Abstract
Objective
We investigated the effect of administration of intra-articular mesenchymal stem cells (MSCs) on cartilage repair at different timings, and the distribution of MSCs in the knee.
Design
A partial thickness cartilage defect (PTCD) was created on the medial femoral condyle in 14-week-old Sprague-Dawley rats. Intra-articular injection of 1 × 106 MSCs was performed at 3 time points, namely at the time of surgery (0w group), at 1 week after surgery (1w group), and at 2 weeks after surgery (2w group). For the control, 50 μL phosphate-buffered saline was injected at the time of surgery. The femoral condyles were collected at 6 weeks after creation of PTCD and assessed histologically. To investigate the distribution of MSCs, fluorescent-labeled MSCs were injected into the knee joint.
Results
In the control group, the cartilage lesion was distinguishable from surrounding cartilage. In the 0w group, hypocellularity and a slight decrease in safranin O stainability were observed around the injured area, but cartilage was restored to a nearly normal condition. In contrast, in the 1w and 2w groups, the cartilage surface was irregular and safranin O stainability in the injured and surrounding areas was poor. Histological score in the 0w group was significantly better than in the control, 1w, and 2w groups. At 1 day postinjection, fluorescent-labeled MSCs were mostly distributed in synovium. However, no migration into the PTCD was observed.
Conclusions
Early intra-articular injection of MSCs was effective in enhancing cartilage healing in a rat PTCD model. Injected MSCs were distributed in synovium, not in cartilage surrounding the PTCD.
Keywords: partial thickness cartilage defects, mesenchymal stem cells, cartilage repair, synovium
Introduction
Osteoarthritis (OA) is the most common form of joint disorder and is a leading cause of joint pain and disability1-3 Aging of a population is accompanied by an increase in the prevalence of OA and an increase in economic burden.2-6 Although the development of disease-modifying osteoarthritis drugs (DMOADs) has long been awaited, few effective treatments are currently available.7
Cartilage defect leads to progressive joint degeneration.8 A cartilage defect is categorized as a full thickness cartilage defect (FTCD) or partial thickness cartilage defect (PTCD). PTCD is a type of injury that does not penetrate the marrow spaces of subchondral bone.9 PTCD does not heal spontaneously and leads to the onset of OA.9-13 Curl et al.14 reported PTCD was commonly encountered in arthroscopy and was considered to occupy a certain percentage of the cause of osteoarthritis. Thus, treatment of PTCD would suppress the onset of OA and lessen the incidence of OA.
Mesenchymal stem cells (MSCs) play an important role in cartilage restoration against PTCD.9 In our previous study, we achieved cartilage restoration with intra-articular injection of MSCs at the time of PTCD creation. Furthermore, synovial-like tissue proliferation and adhesion was observed on the surface of PTCD in the knees injected with MSCs (in submission). In clinical settings, however, treatment immediately after cartilage injury is generally difficult. This emphasizes the importance of understanding the effect of delays in MSC injection on cartilage restoration after PTCD.
We hypothesized that cartilage restoration was worse when MSCs injection occurred later. Here, we investigated differences in cartilage restoration arising from different timing of intra-articular injection of MSCs in a PTCD model. We also evaluated the distribution of MSCs in the knee joint and found that injected MSCs were distributed in synovium.
Material and Methods
Animals and Preparation of Synovial MSCs
A total of 40 male Sprague-Dawley rats (Japan SLC, Inc., Shizuoka, Japan) were used for the experiments at age 14 weeks. All protocols for animal procedures were approved by the Ethics Committee of the Graduate School of Medicine, Chiba University.
MSCs were prepared as described in the literature.15 Briefly, synovium of infrapatella fat pads were obtained from 8 knees of four 14-week-old rats, washed thoroughly, then digested for 1 hour at 37°C with pronase (Merck KGaA, Darmstadt, Germany) and 3 hours with collagenase (Roche Diagnostics GmbH, Mannheim, Germany). After filtration using a 70-μm filter, the sample was cultured in Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 Ham (Sigma-Aldrich Co, St. Louis, MO, USA) supplemented with 20% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA), 200 mM l-glutamine solution (Nacalai Tesque, Kyoto, Japan), 50 mg/mL gentamicin reagent solution (Nacalai Tesque), and 50 g/mL ascorbic acid (Sigma-Aldrich Co) at 37°C. We changed the culture medium once every 2 or 3 days, prepared subcultures on day 7, and obtained cells on day 14 (passage 2). For injection, 1 × 106 synovial MSCs were prepared in 50 μL of phosphate-buffered saline (PBS).
In Vitro Differentiation
For chondrogenesis, 2.5 × 105 MSCs were placed in a 15-mL polypropylene tube and cultured for 21 days in chondrogenic medium which contained chondrogenetic base medium (CCM005, Chondrogenic Base Media, Human/Mouse/Rat, StemXVivo; R&D Systems, Minneapolis, MN, USA) with supplement (CCM020 Rat StemXVivo Chondrogenic Supplement; R&D Systems) according to the manufacturer’s protocol. The pellets were embedded in paraffin, cut into 5-μm sections, and stained with Alcian blue.
For adipogenesis, 1.0 × 106 MSCs were cultured for 21 days in adipogenic medium, which contained adipogenic base media (CCM007 Osteogenic/Adipogenic Base Media, Human/Mouse/Rat, StemXVivo; R&D Systems) with supplement (CCM011 Adipogenic Supplement, Human/Mouse/Rat, StemXVivo; R&D Systems) according to the manufacturer’s protocol. The adipogenic culture was fixed in 4% paraformaldehyde and then stained with oil red-O.
For osteogenesis, 2.5 × 105 MSCs were cultured for 21 days in osteogenic medium, which contained osteogenic base media (CCM007 Osteogenic/Adipogenic Base Media, Human/Mouse/Rat, StemXVivo; R&D Systems) with supplement (CCM009 Osteogenic Supplement, Mouse/Rat, StemXVivo; R&D Systems) according to the manufacturer’s protocol. The osteogenic culture was fixed in 4% paraformaldehyde and then stained with alizarin red.
We conducted experiments twice to confirm multipotential capacities of MSCs described above.
Flow Cytometry
Cell surface staining of MSCs was done using phycoerythrin (PE) anti-rat CD11b/c, Alexa Fluor 647 anti-rat CD45, and Brilliant Violet 421 anti-rat CD90/mouse CD90.1 (Thy-1.1) (BioLegend Inc., San Diego, CA, USA). Fixable viability dye eFluor 780 (eBioscience, San Diego, CA, USA) was used to exclude dead cells from analysis. Flow cytometric analysis was done on a FACSverse flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and the results were analyzed using the Flow Jo software program (Tree Star, Inc., Ashland, OR, USA).
Creation of PTCDs and Injection of MSCs
Rats were anesthetized by intraperitoneal injection of ketamine (0.1 mg/g) and xylazine (0.01 mg/g). Arthrotomy of the left knee was performed through a medial longitudinal parapatellar incision. The patella was displaced laterally and the PTCD was created lineally on the weightbearing region of the medial femoral condyle (MFC) in the sagittal direction using a 100-μm ophthalmic knife (Feather Safety Razor Co., Ltd., Osaka, Japan) as described previously.16 The joint capsule and skin were then closed with 6-0 nylon sutures. Intra-articular injection was performed through the patellar tendon using a 30G needle. Intra-articular injection of 1 × 106 MSCs was performed at 1 of 3 time points from the creation of PTCDs (n = 6 each): at the time of surgery (0w group), 1 week after surgery (1w group), and 2 weeks after surgery (2w group). All the injections were performed on the same day in order to use the same MSCs by creating PTCDs 2 weeks prior to injection, 1 week prior to injection, and on the same day of injection. Thus, injected MSCs were allo-derived cells. For the control, 50 μL of PBS was injected at the time of surgery (n = 6). The rats were allowed to walk freely in their cages.
Histological Assessment
The femoral condyles were collected at 6 weeks after creation of PTCD. The specimens were fixed in 4% paraformaldehyde for 7 days, decalcified in 20% ethylenediamine tetraacetic acid (EDTA) solution for 28 days, then embedded in paraffin. Sections of 5-μm thickness were prepared in the coronal plane and stained with safranin O and fast green. The repair response of cartilage was evaluated using the modified histological classification system of Mukoyama et al.16 This score system consists of 5 aspects: (1) filling of the defect, (2) matrix staining (injured area), (3) matrix staining (surrounding area), (4) surface regularity, and (5) cell morphology and density. The score of each aspect ranges from 0 to 3 points, with a total score of 0 points representing normal tissue and 15 points reflecting the absence of any reparative tissue within the defect. The histological sections were blindly scored twice at an interval of 1 week by 2 evaluators (T.E. and Y.O.). The specimens were also immune-stained for detection of type II collagen. In brief, sections were treated with hyaluronidase for 60 minutes at 37°C. Endogenous peroxidase was blocked with 3% H2O2 for 15 minutes at room temperature. After washing with PBS, sections were treated with blocking reagent (Nacalai Tesque) for 10 minutes at room temperature. The sections were washed again with PBS and primary anti-human collagen II mouse monoclonal antibody clone (dilution of 1:500, Kyowa Pharma Chemical, Toyama, Japan) were applied to the sections and incubated overnight at 4°C. After washing with PBS, the sections were incubated with peroxidase-labeled anti-rabbit or anti-mouse antibody (Histofine Simplestain Max PO; Nichirei, Tokyo, Japan) for 30 minutes at room temperature. The sections were stained with DAB (3,3′-diaminobenzidine) solution and counter stained with Mayer’s hematoxylin.
Detection of MSCs After Intra-Articular Injection
A PKH26 red fluorescent cell linker kit (Sigma-Aldrich, St. Louis, MO, USA) was used for fluorescent labeling of MSCs. PTCDs were created by the same method as described above and 1 × 106 labeled MSCs were injected into the knee joint at the time of surgery (n = 4). The knee joints were then harvested at 1 day or 1 week after treatment and used to create fresh-frozen sections by Kawamoto’s film method.17 An Axioskop 2 plus fluorescent microscope equipped with a Rhodamine filter (Carl Zeiss Japan, Ltd., Tokyo, Japan) was used for detecting fluorescent-labeled MSCs.
Statistical Analysis
Statistical analysis was performed with JMP 13 (SAS Institute Inc., Cary, NC, USA). Histological scores between the 4 groups were analyzed using the Kruskal-Wallis test followed by the Steel–Dwass test. Statistical significance was set at a P value <0.05. Intra- and interrater reliability for histological scores were assessed by the intraclass correlation coefficient.
Results
In Vitro Differentiation and Flow Cytometry of MSCs
MSCs differentiated into chondrocytes ( Fig. 1A ), adipocytes ( Fig. 1B ), and were calcified ( Fig. 1C ) in vitro. Flow cytometric analysis demonstrated that the majority of MSCs expressed CD90 and were negative for CD11b and CD45 ( Fig. 2 ). These findings indicate that the cells derived from synovium demonstrated characteristics of MSCs.
Figure 1.
Multipotency of mesenchymal stem cells (MSCs.) Multipotency of MSCs derived from synovium were confirmed by inducing differentiation into chondrocyte, adipocytes, and osteoblasts. Cartilage tissue was stained with Alcian blue (A), adipose tissue with oil red-O (B), and calcified tissue with alizarin red (C). Scale bars: 25 μm (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
Figure 2.
Flow cytometric analysis of mesenchymal stem cells (MSCs). CD11b, CD45, and CD90 expression are shown as an open plot and isotype control expression as a shaded plot.
Histological Assessment
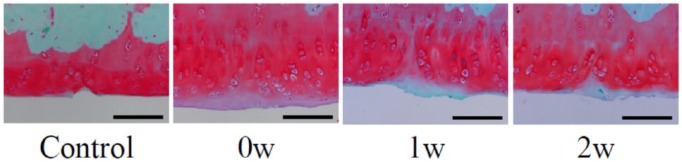
Representative histological sections stained with safranin O and fast green are shown in Figure 3 . In the control group (PBS alone), the cartilage lesion was histologically distinguishable from surrounding cartilage at 6 weeks after PTCD, with scanty restoration reaction and residual damage. In the 0w group, hypocellularity and a slight decrease in safranin O stainability were observed around the injured area, but cartilage was restored to a near-normal condition. In the 1w and 2w groups, in contrast, the cartilage surface was irregular and safranin O stainability in the injured area and surrounding area was poor.
Figure 3.
Histological assessment at 6weeks after partial thickness cartilage defect (PTCD). Representative sections stained with safranin O and fast green following interventions are shown. Control, control group; 0w, 0w group; 1w, 1w group; 2w, 2w group. Scale bars: 100 μm.
The histological scores are shown in Figure 4 . Score in the 0w group was significantly lower than those of the control, 1w, and 2w groups. There was no significant difference between the control, 1w, and 2w groups. Intrarater reliabilities were 0.87 and 0.84, respectively, and interrater reliability was 0.89.
Figure 4.
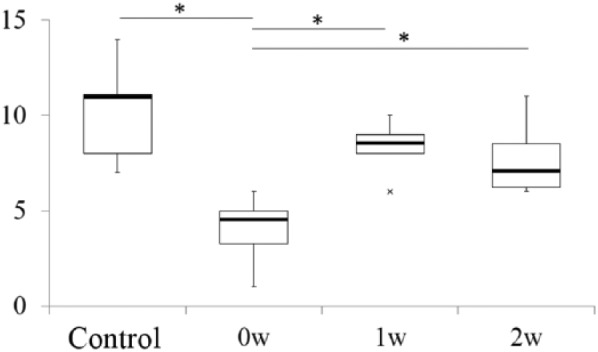
Histological scores of partial thickness cartilage defect (PTCD) following interventions. Box plot showing a comparison of histological scores between the 4 groups. *P < 0.05. Cross (×) represents outliers.
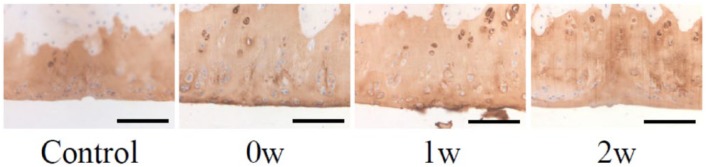
Immunostaining for type II collagen showed that the repaired cartilage was positive for type II collagen in 3 groups ( Fig. 5 ).
Figure 5.
Immunohistochemical staining for type II collagen. Control, control group; 0w, 0w group; 1w, 1w group; 2w, 2w group. Scale bars: 100 μm.
Detection of MSCs After Intra-Articular Injection
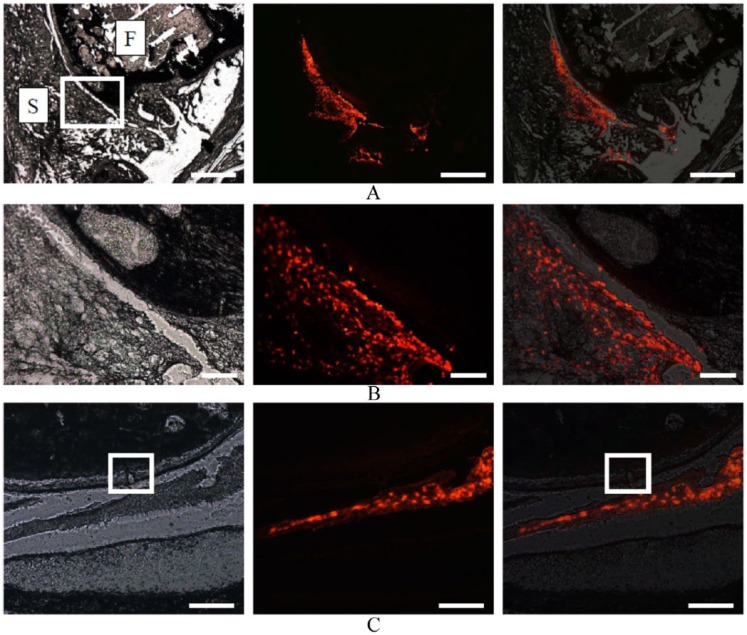
One day postinjection, fluorescent-labeled MSCs were mostly distributed in synovium, but a small number also remained in the joint cavity ( Fig. 6A and B ). However, no migration of fluorescent-labeled MSCs into the PTCD was observed ( Fig. 6C ). One week postinjection, no fluorescent-labeled MSCs were detectable in the knee (not shown).
Figure 6.
Distribution of mesenchymal stem cells (MSCs) at 1 day after intra-articular injection. Images generated with a blank filter (left), a rhodamine filter (center) and merging of a blank filter and a Rhodamine filter (right) are aligned for the same section. (A) Fluorescent-labeled MSCs were distributed in synovium in the sagittal images at a low magnification. F, femoral condyle; S, synovium. Scale bars: 1 mm. (B) High-magnification image of the rectangular area in (A). Scale bars: 200 μm. (C) Coronal images indicating the location of partial thickness cartilage defect (PTCD) by a square. Fluorescent-labeled MSCs were detected in the joint cavity but no migration into the PTCD. Scale bars: 200 μm.
Discussion
In this study of the effect of early intra-articular injection of MSCs in stimulating cartilage healing in a rat PTCD model by time of injection, we found that concurrent intra-articular injection of MSCs at the time of injury was more effective in stimulating cartilage regeneration than PBS injection or MSC injection at later time points. In addition, we confirmed that injected MSCs were absorbed by the synovium membrane of the knee joint. These results suggest that synovium that has absorbed MSCs plays a role in promoting cartilage restoration in the PTCD model.
Regarding the change in cartilage after PTCD, Hembry et al.18 reported the presence of cartilage necrosis adjacent to the defect in a pig model. Lu et al.10 reported that the depth of chondrocyte death significantly increased over time in a sheep model. Zhang et al.19 reported that chondrocyte apoptosis was observed around the cartilage defects. In our present study, cartilage degeneration around the PTCD was observed in the control group. Consistent with a previous study, we considered that chondrocyte death occurring around the PTCD and cartilage degeneration progressed over time. In contrast, intra-articular injection of MSCs at the time of creation of PTCD produced cartilage restoration. Interestingly, this healing effect was limited when the injection of MSCs was delayed to more than 1 week. The reason for restoration of cartilage might be due to the possibility that this intervention was only successful when made before cartilage degeneration had begun.
The effect of intra-articular injection of MSCs was reported to suppress cartilage degeneration in an OA-induced model.20-22 It is well known that intra-articularly injected MSCs are distributed in the synovium.20-23 Using a rat OA model, Ozeki et al.20 reported that MSCs injected at 1 × 106 were present in synovium at 1 day after injection, but not in cartilage or meniscus. After intra-articular injection, the number of viable MSCs rapidly decreased, and could not be detected in the joint at 1 week.20,22 These findings on the distribution of MSCs after intra-articular injection are consistent with those of previous studies. Several studies pointed out that synovium contributes to repair after cartilage injury.9,24,25 Moreover, our previous study reported that the perichondral environment, such as synovium or joint fluid, is indispensable for potentiating the spontaneous restoration of PTCD. Interestingly, cartilage restoration in an ex vivo culture of medial femoral condyles with PTCD of immature rats was not restored spontaneously whereas restoration was achieved in vivo.16 These results suggest that MSCs may secrete a cartilage restoration factor after being absorbed by the synovium.
PTCD was restored naturally in immature rats but was found to be unrestorable in mature rats.16,19 Degenerative change in cartilage progresses after PTCD and gradually progresses to OA.11,12 It is possible that OA onset may be reduced by intervention with PTCD, and we therefore considered that the establishment of PTCD therapy is important.
Our results suggest that a delay in the intra-articular injection of MSCs after creation of a PTCD leads to insufficient cartilage restoration. In clinical settings, however, therapeutic intervention is usually delayed after PTCD. It may be possible to treat these patients by increasing the number of injected MSCs or refreshing or debriding the cartilage around the PTCD prior to MSCs injection.
The present study has several limitations. First, the mechanism of cartilage restoration by MSCs is unknown. A previous study of intra-articular injection of MSCs in an OA model suggested that cytokines related to chondroprotection and anti-inflammation were secreted by MSCs into the knee joint.20 However, the factor which induced the restoration of cartilage remains unknown. In addition, it is unknown whether the synovium contributed to cartilage restoration in this study. Second, MSC was injected at the same time of PTCD surgery in 0w group, which appeared unfeasible in clinical situation. Third, MSC injection was conducted at 3 time points. A healing response may be obtained by injection of MSCs earlier than 1 week after PTCD, but we did not test shorter periods. Forth, detailed tracking of labelled MSCs was not performed. Finally, only 1 × 106 MSCs were injected in this study. Although the healing effect was insufficient with injection of 1 × 106 MSCs at one or two weeks after PTCD, cartilage restoration may be obtained by the injection of greater numbers of MSCs, even when treatment is delayed after PTCD, but we did not test this possibility.
In conclusion, we found that early intra-articular injection of MSCs after injury was effective in stimulating cartilage healing in a rat PTCD model. Injected MSCs were distributed in the synovium, not in the area of the PTCD.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Grant-in Aid for Scientific Research (C) of the Japan Society for the Promotion of Science (JPJS): Grant Number 16K10885.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All protocols for animal procedures were approved by the Ethics Committee of the Graduate School of Medicine, Chiba University.
Animal welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
References
- 1. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41-S47. [PubMed] [Google Scholar]
- 4. Plotnikoff R, Karunamuni N, Lytvyak E, Penfold C, Schopflocher D, Imayama I, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health. 2015;15(1):1195. doi: 10.1186/s12889-015-2529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis. 2011;14(2):113-21. doi: 10.1111/j.1756-185X.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- 6. Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qvist P, Bay-Jensen AC, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): is it in the horizon? Pharmacol Res. 2008;58(1):1-7. doi: 10.1016/j.phrs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21-37. [DOI] [PubMed] [Google Scholar]
- 9. Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78(5):721-33. [DOI] [PubMed] [Google Scholar]
- 10. Lu Y, Markel MD, Swain C, Kaplan LD. Development of partial thickness articular cartilage injury in an ovine model. J Orthop Res. 2006;24(10):1974-82. doi: 10.1002/jor.20249. [DOI] [PubMed] [Google Scholar]
- 11. Marijnissen ACA, van Roermund PM, Verzijl N, Tekoppele JM, Bijlsma JWJ, Lafeber FPJG. Steady progression of osteoarthritic features in the canine groove model. Osteoarthritis Cartilage. 2002;10(4):282-9. doi: 10.1053/joca.2001.0507. [DOI] [PubMed] [Google Scholar]
- 12. Mastbergen SC, Marijnissen AC, Vianen ME, van Roermund PM, Bijlsma JW, Lafeber FP. The canine “groove” model of osteoarthritis is more than simply the expression of surgically applied damage. Osteoarthr Cartil. 2006;14(1):39-46. doi: 10.1016/j.joca.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 13. Jansen EJ, Emans PJ, Van Rhijn LW, Bulstra SK, Kuijer R. Development of partial-thickness articular cartilage injury in a rabbit model. Clin Orthop Relat Res. 2008;466(2):487-94. doi: 10.1007/s11999-007-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries : a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 15. Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, et al. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. 2012;7(9):e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mukoyama S, Sasho T, Akatsu Y, Yamaguchi S, Muramatsu Y, Katsuragi J, et al. Spontaneous repair of partial thickness linear cartilage injuries in immature rats. Cell Tissue Res. 2015;359(2):513-20. doi: 10.1007/s00441-014-2041-3. [DOI] [PubMed] [Google Scholar]
- 17. Kawamoto T, Shimizu M. A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem Cell Biol. 2000;113(5):331-9. doi: 10.1007/s004180000149. [DOI] [PubMed] [Google Scholar]
- 18. Hembry RM, Dyce J, Driesang I, Hunziker EB, Fosang AJ, Tyler JA, et al. Immunolocalization of matrix metalloproteinases in partial- thickness defects in pig articular cartilage. A preliminary report. J Bone Joint Surg Am. 2001;83-A:826-38. [DOI] [PubMed] [Google Scholar]
- 19. Zhang K, Shi J, Li Y, Jiang Y, Tao T, Li W, et al. Chondrogenic cells respond to partial-thickness defects of articular cartilage in adult rats: an in vivo study. J Mol Histol. 2016;47(3):249-58. doi: 10.1007/s10735-016-9668-1. [DOI] [PubMed] [Google Scholar]
- 20. Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24(6):1061-70. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 21. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464-74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 22. Ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64(11):3604-13. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 23. Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354-9. doi: 10.1002/jor.22370. [DOI] [PubMed] [Google Scholar]
- 24. Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63(5):1289-1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 25. Miyamoto A, Deie M, Yamasaki T, Nakamae A, Shinomiya R, Adachi N, et al. The role of the synovium in repairing cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1083-93. doi: 10.1007/s00167-006-0277-5. [DOI] [PubMed] [Google Scholar]