Abstract
Objective
Cetylated fatty acids are a group of naturally occurring fats of plant and/or animal origin. Cetyl myristoleate, in particular, was initially involved in osteoarthritis related research as its therapeutic administration prevented experimentally induced arthritis in Swiss Albino mice. In this context, the aim of our study was to investigate the possible mechanisms of Celadrin cetylated fatty acids action at the cellular level inflammation related pain relief and chondrogenesis.
Design
For this, we tested the effects of the cetylated fatty acids mixture from Celadrin on an in vitro scaffold-free 3-dimensional mesenchymal stem cells culture model of chondrogenesis. Furthermore, we treated stimulated mouse macrophage cells with the cetylated fatty acids mixture to investigate the expression profile of secreted inflammatory cytokines.
Results
The cetylated fatty acids mixture from Celadrin significantly decreased the production of IL-6, MCP-1, and TNF, key regulators of the inflammatory process, in stimulated RAW264.7 mouse macrophage cells. The treatment with cetylated fatty acids mixture initiated and propagated the process of chondrogenesis as demonstrated by the increased expression and deposition of chondrogenic markers by the differentiating mesenchymal cells.
Conclusion
The cetylated fatty acids mixture from Celadrin reduces inflammation in vitro by significantly decreasing the expression of IL-6, MCP-1, and TNF in stimulated RAW264.7 mouse macrophage cells. These compounds facilitate the chondrogenic differentiation process of human adipose-derived stem cells by stimulating the expression of chondrogenic markers under chondrogenic induction conditions.
Keywords: cetylated fatty acids, cetyl myristoleate, chondrogenesis, adipose-derived stem cells, inflammation
Introduction
Cetylated fatty acids are a group of naturally occurring fats of plant and/or animal origin that include cetyl myristoleate, cetyl myristate, cetyl palmitoleate, cetyl laureate, cetyl palmitate, and cetyl oleate. Among the aforementioned compounds, cetyl myristoleate was initially involved in osteoarthritis (OA)-related research as Diehl and May1 had demonstrated in 1994 that its therapeutic administration prevented experimentally induced arthritis in Swiss Albino mice. Although many products containing this compound are now available, the mechanism of its action is still not fully elucidated. Cetylated fatty acids are also used for other types of arthritis including rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis or for autoimmune diseases like: multiple sclerosis, Sjogren’s syndrome, and systemic lupus.
Typically, osteoarthritis (OA), a disease of diarthrodial joints, affects the hands, hips, and knees. OA is a significantly impairing condition because of its chronic character and the resulting pain and lower extremity disability it inflicts on the patient.2 Importantly, as OA is the most prevalent of all arthritis forms, and not fully treatable by available therapeutic options, it represents a significant unmet medical need.3,4 Therefore, continuing efforts are being made for developing therapies able to attenuate disease progression and alleviate symptoms. Among risk factors for OA occurrence, one may mention genetic factors, joint injuries, and overweight.5 Traditionally, OA was defined as a typical “degenerative” joint disease, with joint dysfunction stemming from cartilage degeneration and bone transformation due to formation of osteophytes, largely initiated by mechanical stress and ageing. However, research directed at OA pathophysiology showed that OA patients display gradual alterations at the joint cartilage, the synovium, subchondral bone, ligaments, as well as peri-articular muscles both at the cell and molecule level.5 Indeed, new factors have lately been added to OA pathogenesis, such as the significant role of inflammatory mechanisms and, more specifically, to synovitis,6 which has been associated to OA progression.7 Thus, the histological findings determined in correlation to OA synovitis typically consist of synovial lining hyperplasia, infiltration of macrophages and lymphocytes, neo-angiogenesis, and fibrosis.8 The inflammatory cell infiltration leads to secreting cytokines and chemokines6,9 to the synovial fluid, including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, other common γ-chain cytokines (IL-2, IL-7, IL-15, and IL-21, etc.), which trigger the response from chondrocytes, synovial and other joint cells. Furthermore, a direct correlation between pain and OA-associated synovitis has been established in various studies.10,11 It seems, therefore, that inflammatory cytokines in OA affected joints may act on innervating pseudo-unipolar nociceptors in the joint and thus have direct contribution to generation of pain. Moreover, initial OA pathogenesis is correlated to cartilage extracellular matrix (ECM) remodelling which paves the way for subsequent cartilage tissue degradation.12 Indeed, the changes of ECM constitution are closely correlated to pathologies of the skeletal system.13
Nonsteroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors are commonly used treatments for OA.14 However, NSAIDs were determined to have short-term effects15 whereas their long-term utilization is correlated to substantial morbidity.16 Consequently, patients with OA could benefit from alternatives with improved long-term performance and decreased side effects. Recently, several products containing cetyl myristoleate oil claim to alleviate OA-associated symptoms, but further investigation of the efficacy of these products needs to be performed. Moreover, the European Food Safety Authority (EFSA) published a scientific opinion regarding the safety of cetyl myristoleate complex as a food ingredient. This report summarizes that performed studies exhibit limitations and that potential toxicological effects of cetyl myristoleate have not been fully evaluated, concluding that the safety of “cetyl myristoleate complex” has not been established.17
Celadrin is a patented18 combination of cetylated, esterified fatty acids containing cetyl myristoleate, cetyl myristate, cetyl palmitoleate, cetyl laureate, cetyl palmitate, and cetyl oleate extracted from plant-based source, with oils including palm, palm kernel, olive, nutmeg, coconut, and unsaturated vegetable oils. This dietary supplement is believed to work in a similar way to the essential fatty acids from fish oil, enhancing the cell membranes integrity and reducing inflammation. Hence Celadrin is considered to aid joint mobility and flexibility by reducing pain induced by inflammation.19,20 Several clinical studies have shown that Celadrin is supportive to the lubrication of affected joints.21,22 reduces the production of IL-6 and controls the expression of TNF-α responsible for inflammation.23
In this context, the aim of our study was to investigate the possible mechanisms of Celadrin cetylated fatty acids action at the cellular level on joint mobility improvement and inflammation related pain relief. For this, we tested the effects of the cetylated fatty acids mixture from Celadrin on an in vitro scaffold-free 3-dimensional (3D) stem cells culture model of chondrogenesis. Furthermore, we treated stimulated mouse macrophage cells with cetylated fatty acids mixture from Celadrin to investigate the expression profile of secreted inflammatory cytokines.
Methods
Cell Culture Models
Adipose-Derived Stem Cells for Chondrogenic Process Investigation
Human adipose-derived stem cells (hADSCs) (StemPro Human Adipose-Derived Stem Cells, ThermoFisher) were used in this study as the chondrogenic differentiation process model. After defrosting, the cells were propagated under standard culture conditions (37°C, humidified atmosphere and 5% CO2) in Dulbecco’s modified Eagle medium (DMEM) containing 4.5 g glucose and supplemented with 1.5 g of NaHCO3, 1% antibiotic-antifungal solution, and 10% heat-inactivated fetal bovine serum (FBS).
To investigate the potential effects of cetylated fatty acids mixture from Celadrin on the chondrogenic process, we developed a scaffold-free 3D culture system that optimally mimics the naturally occurring cartilage micro-medium in vitro. Thus, 3D multicellular aggregates, further referred to as spheroids, were generated using the AggreWell plates (StemCells Technology) and following the manufacturer’s instructions. Briefly, 2.25 × 106 cells/well were seeded in order to obtain spheroids of 7.5 × 103 cells after 4 days of culture in DMEM culture medium containing 4.5 g glucose and supplemented with 1.5 g of NaHCO3, 1% antibiotic-antifungal solution, and 10% FBS.
RAW264.7 Mouse Macrophage Cells for Inflammation Profile Investigation
To investigate the anti-inflammatory potential of the cetylated fatty acids mixture from Celadrin, mouse macrophages belonging to the RAW264.7 cell line (American Type Culture Collection (ATCC), Manassas, VA, USA, TIB-71) were used. According to the manufacturer’s datasheet, these cells display typical monocyte morphology. For culture propagation, the cells were cultured in DMEM containing 4.5 g glucose and supplemented with 1.5 g of NaHCO3, 1% antibiotic-antifungal solution, and 10% FBS in sterile culture flasks of 25 cm2 and 75 cm2. The cells were incubated at 37°C, in a humidified atmosphere and 5% CO2.
Preparation of Cetylated Fatty Acids Mixture from Celadrin Emulsion and Dose Screening
The content of the Celadrin capsules was found to be insoluble in any solvent at a nontoxic concentration and consequently it was formulated by Virun (Pomona, CA, USA) into a 25% oil in water emulsion, which was further used in our studies. To determine the optimum working concentration, we performed a dose screening experiment on both hADSCs and RAW264.7 cells using the MTT cell viability assay. For this, we seeded both cell types at an initial cell density of 1.5 × 104 cells/cm2 and after 24 hours of culture, we treated the monolayers with cetylated fatty acids mixture from Celadrin in concentrations ranging from 1 to 0.01 mg/mL. An untreated sample was kept as experimental control for each cell type and the treatments were maintained for 24 hours. To determine cell viability, the monolayers were incubated for 4 hours with 1 mg/mL MTT solution and the formazan crystals that appeared were solubilized in isopropanol. The optic density of the resulting solution was determined at FlexStation III spectrophotometer (Molecular Devices) at 550 nm as a direct measure of cell viability.
In vitro hADSCs Chondrogenic Differentiation
hADSCs are undifferentiated cells isolated from subcutaneous adipose tissue and have the potential to differentiate into several mesenchymal cell types, including adipocytes,24 chondrocytes,25 and osteoblasts.26 In our study, we experimentally induced chondrogenesis by exposing the 4 days developed hADSCs spheroids to chondrogenic induction medium (StemPro Chondrogenesis Differentiation Kit, ThermoFisher).
Cetylated Fatty Acids Mixture from Celadrin Treatment and Experimental Design
To evaluate the chondrogenic process under the treatment with cetylated fatty acids mixture from Celadrin, the hADSCs spheroids were split in 2 groups. The first group was exposed during 21 days to chondrogenic induction medium and served as experimental control, while the second group of hADSCs spheroids was incubated for the same period with chondrogenic induction medium supplemented with 0.7 mg/mL cetylated fatty acids mixture from Celadrin. The efficiency and the dynamics of our in vitro induced chondrogenic process were evaluated during the experimental period of 3 weeks by investigating the expression of the following proteins: Sox-9, collagen, aggrecan, and syndecan-3 as well as glycosaminoglycan (chondroitin sulfate, keratan sulfate) chondrogenic markers.
Immunofluorescence Microscopy
Immunofluorescence microscopy was used to evaluate the initial (T0) as well as weekly (T1-3) expression of the aforementioned chondrogenic markers. For this purpose, at each time point the hADSCs spheroids were fixed with 4% paraformaldehyde (PFA) solution overnight at 4°C and then the cell membranes were permeabilized with 2% bovine serum albumin (BSA) and 0.1% Triton X100 for 2 hours at room temperature. Next, the hADSCs spheroids were incubated overnight at 4°C in the following primary antibodies solutions: anti-Sox-9, anti-aggrecan, anti-chondroitin sulfate, anti-col2a, anti-keratan sulfate, and anti-syndecan-3. After a wash step, the hADSCs spheroids were incubated for 1 hour at room temperature with FITC (fluorescein) and TRITC (tetramethyl-rhodamine) conjugated secondary antibodies and then the nuclei were labeled with DAPI (4′,6-diamidino-2-phenylindole) at room temperature. The samples were inspected under an inverted microscope with fluorescence modulus (Olympus IX73). Images were captured using the CellSense Imaging Software (Olympus, Hamburg, Germany).
In Vitro Investigation of the Inflammatory Profile
RAW264.7 mouse macrophage cell line is traditionally used to simulate the inflammation process in vitro as these cells secrete inflammatory cytokines after stimulation with bacterial lipopolysaccharides (LPS). To investigate the inflammation profile, the RAW264.7 cells were seeded at an initial cell density of 1.5 × 104 cells/cm2 and maintained in standard conditions of culture for 24-hour prior treatments.
Treatments and Experimental Design
The anti-inflammatory potential of the cetylated fatty acids mixture from Celadrin was investigated in comparison with SAID and NSAID. For this purpose, all the RAW264.7 samples were concomitantly exposed to 1 µg/mL LPS and one of the following treatments: 1 mM ibuprofen, 0.5 mM prednisone, 0.5 mM piroxicam, and 0.7 mg/mL cetylated fatty acids mixture. A positive control was prepared by exposing the RAW264.7 cells only to the stimulation of the LPS treatment. After 3 hours (T1) and 24 hours (T2) of treatment, the cell culture media was collected and further analyzed.
Flow Cytometry Investigation of Inflammatory Cytokines Expression Profiles
The inflammation profile of treated versus untreated samples was investigated by flow cytometry using a bead based multiplex assay (BD CBA Inflammation Kit) designed for the analysis of the following cytokines protein levels: IL-6, IL-10, monocyte chemoattractant protein–1 (MCP-1), interferon-γ (IFN-γ), TNF, and IL-12p70. For this purpose, 50 µL of T1 and T2 harvested culture media were incubated for 2 hours at room temperature and darkness with 50 µL of the cytokines mixed Capture Beads and 50 µL Inflammation PE Detection Reagent. After a wash step, all samples were analyzed in a Gallios (Beckman Coulter) flow cytometer using Gallios Software (Beckman Coulter) for sample acquisition and Kaluza 1.5 Software (Beckman Coulter) for data analysis.
Statistical Analysis
The spectrophotometric data were analyzed and interpreted using the GraphPad Prism Software. Data are presented as the average of 3 replicates (mean ± standard deviation) and the statistical analysis was performed using one-way analysis of variance Bonferroni test.
The inflammatory cytokines profiles were obtained after the analysis of the samples acquired in triplicate using a Beckman Coulter Gallios (Beckman Coulter) flow cytometer and the Gallios Software. The data files generated were further analysed for median distribution of the fluorescence intensity using Kaluza 1.5 Software. The graphical representation and statistical analysis was performed using GraphPad Prism Software.
Results
Cetylated Fatty Acids Mixture from Celadrin Working Dose Determination
Both hADSCs and RAW264.7 cells were treated with 10 dilutions of the 25% oil in water emulsion obtained from Celadrin capsules in order to determine the proper working dose. The MTT test was employed to evaluate cell viability after 24 hours of treatment with concentrations ranging from 1 to 0.01 mg/mL cetylated fatty acids mixture. Our results on the hADSCs model show that the treatment with 1, 0.9, and 0.8 mg/mL cetylated fatty acids mixture slightly decreased all cells viability compared to the untreated sample, whereas the concentration of 0.7 mg/mL did not affect these cells viability ( Fig. 1 ). On the other hand, the treatment with 0.3 mg/mL cetylated fatty acids mixture from Celadrin induced a significant increase in cell viability (P < 0.05) as compared with the untreated sample. Lower concentrations induced a slight viability stimulation in both cell types, with no statistical significance (P > 0.05) ( Fig. 1 ). Consequently, we decided to use in our treatments the concentration of 0.7 mg/mL cetylated fatty acids mixture.
Figure 1.
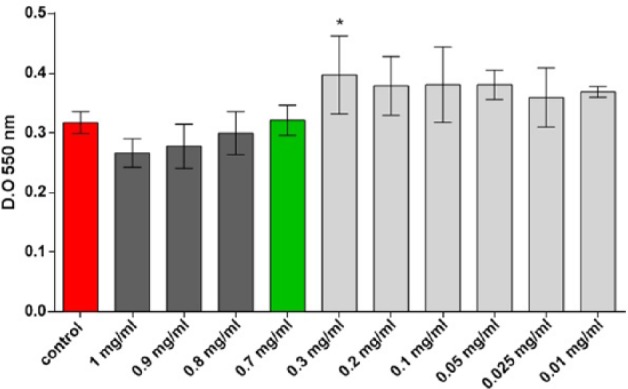
Human adipose-derived stem cells (hADSCs) viability after 24 hours of treatment with cetylated fatty acids mixture from Celadrin as determined by the MTT test (*P < 0.05 control vs. cells treated with 0.3 mg/mL cetylated fatty acids mixture from Celadrin).
Chondrogenic Differentiation Process Under the Treatment with Cetylated Fatty Acids Mixture from Celadrin
The chondrogenic differentiation of the hADSCs in 3D scaffold free culture systems was evaluated after the induction with chondrogenic culture medium in the presence and absence of cetylated fatty acids mixture (0.7 mg/mL). The expression profiles of Sox-9, aggrecan, chondroitin sulfate, keratan sulfate, collagen, and syndecan-3 respective chondrogenic markers were investigated weekly, during 21 days, by fluorescence microscopy.
As presented in Figure 2 , immunofluorescence analysis showed that the chondrogenic process was successfully induced in our experimental model as all the markers investigated displayed a positive expression. Furthermore, we observed that the treatment with 0.7 mg/mL cetylated fatty acids mixture mediated the expression of the chondrogenic process. Thus, as demonstrated in Figure 2 , Sox-9, the master transcription factor of chondrogenesis, was highly expressed at the beginning of the differentiation process (1 week) in both treated and control cells. Treated cells, however, demonstrated higher Sox-9 expression. Both treated and control cells exhibited a decrease of Sox-9 expression after 2 and 3 weeks of chondrogenic culture with treated cells exhibiting higher expression at 2 weeks as compared to control ( Fig. 2 ). The expression of the cartilage ECM components (aggrecan, collagen, chondroitin sulfate) as well as that of the cell-membrane syndecan-3 increased in time during the experimental period of 21 days. The expression of keratan sulfate was not altered by treatment. Furthermore, the double staining of the cells nuclei with DAPI and the ECM components with FITC or TRITC labeled antibodies revealed that the ratio between the cellular and the extra cellular matrix components in our spheroids. As shown by immunofluorescence microscopy images, all the untreated spheroids displayed condensed cells nuclei after 3 weeks of chondrogenic induction, leaving little space between the cells for the ECM components ( Fig. 2 ). However, the treatment with cetylated fatty acids mixture from Celadrin induced obvious modifications in the spheroids architecture as the ECM components hold the majority of the space and the cells nuclei were found dispersed in this well-developed matrix.
Figure 2.
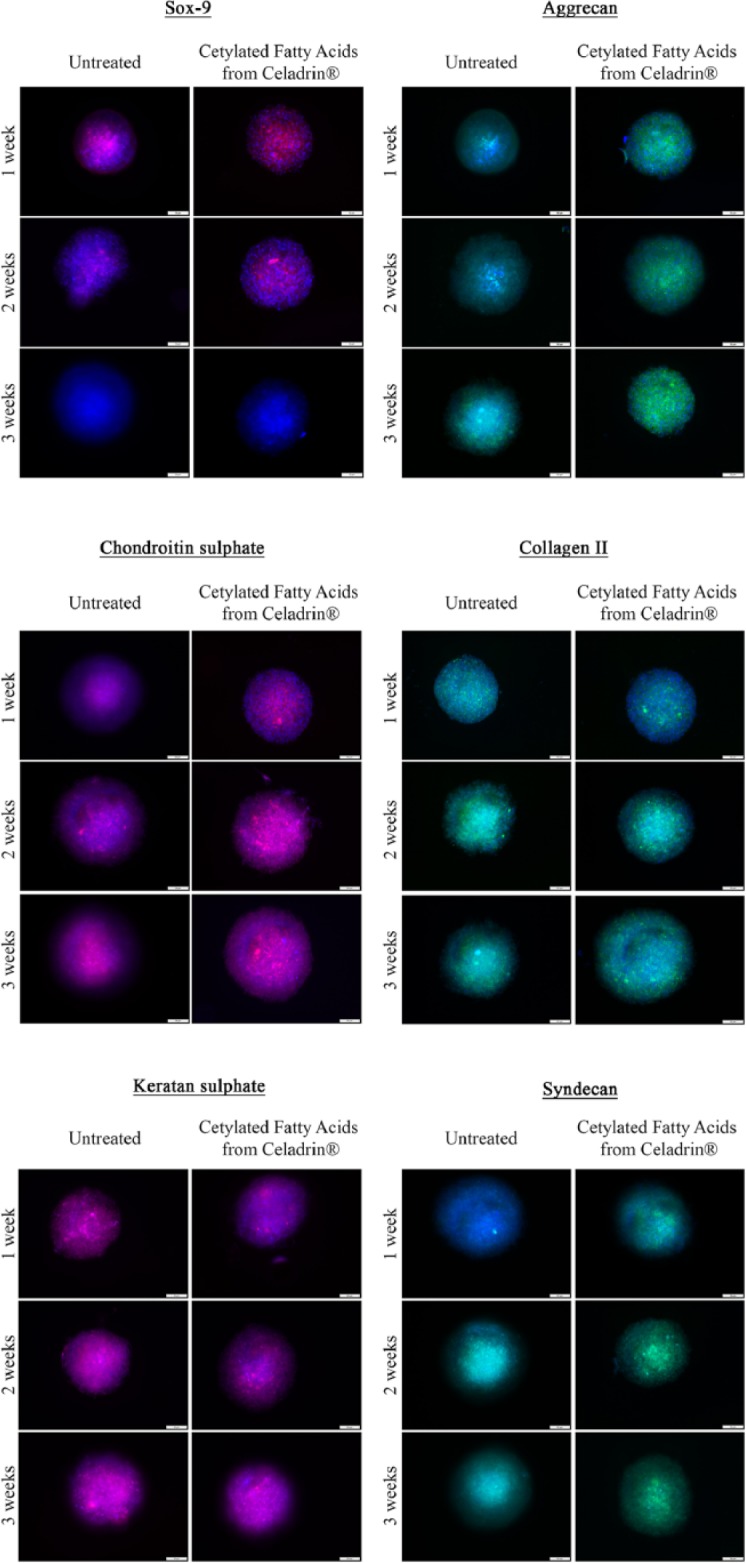
Fluorescence microscopy images of untreated and cetylated fatty acids mixture from Celadrin-treated human adipose-derived stem cells (hADSCs) spheroids labeled with Sox-9, aggrecan, chondroitin sulfate, col2a, keratan sulfate, and syndecan-3 antibodies after 1, 2, and 3 weeks of in vitro chondrogenic induction.
Inflammatory Cytokines Production
The inflammatory response was simulated in vitro by stimulating the RAW264.7 mouse macrophage cells with 1 µg/mL LPS from Escherichia coli for 24 hours. Concomitant with this treatment, the samples were treated with 1 mM ibuprofen, 0.5 mM prednisone, 0.5 mM piroxicam, or 0.7 mg/mL cetylated fatty acids mixture from Celadrin. After 3 and 24 hours of exposure to the treatments, culture media were collected and processed for the quantification of IL-6, IL-10, MCP-1, IFN-γ, TNF, and IL-12p70 by flow cytometry.
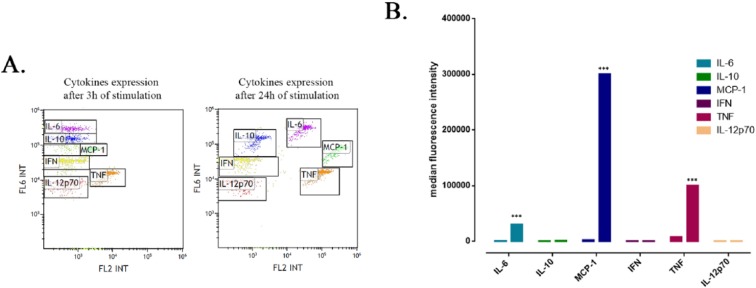
As shown in Figure 3 , there was no inflammatory stimulation after 3 hours of treatment with LPS in our RAW264.7 cell culture. However, after 24 hours of treatment, the mouse macrophage cells produced a significant increased amount of IL-6, MCP-1 and TNF (P < 0.001). No significant expression of IL-10, IFN-γ, or IL-12p70 was detected in culture media even after 24 hours of inflammation induction. Consequently, in further experiments we focused on potential modulation of IL-6, MCP-1, and TNF levels by anti-inflammatory reagents.
Figure 3.
Inflammatory cytokines expression profile after 3 and 24 hours lipopolysaccharide (LPS) stimulation of RAW264.7 mouse macrophage cells. (A) Flow cytometry dot plots revealing the cytokines mixed Capture Beads populations. (B) Graphical representation of the cytokines median fluorescence intensity (***P < 0.001 interleukin-6 (IL-6), monocyte chemoattractant protein–1 [MCP-1], and tumor necrosis factor [TNF] after 24 hours of stimulation vs. 3 hours of stimulation).
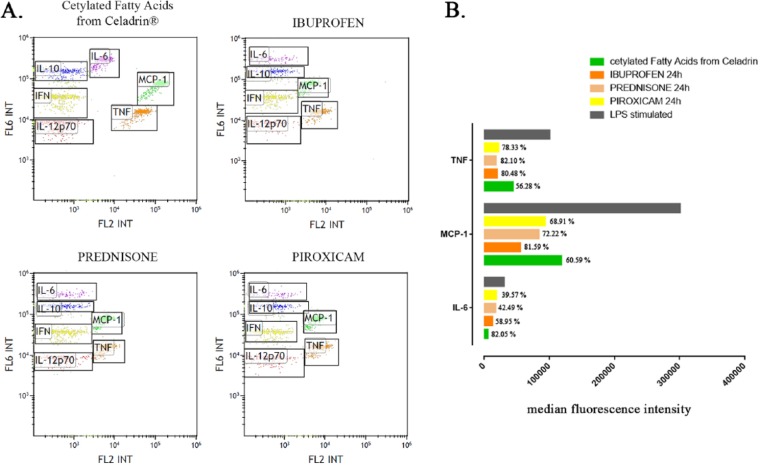
Therefore, next we evaluated the effects of anti-inflammatory mediators (ibuprofren, prednisone, piroxicam) as well as the cetylated fatty acids mixture from Celadrin on these mediator’s levels ( Fig. 4 ). As compared with the control (the LPS stimulated and untreated sample), all anti-inflammatory mediators, including the cetylated fatty acids mixture, significantly decreased the expression of IL-6, MCP-1, and TNF (P < 0.001). In particular, the treatment with 0.7 mg/mL cetylated fatty acids mixture from Celadrin triggered the highest decrease in the expression of IL-6 (82.05% from the control) as compared with ibuprofen (58.95% from the control), prednisone (42.49% from the control), and piroxicam (39.57% from the control). Ibuprofen had the greatest impact on MCP-1 decrease (81.59% from the control) as compared with the cetylated fatty acids mixture from Celadrin (60.59% from the control), prednisone (72.22% from the control), and piroxicam (68.91% from the control). Ibuprofen, prednisone, and piroxicam induced approximatively the same decrease on the expression of TNF (80.48%, 82.1%, and 78.22% from the control, respectively), while the cetylated fatty acids mixture from Celadrin decreased TNF expression only with 56.28% from the control.
Figure 4.
(A) Flow cytometry dot plots revealing the inflammatory cytokines expression profiles after 24 hours of treatment with cetylated fatty acids mixture from Celadrin (0.7 mg/mL), ibuprofen (1 mM), prednisone (0.5 mM), and piroxicam (0.5 mM). (B) Median fluorescence intensity of interleukin-6 (IL-6), monocyte chemoattractant protein–1 (MCP-1), and tumor necrosis factor (TNF) cytokines after 24 hours of treatment with cetylated fatty acids mixture from Celadrin (0.7 mg/mL), ibuprofen (1 mM), prednisone (0.5 mM), and piroxicam (0.5 mM) as compared with the lipopolysaccharide (LPS)-stimulated sample.
Discussion
Fatty acids in the form of food supplements or topical creams have been suggested to alleviate the symptoms of various connective tissue disease. Thus, a number of studies have highlighted the benefits of fish oil in rheumatoid arthritis but there is little information about OA treatment.27-29 Indeed, it was suggested that fish oil supplementation reduced the production of eicosanoids that mediate inflammation as well as cytokines synthesis.30 Furthermore, monounsaturated fatty acids from olive oil inhibited endothelial activation31,32 and reduced tissue responsiveness to cytokines.33-35 Interestingly, cetylated myristoleic acid was proved to confer protection in adjuvant induced arthritis.1
Studies aimed at assessing functional mobility after topical treatment with cetylated fatty acids determined beneficial effects. Thus, topical treatment with cetylated fatty acids significantly increased physical performance such as balance, walking, rising from chair, or stairs-climbing ability in patients with knee OA.19,20 The effects were evident at 30 minutes from initial topic application of creams containing cetylated fatty acids and were further improved after 30 days of treatment. The effects were correlated to pain relief, potentially because of the reduction of inflammation, but mechanisms at the, cellular level were not clear.19,20
The OA-correlated inflammation process is partly because of the release of pro-inflammatory cytokines such as IL-1β and TNF-α. Fatty acids, mainly the n-6 fatty acid group, have the ability to reduce chronic inflammation in rheumatoid arthritis by downregulating or by disrupting of the inflammatory process. This might be propagated by reducing both the secretion of leukotriene B4 from stimulated neutrophils as well as the release of IL-1 by monocytes.36,37 Other suggested mechanisms of action are the direct suppression of leukocyte activity by modulating their ability to migrate or to adhere through the modulation of the adhesion molecule’s expression.36,38 Monounsaturated cetylated fatty acids are believed to affect the inflammatory process through the inhibition of the cyclooxygenase pathway, though this has yet to be confirmed in human subjects.21
In this pilot study on the anti-inflammatory potential of the cetylated fatty acids mixture from Celadrin, we show that these compounds may act on the inflammatory process by significantly decreasing the production of IL-6, MCP-1, and TNF in stimulated RAW264.7 mouse macrophage cells. Importantly, these cytokines are key regulators of the inflammatory process, as IL-6 stimulates the immune response during infection or trauma and is correlated to heightened pain sensitivity in OA patients, whereas MCP-1 is an essential chemokine involved in monocyte and macrophage traffic across endothelial and epithelial barriers. Moreover, TNF is involved in the systemic inflammation, mainly in acute phase and its impaired expression is correlated to pathologies like Alzheimer’s,39 cancer,40 psoriatic arthritis,41 obesity, insulin resistance,42 and so on. Consequently, our results suggest that the cetylated fatty acids mixture from Celadrin can affect the inflammation process through the modulation of IL-6, MCP-1, and TNF production. Furthermore, in our experimental model, we observed that the descendent trend of the IL-6, MCP-1, and TNF production was maintained also in all the samples treated with SAIDs and NSAIDs, suggesting that the cetylated fatty acids from Celadrin may act similarly. However, further studies approaching upstream signaling molecules are to be addressed.
The second point of the present study is the demonstrated stimulatory effect of cetylated fatty acids mixture from Celadrin on chondrogenesis in our model system. This was achieved by upregulating the chondrocyte production of specific chondrogenic markers such as Sox-9, aggrecan, collagen type II, chondroitin sulfate, and syndecan-3.43 Chondrogenesis is a crucial biological process correlated to skeletal development, tissue patterning, and endochondral ossification.44,45 This multistep process requires undifferentiated mesenchymal cells to condense, undergo morphological changes and activate the key genes leading to cartilage formation. The nuclear transcription factor Sox-9 is one of the chondrogenic markers expressed by cells in early stages of condensation. Sox-9 is necessary to activate the genes encoding for specific matrix proteins, such as type II collagen gene. Moreover, Sox-9 is essential for mesenchymal cell commitment toward chondrogenic pathway and protein expression of chondrocyte-specific matrix, including collagen type II, IX, XI and aggrecan. These proteins are required for maintaining the biochemical properties of articular cartilage. Finally, the secreted ECM components are organized in a well-structured network composed mostly of collagens and proteins decorated with glycosaminoglycan chains, proteoglycans, into which the differentiated chondrocytes are embedded.44,45 Indeed, the chondrocytes are the singular resident cells of articular cartilage and therefore uniquely liable for the correct ECM configuration. On the other hand, the metabolism of chondrocytes is regulated by its micro-environment, including inflammatory cells input and this feedback directly affects ECM organization and finally the mechanical properties of cartilage. Thus, the decorated with chondroitin sulfate chains proteoglycan, aggrecan, which is among the most abundant noncollagenous components of the cartilage ECM, forms aggregates and contributes to cartilage mechanical properties, including a good resistance to pressure.44,46 On the other hand, type II collagen, is the most abundant cartilage protein.47 Indeed, in the present study, an increased expression of chondrogenic markers (aggrecan, collagen type II, syndecan-3, and chondroitin sulfate) was demonstrated in spheroids treated with the cetylated fatty acids mixture from Celadrin suggesting that these compounds facilitate the chondrogenesis. There were no changes in keratan sulphate expression in cetylated fatty acids mixture–treated samples, which may be due to conserved, topical distribution of this glycosaminoglycan during joint development.48 Finally, cartilage is a highly specialized tissue that corrects the distribution of load across the joint surface, ensures minimal friction of the bone’s surfaces and allows elastic deformation of the joint surface. Because of the high ECM-producing capability of chondrocytes the ratio between the cellular component and the extracellular matrix is in the favor of the last one. In healthy tissue, articular chondrocytes preserve the articular cartilage by maintaining the balance between ECM synthesis and degradation by proteolytic enzymes, including matrix metalloproteinases (MMPs) and aggrecanases.49 In degenerative joint diseases, including OA an imbalance between anabolic and catabolic processes occurs leading to a developing degradation of the articular cartilage, ultimately resulting in subchondral bone exposure, osteophyte formation, and attenuated joint mobility as well as chronic pain. Therefore, cartilage repair or the prevention of cartilage degradation is needed. Indeed, mesenchymal stem cell–based therapy is an evolving therapeutic strategy to obtain functional replacement of articular cartilage.50
In our experimental model, we have used a scaffold-free 3D culture system, which supplies a permissive environment for the undifferentiated cells to aggregate in order to initiate chondrogenesis. Importantly, the treatment with cetylated fatty acids mixture from Celadrin initiated and propagated the process of chondrogenesis as demonstrated by the increased expression and deposition of chondrogenic markers by the differentiating mesenchymal cells. These initial findings suggest that cetylated fatty acids mixture from Celadrin might have a beneficial role on degenerated cartilage repair.
In conclusion, the cetylated fatty acids mixture from Celadrin reduce inflammation in vitro by significantly decreasing the expression of IL-6, MCP-1, and TNF in stimulated RAW264.7 mouse macrophage cells. These compounds facilitate the chondrogenic differentiation process of hADSCs by stimulating the expression of chondrogenic markers under chondrogenic induction conditions. In perspective, further in vivo studies on animal models and human volunteers should be developed to validate the in vitro findings presented in this article.
Footnotes
Acknowledgments and Funding: The research was financed by Good Days Therapy SRL, distributor of Celadrin in Romania.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Animal Welfare: This study was performed using commercial cell lines and did not involve animals.
Ethical Approval: Ethical approval was not sought for the present study because all the studies were performed on previously purchased cell lines.
References
- 1. Diehl HW, May EL. Cetyl myristoleate isolated from Swiss albino mice: an apparent protective agent against adjuvant arthritis in rats. J Pharm Sci. 1994;83:296–9. [DOI] [PubMed] [Google Scholar]
- 2. Jordan KP, Wilkie R, Muller S, Myers H, Nicholls E; Arthritis Research Campaign National Primary Care Centre. Measurement of change in function and disability in osteoarthritis: current approaches and future challenges. Curr Opin Rheumatol. 2009;21:525–30. [DOI] [PubMed] [Google Scholar]
- 3. Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. [DOI] [PubMed] [Google Scholar]
- 7. Oehler S, Neureiter D, Meyer-Scholten C, Aigner T. Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol. 2002;20:633–40. [PubMed] [Google Scholar]
- 8. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [DOI] [PubMed] [Google Scholar]
- 10. Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther. 2011;13:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rose BJ, Kooyman DL. A tale of two joints: the role of matrix metalloproteases in cartilage biology. Dis Markers. 2016;2016:4895050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem. 2012;287:33926-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:726–37. [DOI] [PubMed] [Google Scholar]
- 15. Bjordal JM, Klovning A, Ljunggren AE, Slørdal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain. 2007;11:125–38. [DOI] [PubMed] [Google Scholar]
- 16. Meek IL, Van de Laar MAFJ, Vonkeman HE. Non-steroidal anti-inflammatory drugs: an overview of cardiovascular risks. Pharmaceuticals (Basel). 2010;3:2146–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the safety of “Cetyl Myristoleate Complex” as a food ingredient. EFSA J. 2010;8:1686. doi: 10.2903/j.efsa.2010.1686. [DOI] [Google Scholar]
- 18. Diehl HW. Method for the Treatment of Osteoarthritis. 1996. Available from http://www.google.com/patents/US5569676.
- 19. Hesslink R, Jr, Armstrong D, 3rd, Nagendran MV, Sreevatsan S, Barathur R. Cetylated fatty acids improve knee function in patients with osteoarthritis. J Rheumatol. 2002;29:1708–12. [PubMed] [Google Scholar]
- 20. Kraemer WJ, Ratamess NA, Anderson JM, Maresh CM, Tiberio DP, Joyce ME, et al. Effect of a cetylated fatty acid topical cream on functional mobility and quality of life of patients with osteoarthritis. J Rheumatol. 2004;31:767–74. [PubMed] [Google Scholar]
- 21. Kraemer WJ, Ratamess NA, Maresh CM, Anderson JA, Volek JS, Tiberio DP, et al. A cetylated fatty acid topical cream with menthol reduces pain and improves functional performance in individuals with arthritis. J Strength Cond Res. 2005;19:475–80. [DOI] [PubMed] [Google Scholar]
- 22. Kraemer WJ, Ratamess NA, Maresh CM, Anderson JA, Tiberio DP, Joyce ME, et al. Effects of treatment with a cetylated fatty acid topical cream on static postural stability and plantar pressure distribution in patients with knee osteoarthritis. J Strength Cond Res. 2005;19:115–21. [DOI] [PubMed] [Google Scholar]
- 23. Hasturk H. The actions of vegetable-derived Celadrin® on monocyte-mediated cytokine response. Available from: https://good-days.ro/files/file/celadrin_vege_boston_university_report.pdf.
- 24. Galateanu B, Dinescu S, Cimpean A, Dinischiotu A, Costache M. Modulation of adipogenic conditions for prospective use of hADSCs in adipose tissue engineering. Int J Mol Sci. 2012;13:15881-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dinescu S, Galateanu B, Radu E, Hermenean A, Lungu A, Stancu IC, et al. A 3D porous gelatin-alginate-based-IPN acts as an efficient promoter of chondrogenesis from human adipose-derived stem cells. Stem Cell Int. 2015;2015:252909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ionita M, Vasile E, Crica LE, Voicu SI, Pandele AM, Dinescu S, et al. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Composites Part B. 2015;72:108–15. [Google Scholar]
- 27. Dyerberg J. Platelet-vessel wall interaction: influence of diet. Philos Trans R Soc Lond B Biol Sci. 1981;294:373–81. [DOI] [PubMed] [Google Scholar]
- 28. Dyerberg J, Bang HO. A hypothesis on the development of acute myocardial infarction in Greenlanders. Scand J Clin Lab Invest Suppl. 1982;161:7-13. [PubMed] [Google Scholar]
- 29. Horrobin DF. Low prevalences of coronary heart disease (CHD), psoriasis, asthma and rheumatoid arthritis in Eskimos: are they caused by high dietary intake of eicosapentaenoic acid (EPA), a genetic variation of essential fatty acid (EFA) metabolism or a combination of both? Med Hypotheses. 1987;22:421–8. [DOI] [PubMed] [Google Scholar]
- 30. Whelan J. Antagonistic effects of dietary arachidonic acid and n-3 polyunsaturated fatty acids. J Nutr. 1996;126(4 Suppl):1086S-10891S. [DOI] [PubMed] [Google Scholar]
- 31. Mata P, Alonso R, Lopez-Farre A, Ordovas JM, Lahoz C, Garces C, et al. Effect of dietary fat saturation on LDL oxidation and monocyte adhesion to human endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 1996;16:1347–55. [DOI] [PubMed] [Google Scholar]
- 32. Pérez-Jiménez F, Castro P, López-Miranda J, Paz-Rojas E, Blanco A, López-Segura F, et al. Circulating levels of endothelial function are modulated by dietary monounsaturated fat. Atherosclerosis. 1999;145:351–8. [DOI] [PubMed] [Google Scholar]
- 33. Granato D, Blum S, Rössle C, Le Boucher J, Malnoë A, Dutot G. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J Parenter Enteral Nutr. 2000;24:113–8. [DOI] [PubMed] [Google Scholar]
- 34. Adam JM, Raju J, Khalil N, Bird RP. Evidence for the involvement of dietary lipids on the modulation of transforming growth factor-β1 in the platelets of male rats. Mol Cell Biochem. 2000;211:145–52. [DOI] [PubMed] [Google Scholar]
- 35. Patrick L, Uzick M. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern Med Rev. 2001;6:248–71. [PubMed] [Google Scholar]
- 36. Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71(1 Suppl):349S-351S. [DOI] [PubMed] [Google Scholar]
- 37. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275:721–4. [DOI] [PubMed] [Google Scholar]
- 38. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(Suppl):S243-7. [DOI] [PubMed] [Google Scholar]
- 39. Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–41. [DOI] [PubMed] [Google Scholar]
- 40. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487-501. [DOI] [PubMed] [Google Scholar]
- 41. Popa OM, Bojinca M, Bojinca V, Dutescu M, Meirosu M, Caisan RE, et al. A pilot study of the association of tumor necrosis factor alpha polymorphisms with psoriatic arthritis in the Romanian population. Int J Mol Sci. 2011;12:5052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827-47. [DOI] [PubMed] [Google Scholar]
- 43. Kosher RA. Syndecan-3 in limb skeletal development. Microsc Res Tech. 1998;43:123–30. [DOI] [PubMed] [Google Scholar]
- 44. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. [DOI] [PubMed] [Google Scholar]
- 45. Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA. Basic science of articular cartilage. Clin Sports Med. 2017;36:413–25. [DOI] [PubMed] [Google Scholar]
- 46. Sanchez C, Bay-Jensen AC, Pap T, Dvir-Ginzberg M, Quasnichka H, Barrett-Jolley R, et al. Chondrocyte secretome: a source of novel insights and exploratory biomarkers of osteoarthritis. Osteoarthritis Cartilage. 2017;25:1199–209. [DOI] [PubMed] [Google Scholar]
- 47. Henrotin Y, Addison S, Kraus V, Deberg M. Type II collagen markers in osteoarthritis: what do they indicate? Curr Opin Rheumatol. 2007;19:444–50. [DOI] [PubMed] [Google Scholar]
- 48. Kavanagh E, Osborne AC, Ashhurst DE, Pitsillides AA. Keratan sulfate epitopes exhibit a conserved distribution during joint development that remains undisclosed on the basis of glycosaminoglycan charge density. J Histochem Cytochem. 2002;50:1039–47. [DOI] [PubMed] [Google Scholar]
- 49. Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. 2012;20:339–49. [DOI] [PubMed] [Google Scholar]
- 50. Mazor M, Lespessailles E, Coursier R, Daniellou R, Best TM, Toumi H. Mesenchymal stem-cell potential in cartilage repair: an update. J Cell Mol Med. 2014;18:2340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]