button or keyboard to trigger Active or Control VNS during upper limb movements (Figure 4(a)). The therapist is blinded to group allocation and will not be able to view the device parameters previously set by the unblinded programmer. The device is programmed to stimulate for 0.5 s on each button push followed by a safety period of 0.5 s during which no stimulation can be delivered. This feature prevents delivery of stimuli in rapid succession. The stimulation output current is set to 0.8 mA for the Active VNS group (or slightly lower, as discussed below) and 0 mA for Control VNS. Stimulation settings for every therapy session are recorded in the IPG.
Figure 4.
Vagus nerve stimulation (VNS) paired with rehabilitation. (a) In-clinic rehabilitation session set-up. The therapist is shown holding the VNS trigger button which delivers a VNS pulse when the participant performs the task-specific movements (e.g. lifting a bottle). Also depicted in the figure is the wireless transmitter on the table connected to the notebook via a USB cable. When the therapist presses the VNS trigger button, the wireless transmitter sends a signal to the implanted device which in turn stimulates the left vagus nerve via a cuff electrode in the participant’s neck. Note: The therapist typically stands near the participant to help adjust tasks and difficulty level. (b) Possible mechanism for VNS paired with movement. Stimulation of the vagus nerve stimulates the deep brain cholinergic nucleus basalis and noradrenergic locus coeruleus neurons (base of green arrow). Stimulation of the vagus nerve during task-specific movements (insert) modulates activity of the motor cortex (blue and red area) in a task-specific manner.
Tolerance
Prior to the start of the first therapy session, the unblinded programmer assesses the participant’s tolerance to VNS by gradually ramping-up stimulation from 0.5 mA to 0.8 mA in 0.1 mA steps. Participants who cannot tolerate 0.8 mA will receive therapy at a slightly lower tolerated intensity, although tolerance will then be assessed throughout the study to determine whether the current can be increased to 0.8 mA. Participants who are unable to feel 0.8 mA will have their perception tested at increasing levels in 0.1 mA steps, up to a maximum of 3.5 mA. This process will help confirm that the device is working correctly by verifying that the participant can feel some level of stimulation. Participants who only perceive currents above 0.8 mA will be told that they have high tolerance to VNS and that the standard study settings are below their perception level. Higher level (above 0.8 mA) will not be used during the rehabilitation therapy during the randomised study.
Participant blinding
At the start of each therapy session during the first few movements, both groups will receive one pulse of 0.8 mA (or slightly lower if required) VNS after which the intensity will be ramped down in 0.2 mA decrements (only four stimulation pulses per session). Thereafter, participants in the Active VNS group will receive 0.8 mA paired with each upper limb movement. Participants in the Control group will receive 0 mA VNS through the rest of the session, i.e. no stimulation current is delivered after the first four push-button presses when the therapist uses the push-button during ongoing rehabilitation. These procedures will help improve blinding by ensuring that Control participants will be treated the same as the Active VNS group, but no continuing stimulation will be delivered.
All participants will be told that they may or may not feel the stimulation during therapy, in fact, only about 25% of the subjects in the US pilot study13 felt 0.8 mA stimulation. Furthermore, participants in both groups will be told that they may initially feel the stimulation but may get acclimated to it over time (as occurs in VNS treatment for epilepsy and depression).16,17 These procedures, along with the stimulation ramp down at the beginning of each therapy session and the fact that all subjects will receive the same type of rehabilitation will help to maintain the blind of participants and therapists. At post-day 1, the primary endpoint time, study blinding will be assessed by asking participants if they know to which group they were assigned.
Rehabilitation exercises
Participants in both groups will receive similar goal-directed and intense upper limb rehabilitation which will follow evidence-based principles of motor learning for stroke rehabilitation.18 The therapy is focused on active movements, task specificity, high number of repetitions, variable practice and active participant engagement. Each task practiced includes movements involving reach, grasp, manipulation and release of objects, specifically related to the target task. There are six functional task categories: reach and grasp, gross movement, object flipping, simulated eating tasks, inserting objects and opening containers. Examples for movements that may be practiced in each category are shown in Table 2. Although tasks are standardised to ensure all participants perform similar movements, tasks are appropriately graded and progressed for each individual to adjust difficulty level and maintain participant engagement within each session and throughout the study. Approximately 30–50 repetitions of each task category are performed at each session. Each movement repetition is associated with a brief burst of VNS triggered by the therapist. The number of stimulations will vary depending on the number of movement repetitions. Since participants will have varying degrees of arm impairment, goals and functional deficits, the exact number of stimulation–movement pairings will vary across participants and sessions. On average, participants typically perform 300–500 repetitions during the in-clinic session. The average number of repetitions will be reported at study completion. Each in-clinic rehabilitation session is expected to last 90 to 120 min.
Table 2.
Paired VNS rehabilitation tasks.
| Task category | Examplea | VNS triggering |
|---|---|---|
| Reach and grasp objects |
|
|
| Gross movement |
|
|
| Flip objects |
|
|
| Eating tasksb |
|
|
| Insert objects |
|
|
| Open and close containers | Open:
|
|
Close:
|
||
| Patient-specific goal (optional) | Handwriting, self-care, playing an instrument | Varies with task |
Note: Each task category will be practiced with a minimum of 30 repetitions at every session.
Examples provided are for illustration purposes only. Letters (a–d) represent each part of the movement sequence to be practiced. Tasks are modified based on subject’s functional level and goals; suggestions for up or downgrading each task are provided to therapists.
Eating-related tasks are simulated to avoid risk of aspiration – subjects are not allowed to eat during therapy.
Home-based rehabilitation
During Stage I, home-based therapy (90 days, blinded) begins after completion of the in-clinic visits. Here, participants are provided a magnet to swipe over the device to self-initiate 30 min of VNS therapy while performing at-home exercises prescribed by the therapist. Each group receives stimulation according to their randomised group (Active 0.8 mA or Control 0.0 mA). Magnet activation of the device will result in a 0.5 s burst of VNS every 10 s for 30 min irrespective of the number of movements or performance speed. Therapists, assessors and participants will remain blinded through this period. In both groups, self-initiated stimulation will start at 0.8 mA and then the intensity will be ramped down for the following four VNS train pulses (similarly to in-clinic therapy). Thereafter, the current amplitude will be 0.8 mA for the Active VNS group and 0 mA for the Control group. All other parameters will be identical to in-clinic therapy (0.5 s duration, 100 µS pulse width and 30 Hz). Stimulations settings for every magnet swipe will be recorded in the IPG. Since most (∼75%) of the subjects do not notice stimulation, blinding will be maintained during this portion through the post-90 assessment; therapists and assessors will not yet know group assignment and continue to be blinded through post-90 visit.
Each participant will be prescribed a daily at-home exercise programme adapted to their functional level and goals. These rehabilitation exercises will follow the same principles as the in-clinic therapy. Participants will practice 30 min of continuous exercises involving three task categories: gross movement, hand/wrist tasks and finger dexterity. Prior to initiation of the upper limb exercises, participants will use a magnet to self-activate the device as described above. The therapists will contact participants on a regular basis to check compliance and adjust the exercise programme as needed. The participants report their exercise history directly to their therapist via phone calls or visits that occur every two weeks during the randomised portion of the study. Magnet use will also be recorded in the IPG as a proxy for compliance.
Outcome measures
Therapeutic outcome will be assessed by measuring the impact of stroke in three domains, including level of impairment, function and quality of life. The assessments have demonstrated reliability, were selected to sample functional arm and hand movements, and have been shown to be sensitive to rehabilitation interventions. The measures include: (a) FMA-UE which will be the primary outcome measure; (b) Wolf motor function test (WMFT; time and functional ability scores); (c) stroke impact scale (SIS); (d) stroke specific quality of life (SS-QOL); (e) EuroQol-5D EQ-5D™; (f) motor activity log (MAL) and (g) BDI. Details for each measure are provided in the online Supplementary Material. It should be noted that the EQ-5D is a general measure of health status and not specifically for stroke or upper limb improvement; it may not be sensitive enough to show differences. Additionally, the SIS, although focused on stroke, collects information broadly across a number of domains including mobility, memory, communication, etc. Since the VNS study is focused on upper limb recovery, specific sub-domains on the SIS (such as hand function and Activities of Daily Living) may be more sensitive to change rather than the overall score. Therefore, upper limb specific sub-domains may provide more relevant information and will be included in the analysis.
Outcomes are assessed at several timepoints throughout the study (Figure 1). In Stage I (Figure 2), assessments occur at the initial screening and pre-implant visit. After implantation, participants will have a pre-therapy baseline assessment prior to starting therapy. Assessments occur again after the end of in-clinic therapy at post-day 1 (primary endpoint analysis timepoint), post-day 30 and post-day 90. All participants, therapists and assessors will be informed of group allocation at the post-day 90 assessment. This visit is the first quarterly assessment for the Active VNS group and the ‘re-baseline’ (active treatment baseline) visit for Control VNS group (visit prior to the cross-over to active VNS therapy). In Stage II, participants in the Active VNS group have quarterly assessments at 6, 9 and 12 months after the end of the in-clinic therapy (Figure 3). Control VNS participants crossover to receive six weeks of in-clinic Active VNS and follow a schedule that is similar to the Active VNS group, i.e. three post therapy assessments (post-day 1, 30 and 90) and quarterly assessments (6, 9 and 12 months) through one year (Figure 3). In Stage III, all participants receive annual follow-up assessments until commercial approval of the device (Figure 3).
Sample size calculation
A sample size of 100 participants total (50 per group) has 80% power with 0.05 alpha to detect a difference of 2.3 with SD = 4.0 on the FMA-UE scale between the two treatment groups. A sample size of 50 per group has over 95% power at 0.05 alpha to show a difference in responders, assuming 75% response in the VNS group and 33% in the control group.13 With respect to safety, a sample of at least 100 participants implanted and receiving VNS allows adequate power to detect the incidence of safety and device events. A sample of 100 participants yields 95% probability that the study will reveal at least one occurrence of all events or complications that occur in participants at a rate of 3% or greater. In addition, implantation and follow-up of 100 participants for six weeks yields 4200 participant-days (600 weeks or over 11 years) of total exposure.
A futility analysis will be overseen by the data safety monitoring board (DSMB) after 40 participants complete six weeks of rehabilitation and the post-day 1 assessment. The conditional power of the two-sample test comparison between treatments groups will be calculated to determine the futility index (1 – conditional power). The study will be stopped if the futility index is greater than 0.90 (at approximately t<–1.25).
Statistical analysis plan
Statistical analyses will be performed and completed by ResearchPoint Global. All efficacy and safety summaries will be performed on the intent-to-treat (ITT) population, defined as all participants who have any surgical portion of the implant procedure attempted, regardless of the treatment to which they are assigned, and have assessments available for analysis. The ITT analysis is performed regardless of amount of rehabilitation completed or VNS therapy being completed. In addition to the ITT population, efficacy analyses will be performed on a per protocol (PP) population defined as participants considered to be compliant with treatment (i.e. subjects completing at least two-thirds of in-clinic therapy sessions) and be without major protocol violations that could impact and/or compromise the safety or efficacy of the treatment. Rehabilitation is verified through completion of case report forms documenting rehabilitation and tasks; VNS is verified through checking of device logs. Exclusion from the PP population will be finalised prior to database lock.
All data will be summarised by treatment group with descriptive statistics, with means and standard deviation for continuous data, and counts and percentages for categorical data. CIs will also be included with the efficacy endpoints. All data analyses and statistical testing will be conducted using SAS Version 9.4 or higher. The null hypothesis shows no difference between the two treatment groups. Unless specified for a specific test, a significance level of α = 0.05 will be used. Analyses outside of this protocol may be performed to supplement results or for research purposes at the discretion of the sponsor.
For the primary efficacy endpoint, control subjects will be compared to active VNS subjects, with null hypothesis of no difference in FMA-UE change from baseline to six weeks between the two groups. An analysis of covariance (ANCOVA) model will be used, with the change from baseline as the dependent variable, and the treatment, region, treatment by region interaction as factors, with age and baseline FMA UE score as covariates.
The secondary endpoints include: (a) Responder analysis at post-day 90: A response to treatment is defined as a six-point or greater improvement in the FMA-UE score10 from pre-therapy to post-day 90. A logistic regression will be used for the responder analysis, with treatment and randomisation strata as factors. (b) WMFT change at post-day 90: Analysis of the change in WMFT scores from pre-therapy to post-day 90. An ANCOVA will be used, with the change from baseline as the dependent variable, and treatment and randomisation strata as factors. (c) FMA-UE change at post-day 90: Analysis of the change in FMA-UE scores from pre-therapy to post-day 90. An ANCOVA model will be used, with the change from baseline as the dependent variable, and treatment and randomisation strata as factors.
The three secondary endpoints will each be tested for significance with 0.05 Type I error (two-sided) in a hierarchical manner in the order as listed above. Significance will be declared for the first secondary endpoint at 0.05, and each subsequent endpoint only if all higher ranked endpoints were significant at 0.05. The SIS, SS-QOL, EQ-5D and MAL are all included as tertiary outcome measures.
Safety analysis
The safety endpoints will be analysed as follows. (a) Adverse events: Adverse events with an onset during the study, including the surgical procedure, will be recorded and tabulated. All adverse events will be tabulated, by body system, first occurrence of the event, maximum severity, and strongest relationship to study treatment and implant surgery. Results will be summarised by treatment group. Furthermore, any adverse events considered serious or resulting in discontinuation of stimulation or device removal will be listed. (b) Device complications: Device complications will be tabulated similarly to the adverse event summaries, with an emphasis on any unanticipated adverse device effects.
Long-term analysis
Long-term analyses will also be performed to compare maintenance of response and changes over time between groups.
Missing data
For the analysis of study endpoints, a ‘last observation carried forward’ approach will be used if an assessment is missing after baseline. To evaluate the effect of missing data, a Mixed Model Repeated Measures (SAS PROC MIXED) will also be performed as a sensitivity analysis to evaluate the full data set. Multiple imputation with missing at random assumptions using SAS PROC MI will also be performed. For the responder analysis, participants with missing results will be imputed as non-responders.
For the analysis of safety, sites will be contacted to confirm that missing data are truly missing and cannot be otherwise assessed. Onset and resolution dates will not be imputed. For severity and relationship, if there is no other information available, relationship will be assessed as ‘possible’, and severity will be assessed as ‘severe’ for summary purposes, unless there is specific justification presented to impute other values.
Dissemination
The results of this pivotal trial will be presented at research conferences and published in peer-review journals.
Data management and clinical monitoring
Data handling
Clinicians will record data on standardised, validated outcome variables and complications, should they occur. Participant confidentiality will be maintained, and each person will be identified only by their study number. Data will be collected using source forms designed specifically for this study and entered into a centralised electronic Case Report Form (a digital database developed by Simplified Clinical). Data are entered from source documents into the electronic database by site personnel (e.g. data entry specialist, coordinator, therapist, clinician or other designated personnel). Company personnel do not enter data.
Monitoring visits
Regular clinical monitoring visits will be conducted by appropriately trained clinical research associates assigned by the sponsor. Monitoring visits occur every 4–8 weeks during the randomised portion of the study. Monitors will be responsible for ensuring that sites maintain up-to-date device accountability logs and subject Case Report Forms. Monitors will also verify compliance with the research protocol, maintenance and accuracy of source documents, and reporting of any adverse events and unanticipated adverse device effects.
Adverse events
Anticipated adverse events related to this clinical trial are listed in Table 3. Adverse event status will be evaluated throughout the study. Collection of adverse events will start after the time of implant and will continue until a participant exits the study. Investigators will obtain all information available to determine the causality, severity, possible relationship to study participation, event outcomes, and any other information to assess whether the event meets the criteria for a serious and/or unexpected event requiring notification to the sponsor, regulatory agency, and as applicable, IRB, within the specified reporting timeframe. Adverse events will be translated from investigator verbatim terms into a standard nomenclature using the Medical Dictionary for Regulatory Activities. The site investigators, sponsor, physician and study director will review all adverse events, device complications, unanticipated adverse device effects and serious adverse events, and take appropriate action (including study termination, if necessary).
Table 3.
Anticipated adverse events during the trial.
| General surgery-related eventsa |
| Side effects from the anaesthesia |
| Blood clot |
| Inflammation |
| Formation of cysts |
| Infection (minor, no explant) |
| Local pain after the operation (including incision pain) |
| Nausea/vomiting |
| Oedema |
| Paraesthesia |
| Haematoma |
| Formation of scar tissue |
| Histotoxicological reaction |
| Irritation of the skin |
| Tissue reaction |
| VNS surgery- or implant-specific events |
| Nerve damage (which may lead to hoarseness, pain, facial weakness, swallowing difficulties and other effects) |
| Infection leading to IV antibiotics or device explant |
| Numbed facial sensation |
| Facial paralysis |
| Hoarseness/vocal cord paresis/paralysis (due to surgery) |
| Bradycardia during the first stimulation at surgery |
| Stimulation-related events |
| Diarrhoea |
| Dyspepsia (indigestion) |
| Dysphagia (swallowing problems) |
| Dyspnoea (problems with breathing) |
| Ear ache |
| Hoarseness (due to stimulation) |
| Hiccup |
| Cough |
| Laryngospasm |
| Muscle twitching during stimulation |
| Nausea and vomiting |
| Pain (especially in the throat or neck) |
| Paraesthesia (numbness or tingling sensation of the skin) |
| Pharyngitis (infection of the throat) |
| Respiratory effects (typically at high output current levels and typically at night during sleep when receiving stimulation) |
These events will not be considered adverse events because they are expected events related to surgical procedures in general.
An independent DSMB will review adverse events and safety information, as well as oversee the futility analysis. The DSMB will describe and compare these events relative to the typical VNS treatment events associated with epilepsy and depression. The review of events will be immediate for any unusual or unexpected serious adverse events and at least yearly for all other events. The DSMB will be comprised of at least three members, including one surgeon with significant VNS surgery experience, one physician with significant VNS therapy experience, and one physician with significant stroke experience. The DSMB includes an independent statistician. The DSMB will also consider and recommend suspension or termination of the study to the sponsor. Any recommendation for suspension or termination of the study will be communicated to the US FDA, the site investigators and IRBs.
Since this is a relatively new device, no significant long-term experience with device longevity or malfunctions is available, other than from the previous two pilot studies (26 total subjects implanted).11,13 All device malfunctions, with the lead, the IPG or both, will be reported and evaluated. Any serious injuries and/or deaths occurring during the procedure will also be evaluated to determine if the device system might have malfunctioned. The evaluation will be done by the sponsor and then reviewed by the site’s investigators and DSMB.
Discussion
The VNS-REHAB trial is a pivotal, double-blind, randomised, sham-controlled study to determine the safety and efficacy of VNS paired with upper limb rehabilitation after chronic stroke.
In rat models of ischaemic stroke, VNS paired forelimb movement demonstrated significantly improved forelimb recovery compared to rats that did only motor training without VNS.5,19 Similar results were obtained for aged rats, haemorrhagic stroke and rats with chronic stroke.5,20,21 VNS also increased synaptic connectivity in residual cortico-spinal tract networks controlling the impaired forelimb, thus providing direct evidence that VNS paired with rehabilitation enhanced plasticity in motor networks after preclinical stroke.6 VNS exerts its action on the cortex by triggering the deep brain release of neuromodulators including acetylcholine and norepinephrine. Release of these neuromodulators timed with task-specific movement increases the salience of the paired movement and promotes clinically useful neural plasticity15 (Figure 4(b)).
VNS is an FDA-approved surgical procedure that has been used over several decades and more than 100,000 patients have been implanted for the treatment of drug-resistant epilepsy and depression22,23 and has a well-documented safety profile.12,24 There are some limitations to this study. First, the study does not include participants with haemorrhagic stroke. The plan is to keep the stroke population fairly uniform for the pivotal study and include broader stroke populations in subsequent post-market studies. It should be noted that in the preclinical studies of haemorrhagic stroke, VNS paired with forelimb movement demonstrated significantly improved recovery compared to rats that did motor training without VNS.25 Second, the study excludes persons with infratentorial (i.e. cerebellar and pontine) strokes. These individuals can also present with upper limb weakness but it is unclear whether the mechanism of action of VNS is similar to cortical and subcortical strokes. Future animal studies are needed to understand the mechanism and effectiveness of paired VNS for infratentorial strokes. Third, the study includes brain imaging at baseline, but not at later time-points. Scientific insights might also benefit from ongoing functional imaging. The device does have conditional magnetic resonance labelling; thus, brain imaging after implant is possible.
Supplemental Material
Supplemental Material for Study protocol for a pivotal randomised study assessing vagus nerve stimulation during rehabilitation for improved upper limb motor function after stroke by Teresa J Kimberley, Cecília N Prudente, Navzer D Engineer, David Pierce, Brent Tarver, Steven C Cramer, David Alexander Dickie and Jesse Dawson in European Stroke Journal
Acknowledgements
The authors thank David Ng (ResearchPoint Global) for formulating the Statistical Analysis Plan for this study and Steven L Wolf, PhD, PT, for initial guidance regarding rehabilitation. The authors also thank the DSMB members: Joe Edmonds, MD (VNS surgery expert); Mark S George, MD (VNS expert); Scott Kasner, MD (stroke expert); Jaye Thompson, PhD (statistician).
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Navzer Engineer, David Pierce, Cecília N Prudente and Brent Tarver are employees of MicroTransponder, Inc. JD has received reimbursement for travel expenses from MicroTransponder for presentation of study data at conferences. Dr Cramer has served as a consultant for Abbvie, Constant Pharmaceutical, MicroTransponder, Neurolutions, Regenera, SanBio, Stemedica and TRCare. David Alexander Dickie has served as a consultant to MicroTransponder for MRI acquisition and analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded by MicroTransponder, Inc., Austin. DAD is funded by a Stroke Association postdoctoral fellowship.
Ethical approval
The study is approved by an institutional review board (IRB) at each institution and subject to appropriate regulatory approvals (US Food and Drug Administration (FDA) Investigational Device Exemption (IDE, #130287) and UK MHRA No. #CI/2015/0011). The principal investigator and IRB for each site are listed below.
United States
Burke Medical Research Institute: Tomoko Kitago, MD, Western Institutional Review Board (WIRB), 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
Emory University School of Medicine: Steven L Wolf, PhD, PT, FAPTA, FAHA, Emory IRB, 1599 Clifton Road NE, 5th Floor, Atlanta, GA 30322
Massachusetts General Hospital Institute of Health Professions: Teresa J Kimberley, PhD, PT, WIRB, 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
Mayo Clinic/Brooks Rehabilitation Research Center: Benjamin L Brown, MD/Lou Demark, PT, DPT, NCS, Mayo Clinic IRB, 201 Building, Room 4-60, 200 First St. SW, Rochester, MN 55905
Medical University of South Carolina: Wayne Feng, MD, MS, WIRB, 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
Perseverance Research Center: Allan Bock, MD, WIRB, 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
Providence Saint John's Health Center: Achal Achrol, M.D, 2200 Santa Monica Blvd. Santa Monica, CA 90404
Rancho Los Amigos Research Institute: Charles Liu, MD, PhD, WIRB, 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
Spectrum Health: Rushna Ali, M.D., 25 Michigan St. NE, Grand Rapids, MI 49503.
The Ohio State University Medical Center: Marcia Bockbrader, MD, PhD, WIRB, 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
The University of Texas Medical School at Houston: Gerard Francisco, MD, UT Health, Committee for the Protection of Human Subjects, 6410 Fannin, Suite 1100, Houston, TX 77030
Thomas Jefferson University: Ashwini D Sharan, MD FACS, WIRB, 1019 39th Avenue SE, Suite 120, Puyallup, WA 98374
University of Texas Southwestern Medical Center: Kathleen Bell, MD, 5323 Harry Hines Blvd., Dallas, TX 75390
Vanderbilt University Medical Center: Peter E Konrad, MD, PhD, 1313 21st Ave., South, Suite 504, Nashville, TN 37232
Weill Cornell Medicine: Michael W O’Dell, MD, Weill Cornell IRB, 1300 York Avenue Box 89, New York, NY 10065
United Kingdom
University of Aberdeen: Mary Joan MacLeod, BSc, MBChB, PhD, FRCP, West of Scotland Research Ethics Service, Clinical Research and Development, West Glasgow Ambulatory Care Hospital, Dalnair Street, Glasgow G3 8SJ
University of East London: Duncan Edwards, PhD/Chris Uff, MD, West of Scotland Research Ethics Service, Clinical Research and Development, West Glasgow Ambulatory Care Hospital, Dalnair Street, Glasgow G3 8SJ
University of Glasgow: Jesse Dawson, MD, West of Scotland Research Ethics Service, Clinical Research and Development, West Glasgow Ambulatory Care Hospital, Dalnair Street, Glasgow G3 8SJ
University of Newcastle: Anand Dixit, MD, West of Scotland Research Ethics Service, Clinical Research and Development, West Glasgow Ambulatory Care Hospital, Dalnair Street, Glasgow G3 8SJ
University of Sheffield: Jessica Redgrave, MD, West of Scotland Research Ethics Service, Clinical Research and Development, West Glasgow Ambulatory Care Hospital, Dalnair Street, Glasgow G3 8SJ
Informed consent
All participants will provide/have provided written informed consent prior to undergoing any study procedures. Written informed consent was obtained from the patient(s) for their anonymised information to be published in this article.
Guarantor
BT.
Contributorship
JD, TJK, BT and NDE contributed to the study design and protocol development. NDE, CNP and BT were responsible for writing the manuscript. SCC helped with outcome assessments and training as well as clinical trial design. DP was involved with study design and implementation. DAD developed the imaging protocol and analysis. All authors provided significant input into reviewing, modifying and editing the manuscript. TJK and JD made final editorial decisions.
MicroTransponder employees are involved in the study design and oversight. Company employees are not involved in the interpretation of data and main data analysis. Ultimate authority for the manuscript resides with Dr Kimberley (TJK) and Dr Dawson (JD).
References
- 1.Feigin VL, Mensah GA, Norrving B, et al. Atlas of the global burden of stroke (1990–2013): the GBD 2013 study. Neuroepidemiology 2015; 45: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics–2019 Update: A Report From the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed]
- 3.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke 2006; 37: 2348–2353. [DOI] [PubMed] [Google Scholar]
- 4.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 2013; 207: 275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodaparast N, Kilgard MP, Casavant R, et al. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil Neural Repair 2016; 30: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers EC, Solorzano BR, James J, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke 2018; 49: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulsey DR, Hays SA, Khodaparast N, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul 2016; 9: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulsey DR, Riley JR, Loerwald KW, et al. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017; 289: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimberley TJ, Pierce D, Prudente CN, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke 2018; 49: 1–12. [DOI] [PubMed] [Google Scholar]
- 10.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012; 92: 791–798. [DOI] [PubMed] [Google Scholar]
- 11.Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016; 47: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol 2001; 18: 415–418. [DOI] [PubMed] [Google Scholar]
- 13.Kimberley TJ, Pierce D, Prudente CN, et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke. Stroke 2018; 49: 1–5. [DOI] [PubMed] [Google Scholar]
- 14.Engineer ND, Riley JR, Seale JD, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011; 470: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter BA, Khodaparast N, Fayyaz T, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex 2012; 22: 2365–2374. [DOI] [PubMed] [Google Scholar]
- 16.Labiner DM, Ahern GL. Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol Scand 2007; 115: 23–33. [DOI] [PubMed] [Google Scholar]
- 17.Bunch S, DeGiorgio CM, Krahl S, et al. Vagus nerve stimulation for epilepsy: is output current correlated with acute response? Acta Neurol Scand 2007; 116: 217–220. [DOI] [PubMed] [Google Scholar]
- 18.Muratori LM, Lamberg EM, Quinn L, et al. Applying principles of motor learning and control to upper extremity rehabilitation. J Hand Ther 2013; 26: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 2013; 60: 80–88. [DOI] [PubMed] [Google Scholar]
- 20.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair 2014; 28: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hays SA, Ruiz A, Bethea T, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging 2016; 43: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terry R. Vagus nerve stimulation: a proven therapy for treatment of epilepsy strives to improve efficacy and expand applications. Conf Proc. Annu Int Conf IEEE Eng Med Biol Soc 2009, IEEE; Minneapolis/St. Paul, Minnesota; 2009: 4631–4634. [DOI] [PubMed]
- 23.Attenello F, Amar AP, Liu C, et al. Theoretical Basis of Vagus Nerve Stimulation. In: Progress in neurological surgery. Slavin KV (ed): Stimulation of the Peripheral Nervous System. The Neuromodulation Frontier. Prog Neurol Surg. Basel, Karger Publishers, 2016, vol 29 pp. 20–28. [DOI] [PubMed]
- 24.Rolston JD, Englot DJ, Wang DD, et al. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus 2012; 32: E14. [DOI] [PubMed] [Google Scholar]
- 25.Hays SA, Khodaparast N, Hulsey DR, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014; 45: 3097–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Study protocol for a pivotal randomised study assessing vagus nerve stimulation during rehabilitation for improved upper limb motor function after stroke by Teresa J Kimberley, Cecília N Prudente, Navzer D Engineer, David Pierce, Brent Tarver, Steven C Cramer, David Alexander Dickie and Jesse Dawson in European Stroke Journal