Abstract
Objective
To perform a systematic review of clinical outcomes following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral allograft transplantation (OCA), and osteochondral autograft transplantation system (OATS) to treat articular cartilage lesions in pediatric and adolescent patients. We sought to compare postoperative improvements for each cartilage repair method to minimal clinically important difference (MCID) thresholds.
Design
MEDLINE, Web of Science, Scopus, and Cochrane Library databases were searched for studies reporting MCID-validated outcome scores in a minimum of 5 patients ≤19 years treated for symptomatic knee chondral lesions with minimum 1-year follow-up. One-sample t tests were used to compare mean outcome score improvements to established MCID thresholds.
Results
Twelve studies reporting clinical outcomes on a total of 330 patients following cartilage repair were identified. The mean age of patients ranged from 13.7 to 16.7 years and the mean follow-up was 2.2 to 9.6 years. Six studies reported on ACI, 4 studies reported on MFX, 2 studies reported on OATS, and 1 study reported on OCA. ACI (P < 0.001, P = 0.008) and OCA (P < 0.001) showed significant improvement for International Knee Documentation Committee (IKDC) scores with regard to MCID while MFX (P = 0.66) and OATS (P = 0.11) did not. ACI (P < 0.001) and OATS (P = 0.010) both showed significant improvement above MCID thresholds for Lysholm scores. MFX (P = 0.002) showed visual analog scale (VAS) pain score improvement above MCID threshold while ACI (P = 0.037, P = 0.070) was equivocal.
Conclusions
Outcomes data on cartilage repair in the pediatric and adolescent knee are limited. This review demonstrates that all available procedures provide postoperative improvement above published MCID thresholds for at least one reported clinical pain or functional outcome score.
Keywords: cartilage injury, pediatric knee, osteochondral allograft, autologous chondrocyte implantation
Introduction
Focal articular cartilage injuries continue to present as challenging clinical problems, particularly when young patients present with large lesions in weight-bearing joints such as the knee. Because of its avascular nature, articular cartilage lacks the ability to spontaneously heal.1 Furthermore, osteochondral defects that result from displaced osteochondritis dissecans lesions can be particularly problematic as they do not have normal subchondral bone architecture. Chondral and osteochondral lesions can result in significant pain and functional impairment.2 If left untreated, these lesions can progress to premature knee osteoarthritis, which is particularly problematic in the active pediatric and adolescent population with high functional demands and long life expectancies.2,3 When nonsurgical management fails, surgical intervention is recommended to restore the articular surface. The surgeon is challenged to address the cartilage lesion in a way that maximizes the integrity of the knee to protect against future degeneration. Advancements in surgical technique have provided biological alternatives for the repair of damaged articular cartilage and subchondral bone. These procedures include microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral allograft transplantation (OCA), and osteochondral autograft transplantation system (OATS).4
Despite a myriad of outcomes reports on these techniques, the fundamental weakness of existing literature is the inconsistent use of validated outcome measures, making it difficult to objectively compare the treatment efficacies of these procedures.5 Indeed, though several surgical options have been proposed based on location, lesion size, and time elapsed since injury, there is no consensus on the ideal indications and outcomes for each procedure. Furthermore, current evidence to guide decision making is mainly in the form of case series rather than prospective randomized controlled trials. When pediatric and adolescent populations are studied alone, even fewer reports are available for comparison to guide treatment decisions. The purpose of this study was to conduct a systematic review of clinical outcomes following MFX, ACI, OCA, and OATS surgery for the treatment of focal knee lesions in pediatric patients. We sought to compare postoperative improvement in functional and activity outcome scores to validated thresholds for minimal clinically important difference (MCID). Our initial hypothesis was that that all available procedures—MFX, ACI, OCA, and OATS—would provide clinically significant improvements in outcomes scores when compared with the current published MCID threshold for each outcome measure.
Materials and Methods
Literature Search
A systematic review was performed using the Web of Science, PubMed, Cochrane Library, and Scopus databases. The review was registered on the PROSPERO database (Registration number: CRD42016052287, University of York, York, United Kingdom). PubMed, Scopus, and Cochrane library databases were searched on December 23, 2015 with the search terms “microfracture” AND “knee.” PubMed, Web of Science, and Cochrane library databases were searched on April 12, 2016 with the search terms “((osteochondral autograft transplantation) OR (osteochondral autografting) OR (osteochondral autograft) OR (OC autograft) OR (mosaicplasty) OR (osteoarticular transfer system) OR (osteochondral cylinder transplantation) OR (osteochondral cylinder) OR (autologous chondrocyte implantation) OR (osteochondral allograft) OR (OC allograft)) AND knee.” Search terms were general to avoid inadvertent exclusion of relevant studies. Duplicate studies and stand-alone abstracts were excluded. The search algorithm was refreshed on August 25, 2017 to ensure inclusion of all literature published in the interim.
Following the primary search, article titles and abstracts were individually reviewed in accordance with the standard PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist. Articles that contained relevant information were identified and systematically analyzed to ensure compliance with the following inclusion criteria: (1) minimum of 5 subjects; (2) pediatric patients aged 19 years and younger; (3) intervention of MFX, ACI, OCA, or OATS; (4) minimum of 1 year of clinical follow-up; (5) clinical outcome reported; and (6) study published in the English language. Review articles, systematic reviews, meta-analyses, cadaveric, in vitro, and animal studies were excluded.
Data Abstraction and Statistical Analysis
Studies that met the inclusion/exclusion criteria were independently reviewed and used to extract cohorts of pediatric patients who had undergone MFX, ACI, OCA, and OATS procedures. Reported data on patient age, gender, lesion size, lesion location, number of lesions, presence of prior surgeries and concurrent procedures in the index knee, time between injury and procedure, length of follow-up, as well as pre- and postoperative clinical outcome scores were collected and reviewed. Clinical outcome scores with validated MCID scores included the International Knee Documentation Committee (IKDC), Lysholm, and visual analog scale (VAS) pain scores.6-8 Two-tailed, 1-sample Student t tests were conducted to evaluate for significance of pre- to postoperative mean improvement compared with current MCID thresholds for each individual study. Statistical analyses were performed using SPSS 21 software (IBM Corp., Armonk, NY).
Results
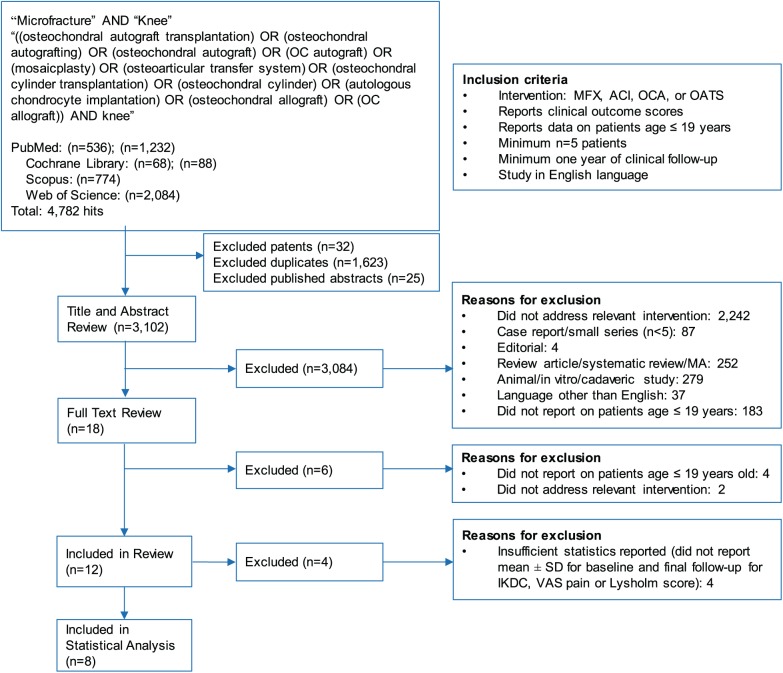
In total, 1,768 articles from PubMed, 156 articles from Cochrane library, 774 articles from Scopus, and 2,084 articles from Web of Science matched our initial search terms, for a total of 4,782 articles. These studies were serially assessed using standardized PRISMA protocol (Fig. 1). After duplicates, patents, and published abstracts were removed, 3,102 articles remained for title and abstract review. After application of exclusion criteria, 18 articles remained for full-text review. Of these 18 articles, 4 did not report on subjects younger than 19 years, and 2 did not address a relevant intervention. Twelve articles were included in our review, but 4 articles did not provide sufficient statistics (measure of center and spread for both baseline and final follow-up for IKDC, VAS pain, or Lysholm score) for MCID analysis ( Table 1 ). Eight articles were ultimately included for statistical analysis. Four of these studies reported on ACI, 3 reported on MFX, 1 reported on OCA, and 1 reported on OATS. All ACI studies and the OCA study included patients with focal cartilage lesions as well as those resulting from juvenile osteochondritis dissecans (JOCD). The OATS study included only patients with JOCD while the MFX studies did not include any patients with JOCD.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram outlining the application of the inclusion and exclusion criteria for the systematic review.
Table 1.
Summary and Characteristics of Studies Identified in Systematic Review.
| Study No. | First Author, Year of Publication | n (Patients) | Male | Female | Age in Years, Mean (Range) | Lesion Size in cm2, Mean (Range) | Lesion Location: Condylar (Fc), Patellar (P), Tibial Plateau (Tp), Trochlear (T) | Follow-up in Years, Mean (Range) | Level of Evidence | Level of Evidence, Study Methodology | ACI | OATS | OCA | MFX | IKDC | Lysholm | VAS Pain | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cvetanovich, 2017 | 37 | 15 | 22 | 16.7 (13-18) | 4 | Fc, P, T | 4.6 (2-10.6) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 2 | Gudas, 2009 | 25 | 15 | 10 | 14.6 (12-18) | 3.2 | Fc | 4.2 (3-6) | Level I | Randomized controlled trial | ✓ | ✓ | ||||||
| 2 | Gudas, 2009 | 22 | 13 | 9 | 14.1 (12-18) | 3.2 | Fc | 4.2 (3-6) | Level I | Randomized controlled trial | ✓ | ✓ | ||||||
| 3 | Lee, 2012 | 5 | 1 | 4 | 14.6 (12-17) | 1.2 (1-1.5) | P | 2.5 (1.3-4.5) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 4 | Macmull, 2011 | 31 | 22 | 9 | 16.3 (14-18) | 5.3 (1.0-15.8) | Fc, P, T | 5.5 (1-10.5) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 5 | Micheli, 2006 | 37 | 22 | 15 | 15.5 (11-17) | 5.2 (1.9-14) | Fc | 4.3 | Level IV | Case series | ✓ | ✓ | ||||||
| 6 | Mithöfer, 2005 | 20 | 15 | 5 | 15.9 (12-18) | 6.4 (2.4-14) | Fc, P, Tp, T | 3.9 (1.9-7.6) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 7 | Murphy, 2014 | 39 | 24 | 15 | 16.4 (11-17.9) | 8.4 (2.2-20.8) | Fc, P, Tp, T | 8.4 (1.7-27.1) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 8 | Niethammer, 2016 | 40 | 24 | 16 | 16.0 (11-19) | 5.3 (1.5-10.5) | Fc, P | 3.0 | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 9 | Ogura, 2017 | 27 | 13 | 14 | 15.9 (13-17) | 6.2 (2.0-23.4) | Fc, P, Tp, T | 9.6 (2-19) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 10 | Salzmann, 2012 | 10 | 8 | 2 | 14.1 (9-16) | 1.2 | Fc, P, Tp, T | 3.5 | Level IV | Case series | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 11 | Sasaki, 2012 | 11 | 9 | 2 | 13.7 (12-16) | 2.7 (1-4.5) | Fc | 2.2 (0.5-5) | Level IV | Case series | ✓ | ✓ | ✓ | |||||
| 12 | Steadman, 2015 | 26 | 12 | 14 | 16.6 (12-18.9) | 1.9 (0.4-5.7) | Fc, P, T | 5.8 (2-13.3) | Level IV | Case series | ✓ | ✓ | ✓ |
ACI = autologous chondrocyte implantation; IKDC, International Knee Documentation Committee; MFX = microfracture; OATS = osteochondral autograft transplantation system; OCA = osteochondral allograft transplantation; VAS = visual analogue scale.
Demographics
Eleven studies were level IV case series and 1 study was a level I randomized controlled trial (RCT). Six studies reported on ACI, 2 studies reported on OATS, 1 study reported on OCA, and 4 studies reported on MFX (Gudas et al.21 reported on 2 procedures, comparing OATS and MFX). There were 330 unique patients identified across all studies; 192 underwent ACI (58.2%), 36 underwent OATS (10.9%), 39 underwent OCA transplantation (11.8%), and 63 underwent MFX (19.1%). Of the entire combined cohort, 58.5% of patients were male. The mean age of all subjects ranged from 13.7 to 16.7 years and mean follow-up ranged from 2.2 to 9.6 years. Average lesion size ranged from 1.2 to 8.4 cm2 ( Table 1 ). Lesions were localized to the medial and lateral femoral condyles, trochlea, patella, and tibial plateau; 251 lesions were condylar (70.1%), 72 were patellar (20.1%), 25 were trochlear (7.0%), and 10 were localized to the tibial plateau (2.8%). Number of lesions treated in each procedure, presence of previous and concurrent surgeries in the index knee, and time between injury and procedure were not uniformly reported across all studies ( Table 2 ). A total of 347 procedures were performed on 330 patients.
Table 2.
Lesion Characteristics and Prior and Concomitant Surgeries for Studies Identified in Systematic Review.
| Study No. | First Author, Year of Publication | Intervention | n (Patients) | Single or Multiple Lesions | No. with Prior Surgeries in Index Knee | No. with Concomitant Procedures in Index Knee |
|---|---|---|---|---|---|---|
| 1 | Cvetanovich, 2017 | ACI | 37 | Not reported | 37/37 patients | 22 |
| 2 | Gudas, 2009 | OATS | 25 | Single lesions | 0/25 patients | 0 |
| 2 | Gudas, 2009 | MFX | 22 | Single lesions | 0/22 patients | 0 |
| 3 | Lee, 2012 | MFX | 5 | Single lesions | 0/5 patients | 2 |
| 4 | Macmull, 2011 | ACI | 31 | 30/31 with single lesions; 1/31 with multiple lesions | Mean 1.4 ± 0.6 surgeries per knee | Not reported |
| 5 | Micheli, 2006 | ACI | 37 | 35/37 with single lesions; 2/37 with multiple lesions | 26/37 patients | Not reported |
| 6 | Mithöfer, 2005 | ACI | 20 | Mean 1.3 ± 0.3 per knee | Mean 2.5 ± 0.3 surgeries per knee | Not reported |
| 7 | Murphy, 2014 | OCA | 39 | 35/39 with single lesions; 4/39 with multiple lesions | Mean 1.5 surgeries per knee | 15 |
| 8 | Niethammer, 2016 | ACI | 40 | 37/40 with single lesions; 3/40 with multiple lesions | Not reported | Not reported |
| 9 | Ogura, 2017 | ACI | 27 | Mean 1.5 ± 1.0 per knee | 22/27 patients | 22 |
| 10 | Salzmann, 2012 | MFX | 10 | Single lesions | 4/10 patients | Not reported |
| 11 | Sasaki, 2012 | OATS | 11 | Single lesions | 0/10 patients | Not reported |
| 12 | Steadman, 2015 | MFX | 26 | Single and multiple lesions | Not reported | Not reported |
ACI = autologous chondrocyte implantation; MFX = microfracture; OATS = osteochondral autograft transplantation system; OCA = osteochondral allograft transplantation.
Functional Outcome Scores
The most commonly reported outcomes measures were IKDC (6/12), Lysholm (4/12), and VAS pain scores (4/12). Four of these 12 studies were incomplete in reporting data on outcome scores (e.g., mean improvement without measure of spread such as standard deviation, only postoperative score, etc.). Eight studies with 189 unique patients and 202 procedures were included in statistical analysis ( Table 3 ). Of these 8 studies, 5 reported complete IKDC scores (2 ACI, 1 MFX, 1 OCA, 1 OATS), two studies reported complete Lysholm outcome scores (1 ACI, 1 OATS), and three studies reported a complete VAS pain outcome score (2 ACI, 1 MFX).
Table 3.
Statistical Analysis of IKDC, Lysholm, and VAS Pain Score Improvements Compared with MCID.
| First Author, Year of Publication | Intervention | n | Follow-up in Years, Mean (Range) | Mean Improvement | SD | P a |
|---|---|---|---|---|---|---|
| IKDC (MCID = 16.7) | ||||||
| Cvetanovich, 2017 | ACI | 37 | 4.6 (2-10.6) | 29.7 | 27.9 | 0.008* |
| Niethammer, 2016 | ACI | 40 | 3.0 | 33.7 | 29.4 | <0.001* |
| Lee, 2012 | MFX | 5 | 2.5 (1.3-4.5) | 12.2 | 21.5 | 0.66 |
| Murphy, 2014 | OCA | 39 | 8.4 (1.7-27.1) | 33.2 | 26.1 | <0.001* |
| Sasaki, 2012 | OATS | 11 | 2.2 (0.5-5) | 24.4 | 15.4 | 0.11 |
| Lysholm (MCID = 10.1) | ||||||
| Mithöfer, 2005 | ACI | 20 | 3.9 (1.9-7.6) | 23.0 | 7.6 | <0.001* |
| Sasaki, 2012 | OATS | 11 | 2.2 (0.5-5) | 21.9 | 13.2 | 0.010* |
| VAS (MCID = 2.7) | ||||||
| Ogura, 2017 | ACI | 27 | 9.6 (2-19) | 3.5 | 2.2 | 0.080 |
| Niethammer, 2016 | ACI | 40 | 3.0 | 4.0 | 3.8 | 0.037* |
| Salzmann, 2012 | MFX | 10 | 3.5 | 6.2 | 2.5 | 0.002* |
ACI = autologous chondrocyte implantation; IKDC = International Knee Documentation Committee; MCID = minimal clinically important difference; MFX = microfracture; OATS = osteochondral autograft transplantation system; OCA = osteochondral allograft transplantation; VAS = visual analog scale.
P value calculated using 2-tailed, 1-sample Student t test.
P ≤ 0.05.
ACI9,10 (P = 0.008, P < 0.001) and OCA11 (P < 0.001) showed improvement of IKDC scores above MCID at follow-up; mean improvement in MFX12 and OATS13 procedures were not significantly above MCID for IKDC. For Lysholm score, both ACI14 (P < 0.001) and OATS13 (P = 0.010) procedures showed significantly greater improvement relative to known MCID values. MFX15 (P = 0.002) showed significantly greater improvement than MCID with regard to VAS pain score while ACI10,16 (P = 0.037; P = 0.070) was equivocal.
Discussion
Most recent reports on the surgical management of articular cartilage lesions focus on adult patients, paralleling the prevalence of these injuries in this population. Pediatric and adolescent patients with articular cartilage defects, though fewer in number, require special attention as these injuries can result in progressive articular cartilage degeneration and functional disability if not properly managed. Common etiologies that cause osteochondral injuries in this patient population include trauma, patellar dislocation, and JOCD. Chondral and osteochondral defects that result from JOCD can be particularly problematic as they do not have normal subchondral bone architecture; thus, traditional MFX or biologically enhanced marrow stimulation techniques may be less effective than in adults. Literature on outcomes following articular cartilage restoration in the pediatric and adolescent population is limited and has not been critically reviewed with regard to objective metrics. This systematic review identified 12 studies and a total of 330 patients reporting clinical outcomes following MFX, ACI, OATS, and OCA for focal lesions of the knee. Here we found that all four of these procedures provided clinically significant improvement in at least one outcomes score when compared to the current published MCID thresholds.6-8
Microfracture
At present, MFX is still considered by many to be the “gold standard” procedure for repair of focal articular cartilage defects of the knee. First described by Pridie in 1959 and later refined by Steadman who popularized the technique, MFX is a marrow stimulation technique wherein an awl is used to arthroscopically induce multiple subchondral fractures to facilitate infiltration of blood and stem cells into a local hematoma.17,18 Theoretically, these stem cells differentiate into fibrocartilage to repair the defect, with the best clinical results seen for patients with lesions <4 cm2 in size.19 While it is a cost-effective procedure, MFX does not treat underlying bone defects and has proved less useful in the management of defects that measure greater than 4 cm2 in size. Similarly, because MFX does not treat abnormal subchondral bone it is less effective for lesions resulting from JOCD.20 In this review, four articles12,15,21,22 focused on MFX as an intervention and 2 of these articles12,15 provided sufficient statistics for MCID analysis. Lee et al.12 retrospectively evaluated the outcomes of five adolescent patients (12-17 years old) who underwent MFX for patellar osteochondral defects (mean size 1.2 cm2) following patellar dislocation. The time between injury and procedure was not explicitly stated; however, patients who presented greater than 4 weeks from injury were excluded from the study. Though they demonstrated statistically significant improvement in postoperative IKDC scores, the mean improvement did not exceed published MCID thresholds in our independent analysis, likely attributable to an underpowered sample size. In their study, they also found that patients treated with MFX had higher KOOS (Knee injury and Osteoarthritis Outcome Score) and IKDC improvements at short-term when compared with patients treated with open fixation of osteochondral fragments. Salzmann et al.15 conducted a retrospective review to study the clinical outcomes of 10 pediatric patients who underwent MFX for knee articular cartilage defects (mean size 1.2 cm2). Mean time from injury to operation was 12.1 ± 13.1 months. Lesions were located on the femoral condyles (5/10), patella (2/10), trochlea (2/10), and tibial plateau (1/10). At an average short-term follow-up of 3.5 years, mean improvement in VAS pain was above the published MCID threshold (P = 0.002). Compared with adult subjects treated with MFX for osteochondral defects at their institution, they reported significantly greater postoperative improvements in Lysholm, IKDC, and Tegner scores. Patients older than 40 years experienced greater deterioration in postoperative score as compared with younger patients.23 This difference in outcome may be attributable to decreased quality of cartilage fill resulting from MFX, fibrocartilage rather than hyaline cartilage, paired with a lower regeneration capacity in aging patients.24
Steadman et al.22 evaluated the effectiveness of MFX for the treatment of full-thickness chondral knee lesions (mean size 1.9 cm2) in 26 adolescent patients (28 knees), of whom 22 were available for follow-up at an average of 5.8 years. Eleven patients underwent MFX within 6 months of injury. Lesions were located on the femoral condyles (17/28), patella (10/28), and trochlea (1/28). Mean Lysholm score was 90 (range 50-100), median Tegner scale was 6 (range 2-10), and median patient satisfaction was 10 (range 1-10). One patient required a revision MFX procedure 1-year postoperatively. This study reported insufficient clinical data for MCID analysis in this review.
Autologous Chondrocyte Implantation
ACI is a 2-stage procedure that aims to provide hyaline-like cartilage in a full-thickness articular cartilage lesion using autologous chondrocytes harvested from the patient.25 This review identified 6 studies9,10,16,26-28 that focused on ACI as an intervention in the pediatric and adolescent knee; 4 of these studies9,10,16,28 provided sufficient statistics for MCID analysis.
In their recently published series, Cvetanovich et al.9 reported clinical outcomes following ACI in 37 adolescent patients with a mean lesion size of 4.0 cm2 and mean follow-up of 4.6 years. The time between injury and ACI was not reported. Lesions were located on the femoral condyles (23/37), patella (7/37), and trochlea (7/37). At final follow-up, mean improvement in IKDC was 29.7 points and mean improvement in KOOS–Quality of Life was 31.0 (P < 0.001). In our statistical analysis, IKDC scores showed significant improvement relative to MCID following ACI (P = 0.008). Of note, the authors reported that 14 of the 37 patients (37.8%) required 1 to 3 subsequent surgeries after ACI, including debridement for graft hypertrophy (54%), meniscectomy (11%), MFX (9%), and loose body removal (9%). In the adult population, reoperation rate averages 37%; however, the indication for reoperation differs: 35% not related to original defect, 29% lysis of adhesions, 19% knee arthroplasty, 19% revision cartilage operation, 6% to 39% graft hypertrophy.29 Reoperation following ACI in young patients may be attributable to a more robust response to graft incorporation as compared with adults. Overall, the reoperation rate for ACI is substantially higher than that reported in the MFX literature.
Mithöfer et al.28 evaluated the clinical efficacy of ACI in the management of full-thickness articular cartilage lesions (mean size 6.4 cm2) of the knee in 20 adolescent athletes (23 knees, average 1.3 lesions per knee). Lesions were located on the femoral condyles (20/27), patella (1/27), trochlea (4/27), and tibial plateau (2/27). The mean time from injury to ACI was 21 ± 17 months. At an average follow-up of 3.9 years, mean improvement in Lysholm score was above the established MCID (P < 0.001). Of note, 96% of patients were routinely engaged in high-impact aerobic sports at a recreational level or higher at follow-up. In their 2016 study, Niethammer et al.10 studied clinical results of ACI in the treatment of full-thickness chondral knee lesions (mean size 5.3 cm2) in 40 children and adolescents (43 knees) with 3-year follow-up. Cartilage defects were the result of OCD in 13 patients, acute trauma (<12 months from injury) in 9 patients, old trauma (>12 months from injury) in 5 patients, and of unclear etiology in 13 patients. Lesions were located on the femoral condyles (17/43) and patella (26/43). In our statistical analysis, ACI10 showed significant improvement in both IKDC (P < 0.001) and VAS pain scores (P = 0.037) with regard to MCID. Ogura et al.16 reviewed clinical outcomes of 27 adolescents (29 knees, average 1.5 lesions per knee) undergoing ACI for knee articular cartilage defects (mean size 6.2 cm2). Mean duration of symptoms prior to ACI was 3.3 years. Lesions were located on the femoral condyles (18/40), patella (10/40), trochlea (6/40), and tibial plateau (6/40). At an average follow-up of 9.6 years, mean improvement in VAS pain score was 3.5 ± 2.2 (P = 0.070). While they demonstrated statistically significant improvement in VAS pain scores, the mean improvement did not exceed published MCID thresholds in our independent analysis. Of note, 20 knees required a total of 29 subsequent procedures; 44.8% (13/29) were graft-related while 55.2% (16/29) were unrelated to the graft. Graft-related complications were more common with the use of periosteum (76.9%) as compared with Bio-Gide (23.1%). The overall failure rate was 20% (4/20). Failure was defined as unresolved or recurrent symptoms paired with MRI confirmation of graft delamination, surgical debridement of more than 25% of graft area, or a second cartilage restoration procedure, including revision ACI, MFX, and autologous bone grafting.
Though they reported insufficient statistical data to perform MCID analysis, 2 other ACI studies deserve mention. Micheli et al.27 noted that 6 of 37 patients (mean size 5.2 cm2) required a revision operation following ACI, and one additional patient had graft failure due to infection and was treated with MFX. Time between injury and ACI was not reported. In their series of 31 patients (mean size 5.3 cm2), Macmull et al.26 reported 1 failure at 4 years following ACI, which was revised with matrix-assisted chondrocyte implantation (MACI), and another patient who had symptomatic periosteal graft hypertrophy that required arthroscopic debridement. Mean time between injury and ACI was 43 months. The average lesion size among the 6 ACI studies was 5.4 cm2. From this body of literature, we conclude that while ACI surpasses published MCID thresholds in postoperative clinical improvement, there is a notably high rate of complications and reoperations in the pediatric/adolescent cohort. While reported reoperation rates are comparable between pediatric and adult populations, 37.8% and 37%, respectively, the majority of pediatric complications are due to graft hypertrophy whereas adult complications are more often unrelated to the initial operation or associated with graft failure.9,29
Osteochondral Allograft Transplantation
OCA transplantation procedures have become increasingly popular for the treatment of large, focal cartilage defects. The goal of OCA is to transplant a size-matched fresh donor allograft, with viable chondrocytes and underlying subchondral bone, into a socket drilled at the site of the recipient’s defect. Advantages of this procedure include the ability to address abnormal subchondral bone and to treat large defects. The notable disadvantage is that fresh allograft is expensive and in limited supply, which can lead to prolonged surgical delays. One study11 focused on OCA as an intervention and provided sufficient statistics for MCID analysis. In this study, Murphy et al.11 reported a case series to evaluate OCA graft survivorship in 39 pediatric patients (43 knees) with large osteochondral defects (mean size 8.4 cm2) at an average follow-up of 8.4 years. The time between injury and OCA was not reported. Lesions were located on the femoral condyles (33/39), patella (3/39), trochlea (2/39), and tibial plateau (1/39). Mean improvement in IKDC score at an average follow-up of 8.4 years was 33.2 ± 26.1. At a median of 2.7 years, 5 grafts had failed. Four of these were successfully managed with a second OCA, while 1 patient underwent total knee arthroplasty after attempted revision OCA approximately 8.6 years after the initial procedure. The authors report a 90% graft survivorship at 10 years and an 88% good/excellent (18-point scale) patient rating at final follow-up. In our statistical analysis, OCA11 showed significant improvement relative to MCID for the IKDC score (P < 0.001).
Osteochondral Autograft Transplantation System
OATS/mosaicplasty involves removing osteochondral bone plugs from an unaffected, low-weight bearing region of the patient’s own knee and transplanting it into the defect location.19 Donor site morbidity imposes size constraints for this procedure, which is preferably used for medium-sized lesions (2.5-4 cm2).19 As with OCA, the radius of curvature of the graft and defect location must be closely matched as incongruity can compromise graft survival due to increased contact pressure.30 Because it is autologous tissue, OATS does not carry the same risk of disease transmission as OCA. Furthermore, OATS is a cost-effective procedure that can be performed during a single open or arthroscopic procedure. Two studies13,21 reported on OATS in the pediatric and adolescent population, one of which13 provided sufficient statistics for MCID analysis; the other21 was an RCT comparing MFX and OATS.
Sasaki et al.13 investigated clinical outcomes of OATS surgery in patients with JOCD. Eleven unique patients with an average lesion size of 2.7 cm2 underwent 12 OATS procedures for condylar lesions. Mean time from symptom onset to OATS was 13.5 months. At an average follow-up of 2.2 years, observed mean improvement in IKDC score and Lysholm score were 24.4 ± 15.4 and 21.9 ± 13.2, respectively. OATS13 showed postoperative improvements significantly greater than the published Lysholm MCID score (P = 0.010), while IKDC scores were not.
The only RCT identified in this review was published by Gudas et al.,21 comparing MFX and OATS as treatment for JOCD defects of the femoral condyles (mean size 3.2 cm2). Mean time from symptom onset to operation was 23.5 ± 4.2 months. International Cartilage Repair Society (ICRS) functional and objective evaluation showed that both OATS and MFX groups had significant clinical improvement (P < 0.05) after 1 year, and maintained significant clinical improvement compared with pretreatment values after 4.2 years. At the 1-year follow-up, 23/25 (92%) patients had excellent or good results following OATS procedure while 19/22 (86%) patients had excellent or good results following the MFX procedure. At mean follow-up of 4.2 years, 19/23 (86%) patients who underwent the OATS procedure maintained excellent or good results as compared with 12/19 (63%) patients who underwent the MFX procedure. There were no failures in the OATS cohort while the failure rate in the MFX group was 41% (9/22 patients) at follow-up.
While this review identifies a series of 12 studies that have documented clinical outcomes of pediatric and adolescent patients that underwent MFX, ACI, OCA, and OATS procedures, the literature remains nonstandardized in its reporting of specific clinical outcome scores.31 This makes the objective comparison of results from different studies near impossible, thereby complicating clinical decision making. Small sample sizes in the remaining studies further complicate analysis by limiting the power of statistical tests. Specific guidelines for the reporting of clinical outcome scores, consistent among all studies, should be established to ensure the ability to compare results with one another. We also note the lack of high-quality level I or II studies (only 1 identified in this review) that would most reliably help guide clinical decision making.32 Finally, the literature is heterogeneous in reporting the etiology of osteochondral lesions of the knee, which is an important consideration given that JOCD has a distinct pathophysiology, natural history, and outcome compared with acute traumatic cartilage injuries.
In summary, our results demonstrate that all available cartilage restoration procedures (MFX, ACI, OCA, and OATS) are effective surgical treatments for pediatric and adolescent patients with symptomatic articular cartilage lesions of the knee. Of note, MFX lesions were on average smaller than ACI, OATS, and OCA lesions (1.9 cm2, 5.4 cm2, 3.0 cm2, and 8.4 cm2, respectively). Additionally, 61/63 MFX lesions were single and only 4/63 patients (6.3%) had prior surgery in the index knee at the time of operation. From this, we conclude that if MFX was performed, it was likely to be applied as first-line treatment for smaller osteochondral lesions. These procedures all provide significant postoperative improvement in functional and activity outcome scores relative to validated MCID. Head-to-head RCTs and larger case series reporting consistent validated outcomes measures are needed to guide clinical decision making in young patients, particularly ones that distinguish between traumatic chondral defects and osteochondral defects resulting from JOCD. Currently, no consensus exists for optimal treatment of articular cartilage lesions in the pediatric population, with algorithms being primarily surgeon specific.
Footnotes
Authors’ Note: This study was primarily performed at the Department of Orthopedic Surgery in the David Geffen School of Medicine at UCLA.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Armin Arshi  https://orcid.org/0000-0002-3391-551X
https://orcid.org/0000-0002-3391-551X
References
- 1. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 2. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994-1009. [DOI] [PubMed] [Google Scholar]
- 3. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321-8. [DOI] [PubMed] [Google Scholar]
- 4. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91(7):1778-90. [PubMed] [Google Scholar]
- 5. Harris JD, Erickson BJ, Abrams GD, Cvetanovich GL, McCormick FM, Gupta AK, et al. Methodologic quality of knee articular cartilage studies. Arthroscopy. 2013;29(7):1243-1252.e5. [DOI] [PubMed] [Google Scholar]
- 6. Badalà F, Nouri-mahdavi K, Raoof DA. NIH public access. Computer (Long Beach Calif). 2008;144(5):724-32. [Google Scholar]
- 7. Irrgang J. Summary of Clinical Outcome Measures for Sports-Related Knee Injuries. Final Report. Rosemont, IL: AOSSM Outcomes Task Force; 2012:1-381. [Google Scholar]
- 8. Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cvetanovich GL, Riboh JC, Tilton AK, Cole BJ. Autologous chondrocyte implantation improves knee-specific functional outcomes and health-related quality of life in adolescent patients. Am J Sports Med. 2017;45(1):70-6. [DOI] [PubMed] [Google Scholar]
- 10. Niethammer TR, Holzgruber M, Gülecyüz MF, Weber P, Pietschmann MF, Müller PE. Matrix based autologous chondrocyte implantation in children and adolescents: a match paired analysis in a follow-up over three years post-operation. Int Orthop. 2017;41(2):343-50. [DOI] [PubMed] [Google Scholar]
- 11. Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635-40. [DOI] [PubMed] [Google Scholar]
- 12. Lee BJ, Christino MA, Daniels AH, Hulstyn MJ, Eberson CP. Adolescent patellar osteochondral fracture following patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1856-61. [DOI] [PubMed] [Google Scholar]
- 13. Sasaki K, Matsumoto T, Matsushita T, Kubo S, Ishida K, Tei K, et al. Osteochondral autograft transplantation for juvenile osteochondritis dissecans of the knee: a series of twelve cases. Int Orthop. 2012;36(11):2243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 15. Salzmann GM, Sah BR, Schmal H, Niemeyer P, Sudkamp NP. Microfracture for treatment of knee cartilage defects in children and adolescents. Pediatr Rep. 2012;4(2):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogura T, Bryant T, Minas T. Long-term outcomes of autologous chondrocyte implantation in adolescent patients. Am J Sports Med. 2017;45(5):1066-74. [DOI] [PubMed] [Google Scholar]
- 17. Pridie KH. A method of resurfacing osteoarthritic knee joints. J Bone Joint Surg Am. 1959;41:618-9. [Google Scholar]
- 18. Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7(4):300-4. [Google Scholar]
- 19. Falah M, Nierenberg G, Soudry M, Hayden M, Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34(5):621-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanders TL, Pareek A, Obey MR, Johnson NR, Carey JL, Stuart MJ, et al. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am J Sports Med. 2017;45(8):1799-1805. [DOI] [PubMed] [Google Scholar]
- 21. Gudas R, Simonaityte R, Cekanauskas E, Tamosiūnas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29(7):741-8. [DOI] [PubMed] [Google Scholar]
- 22. Steadman JR, Briggs KK, Matheny LM, Guillet A, Hanson CM, Willimon SC. Outcomes following microfracture of full-thickness articular cartilage lesions of the knee in adolescent patients. J Knee Surg. 2015;28(2):145-50. [DOI] [PubMed] [Google Scholar]
- 23. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180-6. [DOI] [PubMed] [Google Scholar]
- 24. Verbruggen G, Cornelissen M, Almqvist KF, Wang L, Elewaut D, Broddelez C, et al. Influence of aging on the synthesis and morphology of the aggrecans synthesized by differentiated human articular chondrocytes. Osteoarthritis Cartilage. 2000;8(3):170-9. [DOI] [PubMed] [Google Scholar]
- 25. Brittberg M, Faxén E, Peterson L. Carbon fiber scaffolds in the treatment of early knee osteoarthritis. A prospective 4-year followup of 37 patients. Clin Orthop Relat Res. 1994;(307):155-64. [PubMed] [Google Scholar]
- 26. Macmull S, Parratt MTR, Bentley G, Skinner JA, Carrington RWJ, Morris T, et al. Autologous chondrocyte implantation in the adolescent knee. Am J Sports Med. 2011;39(8):1723-30. [DOI] [PubMed] [Google Scholar]
- 27. Micheli LJ, Moseley JB, Anderson AF, Browne JE, Erggelet C, Arciero R, et al. Articular cartilage defects of the distal femur in children and adolescents: treatment with autologous chondrocyte implantation. J Pediatr Orthop. 2006;26(4):455-60. [DOI] [PubMed] [Google Scholar]
- 28. Mithöfer K, Minas T, Peterson L, Yeon H, Micheli LJ. Functional outcome of knee articular cartilage repair in adolescent athletes. Am J Sports Med. 2005;33(8):1147-53. [DOI] [PubMed] [Google Scholar]
- 29. Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation : a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7(4):298-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patil S, Butcher W, D’Lima DD, Steklov N, Bugbee WD, Hoenecke HR. Effect of osteochondral graft insertion forces on chondrocyte viability. Am J Sports Med. 2008;36(9):1726-32. [DOI] [PubMed] [Google Scholar]
- 31. Jones KJ, Sheppard WL, Arshi A, Hinckel BB, Sherman SL. Articular cartilage lesion characteristic reporting Is highly variable in clinical outcomes studies of the knee. Cartilage. Epub 2018 February 6. doi: 10.1177/1947603518756464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arshi A, Siesener NJ, McAllister DR, Williams RJ, 3rd, Sherman SL, Jones KJ. The 50 most cited articles in orthopedic cartilage surgery. Cartilage. 2016;7(3):238-47. [DOI] [PMC free article] [PubMed] [Google Scholar]