Abstract
The aim of the present European Stroke Organisation guideline document is to provide clinically useful evidence-based recommendation on reversal of anticoagulant activity VKA (warfarin, phenprocoumon and acenocoumarol), direct factor II (thrombin) inhibitors (dabigatran etexilat) and factor-Xa-inhibitors (apixaban, edoxaban and rivaroxaban) in patients with acute intracerebral haemorrhage. The guideline was prepared following the Standard Operational Procedure for a European Stroke Organisation guideline document and according to GRADE methodology. As a basic principle, we defined use of oral anticoagulation pragmatically: oral anticoagulation use is assumed by positive medical history unless relevant anticoagulant activity is regarded unlikely by medical history or has been ruled out by laboratory testing. Overall, we strongly recommend using prothrombin complex over no treatment and fresh-frozen plasma in patients on VKA plus vitamin K. We further strongly recommend using idarucizumab in patients on dabigatran and make a recommendation for andexanet alfa in patients on rivaroxaban and apixaban over no treatment. We make a weak recommendation on using high-dose prothrombin complex concentrate (50 IU/kg) for all patients taking edoxaban and for patients on rivaroxaban or apixaban in case andexanet alfa is not available. We recommend against using tranexamic acid and rFVIIa, outside of trials. The presented treatment recommendations aim to normalise coagulation, there is no or only indirect data on effects on functional outcome or mortality, and only little data from randomised controlled trials.
Keywords: Intracerebral haemorrhage, non-vitamin K antagonists, oral anticoagulants, reversal anticoagulant activity, vitamin K antagonists
Introduction
Oral anticoagulation (OAC) is prescribed in common conditions including atrial fibrillation (AF), deep venous thrombosis and pulmonary embolism, as well as for a number of less frequent indications. The most feared side effect of OAC remains increased risk of bleeding including the life-threatening condition of intracerebral haemorrhage (ICH).
Globally, AF was in 2010 estimated to affect approximately 34 million individuals (21 million men and 13 million women). The burden of AF is increasing: in 1990, the estimated age-adjusted incidence rates were 61 per 100,000 person-years in men and 44 in women, increasing to 78 in men and 60 in women in 2010. The ATRIA-study assessed the increase of AF between 2010 and 2050 to double from 2.6 million to 5.66 million adults.1
Venous thromboembolism (VTE) is also a major global health burden with about 10 million cases occurring every year, thereby representing the third leading vascular disease after acute myocardial infarction and stroke.2 However, the majority of patients with VTE receive anticoagulation for a limited period of 3–6 months, while in AF lifelong anticoagulation is recommended if CHA2DS2-VASc score exceeds 1 point (excluding the item of female sex).3 Although anticoagulation reduces risk of stroke by two-thirds in AF, patients on OAC with AF have a significantly higher risk of bleeding than patients with VTE probably because of co-existing cerebrovascular disease in many patients with AF.4 In trials as well as in clinical practice, the occurrence of ICH during OAC treatment is therefore significantly higher in patients with AF than in patients with VTE.
Annual rates of intracranial bleeding in the phase 3 studies of non-vitamin K antagonist oral anticoagulants (NOAC) versus warfarin ranged from 0.23% to 0.5% in NOACs versus 0.7% to 0.85% in patients on warfarin.5 Risk of ICH varies between individuals, higher HAS-BLED and HEMORR2HAGES score, presence of micro-bleeds and cerebral superficial siderosis predicts higher risk of ICH in persons on OAC.6,7 The increased use of OAC due to the rise in use of NOACs, a potential widening of indications, as well as the demographic change with ageing populations will most likely lead to a further increase of OAC-related ICH despite the relative risk reduction with NOACs.8,9
The aim of the present European Stroke Organisation (ESO) guideline document is to provide evidence-based recommendation on acute treatments aiming to reverse the coagulopathy caused by VKA (warfarin, phenprocoumon and acenocoumarol), direct factor II (thrombin) inhibitors (dabigatran etexilat) and factor-Xa-inhibitors (apixaban, edoxaban and rivaroxaban) in patients with acute ICH.
Methods
The guideline was prepared following the Standard Operational Procedure for an ESO guideline document10 and according to GRADE methodology.11
This GRADE methodology is characterised by a separation of the quality of evidence from the strength of recommendations, transparency in literature search as well as the translation of evidence into recommendations, explicit grading of outcomes, comprehensive criteria for up- and downgrading of quality of evidence, acknowledgement of values and preferences and a clear and pragmatic interpretation of weak versus strong recommendations for clinicians, patients and policy makers.11
In short, the GRADE guideline approach starts with the formulation of PICO questions (Population, Intervention, Comparator and Outcome). The importance of the chosen outcomes is graded from 1 to 9 (from limited to critical importance). A systematic search is formulated to identify available literature; randomised controlled trials (RCTs) are preferred; however, observational data can also be included and assessed according to the GRADE procedure. Eligible studies are selected and data extracted into evidence tables and analysed; in this process, quality of evidence for each outcome is graded.
Quality of evidence is defined as the extent to which one can be confident that an estimate of the effect or association is correct and is up- or downgraded based on criteria referring to design including risk of bias in the selected studies. Quality of evidence is graded into four levels – very low to high – depending on the probability that further evidence will lead to a change of the recommendation.
The recommendation is formulated using a standardised language including the direction (for or against) and its strength. The strength of recommendations has two levels – strong or weak. A strong recommendation is based on the certainty that the desirable effects outweigh the undesirable (while the opposite for a weak recommendation) and the importance of the outcomes. Preferences and values are included into this estimate and health economic aspects may be included at the discretion of the organisation presenting the guideline.
The recommendations are presented based on consensus; the method of consensus is the Delphi method.12
In the preparation of the present guideline document, work group leaders (HC and TS) were selected and their potential conflicts of interests (COI) were assessed by the ESO Guidelines Board and the ESO Executive committee. Work group leaders selected working group members, who were then confirmed by the two committees after assessment of their potential COIs.
Work group members created initial PICO questions and grading of outcomes was done by consensus through repeated Delphi votes (online Appendix 2). PICO questions were divided between work group members (online Appendix 2). Based on the PICO questions, search algorithms were prepared and executed by a professional methodologist (AL).
Literature was selected and assessed for each PICO by two work group members using the Covidence tool,13 and conflicts were solved by discussion. As literature is scarce in the area, both interventional and observational data were accepted. Evidence tables including results from meta-analyses of selected data were based on the identified literature and the grading of outcomes (AL). Recommendations including the strength of the recommendation as well as quality of evidence were prepared by the work group members based on the methodology described in the GRADE handbook.11
As to including values and preferences in the grading of the strength of recommendations, we assumed that in acute life-threatening disease, an active approach to treatment with possible benefits – in the absence of likely harms – is in accordance with generally accepted norms across different cultural backgrounds. This includes interventions where the benefit on patient-important outcomes including, e.g. functional outcome has not been documented or not yet investigated but a potential beneficial effect was shown in non-patient-important outcomes such as laboratory tests. In this process, we followed the principles outlined in GRADE handbook, section 6 to achieve a useful guideline for clinicians, patients and policy makers, transparency as to the limitations in the quality of evidence is covered by this grading.11 Some recommendations were upgraded following the guidance of the GRADE handbook to consider upgrading when low-quality evidence suggests benefit in a life-threatening situation (evidence regarding harms can be low or high).11 Health economic aspects were not included in the grading. No patient representatives were included in the work.
The subject of this guideline is the reversal of effects of anticoagulants in patients with acute ICH related to these anticoagulants. As a basic principle, we defined use of OAC pragmatically: OAC use is assumed by positive medical history unless relevant anticoagulant activity is regarded unlikely by medical history or has been ruled out by laboratory testing; this approach is aligned with the ESO-Karolinska Stroke Update consensus statement 2016.14
The final guideline document was approved by the Guideline Board and the Executive Committee of ESO.
Results
Table 1 presents the summary of recommendations. Online Appendix 1 provides grading of outcome, PICO work group members and search strings; online Appendix 2 contains evidence tables.
Table 1.
Summary of recommendations.
| PICO | Quality of evidence | Strength of recommendation | |
|---|---|---|---|
| 1 | We recommend using PCC (30 IU/kg) in adults with ICH occurring during use of vitamin K antagonists (with an INR above normal) over no treatment to decrease mortality and normalise INR. | Very low | Strong |
| 2 | We recommend using PCC (30 IU/kg) in patients with ICH occurring during use of vitamin K antagonists (with an INR above normal) over FFP (20 mL/kg) to decrease mortality and normalise INR. | Moderate | Strong |
| 3 | In adult patients with ICH occurring during use of vitamin K antagonists (with an INR above normal) we recommend using vitamin K (10 mg IV) in addition to fast reversal strategies including PCC to prevent re-increase of INR to decrease haematoma expansion and decrease mortality | Very low | Strong |
| 4 | In patients with ICH occurring during use of vitamin K antagonists, we recommend against using rFVIIa to improve outcome, decrease haematoma expansion or increase normalisation of INR. | Very low | Strong |
| 5 | In adult patients with ICH occurring during use of vitamin K antagonists (with an INR above normal) we recommend against use of tranexamic acid. | Very low | Strong |
| 6 | In patients with ICH occurring during use of NOAC (fXa inhibitors), we recommend to consider the use of 4-factor PCC (37.5–50 IU/kg) to reverse the anticoagulant effect. | Very low | Weak |
| 7 | In patients with ICH occurring during use of NOAC, we recommend against using FFP to improve outcome, reduce mortality, decrease haematoma expansion or reverse the effects of NOAC. | Very low | Weak |
| 8 | In adult patients with ICH occurring during use of dabigatran, idarucizumab is recommended to reverse effects of dabigatran. | Low | Strong |
| 9 | In adult patients with ICH occurring during use of rivaroxaban or apixaban, andexanet alfa may be considered to reverse the anticoagulant effect. | Low | Weak |
| 10 | We recommend against the administration of ciraparantag outside of clinical trials. | Very low | Strong |
PICO: Population, Intervention, Comparator and Outcome; PCC: prothrombin complex concentrate; ICH: intracerebral haemorrhage; INR: international normalised ratio; IV: intravenously; NOAC: non-vitamin K antagonist oral anticoagulation; FFP: fresh-frozen plasma; IU: international units.
PICO ( P opulation, I ntervention, C omparison, O utcome) 1: For adults with ICH occurring during use of vitamin K antagonists (with an international normalised ratio (INR) above normal), does use of PCC (prothrombin complex concentrate; three factor, four factor) in comparison to placebo decrease mortality, improve functional outcome, decrease occurrence of serious adverse events (SAE), decrease haematoma expansion, or increase normalisation of INR?
No RCTs are available investigating PCC versus placebo.
We retrieved one multicentre retrospective cohort study15 (including 1547 VKA-ICH) comparing no treatment to PCC in VKA-ICH patients. Acute PCC administration was associated with lower probability of mortality within 30 days compared to no treatment (OR 0.37, CI: 0.29 to 0.48). No other outcome measures of interest were presented in that article. As based on observational data, this effect estimate is subject to confounding. The no-treatment group did present with significantly larger baseline haematoma volumes and lower admission Glasgow Coma Scale. No safety data were available for analysis. Consequently, observational data suggest a significant reduction in mortality, but in the absence of RCTs this lacks confirmation.
In conclusion: Observational data and pathophysiology strongly indicate benefit and use of placebo will generally be considered unethical.
Recommendation
In adults with ICH occurring during use of VKA (with an INR above normal), we recommend using PCC to decrease mortality and normalise INR.
Quality of evidence: Very low ⊕
Strength of recommendation: Strong ↑↑
PICO 2: In adult patients with ICH occurring during use of VKA (with an INR above normal), does use of PCC (three factor, four factor) in comparison to FFP decrease mortality during longest follow-up, improve functional outcome during longest follow-up, decrease occurrence of SAE (thrombotic, fluid overload, others), increase INR normalisation, or decrease haematoma expansion?
We identified one RCT investigating the use of four-factor PCC (30 IU/kg) versus FFP (20 mL/kg) in patients with ICH occurring during use of VKA16: The ‘INR Normalisation in Patients with Coumarin-related intracranial Haemorrhages’ (INCH) trial was powered to detect a 75% relative risk reduction of non-corrected INR 3 h after the start of the treatment (INR > 1.2) with a sample size of 74 participants (7% estimated attrition). The competent authority requested the trial terminated prematurely after the inclusion of 50 participants (42 with ICH, 2 with primary intraventricular haemorrhage and 6 with subdural haematoma) based on evidence of more pronounced haematoma expansion in the group allocated to FFP. In analysis of the 42 ICH patients, PCC was superior in correcting INR to the target INR ≤ 1.2 within 3 h after the initiation of treatment (number needed to treat 2, 95% CI: 1 to 3; relative risk 6.65, 95% CI: 1.74 to 25.45, p = 0.006). This finding is consistent with observational data from patients with ICH17 as well as two RCTs conducted in patient populations with other indications for reversal of the VKA-induced coagulopathy.18,19 In the 24-h haematoma expansion secondary outcome, PCC limited haematoma expansion in ICH (relative risk 0.39, CI: 0.17 to 0.95, p = 0.037). In INCH, no group differences were found in patient-important outcomes – quality of life after 3 months, functional outcome (modified Rankin Scale) after 3 months or death.16 However, the trial was not powered to detect such differences. The treatment groups were statistically similar with regard to serious adverse events even though there was a trend towards higher rates of thromboembolic events in the group allocated to PCC, though most of the patients did finally receive PCC as a rescue treatment. No case of fluid overload was reported in either allocation group. The two other RCTs testing PCC versus FFP to reverse VKA-induced coagulopathy in non-ICH patients support the notion that SAEs are distributed equally among the treatment allocations.18,19 They do not support higher rates of thromboembolic events in the PCC group but indicate that fluid overload trends towards being more frequent in patients allocated to FFP.
In conclusion: Available randomised and observational evidence indicate superiority of PCC in normalising the raised INR as well as preventing haematoma expansion. There is insufficient evidence to conclude if PCC is superior in relation to patient relevant outcomes (mortality, functional outcome and quality of life).
Recommendation
In patients with VKA-induced ICH, we recommend PCC over FFP.
Quality of evidence: Moderate ⊕⊕
Strength of recommendation: Strong ↑↑
Additional information on dosing and timing
PCC contains coagulation factors in concentrations up to 25 times greater than plasma obtained from healthy donors.20 Consequently, the total fluid volume used for reversal of VKA-induced coagulopathy is lower with PCC compared to FFP. This will theoretically result in lowering the risk of fluid overload and faster administration of the required dose. PCC is in general stored by room temperature allowing easier administration compared to FFP, which will need to be thawed and warmed before administration.20 As to dosing, PCC was given in the dose of 30 UI/kg in the only RCT on ICH.16 Dosing based on INR with dosages ranging from 25 IU/kg (INR 2–4) up to 50 U/kg in INR above 6 was used in the trial by Sarode et al. conducted in a mixed population of critically bleeding patients.19 The product information for a frequently used four-factor PCC recommends a scaled dosing with four doses (22.5 IU/kg – 47.5 IU/kg) corresponding to INR levels from 2 to >3.5 and suggesting the maximal total dose not to exceed 3000 IU.21 Based on superior data in ICH, the fixed dosing of 30 IU/kg is recommended; however, we cannot otherwise say anything against the other presented option. One a retrospective observational study in VKA-ICH suggested higher risk of thromboembolic events in total doses of PCC > 2000 IU)22; however, doses of 2000 IU or more are needed to reverse INR in the therapeutic interval in a normal weight person. The individual risk benefit ratio should be considered individually and very fast INR is needed to ensure the indication for treatment. PCC should always be combined with the administration of phytomenadione (vitamin K) to maintain normal haemostasis (PICO 3, PICO 4 and PICO 5).
Expert opinion
In patients with VKA-induced ICH, we recommend to use PCC dosages in the range from 20 to 50 IU/kg in the INR ≥ 2 and 10 to 20 IU/kg if 1.3 ≤ INR < 2
Vote: Six of six experts
PICO 3: In adult patients with ICH occurring during use of VKA (with an INR above normal), does the use of vitamin K in comparison to no treatment, PCC or FFP decrease mortality during longest follow-up, improve functional outcome during longest follow-up, decrease occurrence of SAE (thrombotic, fluid overload, others) or decrease haematoma expansion?
No RCTs testing vitamin K versus placebo, or versus FFP, or versus PCC on patients with ICH during the use of VKA were identified.
A small retrospective review of 17 patient cases of major haemorrhages (including 13 ICHs) during warfarin treatment showed that PCC administration with or without vitamin K was more effective in rapidly correcting increased INR levels than vitamin K treatment without PCC.23 However, PCC administration without vitamin K resulted in a rapid decrease of INR but a re-increase of INR 12–24 h after PCC administration in two patients, one of whom developed enlargement of intracerebral haematoma. Early haematoma growth and outcome after one year were analysed in another retrospective study in 55 patients with oral anticoagulant therapy associated ICH (INR ≥ 1.5).24 Incidence and extent of haematoma growth were significantly lower in patients receiving PCCs (19%/44%) compared with FFP (33%/54%) and vitamin K (50%/59%). An early INR reversal (within 2 h) was achieved in 84% of those who received PCCs alone or in combination with FFP or vitamin K, in 39% of the group who received FFP alone or in combination with vitamin K, but none of those who received vitamin K as a monotherapy. In the multivariate analysis, only increased INR levels after 2 h predicted haematoma growth, whereas the administration of PCCs was associated with lack of growth. There were no significant differences between the various groups regarding the outcome. Hanger et al. retrospectively evaluated the reversal protocol of vitamin K, PCC and FFP given alone or in combination to 88 patients with warfarin-related ICH (INR ≥ 1.2).17 In a Kaplan–Meier survival analysis on unadjusted data, non-palliated patients were more likely to survive if they were given PCC (p = 0.007) but not vitamin K or FFP. Further, Cox regression analysis controlled for ICH severity showed that better survival with PCC remained statistically significant. Those who received PCC had significantly improved survival without worsened disability.
Recommendation
In adult patients with ICH occurring during use of VKA (with an INR ≥ 1.3), we recommend the initial use of vitamin K (10 mg intravenously (IV)) in addition to fast reversal strategies including PCC to prevent re-increase of INR, to decrease haematoma expansion and decrease mortality.
Quality of evidence: Very low ⊕
Strength of recommendation: Strong ↑↑
Additional information
Increase in INR 12–24 h after reversal therapy is reported. As a practical approach, vitamin K as a single large bolus (vitamin K 10 mg IV) should be given directly after PCC (30 IU/kg) in the acute phase and INR repeated at 12–24 h to detect re-increase. The target value of INR is <1.3.
PICO 4: In adults with ICH occurring during use of VKA (with an INR above normal), does use of recombinant factor VIIa (rFVIIa) in comparison to placebo or FFP decrease mortality during longest follow-up, improve functional outcome during longest follow-up, decrease occurrence of SAE (thrombotic, fluid overload, others), decrease haematoma expansion or increase normalisation of INR?
We did not identify RCTs comparing rFVIIa and placebo in this indication. None of the non-randomised studies reported outcomes in comparison to placebo. Furthermore, there were no RCTs comparing use of rFVIIa in comparison to FFP. Also, prospective studies on the use of rFVIIa in comparison to placebo or FFP could not be identified. rFVIIa decreases INR in patients on VKA but has no influence on bleeding times.25
Recommendation
We recommend against using rFVIIa to improve outcome, decrease haematoma expansion or increase normalisation of INR in patients with ICH occurring during use of VKA.
Quality of evidence: Very low ⊕
Strength of recommendation: Strong ↑↑
Additional information
Recombinant FVIIa was tested in ICH in the FAST trials; no benefit was observed on three-month outcome though a minor dose-dependent reduction in haematoma expansion was reported. Treatment was associated with increased risk of thromboembolic complications.26,27
A retrospective observational study compared early reversal using FFP or rFVIIa with late reversal28 in a total of 56 patients with VKA-ICH. A significant difference in good outcome (defined as discharge to home) was observed in patients with early reversal therapy (70%) compared to late reversal (29%, p = 0.03) but no difference in mortality between the early and later reversal groups (30% vs. 39%, p = 0.72).28 Based on the small numbers, this does not support safety and efficacy which is also unlikely based on rFVII’s lack of reduction in bleeding time.25
PICO 5: In adult patients with ICH occurring during use of VKA (with an INR above normal), does use of tranexamic acid in comparison to placebo and FFP decrease mortality during longest follow-up, improve functional outcome during longest follow-up, decrease occurrence of SAE (thrombotic, fluid overload and others), decrease haematoma expansion or increase normalisation of INR?
No RCT or observational studies are available investigating tranexamic acid versus fresh-frozen plasma (FFP) or placebo within the relevant patient population. This precludes both assessment of efficacy and safety of the intervention. The only indirect evidence identified in the literature was a study on a murine model anticoagulated with warfarin.29 After the induction of ICH, the coagulopathy was sought to be reversed with saline, murine-FFP or tranexamic acid. Compared to saline, tranexamic acid did not provide significant decrease in INR but did significantly decrease final haematoma volume. FFP decreased both the INR and the final haematoma volume in a statistically significant manner compared to saline. No statistical comparison between tranexamic acid and FFP was offered.29
Recommendation
In adult patients with ICH occurring during use of VKA and INR ≥ 1.3, we recommend against use of tranexamic acid.
Quality of evidence: Very low ⊕
Strength of recommendation: Strong ↑↑
Additional information
Tranexamic acid is an antifibrinolytic agent preventing the degradation of fibrin and hence stabilises the formed blood clot.30 In normal coagulation, epoxid reductase reduces oxidised vitamin K, and this process is inhibited by vitamin K antagonists. As a result, formation of the active forms of the vitamin K dependent coagulation factors (factor II, VII, IX and X) is decreased.31 The vitamin K dependent factors are necessary in order to establish a normal coagulation cascade. Thus, theoretically tranexamic acid cannot by itself be expected to re-establish a normal coagulation nor correct the raised INR in the context of acute VKA-related bleeding as it does not offer replacement of coagulation factors nor facilitate their synthesis. This might be the reason why tranexamic acid has never been studied in relation the VKA-related haemorrhage. Whether tranexamic acid can be an add-on treatment to other VKA-reversal strategies is also currently unknown. An RCT in anticoagulant-naïve ICH-patients found no benefit of tranexamic acid over placebo in terms of functional outcome or death at day 90, although a small reduction in haematoma expansion and seven days mortality was observed.32 Additional on-going RCTs investigate if tranexamic acid can prevent haematoma expansion and improve functional outcome in anticoagulant-naïve ICH-patients (STOP-AUST, NCT01702636; TRAIGE, NCT02625948, TRANSACT, NCT03044184).33 Furthermore, the TICH-NOAC trial (NCT02866838) investigates use of tranexamic acid in NOACs.
PICO 6: In adult patients with ICH occurring during use of NOAC, does use of PCC (three factor or four factor) in comparison to placebo improve functional outcome or decrease mortality, occurrence of SAE or haematoma expansion?
PICO 7: In adult patients with ICH occurring during use of NOAC does use of PCC (three factor or four factor) in comparison to FFP (PICO 7) improve functional outcome or decrease mortality, occurrence of SAE or haematoma expansion?
We did not find any RCT comparing PCC to placebo or to FFP. We identified two observational studies on reversal of coagulation by PCC in acute NOAC-associated non-traumatic ICH that reported outcome parameters including haematoma expansion.34,35 Pooled analysis of the data shows no difference in haematoma enlargement (volume increase of >33%) in NOAC-ICH patients receiving PCC in doses decided by the treating physicians (46/103) compared to no reversal treatment (18/60; OR 1.25 [0.65–2.43]); there was also no difference on functional outcome or mortality at three months.35 Another retrospective pooled study reported no influence of PCC administration on survival in NOAC-ICH compared to VKA-ICH (HR 0.99 [0.57–1.72]).36 Available clinical data did not cover reversal of the later approved edoxaban.
Despite the neutral results regarding clinical efficacy of PCC in patients with ICH, there is some evidence from healthy volunteers that PCC reverses the anticoagulant effects of fXa inhibitors. In case of rivaroxaban, prothrombin time and endogenous thrombin potential were completely reversed by four-factor PCC 50 IU/kg, partially by 37.5 IU/kg but not by 25 IU/kg.37,38 In apixaban, partial and dose-dependent reversal was achieved by PCC 25 IU/kg and 37.5 IU/kg; but data on 50 IU/kg are not available.39 In edoxaban, full reversal was achieved by 50 IU/kg and partially by 10 and 25 IU/kg.40,41 PCC (50 IU/kg) did not reverse the effects of dabigatran.38 No safety data regarding the use of PCC in NOAC-ICH were found.
In summary, there is no evidence to recommend for or against using PCC to improve clinical outcome, reduce mortality or decrease haematoma expansion, but PCC has effects in normalising coagulation tests.
Recommendations
PICO 6: In patients with ICH occurring during use of NOAC and when specific reversal agents are not available we recommend considering the use of four-factor PCC (37.5–50 IU/kg) to normalise coagulation tests.
Quality of evidence: Very low ⊕
Strength of recommendation: Weak ↑
PICO 7: In patients with ICH occurring during use of NOAC, we recommend against using FFP to improve outcome, reduce mortality, decrease haematoma expansion or reverse the effects of NOAC.
Quality of evidence: Very low ⊕
Strength of recommendation: Weak ↓
If NOAC-specific reversal agents are available, these are recommended. In dabigatran-related ICH, idarucizumab should be administered preferably (see PICO 8) and in apixaban- or rivaroxaban-related ICH, andexanet alfa should be administered preferably (see PICO 9).
PICO 8: In adult patients with ICH occurring during use of dabigatran etexilate, does use of idarucizumab (no comparator) have an effect on coagulation tests, functional outcome, on mortality, or on the occurrence of serious adverse events?
Idarucizumab is a Fab antibody fragment that rapidly and specifically binds to and leads to sustained neutralisation (up to 24 h) and elimination of dabigatran in patients with major bleedings or a need for invasive emergency procedures as well as in healthy young and elderly subjects.42
No RCTs have been conducted to evaluate the effect of idarucizumab on mortality or on adverse reactions in comparison with placebo, PCC or FFP.
We identified one prospective study of 503 patients treated with idarucizumab in the context of major bleeding (n = 301) or urgent surgery or intervention (n = 202) – Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD).43 Among the 301 patients with major bleedings, 98 patients had one or more intracranial haemorrhages including 53 ICHs, 39 subdural and 26 subarachnoid haemorrhages. Data regarding haematoma volumes of the 53 patients with ICH are still not available. The primary efficacy end point was the maximum percentage reversal of the anticoagulant effect of dabigatran, determined at any point from the end of the first idarucizumab infusion until 4 h after the end of the second infusion, with the percentage reversal assessed on the basis of the diluted thrombin time or the ecarin clotting time. This endpoint was met in all 98 patients with intracranial haemorrhages. Regarding mortality, 16 patients out of 98 patients with intracranial haemorrhage had died (16%) at 90 days. Serious adverse events were reported in 66 out of 301 patients with major bleedings including 19 thrombotic events within the first 30 days.
Evidence for the effects of idarucizumab on clinical endpoints in dabigatran-related ICH is limited at present. However there is indirect evidence for an effect on mortality comparing the mortality rate of 16% in patients with intracranial bleedings in the RE-VERSE AD-trial and of 35% in the post-hoc analysis of intracranial haemorrhages of the RCT testing dabigatran (150 mg) versus warfarin44 when there was no specific reversal agent available. The non-randomised nature of RE-VERSE AD reduces the value of safety data; fast reversal has been documented by coagulation tests. Prospective controlled studies are needed to guide best management in the future.
Recommendation
We recommend idarucizumab to reverse effects of dabigatran in adult patients with ICH occurring during use of dabigatran. Evidence for effects on clinical endpoints is limited.
Quality of evidence: Low ⊕⊕
Strength of recommendation: Strong ↑↑
PICO 9: In adult patients with ICH occurring during use of a fXa inhibitor, does use of andexanet alfa (no comparator) improve functional outcome, occurrence of SAE (thrombotic, others) or coagulation tests?
Systematic literature search did neither reveal a RCT nor observational studies that had specifically included patient with ICH or intracranial haemorrhage. The search revealed a randomised controlled phase II study that included 101 healthy volunteers.45 Study objectives were the use of rivaroxaban or apixaban and two dose regiments for andexanet alfa (either bolus or bolus plus 2-h infusion). Primary endpoint was the percent change in factor anti-Xa activity. Anti-Xa activity was significantly reduced in both groups treated with andexanet alfa after bolus injection within 2 to 5 min and turned back to similar values as in the placebo group about 2 to 3 h after injection. Similar results were seen after the end of the infusion when volunteers had received bolus followed by a 2-h infusion.
Andexanet alfa was administered in 67 patients (bolus plus 2-h infusion) within up to 18 h after a major bleeding in the ANNEXA-4 study, an ongoing multicentre, prospective, open-label, single-group study.46 Patients had been treated with rivaroxaban (N = 26), apixaban (N = 20) or edoxaban (N = 1). Outcome was measured by two co-primary outcomes: the percent change in the anti-factor Xa activity and the rate of excellent or good haemostatic efficacy at 12 h. The relative decrease of anti-factor Xa activity for rivaroxaban and apixaban at the end of infusion was 86% and 92% and 4 h later 30% and 32%, respectively. Excellent or good efficacy (defined as an increase of haemorrhage volume of <= 35% after 12 h) was observed in 80% (95%CI, 56 to 94) for intracranial bleeding. Twelve of 67 patients (18%) had a thrombotic event during the 30-day follow-up. There are no data on functional outcome. Andexanet alfa was approved by the FDA on 3 May 201847 and a prospective randomised open label blinded endpoint assessment trial (PROBE-design) is ongoing in patients with intracranial haemorrhage on treatment with fXa inhibitors testing andexanet alfa against usual care, primary endpoint being haemostatic efficacy (NCT03661528).
Recommendation
We recommend using andexanet alfa if available – in adult patients with ICH occurring during use of rivaroxaban or apixaban. We also recommend randomising into trials as based on the low quality of evidence, there is significant uncertainty whether desirable outweigh undesirable effects.
Quality of evidence: Low ⊕ ⊕
Strength of recommendation: Weak ↑
PICO 10: In adult patients with ICH occurring during use of a fXa inhibitor, does use of ciraparantag (no comparator) improve functional outcome during longest follow-up, occurrence of SAE (thrombotic, others) or coagulation tests?
One RCT (phase II) was identified. This included 80 healthy volunteers to assess the safety, side effect profile and effect on anticoagulation reversal of ciraparantag (PER977 or aripazine) administered after a 60-mg dose of the factor Xa inhibitor edoxaban.48 To measure the anticoagulant effect of edoxaban and its reversal by ciraparantag, whole-blood clotting time was used. In fact, ciraparantag binds citrate, the reagent into which blood is collected for coagulation testing, which precludes the use of routine tests such as anti-factor Xa activity. Administration of edoxaban made the mean whole-blood clotting time increase by 37% over the baseline value. After a single intravenous dose of ciraparantag (100 to 300 mg), given 3 h after the administration of edoxaban, the whole-blood clotting time decreased to within 10% above the baseline value within 10 min, whereas in healthy volunteers receiving placebo, the time to reach that level was approximately 12 to 15 h. The whole blood clotting time remained within 10% above or below the baseline value for 24 h after the administration of a single dose of ciraparantag. However, the optimal dose is unclear, since apparently the high dose was less effective than the low dose.
No procoagulant activity remained after the administration of ciraparantag as assessed by measurement of levels of D-dimer, prothrombin fragment 1.2 and tissue factor pathway inhibitor.
No RCTs on patients with major bleedings are available.
Recommendation
We recommend against the administration of ciraparantag outside of clinical trials.
Quality of evidence: Very low ⊕
Strength of recommendation: Strong↑↑
Discussion
The purpose of this guideline document is to provide evidence-based and clinically useful recommendations on reversal of anticoagulant activity in acute OAC-related ICH. The presented treatment recommendations aim to normalise coagulation, there is no or only indirect data on effects on functional outcome or mortality, and only little data from RCTs.
Overall, we strongly recommend using prothrombin complex plus vitamin K over no treatment and FFP in patients on VKA. We further strongly recommend using idarucizumab in patients on dabigatran and make a recommendation for andexanet alfa in patients on rivaroxaban and apixaban over no treatment. We make a weak recommendation on using high dose PCC (50 IU/kg) for all patients taking edoxaban and for patients on rivaroxaban or apixaban in case andexanet alfa is not available. We recommend against using tranexamic acid and rFVIIa, outside of trials.
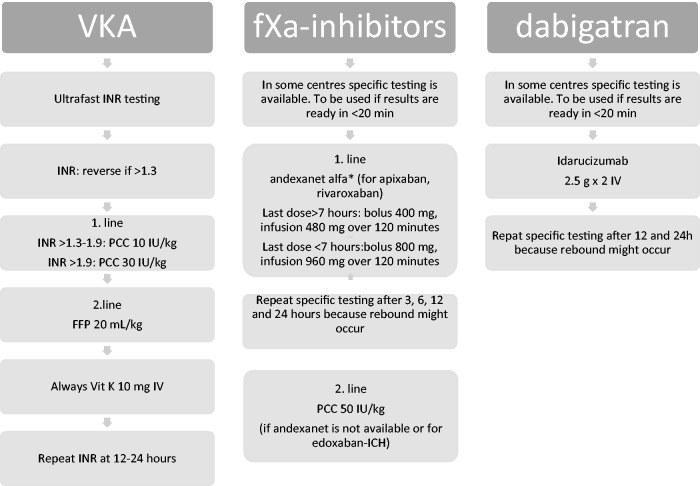
A pragmatic flow chart (Figure 1), guiding on how to treat according to the principles of this guideline, is provided. This is an attempt to provide an easy-to-use clinical tool on how to handle this patient group.
Figure 1.
A pragmatic flowchart to treatment according to this ESO guideline. Consider reversal in patients with sICH who have OAC as an ongoing prescription if no withdrawal of care concept has been started. Reversal should be performed with the same urgency as e.g. thrombolysis. Repeat imaging in case of clinical deterioration and after 24 hours. Control blood pressure (systolic blood pressure to be kept at a target of 140 mmHg) and prevent venous thromboembolism by pneumatic compression stockings. Repeated imaging next day should be considered.
Strength: As described, we weighted preferences and values highly resulting in an inclination towards reversal versus no treatment in the life-threatening hyper-acute condition of intracranial haemorrhage. We aimed to provide clinically useful guidelines despite little available high-quality data. An approach where recommendations were only based on randomised controlled data would have resulted in only recommending PCC over FFP in VKA-related ICH, which would be of little use in clinical practice. The quality of data on which the recommendations are based is, however, transparent as well as the arguments for up-grading and the principles of the GRADE handbook has been followed.
It is physiologically plausible that normalisation of coagulation may reduce haematoma expansion (HE) in ICH. This was supported by the only RTC in the field of reversal of VKA in ICH,16 as well as the FAST and the TICH-2 trial,26,32 investigating rFVIIa and tranexamic acid in spontaneous ICH, respectively. In spite of HE being a strong predictor of poor outcome, no trial has, however, yet translated reduction of HE into a clinical benefit at three months; possibly due to issues of patient selection and statistical power, or small absolute reductions in HE.32
Early timing of reversal is likely to be of crucial importance as HE most frequently occurs in the very first hours after symptom onset,49 and benefit from reversal will only arise from early prevention of HE.
It is well documented that it is possible to reverse OAC within minutes as assessed by coagulation tests; though in some cases these may be misleading as to the actual status of coagulation,25 and documentation of clinically relevant effects on coagulation tests including bleeding times are obvious prerequisites to clinical use.
Vitamin K will reverse VKA but only after 4–6 h, wherefore benefit cannot be assumed in the acute phase. Reversal is complete in PCC used in patients on VKA; however, a late increase in INR is seen if concurrent treatment with vitamin K is omitted.23 INR reversal is superior in 4-PCC compared to 3-PCC (87.5% vs. 45.6%, p < 0.001) also corresponding to a better cost-effectiveness ratio.50 Idarucizumab also completely and permanently reverses dabigatran,42 whereas andexanet alfa provides reversal but the effect returns at placebo levels within 2–3 h after stopping infusion.45 High doses of PCC are needed to reverse the effects of fXa-inhibitors, with some differences between the three drugs (apixaban, rivaroxaban and edoxaban), full reversal not necessarily being provided.37–39,41
Limitations: There are very little data on clinically relevant outcomes including functional outcome and mortality on any reversal strategy; we do not expect that it is likely that such data will be provided either. Further, there are no randomised data to assess clinical harms and benefits from the specific reversal agents for dabigatran and fXa-inhibitors. Further, no data were provided on change of haematoma volume in patients with ICH receiving idarucizumab in the REVERSE trial as control CT scans of the brain was not mandatory.42,43 This is an obvious and severe problem in making recommendations for clinical use and a major limitation of the guideline. Another limitation is that data did not allow for exploring possible sex differences in response to reversal.
As to harms, high-dose PCC (50 IU/kg) as used in fXa reversal is likely to increase risk of thrombosis, though this does not seem to be significant in the doses used in VKA related ICH.16 Rates of thrombotic complications appear higher in andexanet alfa46 than in idarucizumab42,43; however, as both components act on different agents, direct comparison cannot be made. Recombinant FVIIa is associated with a well-recognised dose-dependent risk of thrombotic complications26 and is of no likely benefit. Tranexamic acid is a safe drug,51 but of unlikely benefit as mono-therapy in OAC-related ICH.
OAC-related ICH remains a life-threatening condition and the fact that no clinical benefit has been documented from reversal must be considered. We do not think that any major risk of harm is acceptable in this perspective, which has been taken into consideration in these recommendations.
In conclusion, this guideline document recommends reversal in acute OAC (VKA and NOAC)-related ICH as a treatment principle and recommends the strategies that are best documented as to benefit and harms to provide a clinically useful, though transparent guideline. Data quality was found generally low or very low, thus recommendations have been made with respect to all aspects – but economical – to provide clinically useful guidelines.
Resume in plain language
This guideline deals with the treatment of brain bleeding that occur in association with the use of oral anticoagulants. Oral anticoagulants (blood thinners) are used to reduce the risk of stroke in AF and clots in the veins of the legs and lungs. Currently used blood thinners are vitamin-K antagonists, factor II inhibitor and factor Xa inhibitor. It is estimated that AF alone affects 34 million people globally, and it is estimated that 1 in 200 users of blood thinners have a bleeding in the brain every year. This is a very serious complication leading to death in about half of the affected persons.
This guideline aims to give evidence-based and clinically useful recommendations on the hyperacute treatment of this complication aiming to terminate the blood thinning effects of the blood thinners.
Different treatments are used in clinical practice to reverse the effects of blood thinners. This includes FFP, recombinant, activated coagulation factor VII (rFVIIa), PCC, vitamin K, tranexamic acid, idarucizumab and andexanet alfa.
FFP is a blood product and has to match the receiving patient and be thawed before use; this delays treatment typically by 60 min except in some very large centres and rather large volumes have to be infused (typically 1.5 L) leading to increase in blood pressure and straining of the heart. PCC is a concentrate of substances (mainly coagulation factors) in the blood that induces blood clot formation. It is a commercially available product that can be stored at room temperature, is easily prepared and can be used for all patients independent of blood type. RFVIIa is a commercially available clotting substance used in people lacking this substance and in major trauma. Vitamin K is used in the group of blood thinners that work by opposing the vitamin K derived clotting substances; it however takes hours before it works. Tranexamic acid is a drug that is used extensively in excess bleeding, e.g. in excessive menstrual bleeding and is considered a very safe drug. Idarucizumab and andexanet alfa are new drugs developed to stop the blood thinning effects of specific blood thinners (so called factor IIa- or factors Xa-inhibitors); andexanet alfa has not yet been marketed in Europe.
The side effect of all agents aiming to stop bleeding is the risk of forming blood clots. In an acute life-threatening situation, as bleeding in the brain related to blood thinners, it is considered in the interest of the patient to prioritise risks.
Evidence in the area of this guideline is limited. We have identified best evidence on treating brain bleedings that occur in association with the intake of blood thinners. Our recommendations are based on an assessment of benefits and risks derived from this evidence taking into that the condition we are dealing with is potentially life-threatening and no treatment alternatives are available. We also make suggestions on dosing and observation and have included a flow chart.
Overall, we strongly recommend using PCC over no treatment and FFP, and to use vitamin K in addition to PCC in patients with acute brain bleeding while using vitamin K-antagonists. We further strongly recommend using idarucizumab in patients with brain bleeding on dabigatran and make a weak recommendation for andexanet alfa in patients on rivaroxaban and apixaban over no treatment; if andexanet alfa is not available, we recommend using high-dose PCC in patients on fXa-inhibitors. We recommend against using tranexamic acid and rFVIIa, outside of trials.
Supplemental Material
Supplemental material, Supplemental Material1 for European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage by Hanne Christensen, Charlotte Cordonnier, Janika Kõrv, Avtar Lal, Christian Ovesen, Jan C Purrucker, Danillo Toni and Thorsten Steiner: on behalf of the VISTA-ICH Collaborators in European Stroke Journal
Supplemental Material
Supplemental material, Supplemental Material2 for European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage by Hanne Christensen, Charlotte Cordonnier, Janika Kõrv, Avtar Lal, Christian Ovesen, Jan C Purrucker, Danillo Toni and Thorsten Steiner: on behalf of the VISTA-ICH Collaborators in European Stroke Journal
Acknowledgements
None.
Declarations of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christensen: Travel support and speaker honoraria from Boehringer Ingelheim, Bayer and Merck Sharp & Dohme (MSD); Cordonnier: Speaker honoraria from Pfizer and Boehringer Ingelheim; Korv: Travel support and speaker honoraria from Pfizer, Boehringer Ingelheim and Bayer; Lal: None; Ovesen: Travel support from Merck Sharp & Dohme (MSD); Purrucker: Travel support and speaker honoraria from Pfizer, Boehringer Ingelheim; Toni: Speaker honoraria and advisory board on direct anticoagulants for Boehringer Ingelheim, Bayer, Pfizer Merck Sharp & Dome, Daiichi Sankyo; Steiner: Speaker and consultation fees from Bayer, BMS Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, Research grant from Octapharma; Steiner abstained from the voting process on PICO 2 and 8 due to perceived intellectual conflicts of interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the European Stroke Organisation through funding the salary of the methodologist (AL).
Informed consent
This is a guideline document so this is not relevant as no patients were involved.
Ethical approval
This is a guideline document; consequently, ethical approval was not relevant.
Guarantor
HC.
Contributorship
The work group was chaired by Christensen and co-chaired by Steiner. Lal researched literature based on PICO questions established by the work group. The PICO working groups systematically reviewed results from the search using the Covidence software and working by consensus. Lal extracted data from the finally selected studies. PICO working groups drafted resume of background literature and recommendations to be finalised by group consensus using the Delphi method. HC drafted all other sections of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
PICO working groups – PICO 1: Ovesen and Christensen; PICO 2: Ovesen and Christensen; PICO 3: Korv, Toni and Christensen; PICO 4: Purrucker and Christensen; PICO 5: Ovesen and Steiner; PICO 6: Purrucker and Cordonnier; PICO 7: Purrucker and Cordonnier; PICO 8: Cordonnier and Christensen; PICO 9: Toni and Steiner; PICO 10: Toni and Steiner.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285: 2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2016; 388: 3060–3073. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 4.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996; 348: 423–428. [DOI] [PubMed] [Google Scholar]
- 5.Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med 2016; 129: S33–S40. [DOI] [PubMed] [Google Scholar]
- 6.Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39: 453–460. [DOI] [PubMed] [Google Scholar]
- 7.Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018; 17: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bejot Y, Cordonnier C, Durier J, et al. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain 2013; 136: 658–664. [DOI] [PubMed] [Google Scholar]
- 9.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 10.Ntaios G, Bornstein NM, Caso V, et al. The European Stroke Organisation Guidelines: a standard operating procedure. Int J Stroke 2015; 10 Suppl A100: 128–135. [DOI] [PubMed] [Google Scholar]
- 11.Schünemann H, Brożek J, Guyatt G, et al. (eds). GRADE handbook for grading quality of evidence and strength of recommendations: the GRADE Working Group, guidelinedevelopment.org/handbook (2013, accessed 6 May 2019).
- 12.Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manage Sci 1963; 9: 458–467. [Google Scholar]
- 13.Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, www.covidence.org (accessed 6 May 2019).
- 14.Ahmed N, Steiner T, Caso V, et al. Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 13–15 November 2016. Eur Stroke J 2017; 2: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry-Jones AR, Di Napoli M, Goldstein JN, et al. Reversal strategies for vitamin K antagonists in acute intracerebral hemorrhage. Ann Neurol 2015; 78: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner T, Poli S, Griebe M, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol 2016; 15: 566–573. [DOI] [PubMed] [Google Scholar]
- 17.Hanger HC, Geddes JA, Wilkinson TJ, et al. Warfarin-related intracerebral haemorrhage: better outcomes when reversal includes prothrombin complex concentrates. Intern Med J 2013; 43: 308–316. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JN, Refaai MA, Milling TJ, Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet 2015; 385: 2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarode R, Milling TJ, Jr., Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128: 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus 2010; 8: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The electronic Medicines Compendium (eMC). Octaplex 500 IU, https://www.medicines.org.uk/emc/product/6566/smpc (accessed 6 May 2019).
- 22.Laible M, Jenetzky E, Beynon C, et al. Adverse events following international normalized ratio reversal in intracerebral hemorrhage. Cerebrovasc Dis 2016; 42: 446–454. [DOI] [PubMed] [Google Scholar]
- 23.Yasaka M, Sakata T, Minematsu K, et al. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb Res 2002; 108: 25–30. [DOI] [PubMed] [Google Scholar]
- 24.Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006; 37: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 25.Skolnick BE, Mathews DR, Khutoryansky NM, et al. Exploratory study on the reversal of warfarin with rFVIIa in healthy subjects. Blood 2010; 116: 693–701. [DOI] [PubMed] [Google Scholar]
- 26.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008; 358: 2127–2137. [DOI] [PubMed] [Google Scholar]
- 27.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005; 352: 777–785. [DOI] [PubMed] [Google Scholar]
- 28.Liotta EM, Garg RK, Temes RE, et al. Warfarin-associated intracerebral hemorrhage is inadequately treated at community emergency departments. Stroke 2012; 43: 2503–2505. [DOI] [PubMed] [Google Scholar]
- 29.Illanes S, Zhou W, Schwarting S, et al. Comparative effectiveness of hemostatic therapy in experimental warfarin-associated intracerebral hemorrhage. Stroke 2011; 42: 191–195. [DOI] [PubMed] [Google Scholar]
- 30.Levy JH. Antifibrinolytic therapy: new data and new concepts. Lancet 2010; 376: 3–4. [DOI] [PubMed] [Google Scholar]
- 31.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133: 160S–198S. [DOI] [PubMed] [Google Scholar]
- 32.Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018; 391: 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law ZK, Meretoja A, Engelter ST, et al. Treatment of intracerebral haemorrhage with tranexamic acid – a review of current evidence and ongoing trials. Eur Stroke J 2017; 2: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerner ST, Kuramatsu JB, Sembill JA, et al. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol 2018; 83: 186–196. [DOI] [PubMed] [Google Scholar]
- 35.Purrucker JC, Haas K, Rizos T, et al. Early Clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurol 2016; 73: 169–177. [DOI] [PubMed] [Google Scholar]
- 36.Wilson D, Seiffge DJ, Traenka C, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology 2017; 88: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barco S, Whitney Cheung Y, Coppens M, et al. In vivo reversal of the anticoagulant effect of rivaroxaban with four-factor prothrombin complex concentrate. Br J Haematol 2016; 172: 255–261. [DOI] [PubMed] [Google Scholar]
- 38.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124: 1573–1579. [DOI] [PubMed] [Google Scholar]
- 39.Cheung YW, Barco S, Hutten BA, et al. In vivo increase in thrombin generation by four-factor prothrombin complex concentrate in apixaban-treated healthy volunteers. J Thromb Haemost 2015; 13: 1799–1805. [DOI] [PubMed] [Google Scholar]
- 40.Brown KS, Wickremasingha P, Parasrampuria DA, et al. The impact of a three-factor prothrombin complex concentrate on the anticoagulatory effects of the factor Xa inhibitor edoxaban. Thromb Res 2015; 136: 825–831. [DOI] [PubMed] [Google Scholar]
- 41.Zahir H, Brown KS, Vandell AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation 2015; 131: 82–90. [DOI] [PubMed] [Google Scholar]
- 42.Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373: 511–520. [DOI] [PubMed] [Google Scholar]
- 43.Pollack CV, Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med 2017; 377: 431–441. [DOI] [PubMed] [Google Scholar]
- 44.Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 2012; 43: 1511–1517. [DOI] [PubMed] [Google Scholar]
- 45.Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med 2015; 373: 2413–2424. [DOI] [PubMed] [Google Scholar]
- 46.Connolly SJ, Milling TJ, Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med 2016; 375: 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.U.S. Food and Drug Administration. ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo), https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm606681.htm (accessed 6 May 2019).
- 48.Ansell J, Bakhru S, Laulicht B, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014; 371: 2141–2142. [DOI] [PubMed] [Google Scholar]
- 49.Ovesen C, Christensen AF, Krieger DW, et al. Time course of early postadmission hematoma expansion in spontaneous intracerebral hemorrhage. Stroke 2014; 45: 994–999. [DOI] [PubMed] [Google Scholar]
- 50.DeAngelo J, Jarrell D, Cosgrove R, et al. Comparison of 3-factor versus 4-factor prothrombin complex concentrate with regard to warfarin reversal, blood product use, and costs. Am J Ther 2018; 25: e326–e332. [DOI] [PubMed] [Google Scholar]
- 51.CRASH-2 trial collaborators: effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010; 376: 23–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage by Hanne Christensen, Charlotte Cordonnier, Janika Kõrv, Avtar Lal, Christian Ovesen, Jan C Purrucker, Danillo Toni and Thorsten Steiner: on behalf of the VISTA-ICH Collaborators in European Stroke Journal
Supplemental material, Supplemental Material2 for European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage by Hanne Christensen, Charlotte Cordonnier, Janika Kõrv, Avtar Lal, Christian Ovesen, Jan C Purrucker, Danillo Toni and Thorsten Steiner: on behalf of the VISTA-ICH Collaborators in European Stroke Journal