INTRODUCTION
Development of organ dysfunction is the most important clinical event during sepsis, as it directly relates to mortality and morbidity. Although the new definition of sepsis captures this concept, centering the clinical essence of sepsis on the development of a ‘life-threatening organ dysfunction caused by a dysregulated host response to infection,’1 our understanding of the mechanisms by which sepsis induces organ dysfunction remains incomplete. This knowledge gap is not trivial because mortality from sepsis continues to be very high,2 therapeutic options are limited and nonspecific, and morbidity after sepsis remains a significant burden for patients after hospital discharge.3
In the past 15 years, several new concepts have shifted our understanding of what organ dysfunction means in the context of critical illness, and framed the study of possible mechanisms leading to organ dysfunction. Three of these disruptive ideas are of particular relevance here. The first is that organs can develop dysfunction during sepsis in the absence of decreased oxygen delivery,4,5 suggesting that tissue hypoxia may not be an isolated mechanism. This explains why perfusion-targeted therapeutic efforts may surmount only to partial or to no benefit.6 The second is that organ dysfunction can occur in the absence of significant cell death,7–9 suggesting the lack of function is not due to structural damage but, rather, to a shut-down of usual cellular activities. This has fueled speculation that early on, organ dysfunction may be an adaptive strategy to overwhelming inflammatory injury.10 Of course, should this process become sustained it will become maladaptive and carry the known association with poor prognosis. The third concept is the recognition that the action of the immune system against invading pathogens (also known as resistance capacity) is only part of the body’s defense mechanisms against infection. Only recently was the mechanism known as Tolerance in the fields of plant ecology and biology, and defined as the capacity of the host to limit cellular and tissue injury derived from immune or pathogen action, described in mammals.11 Findings from experimental studies demonstrating that Tolerance mechanisms can confer organ protection and a survival advantage independent of the ability of the host to control the infection (ie, Resistance), provides a framework to investigate organ dysfunction and pathways leading to adaptation versus pathology.
Re-establishing tissue perfusion has been a cornerstone of early therapeutic rescue for patients with septic shock. Microvascular and endothelial dysfunction, autonomic failure, and characteristic bioenergetic and metabolic responses at the cellular level have been observed in multiple studies. Thus, many investigators propose targeting one or more of these mechanisms to reduce the development of sepsis-induced organ dysfunction. Interestingly, some investigators, citing the potential for adaptation (albeit resulting in transient loss of function), have speculated that some of these ‘pathologic’ alterations (eg, bioenergetic responses) may protect organs and tissues in the long run. Therefore, the aim of this review is to examine the current understanding of these various mechanisms in sepsis and their relation to organ dysfunction. Our goals will be to explore explanatory mechanisms as well as potential therapeutic targets.
MICROVASCULAR DYSFUNCTION
An early study by De Backer and colleagues12 demonstrated that septic patients had altered microcirculatory flow by monitoring the sublingual microcirculation with a hand-held orthogonal polarization spectral imaging technique. The characteristic findings were a decrease in the proportion of perfused vessels, an increase in the proportion of vessels with poor flow (ie, intermittent or stopped flow), capillary drop-out (a decrease in total vessel density), and an increase in the heterogeneity of blood flow distribution.12 These findings have now been reported by multiple independent studies, and have been demonstrated in the stomach, small intestine, colon, liver and kidney in animal models.13 Importantly, altered sublingual microcirculatory flow has been linked to organ failure and poor outcome in septic shock.14 What is less well understood is whether these alterations represent the cause or consequence of sepsis-associated organ failure.
Mechanisms of Microvascular Dysfunction: Endothelial Injury and Loss of Autoregulation
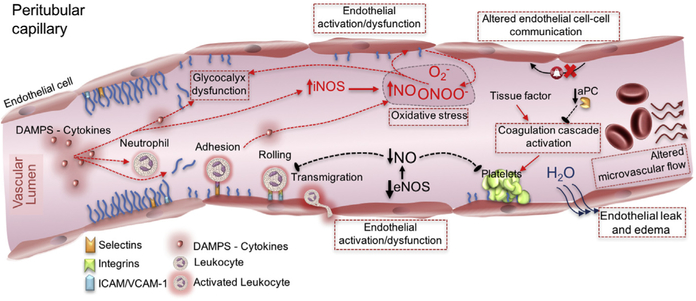
Although the mechanisms leading to microcirculatory dysfunction in sepsis are still incompletely understood, Fig. 1 summarizes the current conceptual framework. Such mechanisms revolve around the loss of autoregulation secondary to endothelial cell injury and altered retrograde endothelial cell-cell communication15; impaired red blood cell deformability16 and increased blood viscosity17; denudation of the glycocalyx (an organized layer of glycosaminoglycans that covers the luminal surface of the endothelium and has key biomechanical activities, including maintenance of blood flow and protection of the barrier function)18; platelet activation; leukocyte adhesion and rolling; and activation of the coagulation and complement systems.19 Nitric oxide (NO) may also contribute to microcirculatory dysfunction. Sepsis is characterized by a global increase in NO production; however, the expression of one of the key catalysts of NO production, inducible NO synthase (iNOS), is heterogeneously distributed.20 Such a distribution may parallel the heterogeneous flow patterns characteristic of septic microvascular dysfunction. Notably, selective inhibition of iNOS in sepsis has been associated with improvement in renal microcirculatory derangements and a decrease in functional renal impairment.21 In addition, endothelial NO synthase–derived NO, which is protective of the endothelium by inhibiting cell aggregation and promoting local vasodilatation, is decreased in sepsis, contributing to microcirculatory dysfunction.22
Fig. 1.
Mechanisms leading to microcirculatory dysfunction: damage- and pathogen-associated molecular patterns (DAMPs and PAMPs), oxidative stress, and altered nitric oxide production contribute to endothelial dysfunction. As a result, glycocalyx denudation alters the colloid osmotic gradient between the capillary lumen and protein-rich area protected by the glycocalyx layer, leading to increased capillary leak and to increased adhesion of platelets and neutrophils. An inducible nitric oxide synthase (iNOS)–dependent decrease in endothelial nitric oxide synthase (eNOS)–derived nitric oxide production results in loss of endothelial protection via loss of direct vasodilation and loss of platelet aggregation and leukocyte activation inhibition and platelet adhesion and coagulation cascade activation in the setting of endothelial dysfunction. VCAM-1, vascular cell adhesion protein 1. (Adapted from Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care 2016;22(6):546–53.)
Disrupted endothelial cell/glycocalyx lining results in interstitial edema, which is directly related to sepsis-induced organ failure.23 The renal glomerular capillary endothelial cell glycocalyx seems to be particularly vulnerable to degradation in sepsis. In a cecal ligation and puncture (CLP) murine study, an increase in urinary albumin and ultrastructural changes of the glomerular filtration barrier were observed with decreased expression of syndecan-1, hyaluronic acid, and sialic acid, all significant glomerular glycocalyx components.24 The cause of these barrier changes was linked to tumor necrosis factor (TNF)-α, as TNF administration to mice resulted in damage similar to lipopolysaccharide (LPS)-associated glomerular ultrastructural changes, whereas mice without TNF receptor-1 seemed resistant to LPS-induced changes in glomerular permeability.25 A similar pathway of glycocalyx destruction has been observed in the lung, suggesting a common injury pathway.26 In the lung, destruction of the alveolar endothelial glycocalyx stimulates increased endothelial cell fluid conductivity and results in pulmonary edema and sepsis-induced lung injury.27 In the liver, sinusoidal endothelial architecture alterations have been linked to sepsis-induced hepatocellular injury and liver dysfunction. In a mouse LPS model, LPS injection resulted in loss of sieve-plate architecture of the hepatic sinusoidal endothelium, with resulting decrease in flow velocities, increase in leukocyte adhesion and sequestration, and increase in flow heterogeneity with supply/demand mismatch.28
Consequences of Altered Microvascular Flow
Altered microvascular flow potentially compromises tissue perfusion. Tissue perfusion depends on 2 essential characteristics: diffusion and convection. Diffusion is contingent on vessel density; as vessel density increases, the distance oxygen will need to diffuse to reach target cells decreases and vice versa. Convection depends on red blood cell velocity and hemoglobin saturation.29 Thus, a decrease in capillary density (ie, capillary dropout) will induce tissue hypoxia by a diffusion-related mechanism, whereas a decline in the proportion of vessels with adequate flow (ie, increase in proportion of vessels with intermittent and stop flow) will induce tissue hypoxia by a convection-related mechanism. Functional capillary density (FCD), estimated as the perfused vessel density (PVD), reflects the number of small vessels (less than 20 μm) that are perfused continuously in a given area.30 It provides a collective estimate of the derangements due to both diffusion (capillary density) and convection (capillary flow). Physiologically, a reduction in FCD/PVD reduces the surface area for capillary exchange, decreases the available oxygen for diffusion, and increases oxygen and nutrient diffusion distance.
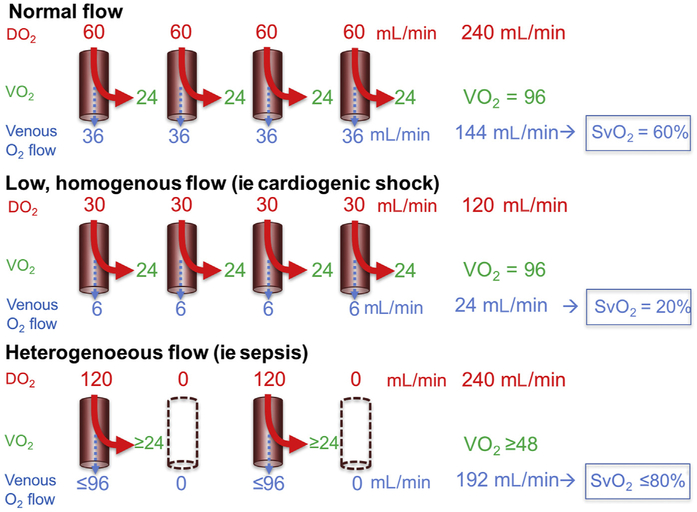
An additional characteristic of the altered microcirculation in sepsis is the increased heterogeneity of blood flow distribution. Although heterogeneity is expected to occur in healthy individuals due to blood flow–metabolic demand coupling, sepsis induces a significant increase in the heterogeneity of flow, presumably uncoupling oxygen delivery from demand and, thus, making oxygen and nutrient delivery less efficient.31 Fig. 2 describes the decreased efficiency of increased heterogeneous flow relative to normal or homogeneously decreased flow conditions, specifically, the impact of cells with capillary flow in excess of demand (luxury flow) relative to cells with capillary flow too distant from perfused vessels (ie, hypoxic). The capillary oxygen saturation of perfused vessels that match metabolic demand is low in sepsis, suggesting sustained tissue uptake of delivered oxygen.32 Because extraction is determined by metabolic demand, capillaries providing luxury flow will contribute to increased venous oxygen saturation (SvO2). The decrease in capillary oxygen tension with an observed preservation or elevation in SvO2 is termed the oxygen partial pressure gap; this indicates both a regional deficiency in oxygen supply and the presence of functional shunting.33 Insufficient blood flow to match the local tissue metabolic demands is also characterized by a decrease in carbon dioxide (CO2) washout with an increase in venous CO2 content and partial pressure (PCO2), resulting in an increase in the venoarterial difference of PCO2 (PCO2 gap). During sepsis, high PCO2 gaps have been documented and an inverse relationship between PCO2 gap and microcirculatory flow has been observed,34 suggesting the presence of areas of stagnant flow with lack of removal of CO2 or, alternatively, at risk of tissue hypoxia. Additionally, capillary leak in sepsis secondary to endothelial and glycocalyx damage limits the potential of crystalloid and colloid administration to reestablish intravascular volume and promotes interstitial edema. Furthermore, interstitial edema may impair blood flow by increasing venous pressure, resulting in areas of microvascular stasis and decreased PVD.35
Fig. 2.
Decreased efficiency of oxygen delivery with heterogeneous flow. In normal microcirculatory flow, cells extract oxygen to meet oxygen consumption (Vo2) requirements. Homogenously decreased flow results in decreased oxygen delivery (DO2) but preserved extraction and Vo2, with a resulting decrease in venous oxygen saturation (SvO2). In the presence of heterogeneous flow, despite preservation of total oxygen delivery, only a portion of capillaries is perfused. Cells too distant from perfused vessels do not receive enough oxygen to meet metabolic requirements and become hypoxic. Therefore, provided no changes in Vo2, hypoxic regions could be found in the presence of an elevated SvO2. (Modified from De Backer D, Ospina-Tascon G, Salgado D, et al. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med 2010;36:1813–25; with permission.)
In addition to the risk of hypoxia, decreased local blood flow velocity may be associated with an amplification of the inflammation signal. In a porcine model of endotoxemia, decreased leukocyte velocity and increased leukocyte transit time were observed in areas of sluggish flow in myocardial capillaries.36 As a prolonged transit is inherently accompanied by an increased time of exposure to cytokine-secreting leukocytes and the associated pathogen- and damage-associated molecular patterns, areas of sluggish flow show increased signs of focal oxidative stress and cellular injury. In a murine CLP model, an increase in reactive oxygen (ROS) and reactive nitrogen species (RNS) was observed within 4 hours of pathogen exposure and predominantly localized to areas of decreased or no blood flow.37
METABOLIC REPROGRAMING
Resistance and Tolerance
The host response against infection has been traditionally attributed to the capacity of the host’s immune system to recognize, target, and eliminate foreign microbial agents with the aim to control pathogen load, a process known as resistance.38 However, an alternative approach to pathogen defense, first described in plants but later described in mammals, is the capacity of the host to limit injury derived either from the infectious agent or from its own immune response. This process is known as tolerance and is characteristically independent of resistance.38 For example, heme oxygenase-1 (HO-1) expression in the setting of malaria infection protects against cell-free hemoglobin-induced organ dysfunction and has been shown to improve survival independent of a persistent parasite burden.39 Expression of HO-1 during sepsis has a similar effect, decreasing sepsis-induced acute kidney injury (AKI) and improving survival, independent of the capacity of the host to control the bacterial burden.40 Although little is known about the specific tolerance pathways triggered to protect a host in response to different threats, this concept lends a framework to understand the potential effect of metabolic adaptations on cell and organ protection and portrays the trade-off in terms of organ dysfunction.41
Metabolic Reprogramming as a Cell Survival Strategy
The cellular metabolic downregulation observed in early sepsis is proposed to be adaptive with the aim to reprioritize energy consumption to limit additional injury, maintain energy balance, prevent DNA damage, and preserve cellular composition.10,42 Hochachka and Guppy43 proposed that cells exposed to persistent hypoxic conditions responded by decreasing O2 demand, a mechanism called oxygen conformance. Buck and Hochachka44 confirmed this by demonstrating that sea turtle hepatocytes downregulated energy consumption up to 10-fold in response to hypoxia, by following a hierarchical shutdown of high-energy consumptive processes, including protein synthesis, while maintaining life-sustaining processes, such as preservation of membrane potentials. Schumacker and colleagues45 demonstrated a similar mechanism in rodent hepatocytes that, in response to chronic (but not acute) hypoxia, suppressed their respiratory rate up to 40% to 60%, demonstrating this is a conserved mechanism across species.
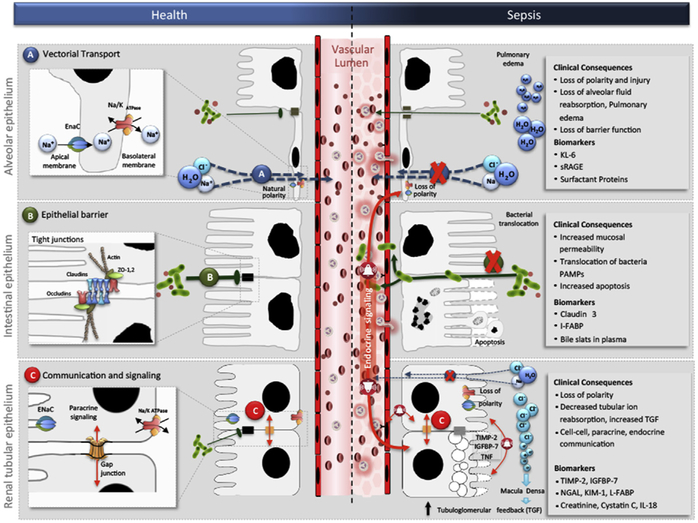
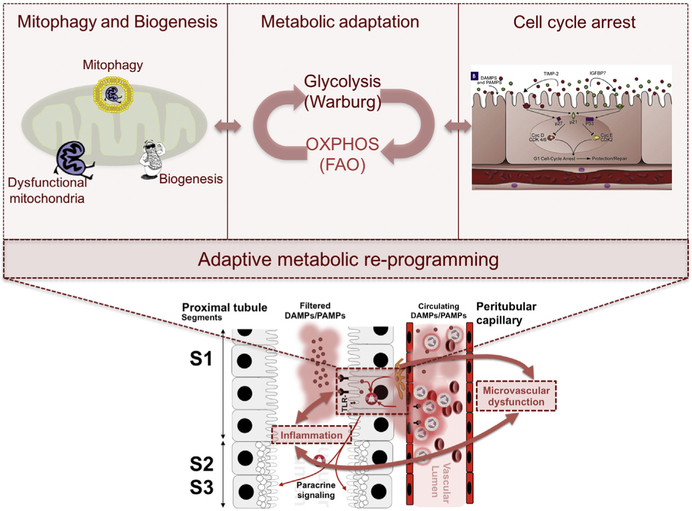
Therefore, transient loss of function seems to be a conserved cellular adaptive mechanism that may explain early organ dysfunction in response to sepsis. In the heart, sepsis induces a loss of contractility without cardiomyocyte death. This loss has been associated with protein downregulation and decreased activity of mitochondrial cytochrome c oxidase.46,47 Improvement of cytochrome c oxidase activity by exogenous cytochrome c restored cardiac contractility,47 suggesting a direct relationship between mitochondrial alterations and loss of organ function. In the lung, energy sinks, such as the sodium and chloride transporters and ATPase-linked transmembrane pumps, are inactivated and internalized during sepsis, avoiding the overtaxing use of energy, while hindering the ability of the alveolar epithelium to clear fluid from the alveolar space and resulting in pulmonary edema (Fig. 3).48 In the kidney, inflammation induced by LPS or proinflammatory cytokines results in downregulation of chloride and sodium channels, the most relevant energy sinks in the tubular epithelial cell (see Fig. 3).49 This mechanism has been suggested to link tubular injury and glomerular filtration in ischemia reperfusion,50 as an increase in tubular chloride concentration (not reabsorbed because of downregulation of channels) beyond the proximal tubule activates tubuloglomerular feedback at the level of the macula densa,51 resulting in reduced glomerular filtration rate by constriction of the afferent arteriole. However, this mechanism has yet to be demonstrated in the context of sepsis.52 In the liver, decreased synthesis of excreted proteins and impaired transformation of exogenous and endogenous toxins is observed in sepsis.53 Hotchkiss and colleagues7 have shown that human sepsis is characterized by a paucity of cell death in several organs, with the exception of the gut, the immune system, and the spleen, where more apoptosis was found to occur relative to other organs. During sepsis, the barrier function of the gut epithelia is altered because of increased epithelial apoptosis54 and decreased crypt proliferation,55 which results in an increase in mucosal permeability and bacterial translocation (see Fig. 3).56 Although the role of energy regulation in the induction of intestinal epithelial apoptosis is unclear, there is evidence to suggest that T CD4+ lymphocytes confer protection from apoptosis57; thus, T CD4+ apoptosis during sepsis may contribute to intestinal epithelial apoptosis. Finally, cellular metabolic reprogramming in response to sepsis may be orches-trated by several coordinated programs, including shifts in metabolic ATP generation (between oxidative phosphorylation [OXPHOS] and glycolysis), inhibition of mitochondrial respiration, activation of quality-control mitochondrial processes, and induction of cell cycle arrest.
Fig. 3.
Common epithelial functions in health and in sepsis. Vectorial transport in the lung becomes altered in sepsis causing loss of polarity and barrier function (A); epithelial barrier function in the intestine becomes impaired in sepsis causing increased permeability and bacterial translocation (B); and communication and signaling in the kidney becomes associated with cell-cell, paracrine, and endocrine communication in sepsis (C). Cl, chloride; H2O, water; I-FABP, intestinal-fatty acid binding protein; IGFBP-7, insulinlike growth factor binding protein-7; IL-18, interleukin-18; K, potassium; KIM-1, kidney injury molecule-1; KL-6, Krebs Von den Lungen-6; Na, sodium; NGAL, neutrophil gelatinase–associated lipocalin; PAMPs, pathogen-associated molecular patterns; sRAGE, soluble receptor of advanced glycation end products; TGF, transforming growth factor; TIMP-2, tissue inhibitor of metalloproteinases-2; ZO, zonula occludens. (From Acute dialysis quality initiative 14. Available at: www.adqi.org. Accessed June 7, 2017; with permission.)
Metabolic Reprogramming: From Oxidative Phosphorylation to Aerobic Glycolysis
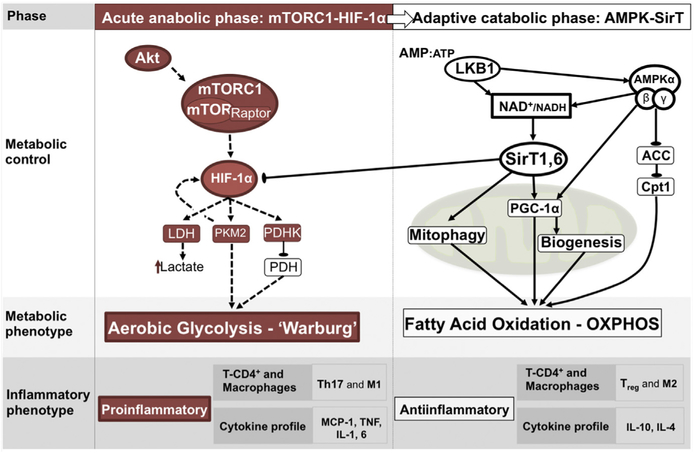
The preferential oxidation of glucose through glycolysis despite the availability of oxygen (ie, aerobic glycolysis), a phenomenon known as the Warburg effect, is of particular interest. Although largely observed in cancer cells, immune cells also use this mechanism in response to inflammatory stimulation.58 Using this mechanism, immune cells reprogram the pathways of energy generation such that housekeeping functions are sustained through OXPHOS, whereas the energy required for activation is derived from aerobic glycolysis. Furthermore, experimental models of sepsis have shown an early response characterized by a metabolic shift toward aerobic glycolysis in renal tissue of rodents exposed to CLP.59 Despite being less energetically efficient than OXPHOS, there are at least 2 advantages of using glycolysis for activation roles. First, it allows for the production of essential structural components, such as fatty acids, amino acids, and nucleotides, while producing sufficient energy for cell survival.60 Second, because of shunting of glycolytic intermediaries through the pentose phosphate pathway, increased glycolysis results in an increase in nicotinamide adenine dinucleotide phosphate (NADPH),61 which is key to reducing oxidative damage from the production of mitochondrial ROS. On the other hand, failure to restore OXPHOS at later stages of experimental sepsis has been shown to perpetuate a proinflammatory state that limits organ function and survival.62 Accordingly, multiple studies have shown that the stimulation of OXPHOS promoters in experimental sepsis was protective against organ damage and improved survival.62–64 The shifts in cellular metabolism in inflammatory cells have been shown to be secondary to the complex interaction of important cellular regulation nodes that include the Akt/mammalian target of rapamycin complex 1 (mTORC1)/hypoxia inducible factor 1 alpha (HIF-1α) pathway driving the initial shift toward glycolysis,65 and sirtuins (particularly Sirt1 and 6) driving the switch back to OXPHOS at a later stage (Fig. 4).66
Fig. 4.
Inflammation-induced metabolic shift from OXPHOS to aerobic glycolysis. In monocytes, the shift toward aerobic glycolysis has been attributed to the activation of HIF-1 α. This shift results in increased expression of cytoplasm glucose transporters, enhanced activity of glycolytic enzymes, expression of pyruvate kinase isoform M2 (PKM2, slows conversion of phos-phoenolpyruvate to pyruvate), and expression of pyruvate dehydrogenase kinase (PDHK, limits pyruvate entrance into Krebs cycle). A shift back to OXPHOS has been attributed to the nicotinamide adenine dinucleotide (NAD)–dependent deacetylases (sirtuins) SirT1 and SirT6, which blocks the HIF-1α axis. ACC, acetyl CoA carboxylase; Akt, serine/threonine-specific protein kinase; AMP, adenosine monophosphate; ATP, adenosine triphosphate; cpt1, carnitine palmitoyl transferase; HIF-1a, hypoxia inducible factor-1 alpha; IL, interleukin; IL-1, IL-4, IL-6, and IL-10, interleukin 1, 4, 6 and 10, respectively; LDH, lactate dehydrogenase; M1, macrophage activation phenotype with inflammatory functions; M2, macrophage activation phenotype with anti-inflammatory functions; MCP-1, monocyte chemoattractant protein-1; mTOR, mammalian target of rapamicin; mTORC1, mammalian target of rapamicin complex; NAD+/NADH, oxidized/reduced nicotinamide adenine dinucleotide; PDH, pyruvate dehydrogenase; PGC-1a, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Sirt1,6, sirtuin 1 and 6; T-CD4+, T lymphocyte-cluster of differentiation 4; Th17, T helper 17 cell; TNF, tumor necrosis factor; Treg, regulatory T cell. (Adapted from Gómez H, Kellum JA, Ronco C, et al. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 2017;13(3):143–51.)
Respiratory Electron Transport Chain Inhibition
The effect of sepsis on mitochondrial respiration and on specific electron transport chain complexes has been documented in both animal models42 and human sepsis.67 Complex I and IV activity of the mitochondrial electron transport chain was significantly reduced in skeletal muscle biopsies of critically ill patients, with nonsurvivors demonstrating a more profound reduction in activity and a concomitantly greater reduction in tissue ATP.67,68 These events have also been documented in the liver in rodent models of sepsis,42 suggesting this is not specific to skeletal muscle but rather a potentially universal mechanism. Mitochondrial complexes I and IV are susceptible to persistent inhibition by nitrosylation, a reaction facilitated by RNS, that is elevated in the setting of sepsis,69,70 limits cell death, and, importantly, is reversible to reestablish normal organ function.
Cellular Regulation of Mitochondria: Mitophagy and Biogenesis
Increased autophagy, the cellular digestion of unnecessary or dysfunctional components, has been observed in multiple organs during the early stages of experimental sepsis71–73 and has been documented in human sepsis.71 Autophagy occurs during sepsis in response to Toll-like receptor 4 (TLR-4)-mediated inflammation,74 oxidative stress,75 and mitochondrial membrane depolarization due to uncoupled respiration from ATP production in the electron transport chain.76 In this context, sepsis-induced inhibition of electron transport chain complexes may serve as a signaling mechanism to activate autophagy. Activation of autophagy is clinically relevant because lack of initiation of the autophagic response is associated with prolonged critical illness and lack of recovery from organ dysfunction.77 Furthermore, pharmacologic enhancement of autophagy by inhibiting mTOR with temsirolimus or epirubicin results in protection from AKI78 and in improved survival,79 respectively, in murine models of LPS-induced sepsis. By contrast, inhibition of autophagy by blocking vacuolar sorting protein 34 (VPS34), a central protein in promoting autophagic signaling, resulted in increased liver dysfunction.72 The autophagic response has also been suggested to be an integral mechanism of metabolic reprogramming in response to inflammation because removing dysfunctional mitochondria and decreasing mitochondrial mass can decrease ROS-induced injury and OXPHOS, particularly in the acute shift toward aerobic glycolysis.41
Mitophagy is coupled to mitochondrial biogenesis by redox pathways and TLR-9–dependent mechanisms.80 Replenishment of the mitochondrial pool may be integral in recovery from sepsis. Accordingly, Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α), a transcriptional coactivator of mitochondrial biogenesis, was shown to be increased in muscle biopsies of sepsis survivors as compared with nosurvivors.68 Furthermore, exogenous activation of mitochondrial biogenesis with inhaled carbon monoxide during experimental sepsis was protective from hepatic injury.81,82
Regulation of the Cell Cycle
Mitochondria are also involved in regulation of the cell cycle76 and may induce cell cycle arrest as a protective mechanism. In its simplest terms, the cell cycle is the progression of the cell through specific phases in preparation for cell division (including G0, G1, S, G2, and M for mitosis). During this progression, the cell seems to use specific checkpoints to verify it is ready to advance to the next stage. The G1-S checkpoint seems to be important from an energy standpoint because it is at this stage that mitochondria coalesce into a giant mesh,83 presumably to increase energy availability for replication of DNA during G2. During these checkpoints, the cell seems to verify if it is ready to advance to the next stage. If not ready, cell cycle arrest can prevent the energetic cost of replication but can also prevent cell death in the setting of cell injury (Fig. 5). This prevention is supported by the findings of Yang and colleagues84 who demonstrated that G1-S cell cycle arrest was associated with AKI after CLP-induced sepsis, and recovery, with progression to G2. Interestingly, the recently approved AKI biomarkers, tissue inhibitor of metalloproteinases-2 and insulinlike growth factor-binding protein 7, are known to induce G1 cell cycle arrest. These biomarkers were superior to other markers for identifying the risk of AKI during critical illness, including sepsis.85
Fig. 5.
Inflammation-induced metabolic reprogramming. Exposure of tubular epithelial cells to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) leads to changes in metabolic regulation within the cell that can impact cell survival, organ function and, possibly, repair events after injury subsides. The image shows 3 possible domains of acute-phase metabolic regulation, including triggering of mitochondrial quality control processes including mitophagy and biogenesis, shifting metabolism from OXPHOS toward glycolysis, and inducing cell cycle arrest. FAO, fatty acid oxidation; IGFBP7, insulinlike growth factor-binding protein 7; OXPHOS, oxidative phosphorylation; TIMP-2, tissue inhibitor of metalloproteinases-2; TLR-4, toll-like receptor-4. (Adapted from Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care 2016;22(6):546–53.)
ORGAN CROSSTALK
Dysfunctional organs may impact other remote organs through complex, and incompletely understood, biological communication processes known as organ crosstalk. Kidney-brain crosstalk was observed in animal models of AKI in which local renal inflammation secondary to TNFα administration was associated with a disrupted, more permeable blood-brain barrier and activation of brain astrocytes.86 Glial fibrillary acidic protein, a cellular marker of brain inflammation, was elevated in animal models of AKI but was not elevated in animal models of liver injury, suggesting specificity for AKI.86 In other animal models, AKI has been associated with alterations in cerebral neurotransmitter concentrations and depletion of brain catecholamine concentrations.87,88 Lung-brain crosstalk occurs in patients following lung injury with subsequent development of brain damage despite otherwise normal prior neurologic function.89 This neuropathology was shown to be independent of hypoxia in a porcine model of acute lung injury in which similar levels of hypoxemia were achieved by pulmonary lavage and decreased fraction inspired oxygen, with lavage animals ultimately showing greater brain damage.90
Differentiating crosstalk from other inflammatory syndromes is difficult. For example, LPS-challenged rats subjected to moderate positive end expiratory pressure (7 cm H2O) and low tidal volume ventilation showed decreased lung injury and systemic inflammation compared with rats ventilated with high tidal volumes; yet, expression of c-Fos, an early marker of neuronal activation in the brain, was similarly elevated in the same regions of the brain in both LPS and high tidal volume groups.91,92 Inflammatory crosstalk via systemic cytokines between the kidney and lungs has been observed in preclinical and clinical studies with ventilator-induced lung injury (VILI) associated with high tidal volume mechanical ventilation contributing to the development of AKI.93 There seems to be a unique pattern of renal inflammation in kidney-lung crosstalk. A mouse model of AKI with subgroups exposed to CLP, VILI, and sham demonstrated an elevation in vascular endothelial growth factor, an angiogenic and endothelium activating protein, and vascular cell adhesion protein 1, a leukocyte adhesion molecule, in only those animals within the VILI subgroup.94 In a murine model of AKI and bacterial pneumonia, AKI alone did not cause clinically significant acute lung injury; however, in the setting of bacterial pneumonia, AKI did attenuate pulmonary neutrophil recruitment and increase bacterial load with resulting compromised oxygenation.95
Although not solid organs, the coagulation and complement systems influence each other through interrelated pathways in the setting of sepsis. This relationship is observed in early sepsis with coinciding robust complement activation and the potential for disseminated intravascular coagulation. Complement end products increase thrombogenicity of the blood, induce procoagulant and antifibrinolytic proteins, and inhibit anticoagulant pathways.96,97 C3 convertase inhibition in bacteria-induced sepsis in baboons prevented sepsis-induced complement activation, decreased thrombocytopenia and coagulopathy, and preserved endothelial anticoagulant properties.98 Coagulation factors also impact complement activation. Thrombin and coagulation factor Xa directly activate components of the complement cascade99; protein C inhibits complement activation,100 and coagulation factor XIIa directly activates the classic complement pathway.101
Autonomic Nervous System
A conceptual extension of this crosstalk involves communication between the autonomic nervous system and immune system in the setting of inflammation. Increased circulating catecholamines in early sepsis enhances the initial inflammatory response. In addition to autonomic neurons, leukocytes can synthesize catecholamines102 and express adrenergic receptors, demonstrating neurotransmitter-influenced lymphocytic trafficking, vascular perfusion, and immune cellular proliferation and apoptosis.103 Furthermore, portal venous drainage of gut-derived catecholamines can alter the functional state of the liver Kupffer cells and hepatocytes through α2-adrenergic receptor signaling.104,105
Vagus afferents stimulated by cytokines and inflammatory molecules promote vagal nerve efferent cholinergic signaling, inhibiting the excessive release of TNF and other proinflammatory cytokines.106 This cholinergic signaling is communicated via α-7 nicotinic acetylcholine receptors found on macrophages and other immune cells.107 This inflammatory reflex for excessive proinflammatory signaling also extends to efferent vagal communication with the splenic nerve.108 Vagal nerve stimulation 24 hours after CLP-induced sepsis in a murine model improved survival.109 On the other hand, vagotomy was associated with increased proinflammatory cytokine levels in endotoxemic animals.106
SUMMARY
Sepsis is a complex syndrome characterized by significant clinical heterogeneity, in which morbidity and mortality are driven by organ dysfunction. Importantly, organ dysfunction in sepsis is now recognized to be more than just the consequence of decreased tissue oxygen delivery and instead involves multiple responses to inflammation, including endothelial and microvascular dysfunction, immune and autonomic dysregulation, and cellular metabolic reprogramming. Experimental data suggest that targeting these mechanisms may result in organ protection and offer survival advantage. However, it also underscores that the effect of targeting these mechanistic pathways on short- and long-term outcomes depends highly on the timing of therapeutic intervention. In moving forward, efforts to understand the adaptive or maladaptive character of these mechanisms, to discover phase-specific biomarkers to guide therapy and to conceptualize these mechanisms in terms of resistance and tolerance will provide a structured research platform, a translational gateway, and an opportunity to improve patient outcomes.
KEY POINTS.
Organ dysfunction in sepsis involves multiple mechanisms, including endothelial and microvascular dysfunction, immune and autonomic dysregulation, and cellular metabolic reprogramming.
Both adaptive and pathogenic responses result in decreased organ function; the clinical phenotype involves a mixture of these responses in a complex, time-dependent way.
The concept of resistance is well engrained in medicine; but tolerance is less well understood and potentially as important, especially for the critically ill and injured.
Multiple forms of organ crosstalk have been identified, helping to explain the multiple organ dysfunction that is characteristic of sepsis.
Footnotes
Disclosure Statement: The authors have no disclosures.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369:840–51. [DOI] [PubMed] [Google Scholar]
- 3.Yende S, Iwashyna TJ, Angus DC. Interplay between sepsis and chronic health. Trends Mol Med 2014;20:234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langenberg C, Wan L, Egi M, et al. Renal blood flow in experimental septic acute renal failure. Kidney Int 2006;69:1996–2002. [DOI] [PubMed] [Google Scholar]
- 5.Prowle JR, Ishikawa K, May CN, et al. Renal blood flow during acute renal failure in man. Blood Purif 2009;28:216–25. [DOI] [PubMed] [Google Scholar]
- 6.The ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 1999;27: 1230–51. [DOI] [PubMed] [Google Scholar]
- 8.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013;187: 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med 2010;36:471–80. [DOI] [PubMed] [Google Scholar]
- 10.Singer M, De Santis V, Vitale D, et al. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 2004;364:545–8. [DOI] [PubMed] [Google Scholar]
- 11.Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007;318:812–4. [DOI] [PubMed] [Google Scholar]
- 12.De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98–104. [DOI] [PubMed] [Google Scholar]
- 13.Krejci V, Hiltebrand L, Banic A, et al. Continuous measurements of microcirculatory blood flow in gastrointestinal organs during acute haemorrhage. Br J Anaesth 2000;84:468–75. [DOI] [PubMed] [Google Scholar]
- 14.Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825–31. [DOI] [PubMed] [Google Scholar]
- 15.Tyml K, Wang X, Lidington D, et al. Lipopolysaccharide reduces intercellular coupling in vitro and arteriolar conducted response in vivo. Am J Physiol Heart Circ Physiol 2001;281:H1397–406. [DOI] [PubMed] [Google Scholar]
- 16.Katz SD, Khan T, Zeballos GA, et al. Decreased activity of the N-arginine–nitric oxide metabolic pathway in patients with congestive heart failure. Circulation 1999;99:2113–7. [DOI] [PubMed] [Google Scholar]
- 17.Astiz ME, DeGent GE, Lin RY, et al. Microvascular function and rheologic changes in hyperdynamic sepsis. Crit Care Med 1995;23:265–71. [DOI] [PubMed] [Google Scholar]
- 18.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007;9:121–67. [DOI] [PubMed] [Google Scholar]
- 19.De Backer D, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care 2011;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha FQ, Assreuy J, Moss DW, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology 1994;81:211–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari MM, Brock RW, Megyesi JK, et al. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol 2005;289:F1324–32. [DOI] [PubMed] [Google Scholar]
- 22.Ince C, Mayeux PR, Nguyen T, et al. The endothelium in sepsis. Shock 2016;45: 259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2006;290:H2247–56. [DOI] [PubMed] [Google Scholar]
- 24.Adembri C, Sgambati E, Vitali L, et al. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit Care 2011;15:R277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Chang A, Hack BK, et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int 2014;85:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012;18:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care 2008;14:22–30. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Abril ER, Bethea NW, et al. Mechanisms and pathophysiological implications of sinusoidal endothelial cell gap formation following treatment with galac-tosamine/endotoxin in mice. Am J Physiol Gastrointest Liver Physiol 2006;291: G211–8. [DOI] [PubMed] [Google Scholar]
- 29.Ince C The rationale for microcirculatory-guided fluid therapy. Curr Opin Crit Care 2014;20:301–8. [DOI] [PubMed] [Google Scholar]
- 30.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care 2007;11(5):R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman D, Bateman RM, Ellis CG. Effect of decreased O2 supply on skeletal muscle oxygenation and O2 consumption during sepsis: role of heterogeneous capillary spacing and blood flow. Am J Physiol Heart Circ Physiol 2006;290: H2277–85. [DOI] [PubMed] [Google Scholar]
- 32.Ellis CG, Bateman RM, Sharpe MD, et al. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am J Physiol Heart Circ Physiol 2002;282:H156–64. [DOI] [PubMed] [Google Scholar]
- 33.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med 1999;27:1369–77. [DOI] [PubMed] [Google Scholar]
- 34.De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 2006;34:403–8. [DOI] [PubMed] [Google Scholar]
- 35.Rajendram R, Prowle JR. Venous congestion: are we adding insult to kidney injury in sepsis? Crit Care 2014;18:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goddard CM, Allard MF, Hogg JC, et al. Prolonged leukocyte transit time in coronary microcirculation of endotoxemic pigs. Am J Physiol 1995;269(4 Pt 2): H1389–97. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 2012;180:505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 2008;8:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira A, Balla J, Jeney V, et al. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med 2008;86:1097–111. [DOI] [PubMed] [Google Scholar]
- 40.Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2010;2:51ra71. [DOI] [PubMed] [Google Scholar]
- 41.Gomez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 2017;13:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 2004;286:R491–7. [DOI] [PubMed] [Google Scholar]
- 43.Hochachka PW, Guppy M. Animal anaerobes In: Hochachka PW, Guppy M, editors. Metabolic arrest and the control of biological time. Cambridge (MA): Harvard Univ Press; 1987. p. 10–35. [Google Scholar]
- 44.Buck LT, Hochachka PW. Anoxic suppression of Na+-K+-ATPase and constant membrane potential in hepatocytes: support for channel arrest. Am J Physiol 1993;265(5 Pt 2):R1020–5. [DOI] [PubMed] [Google Scholar]
- 45.Schumacker PT, Chandel N, Agusti AG. Oxygen conformance of cellular respiration in hepatocytes. Am J Physiol 1993;265(4 Pt 1):L395–402. [DOI] [PubMed] [Google Scholar]
- 46.Levy RJ, Piel DA, Acton PD, et al. Evidence of myocardial hibernation in the septic heart. Crit Care Med 2005;33:2752–6. [DOI] [PubMed] [Google Scholar]
- 47.Piel DA, Gruber PJ, Weinheimer CJ, et al. Mitochondrial resuscitation with exogenous cytochrome c in the septic heart. Crit Care Med 2007;35:2120–7. [DOI] [PubMed] [Google Scholar]
- 48.Vadasz I, Dada LA, Briva A, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and humans by promoting Na,K-ATPase endocytosis. J Clin Invest 2008;118:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt C, Hocherl K, Schweda F, et al. Proinflammatory cytokines cause downregulation of renal chloride entry pathways during sepsis. Crit Care Med 2007; 35:2110–9. [DOI] [PubMed] [Google Scholar]
- 50.Singh P, Blantz RC, Rosenberger C, et al. Aberrant tubuloglomerular feedback and HIF-1a confer resistance to ischemia after subtotal nephrectomy. J Am Soc Nephrol 2012;23:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnermann J, Ploth DW, Hermle M. Activation of tubule-glomerular feedback by chloride transport. Pflugers Arch 1976;362:229–40. [DOI] [PubMed] [Google Scholar]
- 52.Matejovic M, Ince C, Chawla LS, et al. Renal hemodynamics in AKI: in search of new treatment targets. J Am Soc Nephrol 2016;27:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim PK, Chen J, Andrejko KM, et al. Intraabdominal sepsis down-regulates transcription of sodium taurocholate cotransporter and multidrug resistance-associated protein in rats. Shock 2000;14:176–81. [DOI] [PubMed] [Google Scholar]
- 54.Perrone EE, Jung E, Breed E, et al. Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock 2012;38:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coopersmith CM, Stromberg PE, Davis CG, et al. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med 2003;31:1630–7. [DOI] [PubMed] [Google Scholar]
- 56.Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care 2017;23:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stromberg PE, Woolsey CA, Clark AT, et al. CD4+ lymphocytes control gut epithelial apoptosis and mediate survival in sepsis. FASEB J 2009;23:1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002;16:769–77. [DOI] [PubMed] [Google Scholar]
- 59.Waltz P, Carchman E, Gomez H, et al. Sepsis results in an altered renal metabolic and osmolyte profile. J Surg Res 2016;202:8–12. [DOI] [PubMed] [Google Scholar]
- 60.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci 2014;39:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang L, Xie M, Yang M, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 2014;5:4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Opal S, Ellis JL, Suri V, et al. Sirt1 activation markedly alters transcription profiles and improves outcome in experimental sepsis. Shock 2016;45:411–8. [DOI] [PubMed] [Google Scholar]
- 64.Vachharajani VT, Liu T, Brown CM, et al. SIRT1 inhibition during the hypoinflam-matory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol 2014;96:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng SC, Quintin J, Cramer RA, et al. mTOR-and HIF-1-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014;345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu TF, Vachharajani VT, Yoza BK, et al. NAD+ dependent Sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 2012;287:25758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360: 219–23. [DOI] [PubMed] [Google Scholar]
- 68.Carré JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 2010;182: 745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuzzocrea S, Mazzon E, Di Paola R, et al. A role for nitric oxide-mediated per-oxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther 2006;319:73–81. [DOI] [PubMed] [Google Scholar]
- 70.Beltran B, Orsi A, Clementi E, et al. Oxidative stress and S-nitrosylation of proteins in cells. Br J Pharmacol 2000;129:953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe E, Muenzer JT, Hawkins WG, et al. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab Invest 2009;89:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carchman EH, Rao J, Loughran PA, et al. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 2011;53:2053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsiao HW, Tsai KL, Wang LF, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 2012;37:289–96. [DOI] [PubMed] [Google Scholar]
- 74.Waltz P, Carchman EH, Young AC, et al. Lipopolysaccharide induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy 2011;7:315–20. [DOI] [PubMed] [Google Scholar]
- 75.Frank M, Duvezin-Caubet S, Koob S, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 2012;1823:2297–310. [DOI] [PubMed] [Google Scholar]
- 76.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011;333:1109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanhorebeek I, Gunst J, Derde S, et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab 2011;96:E633–45. [DOI] [PubMed] [Google Scholar]
- 78.Howell GM, Gomez H, Collage RD, et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One 2013;8:e69520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Figueiredo N, Chora A, Raquel H, et al. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity 2013;39: 874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carchman EH, Whelan S, Loughran P, et al. Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver. FASEB J 2013;27:4703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fredriksson K, Hammarqvist F, Strigard K, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol 2006;291:E1044–50. [DOI] [PubMed] [Google Scholar]
- 82.MacGarvey NC, Suliman HB, Bartz RR, et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med 2012;185(8):851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitra K, Wunder C, Roysam B, et al. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A 2009;106:11960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang QH, Liu DW, Long Y, et al. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect 2009;58:459–64. [DOI] [PubMed] [Google Scholar]
- 85.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 2008;19:1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palkovits M, Sebekova K, Gallatz K, et al. Neuronal activation in the CNS during different forms of acute renal failure in rats. Neuroscience 2009;159:862–82. [DOI] [PubMed] [Google Scholar]
- 88.Haase-Fielitz A, Haase M, Bellomo R. Decreased catecholamine degradation associates with shock and kidney injury after cardiac surgery. J Am Soc Nephrol 2009;20:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharma-cological implications. Pharmacol Ther 2011;130:226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fries M, Bickenbach J, Henzler D, et al. S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury. Anesthesiology 2005;102: 761–7. [DOI] [PubMed] [Google Scholar]
- 91.Markiewski MM, Nilsson B, Ekdahl KN, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol 2007;28:184–92. [DOI] [PubMed] [Google Scholar]
- 92.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor crosstalk in neutrophils links innate immunity to coagulation pathways. J Immunol 2006;177:4794–802. [DOI] [PubMed] [Google Scholar]
- 93.Silasi-Mansat R, Zhu H, Popescu NI, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood 2010;116:1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med 2006;12:682–7. [DOI] [PubMed] [Google Scholar]
- 95.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol 2012;34:107–25. [DOI] [PubMed] [Google Scholar]
- 96.Ghebrehiwet B, Randazzo BP, Dunn JT, et al. Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J Clin Invest 1983;71:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quilez ME, Fuster G, Villar J, et al. Injurious mechanical ventilation affects neuronal activation in ventilated rats. Crit Care 2011;15:R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quilez ME, Rodríguez-González R, Turon M, et al. Moderate PEEP after tracheal lipopolysaccharide instillation prevents inflammation and modifies the pattern of brain neuronal activation. Shock 2015;44:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuiper JW, Vaschetto R, Della Corte F, et al. Bench-to-bedside review: ventilation-induced renal injury through systemic mediator release–just theory or a causal relationship? Crit Care 2011;15:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hepokoski M, Englert JA, Baron R. Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am J Physiol Renal Physiol 2016;312:F654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singbartl K, Bishop JV, Wen X, et al. Differential effects of kidney-lung cross talk during acute kidney injury and bacterial pneumonia. Kidney Int 2011;80:633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flierl MA, Rittirsch D, Nadeau BA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007;449:721–5. [DOI] [PubMed] [Google Scholar]
- 103.Kradin R, Rodberg G, Zhao LH, et al. Epinephrine yields translocation of lymphocytes to the lung. Exp Mol Pathol 2001;70:1–6. [DOI] [PubMed] [Google Scholar]
- 104.Zhou M, Das P, Simms HH, et al. Gut- derived norepinephrine plays an important role in up-regulating IL-1b and IL-10. Biochim Biophys Acta 2005;1740: 446–52. [DOI] [PubMed] [Google Scholar]
- 105.Yang S, Zhou M, Chaudry IH, et al. Norepinephrine-induced hepatocellular dysfunction in early sepsis is mediated by activation of a2-adrenoceptors. Am J Physiol Gastrointest Liver Physiol 2001;281:G1014–21. [DOI] [PubMed] [Google Scholar]
- 106.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–62. [DOI] [PubMed] [Google Scholar]
- 107.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- 108.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 2008;105:11008–110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huston JM, Gallowitsch-Puerta M, Ochani M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med 2007;35:2762–8. [DOI] [PubMed] [Google Scholar]