Abstract
Objective
To compare the progression of biochemical biomarkers of osteoarthritis (OA), knee pain, and function between nonobese patients (NON), obese patients without depression (OBESE), and obese patients with comorbid depression (O + D).
Design
Utilizing the FNIH OA Biomarkers Consortium dataset, we categorized knee OA patients into NON, OBESE, and O + D groups based on body mass index and Center for Epidemiological Studies–Depression (CES-D) scores. Subjective symptoms (Knee injury and Osteoarthritis Outcome Score Quality of Life subscale (KOOS QOL), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain and Physical Function scores, and the Short Form–12 (SF-12) Physical Component Score [PCS]) and objective measures of cartilage degradation and bone remodeling (urinary CTXII and CTXIα) were compared among groups at baseline and 2-year follow-up.
Results
Of the 600 patients, 282 (47%) were NON, 285 (47.5%) OBESE, and 33 (5.5%) O + D. The O + D group had significantly worse pain and function both at baseline and 2-year follow-up (P < 0.001 for all comparisons) as evidenced by self-reported measures on KOOS QOL, WOMAC Pain, WOMAC Physical Function, and SF-12 PCS. The O + D group also demonstrated significant increases in CTXII (P = 0.01) and CTXIα (P = 0.005), whereas the NON and OBESE groups did not.
Conclusions
The combination of inferior knee pain, physical function, and significantly greater increases in biomarkers of cartilage degradation and bony remodelling suggest a more rapid progression for obese OA patients with comorbid depression. The link between systemic disease, inflammatory burden, and progressive cartilage degradation is in line with increasing concerns about a degenerative synovial environment in early osteoarthritic knees that progress to treatment failure with biologic restoration procedures.
Keywords: osteoarthritis, knee, obesity, depression, biomarker, pain
Introduction
For the majority of patients with knee osteoarthritis (OA), the disease progresses slowly over the course of multiple years. However, there is a subset of approximately one-third of knee OA patients who experiences a more rapid progression of cartilage degradation, knee pain, and disability.1 Rapid progression of knee OA is not only associated with increased pain, reduced mobility, and reduced quality of life, but also with greater utilization of health care resources.2-4 As such, there is an unmet need to identify patient subsets at greatest risk of rapid OA progression in order to prevent or slow its occurrence.
Depression and obesity are highly comorbid5 and each is individually associated with increased pain and decreased physical function in patients with knee OA,6,7 However, neither obesity nor depression alone has been identified to be solely responsible for rapid OA progression.1,8 Depression has been prospectively associated with worsening pain and function in knee OA patients,6 but has not been linked to structural knee OA progression.8 On the contrary, structural progression of knee OA has been linked to obesity, as the lifetime risk of developing symptomatic knee OA is 60% for patients with a body mass index (BMI) ≥30 kg/m2.5
While depression and obesity have been individually assessed as risk factors of rapid OA progression, it remains unknown if the combination of obesity and comorbid depression results in progressive worsening of both subjective symptoms of pain and function, and objective measures of cartilage degradation and bone remodeling. Hence, the purpose of this study was to compare the progression of biochemical biomarkers of OA and knee pain, symptoms, and function between nonobese patients (NON), obese patients without depression (OBESE), and obese patients with comorbid depression (O + D). We hypothesized that the O + D group would demonstrate significantly worse progression of both OA-related biomarkers and subjective symptoms and function than the other 2 groups.
Methods
Data used in the preparation of this article were obtained from the Osteoarthritis Initiative (OAI) database, which is available for public access at http://www.oai.ucsf.edu/ (Clinicaltrials.gov Identifier: NCT00080171). We performed an analysis of the FNIH (Foundation for the National Institutes of Health) OA Biomarkers Consortium dataset9 to determine if patients with combined obesity and depression demonstrate a more rapid progression of knee OA biomarkers and symptoms. The FNIH OA Biomarkers Consortium is a subset of the OAI database that is a publically available and includes prospectively collected patient demographics and health history, subjective knee symptoms, biochemical biomarkers and imaging measurements of knee OA in a series of 600 patients.9-11 As part of the original study, patients were followed over a 2-year period with patients completing a series of patient-reported outcomes both at baseline and 2-year follow-up. BMI at baseline was used to identify the presence of obesity, and the Center for Epidemiological Studies–Depression (CES-D) scale was used to quantify depressive symptoms at baseline.8,12 Consistent with previous studies, obesity was defined as a BMI ≥30 kg/m2 and the presence of clinically significant depression was defined as CES-D scores ≥16.8,12
For the current study, the following self-administered patient-reported outcomes were included for analysis: Knee injury and Osteoarthritis Outcome Score Quality of Life subscale (KOOS QOL), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain and Physical Function scores, and the Medical Outcomes Study Short Form–12 (SF-12) Physical Component Scale (PCS).
KOOS QOL: The KOOS QOL is a validated 4-item instrument specifically developed to assess knee-related quality of life for patients with knee conditions.13,14 Subscale scores range from 0 to 100, with greater values indicative of increased QOL.15 In knee OA patients, the KOOS QOL has demonstrated excellent test-retest reliability (intraclass correlation coefficient [ICC] = 0.83-0.95), acceptable levels of floor and ceiling effects, and minimal detectable change values ranging from 7.0 to 7.2 points.15 Changes in the KOOS QOL have been demonstrated to coincide with the progression of radiographic knee OA.16,17
WOMAC: The WOMAC OA Index is a 22-item scale, which was also developed and validated for use with OA patients in order to assess the course of OA progression.18 The WOMAC Pain score ranges from 0 to 20 with greater scores indicating worse, more severe pain. Test-retest reliability (ICC) of the WOMAC Pain score ranges from 0.65 to 0.98, with minimal detectable change values ranging from 18.8 to 22.4.15 WOMAC Physical Function scores range from 0 to 68, again with greater scores indicating worse function. Test-retest reliability (ICC) WOMAC Physical Function score ranges from 0.86 to 0.93, with minimal detectable change values ranging from 10.6 to 15.0.15 The WOMAC Pain and Physical Function scores are the 2 most commonly used patient-reported outcome tools used in knee OA patient populations.19
SF-12 PCS: The SF-12 PCS ranges from 0 to 100 where greater values are associated with increased physical functioning. Like the WOMAC, the SF-12 PCS has been extensively used in the knee OA patient population to assess physical function.19-21 It consists of 12 items, with PCS scores calculated by summing factor-weighted scores derived from a US-based general population sample.22
To quantify the progression of knee OA, we opted to use biochemical biomarkers of cartilage degradation and bone remodeling as opposed to radiographic measures. Radiographic joint space narrowing has long been considered the gold standard for determining the progression of OA. Unfortunately, joint space narrowing has limited responsiveness and often requires longer follow-up times before measurable differences can be detected.9 As such, we opted to use 2 biochemical biomarkers that have been demonstrated to be predictive of radiographic OA progression.11
C-terminal crosslinked telopeptide of type II collagen (CTXII): Following cartilage degradation, CTXII is released into the synovial fluid and the circulation. CTXII has been identified as a biomarker for the diagnosis, staging, and evaluating the prognosis of hip and knee OA.11,23 We have also reported that CTXII correlates with the degree of joint destruction and increases significantly within one month after anterior cruciate ligament injury.24,25 CTXII was measured in the urine by enzyme-linked immunosorbent assay (ELISA; Cartilaps (CTX-II); Immunodiagnostic Systems, Inc., Fountain Hills, AZ),24,25 and was normalized to urinary creatinine levels which were also measured by ELISA (Quidel, San Diego, CA).11,26
Nonisomerized alpha version of the C-terminal crosslinked telopepide of type I collagen (CTXIα): In OA knees, CTXIα is localized to areas of high turnover of subchondral bone,26 and has been found to be predictive of OA symptom and radiographic progression.11 CTXIα was measured in the urine by sandwich ELISA (Immunodiagnostic Systems, Inc., Fountain Hills, AZ), and was normalized to urinary creatinine levels also measured by ELISA (Quidel, San Diego, CA).11,26 LabCorp Clinical Trials performed both biomarker assays.9-11
Statistical Analyses
Patients were categorized into 3 groups based on BMI and CES-D scores: nonobese patients (NON; BMI <30 kg/m2), obese patients without depression (OBESE; BMI ≥30 kg/m2 and CES-D <16), and obese patients with depression (O + D; BMI ≥30 kg/m2 and CES-D ≥16). Separate repeated measures analyses of variance (ANOVAs) were used to compare baseline and 2-year values between groups for the following variables: KOOS Quality of Life, WOMAC Pain, WOMAC Physical Function, SF-12 Physical Component Score, CTXII, and CTXIα. For all analyses, patient age, biological sex, race, and OA grade at baseline were included as covariates,10 and the location of significant differences was determined using post hoc tests with a Bonferroni correction. Biological sex, race, and OARSI (Osteoarthritis Research Society International) grade at baseline were compared between the 3 groups using chi-square tests, and a 1-way ANOVA was used to compare age between groups. An α-level of 0.05 was considered to be statistically significant, and all analyses were performed using SPSS Statistics version 24 (IBM, Armonk, NY).
Results
Of the 600 patients, 282 (47%) were not obese, 285 (47.5%) were obese without depression, and 33 (5.5%) were obese with comorbid depression at the baseline visit ( Table 1 ). While BMI remained stable for all 3 groups over the 2-year study period, there was a significant group × time interaction for CES-D scores, with the O + D group demonstrating a reduction in the severity of depressive symptoms over this period ( Table 1 ). KOOS QOL, WOMAC Pain, WOMAC Physical Function, and SF-12 PCS were significantly worse for the O + D group compared with the other 2 groups, both at baseline and 2-year follow-up ( Table 2 ).
Table 1.
Comparison of Baseline Characteristics (mean ± SD) between Nonobese Patients (NON), Obese Patients without Depression (OBESE), and Obese Patients with Comorbid Depression (O + D).
| Variable | NON | OBESE | O + D | P |
|---|---|---|---|---|
| N | 282 | 285 | 33 | — |
| Sex, male/female, (% female) | 133/149 (52.8) | 109/176 (61.8) | 9/24 (72.7) | 0.02 |
| Age, years, mean ± SD | 63.1 ± 9.1 | 60.2 ± 8.4 | 59.0 ± 8.3 | <0.001 |
| BMI, kg/m2, mean ± SD | <0.001 | |||
| Baseline | 26.7 ± 2.5 | 33.9 ± 3.2 | 35.9 ± 4.6 | |
| 2-year | 27.3 ± 3.1 | 33.6 ± 3.7 | 35.2 ± 5.3 | |
| CES-D,a mean ± SD | <0.001 | |||
| Baseline | 6.5 ± 7.9 | 5.4 ± 4.2 | 24.3 ± 6.9 | |
| 2-year | 6.4 ± 6.6 | 5.9 ± 5.7 | 17.3 ± 8.7 | |
| Race, % Caucasian | 87.2 | 71.6 | 63.6 | <0.001 |
| Baseline OARSI grade, n (%) | 0.01 | |||
| 0 | 33 (11.7) | 17 (6.0) | 4 (12.1) | |
| 1 | 92 (32.6) | 67 (23.5) | 8 (24.2) | |
| 2 | 64 (22.7) | 99 (34.7) | 9 (27.3) | |
| 3 | 70 (24.8) | 80 (28.1) | 11 (33.3) | |
| 4 | 23 (8.2) | 22 (7.7) | 1 (3.0) |
BMI = body mass index; CES-D = Center for Epidemiological Studies–Depression scale; OARSI, Osteoarthritis Research Society International.
There was a significant group × time interaction for CES-D as the O + D group demonstrated a significant reduction in CES-D scores at 2 years. The mean CES-D score at 2 years for the O + D group was >16 suggesting that clinically significant depressive symptoms persisted for the majority of patients in this group.
Table 2.
Baseline and 2-Year Patient-Reported Outcomes (mean ± SD) between Nonobese Patients (NON), Obese Patients without Depression (OBESE), and Obese Patients with Comorbid Depression (O + D).
| Variable | NON | OBESE | O + D | P |
|---|---|---|---|---|
| KOOS QOL | <0.001a | |||
| Baseline | 66.8 ± 20.7 | 64.1 ± 19.7 | 48.7 ± 24.6 | |
| 2-year | 68.7 ± 21.9 | 66.3 ± 21.8 | 44.3 ± 21.6 | |
| WOMAC Pain | <0.001a | |||
| Baseline | 2.1 ± 2.9 | 2.4 ± 3.0 | 5.5 ± 4.6 | |
| 2-year | 2.7 ± 3.3 | 2.9 ± 3.2 | 7.1 ± 4.6 | |
| WOMAC ADL | <0.001a | |||
| Baseline | 6.8 ± 9.2 | 9.1 ± 10.3 | 20.7 ± 16.1 | |
| 2-year | 7.9 ± 9.9 | 9.4 ± 10.4 | 25.1 ± 14.4 | |
| PCS | <0.001a | |||
| Baseline | 50.1 ± 8.3 | 48.0 ± 8.7 | 41.0 ± 9.0 | |
| 2-year | 48.3 ± 9.3 | 46.9 ± 8.9 | 37.6 ± 10.0 |
KOOS QOL = Knee injury and Osteoarthritis Outcome Score Quality of Life subscale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; ADL, activities of daily living; PCS, physical component summary.
O + D group significantly differed from NON and OBESE groups at both time points.
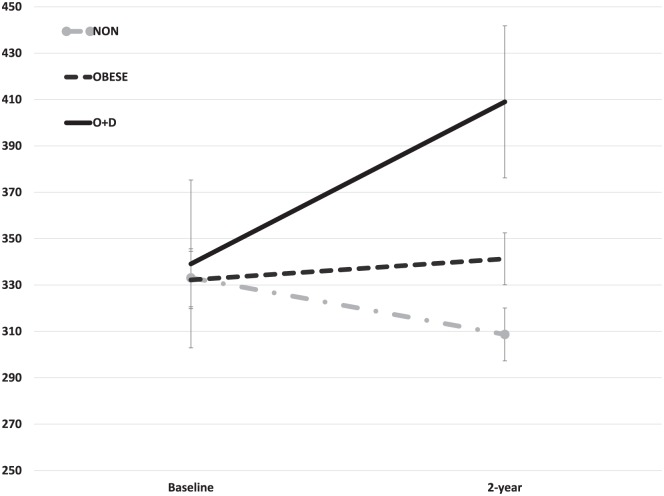
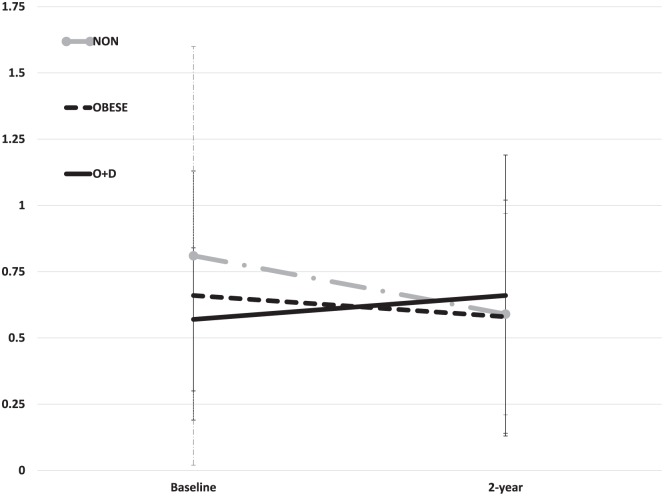
The O + D group demonstrated significantly greater increases in both CTXII and CTXIα between baseline and 2 years when compared with the NON and OBESE groups (P = 0.03 and P = 0.005, respectively; Figs. 1 and 2 ). CTXII for the O + D group was 339.1 ± 36.2 ng/mmol creatinine (Cr) at baseline and increased to 409.0 ± 32.8 ng/mmol Cr at 2 years. In comparison, CTXII in the OBESE group remained relatively unchanged from baseline (332.2 ± 12.4 ng/mmol Cr) to 2 years (341.3 ± 11.2 ng/mmol Cr) whereas the NON group demonstrated a decrease in CTXII over this period (baseline = 333.1 ± 12.5 ng/mmol Cr, 2 year = 308.7 ± 11.4 ng/mmol Cr). Similarly, CTXIα increased for the O + D group between the 2 time points (baseline = 0.57 ± 0.27 µg/mmol Cr, 2 year = 0.66 ± 0.53 µg/mmol Cr) whereas CTXIα decreased over time for both the NON (baseline = 0.81 ± 0.79 µg/mmol Cr, 2 year = 0.59 ± 0.38 µg/mmol Cr) and OBESE groups (baseline = 0.66 ± 0.47 µg/mmol Cr, 2 year = 0.58 ± 0.44 µg/mmol Cr).
Figure 1.
Significantly greater increases in CTXII (mean ± standard error) were noted between baseline to 2-year follow-up for the group of patients with combined obesity and depression (O + D). Values normalized to urinary creatinine concentration and expressed as ng/mmol Cr.
Figure 2.
Significantly greater increases CTXIα (mean ± standard error) were noted between baseline to 2-year follow-up for patients with combined obesity and depression (O + D). Values normalized to urinary creatinine concentration and expressed as µg/mmol Cr.
Discussion
The purpose of this study was to compare the progression of biochemical biomarkers of OA and knee pain and symptoms between nonobese patients, obese patients without depression, and obese patients with comorbid depression. This study was not without limitation. First and foremost, only 33 patients (5.5%) in the current study presented with combined obesity and depression. The low prevalence of combined obesity and depression was driven in large part by a lower prevalence of depression in the FNIH OA Biomarkers dataset than previously demonstrated in other series of OA patients. It has been previously reported that approximately 20% of OA patients have comorbid depression,27,28 and additional study is necessary to determine the prevalence of combined obesity and depression in the larger OA population. Second, we opted to treat obesity and depression as categorical variables instead of using BMI and CES-D scores as continuous variables. This was done in an attempt to make the findings easier to transition in the clinical setting, as using predefined thresholds for both obesity and CES-D scores would offer simple, straightforward methods to identify at risk patients in the clinical setting. However, even symptoms of depression that do not meet the clinical significance threshold may affect progression of knee OA in obese individuals. Third, synovial fluid samples were not collected and the current analysis was limited to urinary biomarkers of OA. While not a 1:1 representation of the intra-articular environment, both urinary CTXII and CTXIα have been identified as being predictive of radiographic OA progression.11 Finally, other comorbid conditions associated with increased systemic inflammation such as diabetes or chronic obstructive pulmonary disorder were not assessed in the current study, and future studies are necessary to determine if there is an additive effect of obesity and other comorbidities in terms of the total systemic inflammatory burden. Despite these limitations, by and large the results supported our hypothesis that the group with combined obesity and depression would demonstrate significantly worse progression of both OA biomarkers and subjective symptoms than the other 2 groups.
Rationale for Worsening OA for Obese Patients with Comorbid Depression
Knee OA is a chronic inflammatory condition.29,30 Cytokines, monocytes, and macrophages act as mediators in the cycle of cartilage degradation in knee OA. Synovial macrophages activated by proinflammatory cytokines (IL-1β and others) as a result of cartilage breakdown produce proinflammatory cytokines, which then activate chondrocytes and production of matrix metalloproteinases (MMPs), thereby creating the cyclical process of cartilage degradation.29 However, this local inflammatory process within the knee may be mediated by the level of inflammation and IL-1β expression within the entire body.31 This systemic, extra-articular process is not benign as patients with increased peripheral expression of IL-1β demonstrate a more rapid progression of knee OA.1
Like knee OA, obesity and depression are also associated with increased systemic inflammation and expression of peripheral IL-1β.29,31-33 Both obesity and depression have been individually identified to increase the risk of systemic conditions ranging from rheumatoid arthritis to leukemia.34-37 Obesity and depression have each been shown to directly affect the knee, as the risk of surgical site infections is significantly greater for knee arthroscopy patients with comorbid depression and/or obesity.38 It has been proposed that these increased risks may be due to increased systemic inflammation creating a physiological environment that promotes the development of additional inflammatory comorbidities.39 Furthermore, there may be an additive effect of combined inflammatory comorbidities as the severity of depression and anxiety in both knee osteoarthritis and rheumatoid arthritis patients correlated with serum concentrations of pro-inflammatory cytokines.40,41 As such, in the current study the increased cartilage degradation and bone remodeling demonstrated by the subset of obese patients with comorbid depression may perhaps be due to an increased systemic inflammatory burden when compared with either nonobese patients or obese patients without depression.
However, the rationale for the increased cartilage degradation and bone remodeling in the subset with combined obesity and depression may not be solely biological, as there could also be a mechanical link in the cycle of obesity, depression, and OA progression. Both pain and functional limitations are lessened for OA patients that routinely engage in physical activity and other management strategies.42 On the contrary, depressed OA patients have a greater likelihood of reduced physical activity, which may contribute, in part, to progressive cartilage degradation.43 In addition to the proposed biological mechanism of OA progression associated with the combination of obesity and depression, the reduction in physical activity may result in reduced loading and subsequent deconditioning of the articular cartilage. There appears to be an optimal window in terms of mechanical loading of articular cartilage. It is logically intuitive that excessive cyclical loading of the cartilage can result in degradation and/or injury44,45; however, there may also be a lower bound or threshold of loading below which cartilage degradation may occur.30 Depression, obesity, and knee OA are individually associated with sedentary behaviors and reduced physical activity,43 thereby creating a theoretical cycle of pain, inactivity, and cartilage degradation that may coincide with cartilage degradation secondary to the systemic inflammatory burden for the subset of obese OA patients with comorbid depression.
Clinical Implications
In the current study, obese OA patients with comorbid depression demonstrated increased knee pain and inferior physical function both at baseline and 2-year follow-up when compared with nonobese patients or obese OA patients without depression. This subset of patients also demonstrated significantly greater increases in biomarkers of cartilage degradation and bony remodeling consistent with rapid OA progression. The current results can help clinicians identify the subgroup of patients with O+D who are at the highest risk of rapid progression and justify the need for educational information and potential referrals to address depression and obesity in addition to usual care treatments in orthopedic practices. This study also highlights the need to develop interventions that can directly target comorbid depression in obese OA patients.
BMI is already routinely collected as part of the clinical care for those with knee OA, with obese patients often counselled on the benefits of weight loss and/or physical activity programs. The current results further suggest that the orthopedic community may need to consider administering a depression scale as part of clinical care to identify patients who may benefit from adjunctive behavioral interventions. Depressed patients, whether or obese or not, are less adherent to treatment recommendations and demonstrate reduced benefits even when treatment regimens are followed.46-48 Behavioral interventions to specifically lessen depressive symptoms have also been demonstrated to reduce knee OA pain and symptoms,49 but it remains unknown if depression-related behavioral interventions also promote greater adherence with weight loss and/or physical activity programs. Breaking the cycle of depression and obesity may reduce OA symptoms,42,49 and future studies are necessary to determine if this may also slow the progression of cartilage degradation by reducing systemic inflammation and/or creating more optimal mechanical loading through increased physical activity. Failing to reduce depressive symptoms and body weight may not only predispose patients to a more rapid OA progression but also increases the likelihood of a poor postoperative outcome after arthroplasty procedures for those that progress to end-stage OA.50,51 Finally, this investigation opens the door to a potential link between systemic disease and inflammatory burden in relation to progressive cartilage degradation for those with knee OA. This is in line with increasing concerns about a degenerative synovial environment that is being identified in early osteoarthritic knees that progress to treatment failure with biologic restoration procedures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The OAI is a public-private partnership composed of 5 contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This article was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. Data provided from the FNIH OA Biomarkers Consortium Project are made possible through grants and direct or in-kind contributions by: AbbVie; Amgen; Arthritis Foundation; Artialis; Bioiberica; BioVendor; DePuy; Flexion Therapeutics; GSK; IBEX; IDS; Merck Serono; Quidel; Rottapharm | Madaus; Sanofi; Stryker; the Pivotal OAI MRI Analyses (POMA) study, NIH HHSN2682010000 21C; and the Osteoarthritis Research Society International.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors’ Note: The study was performed at the University of Kentucky. This study is an analysis of a publicly available dataset from the Osteoarthritis Initiative (OAI; Clinicaltrials.gov Identifier: NCT00080171).
Ethical Approval: Ethical approval was not sought for the present study because it involved secondary analysis of a publically-available and de-identified dataset.
Informed Consent: Informed consent was not sought for the present study because it involved secondary analysis of a publically-available and de-identified dataset. Patients did provide written informed consent as part of the original data collection performed as part of the Osteoarthritis Initiative.
Trial Registration: Current study was not registerred as it was a secondary anlaysis of existing data; however, the Osteoarthritis Initiatve is registered at clinicaltrials.gov (NCT00080171).
References
- 1. Attur M, Belitskaya-Levy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, et al. Increased interleukin-1b gene expressin in peripheral blood leukocytes is associated with increased pain and predicts risk of progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63(7):1908-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desmeules F, Dionne CE, Belzile E, Bourbonnais R, Fremont P. The burden of wait for knee replacement surgery: effects on pain, function, and health-related quality of life at the time of surgery. Rheumatol. 2010;49(5):945-54. [DOI] [PubMed] [Google Scholar]
- 3. Ackerman IN, Bennell KL, Osborne RH. Decline in health-related quality of life reported by more than half of those waiting for joint replacement surgery: a prospective cohort study. BMC Musculoskelet Disord. 2011;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bedard NA, Dowdle SB, Anthony CA, DeMik DE, McHugh MA, Bozic KJ, et al. The AAHKS Clinical Research Award: what are the costs of knee osteoarthritis in the year prior to total knee arthroplasty? J Arthroplasty. 2017;32:S8-S10.e1. [DOI] [PubMed] [Google Scholar]
- 5. Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riddle DL, Kong X, Fitzgerald GK. Psychological health impact on 2-year changes in pain and function in persons with knee pain: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:1095-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosemann T, Backenstrass M, Joest K, Rosemann A, Szecsenyi J, Laux G. Predictors of depression in a sample of 1021 primary care patients with osteoarthritis. Arthritis Rheum. 2007;57(3):415-22. [DOI] [PubMed] [Google Scholar]
- 8. Rathbun AM, Yau MS, Shardell M, Stuart EA, Hochberg MC. Depressive symptoms and structural disease progression in knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2017;36:155-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps toward further validation. Best Practice Res Clin Anaesthesiol. 2014;28(1):61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraus VB, Hargrove DE, Hunter DJ, Renner JB, Jordan JM. Establishment of reference intervals for osteoarthritis-related soluble biomarkers: the FNIH/OARSI OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):179-85. [DOI] [PubMed] [Google Scholar]
- 11. Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. ; OA Biomarkers Consortium. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):186-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rathbun AM, Stuart EA, Shardell M, Yau MS, Baumgarten M, Hochberg MC. Dynamic effects of depressive symptoms on osteoarthritis knee pain. Arthritis Care Res (Hoboken). 2018;70(1):80-8. doi: 10.1002/acr.23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. [DOI] [PubMed] [Google Scholar]
- 14. Collins NJ, Prinsen CAC, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24:1317-29. [DOI] [PubMed] [Google Scholar]
- 15. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011;63(Suppl 11):S208-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis. a sixteen-year followup of menisectomy with matched controls. Arthritis Rheum. 2003;48(8):2178-87. [DOI] [PubMed] [Google Scholar]
- 17. Schiphof D, Waarsing JH, Oei EH, Bierma-Zeinstra SMA. KOOS subscale and individual items associate with onset of structural knee osteoarthritis within five years. Osteoarthritis Cartilage. 2016;24:S438. [Google Scholar]
- 18. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapies in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40. [PubMed] [Google Scholar]
- 19. Juhl C, Lund H, Roos EM, Zhang W, Christensen R. A hierarchy of patient-reported outcomes for meta-analysis of knee osteoarthritis trials: empirical evidence from a survey of high impact journals. Arthritis. 2012;2012:136245. doi: 10.1155/2012/136245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Waal JM, Terwee CB, van der Windt DAWM, Bouter LM, Dekker J. The impact of non-traumatic hip and knee disorders on health-related quality of life as measured with the SF-36 or SF-12. A systematic review. Qual Life Res. 2005;14(4):1141-55. [DOI] [PubMed] [Google Scholar]
- 21. Webster KE, Feller JA. Comparison of the Short Form–12 (SF-12) health status questionnaire with the SF-36 in patients with knee osteoarthritis who have replacement surgery. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2620-6. [DOI] [PubMed] [Google Scholar]
- 22. Ware JE, Jr, Kosinski MA, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric; 2005. [Google Scholar]
- 23. Nepple JJ, Thomason KM, An TW, Harris-Hayes M, Clohisy JC. What is the utility of biomarkers for assessing the pathophysiology of hip osteoarthrits? A systematic review. Clin Orthop Related Res. 2015;473:1683-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lattermann C, Jacobs CA, Bunnell PM, Huston LJ, Gammon LG, Johnson DL, et al. A multicenter study of early anti-inflammatory treatment in patients with acute ACL tear. Am J Sports Med. 2017;45(2):325-33. [DOI] [PubMed] [Google Scholar]
- 25. Lattermann C, Jacobs CA, Bunnell PM, Jochimsen KN, Abt JP, Reinke EK, et al. Logistical challenges and design considerations for studies using acute anterior cruciate ligament injury as a potential model for early posttraumatic arthritis. J Orthop Res. 2017;35(5):641-50. [DOI] [PubMed] [Google Scholar]
- 26. Huebner JL, Bay-Jensen AC, Huffman KM, He Y, Leeming DJ, McDaniel GE, et al. ALPHA-CTX is associated with subchondral bone turnover and predicts progression of joint space narrowing and osteophytes in osteoarthritis. Arthritis Rheum. 2014;66(9):2440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mossey JM, Gallagher RM. The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med. 2004;5(4):335-48. [DOI] [PubMed] [Google Scholar]
- 28. Sale JE, Gignac M, Hawker G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J Rheumatol. 2008;35(2):335-42. [PubMed] [Google Scholar]
- 29. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21:16-21. [DOI] [PubMed] [Google Scholar]
- 30. Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol Aging Age Relat Dis. 2012;2(2012):17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loukov D, Karampatos S, Maly MR, Bowdish DME. Monocyte activation is elevated in women with knee-osteoarthritis and assocaited with inflammation, BMI and pain. Osteoarthritis Cartilage. 2018;26(2):255-63. doi: 10.1016/j.joca.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 32. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70:185-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122(6):1418-21. [DOI] [PubMed] [Google Scholar]
- 36. Dar L, Tiosano S, Watad A, Bragazzi NL, Zisman D, Comeneshter D, et al. Are obesity and rheumatoid arthritis interrelated? Int J Clin Pract. 2018;72(1). doi: 10.1111/ijcp.13045. [DOI] [PubMed] [Google Scholar]
- 37. Vallerand I, Lewinson R, Lowerison M, Frolkis A, Kaplan G, Bulloch A, et al. Depression as a risk factor for the development of rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2017;69(Suppl 10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cancienne JM, Mahon HS, Dempsey IJ, Miller MD, Werner BC. Patient-related risk factors for infection following knee arthroscopy: an analysis of over 700 000 patients from two large databases. Knee. 2017;24:594-600. [DOI] [PubMed] [Google Scholar]
- 39. Ursum J, Nielen MMJ, Twisk JWR, Peters MJL, Schellevis FG, Nurmohamed MT, et al. Increased risk for chronic comorbid disorders in patients with inflammatory arthritis: a population based study. BMC Fam Pract. 2013;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El-Tantawy AM, El-Sayed AE, Kora BA, Amin RT. Pyschiatric morbidity associated with some cytokines (IL-1β, IL-12, IL-18, TNF-α) among rheumatoid arthritis patients. Egypt J Immunol. 2008;15(1):1-11. [PubMed] [Google Scholar]
- 41. Shimura Y, Kurosawa H, Tsuchiya M, Sawa M, Kaneko H, Liu L, et al. Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin Rheumatol. 2017;36(12):2781-7. [DOI] [PubMed] [Google Scholar]
- 42. Kelley GA, Kelley KS, Hootman JM, Jones DL. Effects of community-deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: a meta-analysis. Arthritis Care Res (Hoboken). 2011;63:79-93. [DOI] [PubMed] [Google Scholar]
- 43. White DK, Tudor-Locke C, Zhang Y, Niu J, Felson DT, Gross KD, et al. Prospective change in daily walking over two years in older adults with or at risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24(2):246-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kiviranta I, Tammi M, Jurvelin J, Arokoski J, Saamanen AM, Helminen HJ. Articular cartilage thickness and glycosaminoglycan distribution in the canine knee joint after strenuous running exercise. Clin Orthop Relat Res. 1992;283:302-8. [PubMed] [Google Scholar]
- 45. Mithoefer K, Hambly K, Logerstedt D, Ricci M, Silvers H, Villa DS. Current concepts for rehabilitation and return to sport after knee articular cartilage repair in the athlete. J Orthop Sports Phys Ther. 2012;42(3):254-73. [DOI] [PubMed] [Google Scholar]
- 46. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-7. [DOI] [PubMed] [Google Scholar]
- 47. Wing RR, Phelan S, Tate D. The role of adherence in mediating the relationship between depression and health outcomes. J Pyschom Res. 2002;53(4):877-81. [DOI] [PubMed] [Google Scholar]
- 48. Axford J, Heron C, Ross F, Victor CR. Management of knee osteoarthritis in primary care: pain and depression are the major obstacles. J Psychosom Res. 2008;64(5):461-7. [DOI] [PubMed] [Google Scholar]
- 49. O’Moore KA, Newby JM, Andrews G, Hunter DJ, Bennell K, Smith J, et al. Internet cognitive behaviour therapy for depression in older adults with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res (Hoboken). 2018;70(1):61-70. doi: 10.1002/acr.23257. [DOI] [PubMed] [Google Scholar]
- 50. Blackburn J, Qureshi A, Amirfeyz R, Bannister G. Does preoperative anxiety and depression predict satisfaction after total knee replacement? Knee. 2012;19(5):522-4. [DOI] [PubMed] [Google Scholar]
- 51. Scott CEH, Howie CR, MAcDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J Bone Joint Surg Br. 2010;92(9):1253-8. [DOI] [PubMed] [Google Scholar]