Abstract
Objective
To investigate survival of cartilage repair in the knee by microfracture (MFX; n = 119) or mosaicplasty osteochondral autograft transfer (OAT; n = 84).
Design
For survival analyses, “failure” was defined as the event of a patient reporting a Lysholm score <65 or undergoing an ipsilateral knee replacement. The Kaplan-Meier method was used for construction of a survival functions plot for the event “failure.” Log rank (Mantel-Cox) test was used for comparison of survival distributions in the 2 groups.
Results
The long-term failure rate (62% overall) was significantly higher in the MFX group (66%) compared with the OAT group (51%, P = 0.01). Furthermore, the mean time to failure was significantly shorter (P < 0.001) in the MFX group, 4.0 years (SD 4.1) compared with the OAT group, 8.4 years (SD 4.8). In the OAT group, the survival rate stayed higher than 80% for the first 7 years, and higher than 60% for 15 years, while the survival rate dropped to less than 80% within 12 months, and to less than 60% within 3 years in the MFX group, log rank (Mantel-Cox) 20.295 (P < 0.001). The same pattern was found in a subgroup of patients (n = 134) of same age (<51 years) and size of treated lesion (<500 mm2), log rank (Mantel-Cox) 10.738 (P = 0.001). The nonfailures (48%) were followed for median 15 yeas (1-18 years).
Conclusions
MFX articular cartilage repairs failed more often and earlier than the OAT repairs, both in the whole cohort and in a subgroup of patients matched for age and size of treated lesion, indicating that the OAT repair is the more durable.
Level of evidence
Therapeutic study, Level III.
Keywords: microfracture, mosaicplasty, knee, articular cartilage defects, survival analyses
Introduction
Intact hyaline cartilage and synovial fluids provide the basis for a low-friction pain-free movement of synovial joints, and chondral lesions disrupt this homeostasis. Unfortunately, articular cartilage defects are common, seen in up 19% of patients undergoing knee arthroscopies.1 With their debilitating effect on knee function and quality of life2,3 and little potential for healing they continue to represent a challenge for orthopedic surgeons.4
Various cartilage repair techniques, such as microfracture (MFX)5,6 and osteochondral autograft transfer (OAT)7 have displayed acceptable short-term results in many patients. However, few patients regain a normal pain-free function.8,9 Several studies have also indicated that results seem to deteriorate with time.10-13 Thus, there is a need for further studies analyzing results over time, assessing patient-related outcomes and secondary surgery, including the need for knee replacement.14-18 Until date, few comparative studies on MFX and OAT have been published with long-term results.17,19-21 Furthermore, utilization of survival analyses of the cartilage repair has been used sparingly.17
The present work aimed to compare the long-term survival of the cartilage repair by the 2 methods (MFX and OAT). By use of the Kaplan-Meier method, a survival functions plot was constructed for the event “failure,” defined as a poor Lysholm score (<65 points)22 or a knee replacement. Log rank (Mantel-Cox) test was used for comparison of survival distributions in the 2 groups. The null hypothesis was that occurrence of failure did not differ between the 2 methods.
Materials and Methods
All patients undergoing a cartilage repair procedure at our institution from 1998 to 2003 were registered prospectively. Data were acquired from standardized forms completed by both the patient and the surgeon. The form contained details about preoperative symptoms and function (including the Lysholm score), and details about the articular cartilage procedure, including localization and size of the articular cartilage defect, similar to the system recommended by the International Cartilage Repair Society.23 The data were stored in a local database (Access, Microsoft Corporation, Redmond, WA, USA).
Patients of age 60 years or younger at the time of surgery; with 1 to 3 symptomatic focal full-thickness articular chondral defects of the knee (verified by arthroscopic examination) treated with either MFX or OAT technique were included in the study. Exclusion criteria (applied at the time of surgery) were the following: joint space narrowing (to a space <4 mm) on standard anteroposterior radiographs, more than 5° varus or valgus malalignment, previous or concurrent realignment surgery, ligament instabilities, or the inability to follow the rehabilitation protocol.
Outcome evaluation was performed by the Lysholm score22,24 and any report of the patient undergoing a knee replacement of the same knee (after the index surgery). Data were prospectively collected before the operation (baseline) and at several time points after the surgery. For the first few years data were collected at routine check-ups at the outpatient department, thereafter by the patients completing and returning standardized questionnaires sent by mail every 2 to 3 years until 2017.
Surgical Technique
After an arthroscopic evaluation, an MFX or an OAT procedure was performed. The choice of procedure was based on the surgeon’s preference, and patient’s wishes, in the current case. The lesion was debrided down with curettes to subchondral bone, and around the edges until only healthy surrounding cartilage remained. The size of the lesion was calculated as millimeters squared after measuring the length and width using a meniscal probe.1
The MFX procedure was performed as described by the Steadman et al.6 Angled awls were used for piercing the subchondral bone plate, placing microfracture holes 3 to 4 mm apart. The inflow was stopped, and the flow of marrow elements from the openings was verified. The OAT procedure (Smith and Nephew Inc., Andover, MA, USA) was performed as described by Hangody et al.7,25 Grafts were harvested from the periphery of the femoral condyles at the level of the patellofemoral joint and transplanted to corresponding burr holes in the defect. The procedure was performed using an arthroscopic approach, a mini-arthrotomy (in most cases; allowing both harvesting and transplanting through the same incision) or, when mandatory, by a full arthrotomy.
Rehabilitation
Both procedures received the same rehabilitation protocol. Continuous passive motion was started within a few hours after the operation and was continued for the duration of the stay in hospital (4-7 days). The patients were instructed in the use of crutches by a physiotherapist and maintained foot-touch weightbearing for 6 weeks. Thereafter, full weightbearing was gradually introduced. Physiotherapy was continued after discharge. Initial exercises included stretching, straight-leg rises, and passive motion, further progressing gradually through active closed-chain exercises, including stationary bicycling to dynamic weight training.26 The ethical committee at our institution reviewed and approved of the study (HDS ID 1998-0201). All patients gave their informed consent prior to inclusion.
Statistical Analyses
Statistical analyses were performed with the Statistical Package for the Social Sciences (IBM Corporation, Armonk, NY, USA) on a personal computer. An a priori P value less than 0.05 was considered statistically significant. As measures of central location and spread of data, mean and standard deviation (SD) or median and range were calculated. A 2-tailed unpaired t test was used to compare the sets of continuous data between subgroups of the patient population. For comparing binominal data of subgroups, the chi-square test was used.
For survival analyses, “failure” was defined as the patient reporting a poor Lysholm score <65 points (at the 1-year follow-up, or later)22 and/or undergoing an ipsilateral knee replacement procedure.18 Time from the index cartilage surgery until (a) the event of failure (regardless of whether a poor Lysholm score or a knee replacement was reported first) or (b) the last follow-up with survival of the repair, was recorded and used for analyses. The Kaplan-Meier method was used for construction of a survival functions plot for the event “failure.” Log rank (Mantel-Cox) test was used for comparison of survival distributions in the 2 groups (MFX and OAT). Analyses were conducted for the whole patient population (n = 203) as well as for a subgroup of patients aged 50 years or younger, with the repair of a total defect area <500 mm2 (n = 134).
Results
A total of 203 patients were eligible for inclusion. The time of failure of the cartilage repair, or a latest known time of nonfailure (censored data) were available on all eligible patients. Thus, 118 males and 85 females, with a median age at surgery 36 years (range 15-60 years) were included. A total of 119 patients were treated by the MFX technique, while 84 cases had an OAT procedure performed ( Table 1 ). At the time of surgery, median symptom duration was 60 months (range 1-360 months). The right knee (62%) was more often treated than the left knee (38%). The treated lesion, or the largest of multiple treated lesions, was located on the medial femoral condyle (58%), patella (15%), trochlea (14%), lateral femoral condyle (7%), or lateral tibial plateau (6%). We treated 1 (75%), 2 (21%), or 3 (4%) lesions with a median defect size of 350 mm2 (range 100-1700 mm2).
Table 1.
Patient Demographics and Baseline Characteristics (Total Study Population n = 203).a
| Microfracture (n = 119) | Mosaicplasty (n = 84) | P | |
|---|---|---|---|
| Male/female, n | 69/50 | 49/35 | 0.96 (n.s.) |
| Age at surgery, years | 38 (11) | 35 (9) | 0.03* |
| Duration, months | 77 (65) | 78 (73) | 0.95 (n.s.) |
| No. of previous surgeries | 1.7 (1.5) | 1.8 (1.4) | 0.58 |
| Right/left knee, n | 68/51 | 57/27 | 0.12 (n.s.) |
| Treated area, mm2 | 480 (290) | 300 (110) | <0.001* |
| Patellofemoral joint, % | 27 | 31 | 0.55 (n.s.) |
| Baseline Lysholm score | 47 (18) | 47 (16) | 0.76 (n.s.) |
s. = not significant.
Values are presented as mean (SD) unless otherwise indicated.
Statistically significant difference.
A total of 125 (62%) cartilage repairs were noted as failures at median 4 years (range, 1-18 years) after the surgery. The nonfailures (48%) were followed for median 15 years (range, 1-18 years). This latter group of repairs (with censored data) included 6 patients that were deceased during the study period, 4 in the MFX group and 2 in the OAT group (not significance). The reported failure rate was significantly higher in the MFX group (66%) compared with the OAT group (51%, P = 0.011). Furthermore, mean time to failure was significantly shorter (P < 0.001) in the MFX group, 4.0 years (SD 4.1) compared with the OAT group, 8.4 years (SD 4.8). The difference in failure rate was greatest at 5 years. Thereafter, the difference diminished (Fig. 1). In the OAT group, the survival rate stayed higher than 80% for the first 7 years, and higher than 60% for 15 years, while the survival rate dropped to less than 80% within 12 months, and to less than 60% within 3 years in the MFX group (Fig. 1), log rank (Mantel-Cox) 20.295 (P < 0.001).
Figure 1.
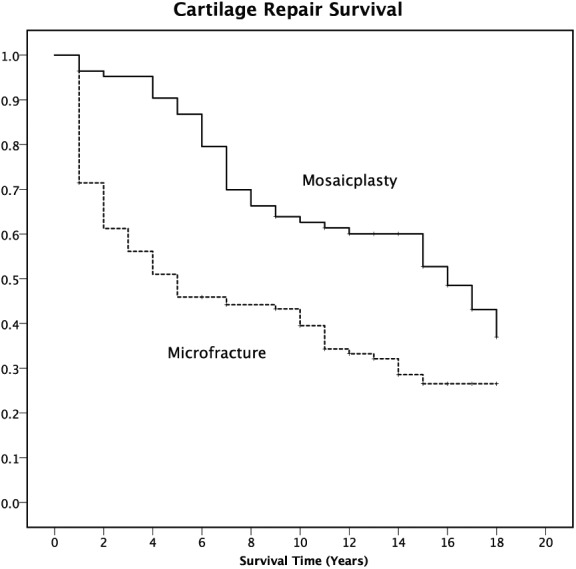
Kaplan-Meier survival functions plot for the event “failure” (a knee replacement procedure in the same knee or Lysholm score <65) after cartilage repair surgery (Mosaicplasty solid line; Microfracture dashed line) of the total study population (n=203).
In the total study group, the mean age was higher (P = 0.03) and the mean treated area was larger (P < 0.001) in the MFX group compared with that of the OAT group ( Table 1 ). Thus, to compare groups with similar predictive characteristics, the same analyses were repeated for a subgroup of patients (n = 134) of same age (<51 years) and size of treated lesion (<500 mm2) ( Table 2 ). The survival analyses of the repair, as displayed by a Kaplan-Meier plot (Fig. 2) showed very similar results to that of the total study group, in favor of the OAT procedure, with a log rank (Mantel-Cox) 10.738 (P = 0.001).
Table 2.
Patient Demographics and Baseline Characteristics of a Subgroup of Patients Aged 51 Years or Younger Treated for Defects with a Total size <500 mm2 (n = 134).
| Microfracture (n = 61) | Mosaicplasty (n = 73) | P | |
|---|---|---|---|
| Male/female | 34/27 | 43/30 | 0.71 (n.s.) |
| Age at surgery, years | 33 (9) | 34 (8) | 0.72 (n.s.) |
| Duration, months | 69 (64) | 82 (74) | 0.41 (n.s.) |
| No. of previous surgeries | 1.7 (1.5) | 1.9 (1.5) | 0.41 (n.s.) |
| Right/left knee, n | 40/21 | 48/25 | 0.12 (n.s.) |
| Treated area, mm2 | 310 (100) | 280 (99) | 0.10 (n.s.) |
| Patellofemoral joint, % | 23 | 30 | 0.35 (n.s.) |
| Baseline Lysholm score | 48 (19) | 47 (15) | 0.84 (n.s.) |
s. = not significant.
Values are presented as mean (SD) unless otherwise indicated.
Figure 2.
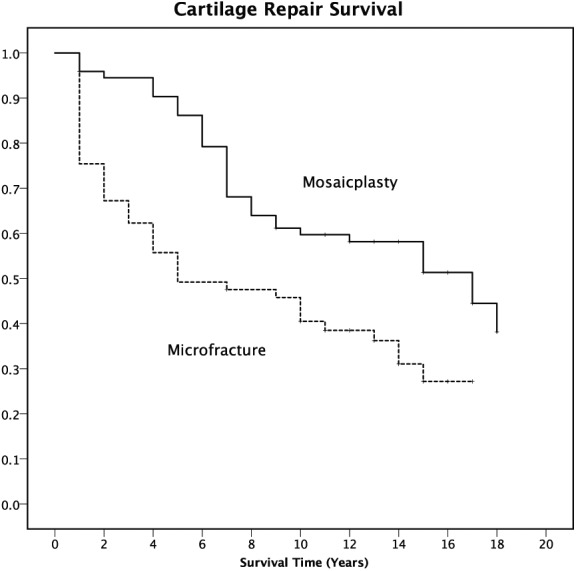
Kaplan-Meier survival functions plot for the event “failure” (a knee replacement procedure in the same knee or Lysholm score <65) after cartilage repair surgery (Mosaicplasty solid line; Microfracture dashed line) of a subgroup stratified for age and size of treated lesion (n=134).
Discussion
We have previously published medium-term11,27 and 10- to 14-year follow-up28,29 data for the MFX and OAT procedures, separately. Furthermore, we have recently compared long-term >15 years outcome for the 2 methods in subgroups of the present material.20,21 In the present study, we performed survival analyses for the total cohort of patients that had undergone MFX or OAT cartilage repair procedures at our clinic up to 18 years earlier. Thus, the study spans nearly 18 years. All patients included in the study are accounted for; either by the time of failure (in which case no more follow-up was needed), or by the last known point of time (the number of years since the surgery) that the individual was still a nonfailure. This latter group (with censored data) were followed for median 15 years (1-18 years) and included 6 deceased patients.
The most important finding was that MFX articular cartilage repair failed more often and earlier than OAT repairs, both in the whole study population (n = 203) as well in a subgroup of patients (n = 134) of same age (<51 years) and size of treated lesion (<500 mm2). In the OAT group, the survival rate was higher than 80% for the first 7 years, and higher than 60% for 15 years, while the survival rate dropped to less than 80% within 12 months, and to less than 60% within 3 years in the MFX group. Thus, based on the results of the present (non-randomized) survival study, it seems that the OAT technique results in a more durable cartilage repair.
In the present study, the Lysholm score22 was used for evaluating symptoms and function of the knee. The Lysholm score has been used in many studies of cartilage surgery, including microfracture and mosaicplasty/OAT18,19,28-30 and (the score) has demonstrated good psychometric performance for outcome assessment of various chondral disorders of the knee.24 Still, we recognize that other researchers may have preferences for other/newer knee score systems. In response, we would like to point out that the choice of the outcome measures was made almost 20 years, and that new knowledge on outcome evaluation will always be a challenge for studies that span over a long period of time.
One of the main difficulties with assessing the outcome in long-term clinical studies on articular cartilage repair, is that an increasing percentage of the patients are having their (ipsilateral) knee replaced as osteoarthritis develops and progresses as the years pass by.18 As a knee score is often the main outcome variable, authors tend to exclude the replacement (failure) cases, acknowledging that the score represents the knee replacement and not the original cartilage report.18 However, by reporting the average score for only the nonfailures, a large bias (toward reporting too optimistic results) is introduced.
Furthermore, “failure” is often restricted to the occurrence of a knee replacement, disregarding the fact that many patients experience a poor result without undergoing a knee replacement.18,31 Thus, we have previously introduced the term “failed cartilage repair” (or “poor result”) defined as the occurrence of knee replacement surgery and/or a Lysholm score <65.28 For the survival analyses, the occurrence of any of the 2 latter events (whichever occurring first) identified “failure.” Other authors have included other more minor reinterventions (than a knee replacement) as a failure.17 However, by including the event of the Lysholm score dropping to less than 65 points as a failure, any reintervention would generally be picked up, as it would be predated (and initiated) by a poor outcome (even if the new procedure improved the symptoms and precluded/delayed a knee replacement).
Another important difficulty in long-term clinical studies on articular cartilage repair, is the increasing difficulty in maintaining a high follow-up rate, as the patients move; get bored answering the questionnaires; get old and mentally reduced; or die. Using survival analyses solves this issue by introducing “censored data,” the recording of the latest point of time of survival (in patients with repair that do not fail during the study period). Furthermore, when the repair fails, the time is recorded and no more follow-up is needed to perform the calculations.
Events of failure accumulated steadily from 12 months up to 18 years after surgery (more so in the MFX group), supporting the notion that long-term studies are important to display outcomes over time after an intervention. Still, most comparative studies on MFX and OAT to date have only short- to mid-term follow-up evaluation and we are aware of only one previously study reporting the long-term survival of cartilage repair after MFX procedure versus OAT.17 The patterns of the survival curves are strikingly similar in the randomized study by Gudas et al.17 and the present, nonrandomized, work. In both studies, MFX repairs start to fail the first few years whereas the OAT repairs seem to be more durable.
The better outcome by the OAT procedure (vs. MFX) could possibly be related to the type of repair tissue formed in the treated defect. The MFX technique rely on the release of pluripotent mesenchymal stem cells being released from the bone marrow. Although this can sometimes lead to a hyaline-like cartilage repair,26 the procedure generally results in fibrocartilage or fibrous repair tissue.26,32,33 This latter type of tissue offers little mechanical protection of the underlying—exposing pain-fiber innervated bone34 and making it less resistant to wear and tear. In contrast, by the OAT procedure, the articular cartilage defect is replaced, at the time of surgery, by a mosaic of transplants of normal, healthy, hyaline cartilage and subchondral bone.7,32 However, the long-term results may still be hampered by donor defect morbidity; inadequate bonding between the cylinder grafts and the normal surrounding cartilage; and any misplacement of cartilage cylinders.
The strengths of the current study include a high follow-up rate; a large patient population; a long follow-up time; and the use of prospective data collection. The weaknesses include the lack of randomization of treatment type and a control group with a nonsurgical treatment strategy; and not including newer knee rating devices in addition to the Lysholm score. Furthermore, neither a routine second-look arthroscopy nor a magnetic resonance imaging examination was performed to evaluate the repair. Finally, the study does not include routine radiological evaluation of the development of osteoarthritis.
Conclusion
In 2 cohorts of different cartilage repair methods used, a nonrandomized comparison was performed. The MFX articular cartilage repairs failed more often and earlier than the OAT repairs. The same finding was evident in a selected subgroup of patients of same age and size of treated lesion across the 2 methods. The results indicated the need for continued follow-up evaluation after surgical treatment for articular cartilage defects and, although limitations apply to the current work, the results indicate that OAT repair is the more durable of the 2 evaluated methods.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The ethical committee at our institution reviewed and approved of the study (HDS ID 1998-0201).
Informed Consent: All patients gave their informed consent prior to inclusion.
Trial Registration: Not applicable.
ORCID iD: Eirik Solheim  https://orcid.org/0000-0001-6799-1636
https://orcid.org/0000-0001-6799-1636
References
- 1. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. [DOI] [PubMed] [Google Scholar]
- 2. Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231-7. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 3. Solheim E, Krokeide AM, Melteig P, Larsen A, Strand T, Brittberg M. Symptoms and function in patients with articular cartilage lesions in 1000 knee arthroscopies. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1610-6. doi: 10.1007/s00167-014-3472-9. [DOI] [PubMed] [Google Scholar]
- 4. Hunziker EB, Lippuner K, Keel MJB, Shintani N. An educational review of cartilage repair: precepts & practice—myths & misconceptions—progress & prospects. Osteoarthritis Cartilage. 2015;23(3):334-50. doi: 10.1016/j.joca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 5. Rodrigo JJ, Steadman JR, Silliman JF, Fulstone HA. Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg. 1994;7(3):109-16. [Google Scholar]
- 6. Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7(4):300-4. [Google Scholar]
- 7. Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5(4):262-7. [DOI] [PubMed] [Google Scholar]
- 8. Løken S, Heir S, Holme I, Engebretsen L, Arøen A. 6-year follow-up of 84 patients with cartilage defects in the knee. Knee scores improved but recovery was incomplete. Acta Orthop. 2010;81(5):611-8. doi: 10.3109/17453674.2010.519166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goyal D, Keyhani S, Lee EH, Hui JHP. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579-88. doi: 10.1016/j.arthro.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 10. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-25. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11. Solheim E, Hegna J, Øyen J, Austgulen OK, Harlem T, Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 2010;17(1):84-7. doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 12. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. doi: 10.1007/s00167-013-2676-8. [DOI] [PubMed] [Google Scholar]
- 13. Filardo G, Kon E, Perdisa F, Tetta C, Di Martino A, Marcacci M. Arthroscopic mosaicplasty: long-term outcome and joint degeneration progression. Knee. 2015;22(1):36-40. doi: 10.1016/j.knee.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 14. Steadman JR, Ramappa AJ, Maxwell RB, Briggs KK. An arthroscopic treatment regimen for osteoarthritis of the knee. Arthroscopy. 2007;23(9):948-55. doi: 10.1016/j.arthro.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 15. Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S-19S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 16. Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Südkamp NP, Niemeyer P. Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med. 2012;40(1):58-67. doi: 10.1177/0363546511423522. [DOI] [PubMed] [Google Scholar]
- 17. Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 18. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Ludvigsen TC, Løken S, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332-9. doi: 10.2106/JBJS.15.01208. [DOI] [PubMed] [Google Scholar]
- 19. Ulstein S, Arøen A, Røtterud JH, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-15. doi: 10.1007/s00167-014-2843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solheim E, Hegna J, Inderhaug E. Long-term clinical follow-up of microfracture versus mosaicplasty in articular cartilage defects of medial femoral condyle. Knee. 2017;24(6):1402-7. doi: 10.1016/j.knee.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 21. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826-31. doi: 10.1177/0363546517745281. [DOI] [PubMed] [Google Scholar]
- 22. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-9. [PubMed] [Google Scholar]
- 23. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58-69. [DOI] [PubMed] [Google Scholar]
- 24. Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg Am. 2004;86-A(6):1139-45. [DOI] [PubMed] [Google Scholar]
- 25. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A(Suppl 1):65-72. [PubMed] [Google Scholar]
- 26. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 27. Solheim E, Øyen J, Hegna J, Austgulen OK, Harlem T, Strand T. Microfracture treatment of single or multiple articular cartilage defects of the knee: a 5-year median follow-up of 110 patients. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):504-8. doi: 10.1007/s00167-009-0974-y. [DOI] [PubMed] [Google Scholar]
- 28. Solheim E, Hegna J, Øyen J, Harlem T, Strand T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee. 2013;20(4):287-90. doi: 10.1016/j.knee.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 29. Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1587-93. doi: 10.1007/s00167-014-3443-1. [DOI] [PubMed] [Google Scholar]
- 30. Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87(10):2232-9. doi: 10.2106/JBJS.D.02904. [DOI] [PubMed] [Google Scholar]
- 31. Bae DK, Song SJ, Yoon KH, Heo DB, Kim TJ. Survival analysis of microfracture in the osteoarthritic knee—minimum 10-year follow-up. Arthroscopy. 2013;29(2):244-50. doi: 10.1016/j.arthro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 32. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241(2):407-14. doi: 10.1148/radiol.2412051750. [DOI] [PubMed] [Google Scholar]
- 33. DiBartola AC, Everhart JS, Magnussen RA, Carey JL, Brophy RH, Schmitt LC, et al. Correlation between histological outcome and surgical cartilage repair technique in the knee: a meta-analysis. Knee. 2016;23(3):344-9. doi: 10.1016/j.knee.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 34. Nencini S, Ivanusic JJ. The physiology of bone pain. How much do we really know? Front Physiol. 2016;7:157. doi: 10.3389/fphys.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]


