Abstract
Background
Dry eye disease (DED) affects more than 14% of the elderly population causing decrease of quality of life, high costs and vision impairment. Current treatments for DED aim at lubricating and controlling inflammation of the ocular surface. Development of novel therapies targeting different pathogenic mechanisms is sought-after. The aim of this study is to evaluate safety and efficacy of recombinant human nerve growth factor (rhNGF) eye drops in patients with DED.
Methods
Forty consecutive patients with moderate to severe DED were included in a phase IIa, prospective, open label, multiple-dose, clinical trial to receive rhNGF eye drops at 20 µg/mL (Group 1: G1) or at 4 µg/mL (Group 2: G2) concentrations, two times a day in both eyes for 28 days (NCT02101281). The primary outcomes measures were treatment-emerged adverse events (AE), Symptoms Assessment in Dry Eye (SANDE) scale, ocular surface staining and Schirmer test.
Results
Of 40 included patients, 39 completed the trial. Both tested rhNGF eye drop concentrations were safe and well tolerated. Twenty-nine patients experienced at least one AE (14 in G1 and 15 in G2), of which 11 had at least 1 related AE (8 in G1 and 3 in G2). Both frequency and severity of DED symptoms and ocular surface damage showed significant improvement in both groups, while tear function improved only in G1.
Conclusions
The data of this study indicate that rhNGF eye drops in both doses is safe and effective in improving symptoms and signs of DED. Randomised clinical trials are ongoing to confirm the therapeutic benefit of rhNGF in DED.
Trial registration number
Keywords: clinical trial, ocular surface, pharmacology, tears
Introduction
Nerve growth factor (NGF), the first identified and most well-known member of the family of neurotrophins, plays a crucial role in modulating function of central and peripheral nervous systems, endocrine, immune and visual system.1 2 Since the discovery of NGF in the 1950s, increasing experimental and clinical evidence has shown that NGF is an essential factor for the trophism, sensitivity and healing of the cornea and the conjunctiva.3–5 Preliminary clinical studies have proven the safety and efficacy of topical treatment with murine NGF (mNGF) eye drops in patients with neurotrophic keratitis and autoimmune corneal ulcers.6–8 Furthermore, data from both human and animal studies demonstrate that exogenously administered NGF stimulates ocular surface wound healing and sensitivity and increases tear production and conjunctival goblet cells density indicating that NGF may represent a potential treatment for dry eye disease (DED).5 9–11
Currently, treatments for dry eye aim at lubricating the exposed epithelia with tear substitutes and at controlling ocular surface inflammation with immunosuppressive drugs and steroids.12 Novel therapeutic approaches targeting different mechanisms of DED, such as impairment of ocular surface sensitivity and epithelial damage, are highly required to improve clinical outcomes.
Recently, a novel recombinant human NGF (rhNGF) produced in Escherichia coli for the treatment of ocular diseases has been introduced. rhNGF eye-drop formulation has been successfully tested in a phase I clinical trial showing a good safety and tolerability profile.13 In July 2017, European Medicine Agency licensed full market authorisation for the use of rhNGF (Cenegermin) eye drops for the treatment of moderate and severe neurotrophic keratitis, a rare ocular disease characterised by impairment of corneal sensitivity, healing and tear production (EMA/351805/2017; Committee for Medicinal Products for Human Use). Here, we present the results of the first clinical trial using rhNGF eye drops in patients with moderate to severe DED.
Methods
This was an open-label, single-centre, two-group, efficacy and safety study to test two doses of rhNGF eye drop solution in patients with DED. The study was performed at the Department of Clinical Pharmacology, Medical University and General Hospital of Vienna, Austria. The study was approved by the local Ethics Committee (Ethik-Kommission der Medizinischen Universität Wien und des Allgemeines Krankenhauses der Stadt Wien AKH, Vienna) and by the Federal Health Authorities. The study was performed in accordance with the relevant guidelines and the Declaration of Helsinki and was carried out according to the general principles of: ‘ICH Harmonised Tripartite Guidelines for Good Clinical Practice’ (ClinicalTrials.gov Identifier: NCT02101281).
Patients
Following written informed consent, only adult patients with a diagnosis of moderate to severe DED and no restriction of gender or ethnicity were enrolled in this study.14 Inclusion criteria were: (1) lissamine green (LG) staining score greater than three in both cornea and conjunctiva (National Eye Institute—NEI—grading system) in the worse eye (study eye); (2) Schirmer test type I without anaesthesia ≤10 mm/5 min and Tear Film Break-Up Time—TFBUT—≤10 s. In addition, patients were required to have a history of artificial tears' use for dry eye within the 3 months prior to study enrolment, and to have an average Visual Analogue Scale (VAS) score for typical symptoms of dry eye (foreign body sensation, burning/stinging, itching, pain, sticky feeling, blurred vision and photophobia)≥25 mm in a 100 mm scale. For each treatment group, it was mandatory that at least six patients with severe or very severe dry eye (stage 3 or 4 according to the 2007 Report of the International Dry Eye Workshop—DEWS) were included.14
Exclusion criteria were: presence of any ocular disease other than dry eye requiring treatment with topical medications; use of topical ciclosporin, topical corticosteroids or any other topical medication for the treatment of DED; use of therapeutic or refractive contact lenses within 30 days from enrolment in the study; any active ocular infection or inflammation unrelated to DED in either eye; history of ocular surgery in the study eye including corneal refractive procedures, within 90 days from enrolment in the study; presence or history of any systemic or ocular condition that could interfere with the conduct of the required study procedures or the interpretation of the study results; presence or history of serious adverse reaction or significant hypersensitivity to any drug or chemically related compounds; significant allergy to drugs, foods, amide, local anaesthetics or other materials including commercial artificial tears containing hypromellose.
Study design
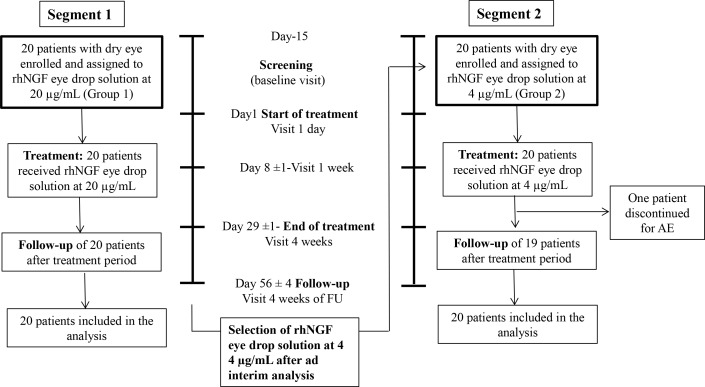
This study was designed to evaluate safety and tolerability of rhNGF eye drop administration in patients with dry eye and to identify the best dose regimen to be tested in following randomised, controlled, masked phase II studies. To achieve these objectives, two different cohorts were included: in the first cohort, 20 consecutive patients (Group 1 [G1]) received rhNGF eye drop solution (Dompé farmaceutici s.p.a., Italy) at a dose of 20 µg/mL, 1 drop in each eye (35 µL, corresponding to 0.70 µg of rhNGF) two times a day for 28 days. After completion of all subjects from Group 1, an interim analysis of the on primary efficacy and safety variables was performed to identify the dose for group 2 (G2). In the protocol, two alternative doses were foreseen: the planned dose of rhNGF eye drops solution for cohort 2 was either 4 µg/mL or 60 µg/mL concentration two times a day for 28 days, depending on the results of cohort 1 (figure 1).
Figure 1.
Study design. AE, adverse events; rhNGF, recombinant human nerve growth factor.
Before inclusion of the patients, a screening visit was performed between day −15 and day −1. After collection of systemic and ocular history, a full physical examination, including body weight, height and vital signs (blood pressure and pulse rate) was performed. Furthermore, ocular examinations were performed in the following order: assessment of ocular tolerability (VAS), assessment of frequency and severity of DED symptoms, Ocular Surface Disease Index (OSDI), best-corrected distance visual acuity (ETDRS), slit lamp examination and tear osmolarity. After a 10-min break, tear film break-up time (TFBUT) was measured. Again separated by a 5-min break, Schirmer test I (without anaesthesia) and corneal sensitivity (Cochet-Bonnet) were assessed. After another break of 10 min, ocular surface staining with LG according to the NEI score was performed. Finally, after a 10-min break, applanation tonometry for intraocular pressure (IOP) was performed.
In all patients both eyes were treated, whereas only the ‘worse eye’ was defined as the study eye and included in the statistical analysis.
Patients were evaluated as described above at the screening visit (from day −15 to baseline visit at −1 day from treatment initiation), started treatment at day 1 (day one visit) and were evaluated at day 8±1 and day 29±1. Patients were also evaluated 4 weeks after the end of treatment (day 56±4).
The use of artificial tears Prosicca Sine monodose solution (Agepha Pharmaceuticals GmbH, Austria) was allowed during the whole study and the number of daily instillations was recorded.
Outcome variables
The objective of this study was to assess the efficacy and safety of different doses of rhNGF eye drops in patients with DED.
The primary outcomes were clinical efficacy and safety parameters including:
Treatment-emerged adverse events (TEAEs), assessed throughout the study.
Frequency and severity of DED symptoms evaluated by the Symptoms Assessment in Dry Eye (SANDE) scale.
Ocular surface staining evaluated by LG using the NEI scales.
Tear production evaluated by Schirmer test type I (without anaesthesia).
Secondary outcomes included:
VAS for ocular tolerability evaluating foreign body sensation, burning/stinging, itching, pain, sticky feeling, blurred vision and photophobia.
Tear function evaluated by TFBUT and tear film osmolarity.
Corneal sensitivity measured by Cochet-Bonnet aesthesiometry.
OSDI.
Frequency of artificial tears use.
Safety variables:
IOP.
Best-corrected visual acuity (BCVA).
Methods of assessment of clinical outcomes are available at online supplementary file 1.
bjophthalmol-2018-312470supp001.pdf (114.7KB, pdf)
Statistical analysis
As this was an exploratory, phase IIa study, no formal statistical sample size calculation was performed. Considering a 20%–25% dropout rate, 40 patients (20 per treatment for each cohort) were planned to be enrolled in the study. A total of 15 evaluable patients per group was considered to be appropriate for accomplishing the study objective. For each treatment group, a minimum of six patients had to have a severe dry eye (score 3–4) condition according to 2007 DEWS report. All 40 patients that were enrolled and received at least one dose of study drug were included in the analysis.
Efficacy parameters were evaluated as change from baseline (screening visit). Efficacy variables of the study eyes were compared within each dose group and evaluation visit versus baseline values by a two-sided Wilcoxon signed-rank test with a nominal α level of 0.05.
Wilcoxon rank-sum test and Hodges-Lehmann CI were used for the comparison between group 1 (20 µg/mL group) and group 2 (4 µg/mL group). Changes from baseline of corneal and conjunctival LG staining (NEI scale), Schirmer test type I and TFBUT were assessed. The statistical analysis was performed using SAS V.9.3 TS1M1 software.
The number and percentage of patients with any TEAE (coded by System Organ Class and Preferred Term), the number of TEAEs, the number and percentage of patients with any TEAE by severity (see online supplementary file 2), the number and percentage of patients with any TEAE related to study drug are presented.
bjophthalmol-2018-312470supp002.pdf (37.7KB, pdf)
Results
Forty patients with DED were recruited in the study. All patients were diagnosed with Non-Sjogren Dry Eye. The first group (G1) of 20 consecutive patients (16 females, 4 males, mean age 48.4±12.0 years) was treated with rhNGF eye drops at 20 µg/mL two times a day for 28 days. Since no safety concerns emerged, and the ad interim analysis showed that all the three primary efficacy parameters significantly improved, the study proceeded with the evaluation of the lower dose of rhNGF eye drop solution at 4 µg/mL, two times a day for 28 days in the second group (G2) of 20 consecutive patients with DED (17 females, 3 males, mean age 55.9±14.8 years) (figure 1). Thirty-nine patients completed all study visits, and only one patient (Group 2) discontinued the treatment due to an AE. Statistical analysis was performed for all 40 patients since all received at least one dose of the study drug, on a full data set basis (table 1).
Table 1.
Demographic and clinical characteristics of patients included in the study
| rhNGF 20 µg/mL | rhNGF 4 µg/mL | P value | |
| Gender—N (%) | |||
| Female | 16 (80%) | 17 (85%) | NSS |
| Male | 4 (20%) | 3 (15%) | |
| Age | |||
| Years (mean±SD) | 48.4±12.0 | 55.9±14.8 | NSS |
| Race | |||
| Caucasian—N (%) | 20 (100%) | 20 (100%) | NSS |
| Body weight | |||
| kg (mean±SD) | 70.7±8.7 | 73.2±18.3 | NSS |
| Height | |||
| cm (mean±SD) | 167.7±8.2 | 165.1±7.3 | NSS |
| Vital signs | |||
| Blood pressure | |||
| Systolic/Diastolic blood pressure mm Hg (mean±SD) | 126.2±15.1/78.5±8.5 | 123.1±18/75.7±10 | NSS |
| Pulse rate beats/min (mean±SD) | 71.5±7.4 | 76.1±11 | NSS |
| Dry eye severity (DWES classification-N) | |||
| Mild | 0 | 0 | NSS |
| Moderate | 11 | 13 | |
| Severe | 8 | 5 | |
| Very severe | 1 | 2 | |
| Frequency of DED symptoms (SANDE scale) | |||
| Mean±SD | 55.3±27.3 | 52.8±23.2 | NSS |
| Severity of DED symptoms (SANDE scale) | |||
| Mean±SD | 59.7±29.2 | 60.1±29.6 | NSS |
| OSDI score | |||
| Mean±SD | 55.52±21.81 | 52.48±21.84 | NSS |
| Frequency of AT use | |||
| Number of daily administration (mean±SD) | 2.9±3.9 | 3.3±1.9 | NSS |
| BCVA | |||
| ETDRS number of letters (mean±SD) | 85.5±5.5 | 74.6±21.9 | NSS |
| Ocular surface staining | 5.2±2 | 5.9±2.7 | NSS |
| (NEI scale) total score cornea (mean±SD) total score | 8.1±3.5 | 6.8±2.7 | NSS |
| conjunctiva (mean±SD) total score (mean±SD) | 13.3±4.9 | 12.6±5.1 | NSS |
| Cornea sensitivity | |||
| (Cochet-Bonnet esthesiometer) mm (mean±SD) | 5.6±0.9 | 5.4±1.2 | NSS |
| Schirmer test type I | |||
| mm/5 min (mean±SD) | 4.1±3.3 | 5.2±3.7 | NSS |
| Break-up time | |||
| s (mean±SD) | 3.4±2 | 3.0±2.4 | NSS |
| Tear osmolarity (Tear Lab) | |||
| mOsm/L (mean±SD) | 313.6±13.7 | 313.6±16.1 | NSS |
AT, artificial tears; BCVA, best-corrected visual acuity; DED, dry eye disease; NEI, National Eye Institute; NSS, not statistically significant; OSDI, Ocular Surface Disease Index.
DEWS, Dry Eye Workshop
DEWS, Dry Eye Workshop.
No changes of vital signs out of the normal range were observed throughout the entire study. One patient experienced a SAE (commotio cerebri) that did not lead to study drug discontinuation and was considered unrelated to the study drug. A total of 29 subjects experienced at least one AE (14 in G1 and 15 in G2), of which 11 had at least one related AE (eight in G1 and 3 in G2). All AEs were classified as mild except one in the lower concentration group, that was classified as moderate. Among the 101 AEs recorded, 77% were classified as eye disorders (32 AEs in G1 and 46 AEs in G2) (table 2). Altogether, the investigator deemed only 15 of the 101 reported TEAEs as related to the treatment (abnormal sensation in eye 3 AEs in G1; eye pain 2 AEs in G1 and 2 in G2; eye irritation 2 AEs in G1 and 1 AE in G2; eye pruritus 1 AE in G1; vision blurred 1 AE in G1; eye discharge 1 AE in G1; eyelid pain 1 AE in G1; eyelid sensory disorder 1 AE in G2)
Table 2.
Number of subjects reporting and number of reported TEAEs by SOC and PT
| MedDRA* description | rhNGF 20 µg/mL N=20 n (%) (n AE) |
rhNGF 4 µg/mL N=20 n (%) (n AE) |
Overall N=40 n (%) (n AE) |
|||
| SOC | AEs n | Subjects n (%) |
AEs n | Subjects n (%) |
AEs n | Subjects n (%) |
| PT | ||||||
| All SOCs | 36 | 14 (70%) | 65 | 15 (75%) | 101 | 29 (72.5%) |
| Eye disorders | 32 | 12 (60%) | 46 | 15 (75%) | 78 | 27 (67.5%) |
| Abnormal sensation in eye | 4 | 4 (20%) | 9 | 8 (40%) | 13 | 12 (30%) |
| Eye pain | 7 | 5 (25%) | 7 | 6 (30%) | 14 | 11 (27.5%) |
| Eye irritation | 6 | 3 (15%) | 7 | 7 (35%) | 13 | 10 (25%) |
| Eye pruritus | 4 | 2 (10%) | 5 | 5 (25%) | 9 | 7 (17.5%) |
| Vision blurred | 4 | 3 (15%) | 2 | 2 (10%) | 6 | 5 (12.5%) |
| Foreign body sensation in eyes | 0 | 0 | 4 | 4 (20%) | 4 | 4 (10%) |
| Photophobia | 2 | 1 (5%) | 3 | 3 (15%) | 5 | 4 (10%) |
| Lacrimation increased | 0 | 0 | 3 | 3 (15%) | 3 | 3 (7.5%) |
| Visual impairment | 1 | 1 (5%) | 1 | 1 (5%) | 2 | 2 (5%) |
| Asthenopia | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Erythema of eyelid | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Eye discharge | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Eye disorder | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Eyelid pain | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Eyelid sensory disorder | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Ocular discomfort | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Ocular hyperaemia | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Vitreous detachment | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Infections and infestations | 2 | 2 (10%) | 5 | 5 (25%) | 7 | 7 (17.5%) |
| Rhinitis | 0 | 0 | 2 | 2 (10%) | 2 | 2 (5%) |
| Conjunctivitis bacterial | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Herpes simplex | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Nasopharyngitis | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Sinusitis | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Urinary tract infection | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Nervous system disorders | 1 | 1 (5%) | 9 | 5 (25%) | 10 | 6 (15%) |
| Headache | 0 | 0 | 9 | 5 (25%) | 9 | 5 (12.5%) |
| Migraine | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 2 | 2 (10%) | 2 | 2 (5%) |
| Back pain | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Neck pain | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Ear and labyrinth disorders | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Tinnitus | 1 | 1 (5%) | 0 | 0 | 1 | 1 (2.5%) |
| Gastrointestinal disorders | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Flatulence | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Injury, poisoning and procedural complications | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Concussion | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
| Fall | 0 | 0 | 1 | 1 (5%) | 1 | 1 (2.5%) |
AE, adverse events; MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SOC, system organ class; TEAEs, treatment-emerged adverse events.
All the ocular AEs were mild and transient and one patient discontinued treatment due to an infection (bacterial conjunctivitis) (table 2).
No safety concerns were observed in both groups of rhNGF treatment (table 3). Neither an increase in IOP nor a decrease in BCVA was observed. In addition, both higher and lower rhNGF eye drop concentrations were well tolerated with significant improvement of all symptoms investigated by VAS scale.
Table 3.
Safety and tolerability parameters evaluated during the study period
| Dose rhNGF eye drop (µg/mL) | Baseline (from day −15 to day −1) |
Day 1 | Day 8±1 | Day 29±1 (end of treatment) |
Day 56±4 (end of follow-up) |
|
| IOP: mm Hg (mean±SD) | 20 | 15.9±1.9 | 15±1.6* | 14.1±1.6* | 14.4±1.9* | 14.2±2.7* |
| 4 | 12.7±2.3 | 12.9±2.4 | 13.2±2.9 | 14.1±2.4 | 13.6±2.5 | |
| Frequency of AT use: number of daily administration (mean±SD) | 20 | 2.9±3.85 | – | 1.81±1.9 | 2.13±2.81 | 2.87±2.96 |
| 4 | 3.3±1.89 | – | 1.083±1.54 | 1.27±1.33 | 2.46±2.86 | |
| BCVA: number of letters ETDRS (mean±SD) | 20 | 85.5±5.5 | 86.1±5.1 | 85.9±5.1 | 86.7±4.4 | 86.6±3.7 |
| 4 | 74.6±21.9 | 76.2±20.9 | 78±19.4* | 78.9±15.4* | 81.3±13.3* | |
|
VAS:
Foreign body sensation mm (mean±SD) |
20 | 48.0±27.8 | 29.6±26.5* | 22.8±25.3* | 16.6±24.7* | 17.4±25.8* |
| 4 | 48.3±26.2 | 47.1±28.6 | 28.4±27.4* | 18.7±19.5* | 18.4±25.9* | |
|
VAS:
Burning/stinging mm (mean±SD) |
20 | 48.2±23.6 | 26.9±24.8* | 22.4±24.2* | 15.5±24.7* | 18.0±25.7* |
| 4 | 46.8±28.6 | 39.4±28.6 | 33.1±28.6 | 21.2±23.4* | 16.9±23.6* | |
| VAS: itching mm (mean±SD) | 20 | 43.3±23.5 | 28.7±26.2* | 19.6±18.1* | 12.7±21.0* | 17.4±26.4* |
| 4 | 54±26.9 | 40±30* | 29.9±29.9* | 19.1±22.4* | 18.8±21.9* | |
| VAS: pain mm (mean±SD) | 20 | 40.7±29.5 | 21.5±24.6* | 25.3±34.2* | 16.3±28.2* | 15.8±27.0* |
| 4 | 29.5±26.2 | 27±25 | 25.4±27.8 | 16.1±24.9* | 12.6±20.3* | |
|
VAS:
Sticky feeling mm (mean±SD) |
20 | 30.2±25.6 | 21.0±22.0* | 18.5±21.9* | 17.5±28.9* | 17.1±30.1* |
| 4 | 38.8±26.7 | 37.4±30.9 | 21.9±27.3* | 16.6±26.4* | 16.2±23.6* | |
|
VAS:
Blurred vision mm (mean±SD) |
20 | 58.7±22.8 | 34.7±25.6* | 28.8±28.5* | 17.7±26.5* | 19.3±27.6* |
| 4 | 54.5±31.8 | 47.5±31.7 | 37.3±32.5* | 25.7±28* | 26.1±27.9* | |
| VAS: photophobia mm (mean±SD) | 20 | 59.9±20.8 | 38.9±30.6* | 32.9±30.5* | 28.4±31.2* | 27.7±32.3* |
| 4 | 48.1±31.6 | 53.1±32.5 | 37.3±31.7 | 29.8±34.8* | 25.1±26.8* |
*P<0.05 Wilcoxon signed-rank test.
AT, artificial tears; BCVA, best-corrected visual acuity;IOP, intraocular pressure; VAS, Visual Analogue Scale.
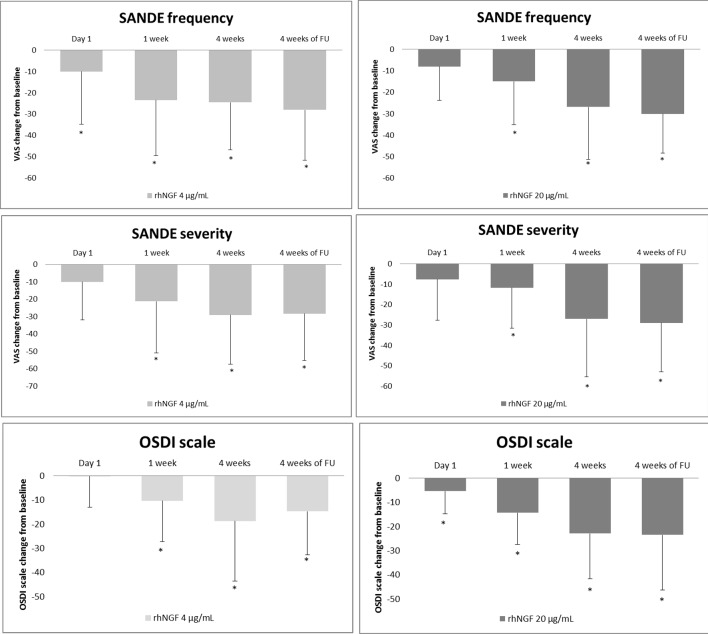
Both concentrations of rhNGF eye drops induced a significant improvement in signs and symptoms of DED at the end of treatment period that partially lasted to the end of the follow-up. Specifically, a significant improvement in both frequency (G1 baseline: 55.3±27.3, 4 weeks 28.4±26.2, p<0.0001; G2 baseline 59.7±29.2, 4 weeks 33.5±26.4, p<0.0001) and severity (G1 baseline 52.8±23.2, 4 weeks 25.9±26.9, p=0.0005; G2 baseline 60.1±29.6, 4 weeks 31.5±25.5; p=0.0002) of DED symptoms was observed in both groups (figure 2). In addition, OSDI scores showed a significant improvement (G1 baseline 55.5±21.8, 4 weeks 32.6±16.2, p<0.001, G2 baseline: 52.4±21.8, 4 weeks: 35.7±22.8, p=0.0035) at the end of treatment period (figure 2).
Figure 2.
Both high and low doses of rhNGF eye drops treatments showed a significant improvement of DED symptoms evaluated by SANDE and OSDI scales (*p<0.05). DED, dry eye disease; rhNGF, recombinant human nerve growth factor; OSDI, Ocular Surface Disease Index; SANDE, Symptoms Assessment in Dry Eye.
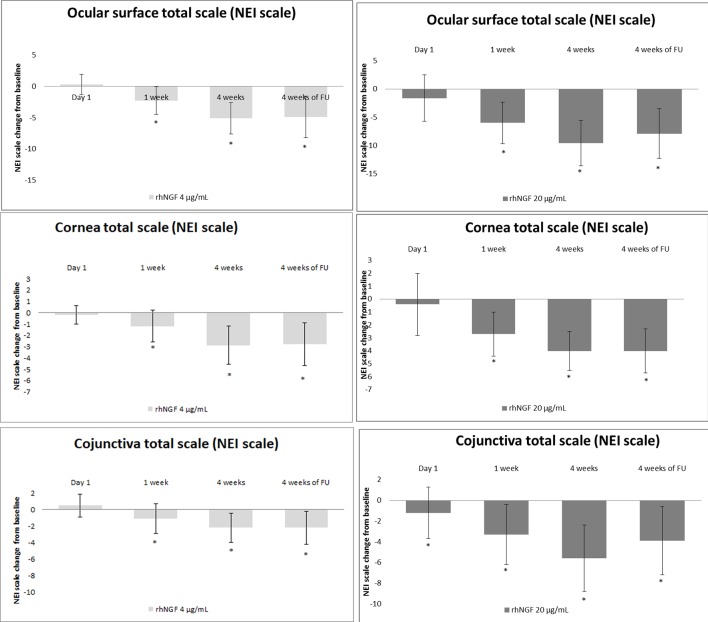
Both doses of rhNGF eye drops improved ocular surface damage at the end of treatment period, as observed by LG staining in both group 1 (NEI cornea total score: baseline: 5.2±2.0, 4 weeks: 1.3±2.2, p<0.001; NEI conjunctiva total score baseline: 8.1±3.5, 4 weeks 2.5±1.4, p<0.001) and group 2 (NEI cornea total score: baseline: 5.9±2.7, 4 weeks: 3.0±3.3, p<0.001; conjunctiva total score baseline 6.8±2.7 vs 4 weeks 4.5±2.1, p<0.001) (figure 3).
Figure 3.
Both high and low doses of rhNGF eye drops treatments showed a significant improvement of ocular surface damage evaluated by lissamine green staining (NEI score) (*p<0.05). NIE, National Eye Institute; rhNGF, recombinant human nerve growth factor.
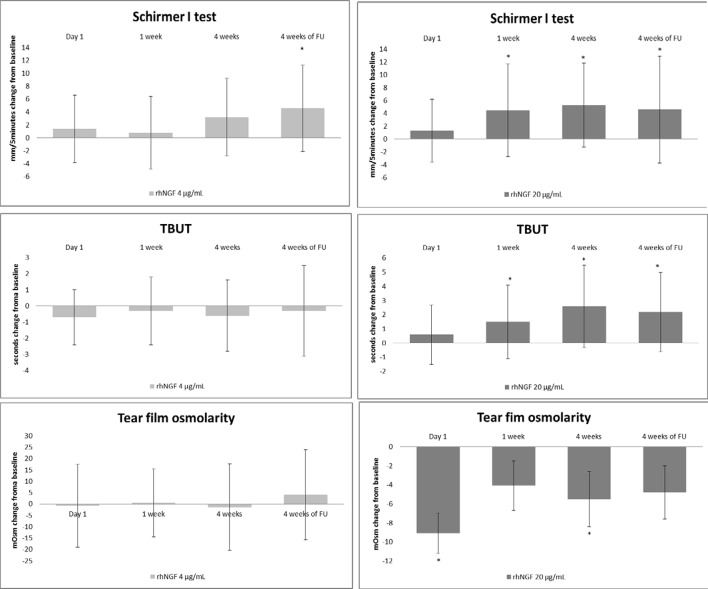
These effects were associated with a significant improvement of tear film production and function in G1, but not in G2. Specifically, a significant increase of tear film parameters was observed in group 1 (Schirmer test I: baseline 4.1±3.3 mm/5 min, 4 weeks 9.4±7.8 mm/5 min, p=0.0006; TFBUT baseline 3.4±2 s vs 4 weeks 6±2.5 s, p=0.0015; tear osmolarity baseline: 313.6±13.7 mOsm/L, 4 weeks 308.2±12.3 mOsm/L, p=0.0427) but not in the group 2 (Schirmer test type I: baseline 5.2±3.7 mm/5 min, 4 weeks 8.2±6.8 mm/5 min, p=0.0734, TFBUT baseline 3±2.4 s, 4 weeks 2.5±1.3 s, p=0.4087; tear osmolarity: 313.6±16.1 mOsm/L, 4 weeks 312.5±12.3 mOsm/L; p=0.6144) (figure 4, online supplementary table 1).
Figure 4.
The higher dose of rhNGF at 20 µg/mL concentration showed improvement of lacrimal function at each time point (*p<0.05). rhNGF, recombinant human nerve growth factor; TBUT, tear film break-up time.
bjophthalmol-2018-312470supp003.pdf (22.9KB, pdf)
A long-lasting effect of rhNGF eye drop treatments was observed at the follow-up visit (4 weeks after the end of treatment), as compared with baseline values. Specifically, a significant improvement of DED symptoms was detected by both SANDE (frequency: G1 25.2±26.5, p<0.0001 and G2 31.7±26.8, p<0.0001; severity: G1 23.9±28.1, p=0.0002 and G2 31.9±27.9, p=0.0003) and OSDI (G1 32.±21.4, p<0.0001; G2 37.7±20.1, p=0.0021) scales. Clinical signs of DED were also significantly improved during follow-up as shown by ocular surface staining with LG (NEI cornea total score G1: 1.2±1.6, p<0.0001 and G2: 3.1±2.9, p<0.0001 and NEI conjunctival total score G1 4.3±2, p<0.0001; G2 4.6±2.9, p=0.0004). Similarly, the improvement of tear function in G1 persisted during follow-up as demonstrated by Schirmer I test (8.7±10.2 mm/5 min, p=0.0428) and TFBUT (5.6±2.4 s, p=0.0032). Interestingly, the increase in corneal sensitivity values reached statistical significance at the final visit (G1 baseline: 5.6±0.91 mm, 56 days: 5.88±0.39 mm, p=0.0313, G2 baseline: 5.43±1.2 mm, 56 days: 5.93±0.18 mm, p=0.0391).
Comparison of clinical effects of the two rhNGF doses showed a significant improvement of NEI conjunctival total score at the end of treatment period (p=0.0012; 95% CI 1.0 to 5.0) and of TBUT values at both end of treatment period (4 weeks, p=0.0019; 95% CI −5.0 to −1) and follow-up (4 weeks of follow-up, p=0.0166; 95% CI 1 to 4) in G1 when compared with G2.
Discussion
This is the first study that demonstrates the safety and efficacy of topically applied rhNGF in patients with DED. Specifically, topical ophthalmic formulation of rhNGF at both 4 µg/mL and 20 µg/mL revealed no local or systemic safety concerns and showed good tolerability in patients with moderate to severe DED. The majority of AEs were mild in severity and resolved without treatment, and no patients were discontinued for AEs related to rhNGF eye drop treatment. Our results are in line with the safety results observed in the phase II clinical trials performed in patients with neurotrophic keratoconjunctivitis treated with rhNGF eye drop at concentrations of 20 µg/mL or 10 µg/mL for 8 weeks. Similar to our study, the results of this studies showed a good safety profile of treatment with rhNGF eye drop at 20 µg/mL, with eye pain being the most frequent treatment-related AE and no new AEs reported as compared with other studies.15–17
In this study, 4 weeks treatment with rhNGF eye drops at both 4 µg/mL and 20 µg/mL was effective in improving ocular surface damage and dry eye symptoms. These findings are supported by clinical and experimental evidence showing that NGF is able to stimulate corneal and conjunctival healing in vitro, in animal models of ocular diseases, as well as in patients affected by neurotrophic and autoimmune corneal ulcers.5–7 9–11 18 The involvement of NGF in patients with dry eye has been previously reported, since in this study tear levels of NGF were associated to the severity of the ocular surface damage.11 19 In fact, an increase of NGF levels represents a physiological response to tissue damage, in order to stimulate ocular surface epithelial healing.5 11 In addition, NGF exerts an antiapoptotic effect on human corneal cells, as demonstrated by Chang et al showing an upregulation of NGF in corneal epithelial cells under hyperosmolar stress, a typical pathogenic mechanism of dry eye.20 Experimental evidence also showed that topical mNGF administration in a dog model of dry eye was able to improve ocular surface healing and to increase tear production and conjunctival goblet cell density.9
In addition, our data indicate that rhNGF treatment at the higher concentration significantly increased tear film production and function. Specifically, the therapeutic effect on TBUT is in line with the in vitro studies showing that NGF induces an increase of human goblet cells density and stimulates production and release of Mucin-5AC, which regulates tear film stability (clinically assessed by TBUT).10 Our results also showed an increase of tear production, as demonstrated by the increase of Schirmer test values. This finding may be related with the well-known activity of NGF in increasing ocular surface sensitivity and stimulating the tear reflex.3
Our results showed that rhNGF eye drop treatment induced an improvement of both signs and symptoms in patients with DED observed at the first time point (1 week). This rapid effect may be related to the well-known mechanisms of action of NGF in stimulating epithelial healing and modulating nerve function.5 21
The long-lasting therapeutic effect of rhNGF observed in this study is an intriguing observation that is potentially explained by its typical neurotrophin-related activities. In fact, NGF exerts a well-characterised trophic, tropic and protective action on peripheral sensitive nerves, including the ocular surface trigeminal supply.1 2 In line with this, experimental and clinical studies clearly demonstrated the efficacy of NGF administration in restoring ocular surface sensitivity and corneal epithelial integrity in patients with neurotrophic keratopathy, a degenerative cornea disease caused by damage of corneal innervation.7 22 We can speculate that the long-lasting effect of rhNGF treatment in patients with dry eye could also be related to an improvement in corneal sensitivity, as demonstrated by Cochet-Bonnet evaluation, representing an additional mechanism in the regulation of tear production. Although Cochet-Bonnet esthesiometer assesses only mechanical sensitivity and it has some limitations, including a low reproducibility, our results suggest that local innervation may represent a novel therapeutic target to improve dryness in the eye, complained by more than 30% of the elderly population, and frequently associated with diabetes and autoimmune diseases.23 24 Further studies should be performed to evaluate changes of corneal nerves in terms of function and morphology during treatment with rhNGF eye drop in patients with DED.
Several limitations have to be considered when interpreting our results including the open-label study design, the low sample size and the lack of patients with Sjogren’s syndrome dry eye. In addition, we cannot exclude that the observed improvement of symptoms and signs after rhNGF eye drop treatment may be due, at least in part, to a placebo effect.25 The observation that rhNGF eye drop treatment at the higher concentration was significantly more effective in improving tear film stability (TBUT) and conjunctival damage when compared with the rhNGF treatment at lower concentration suggests a dose dependent rhNGF therapeutic effect. In conclusion, the results of this study demonstrate that topical rhNGF is safe and well tolerated in patients with moderate to severe DED. When available, results of recently completed randomised, controlled clinical trials on larger population of patients with DED will provide additional information on the efficacy of topical treatment with rhNGF on DED signs and symptoms (NCT03019627, NCT03035864).
Footnotes
Contributors: MS: study design, data analysis and interpretation, writing manuscript and final approval. AL: study design and conception, data interpretation, writing manuscript and final approval. DS: patients evaluation and data acquisition, manuscript revision and final approval. LS: patients evaluation and data acquisition, manuscript revision and final approval. MF: data analysis, study design and manuscript writing and final approval. FM: study design and conception, manuscript revision and final approval. MA: study design and manuscript revision and final approval. GG: study design and conception, data interpretation, manuscript revision and final approval.
Funding: The study was sponsored by Dompe Farmaceutici spa, Via Santa Lucia, 6, 20122 Milan, Italy.
Competing interests: MS and AL: Consultant—Dompé Farmaceutici SpA. MA, FM and MF: Employee—Dompé Farmaceutici SpA. MS, AL: report personal fees from Dompé Farmaceutici SpA, Italy, outside the submitted work. MA, MS, MF, GG: report personal fees from Dompe Farmaceutici spa, during the conduct of the study.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Levi-Montalcini R. The nerve growth factor 35 years later. Science 1987;237:1154–62. 10.1126/science.3306916 [DOI] [PubMed] [Google Scholar]
- 2. Lambiase A, Mantelli F, Sacchetti M, et al. Clinical applications of NGF in ocular diseases. Arch Ital Biol 2011;149:283–92. 10.4449/aib.v149i2.1363 [DOI] [PubMed] [Google Scholar]
- 3. Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol 2012;23:296–302. 10.1097/ICU.0b013e3283543b61 [DOI] [PubMed] [Google Scholar]
- 4. Bonini S, Aloe L, Bonini S, et al. Nerve growth factor (NGF): an important molecule for trophism and healing of the ocular surface. Adv Exp Med Biol 2002;506:531–7. [DOI] [PubMed] [Google Scholar]
- 5. Lambiase A, Manni L, Bonini S, et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci 2000;41:1063–9. [PubMed] [Google Scholar]
- 6. Bonini S, Lambiase A, Rama P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000;107:1347–51. discussion 51-2 10.1016/S0161-6420(00)00163-9 [DOI] [PubMed] [Google Scholar]
- 7. Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med 1998;338:1174–80. 10.1056/NEJM199804233381702 [DOI] [PubMed] [Google Scholar]
- 8. Lambiase A, Bonini S, Aloe L, et al. Anti-inflammatory and healing properties of nerve growth factor in immune corneal ulcers with stromal melting. Arch Ophthalmol 2000;118:1446–9. 10.1001/archopht.118.10.1446 [DOI] [PubMed] [Google Scholar]
- 9. Coassin M, Lambiase A, Costa N, et al. Efficacy of topical nerve growth factor treatment in dogs affected by dry eye. Graefes Arch Clin Exp Ophthalmol 2005;243:151–5. 10.1007/s00417-004-0955-2 [DOI] [PubMed] [Google Scholar]
- 10. Lambiase A, Micera A, Pellegrini G, et al. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Invest Ophthalmol Vis Sci 2009;50:4622–30. 10.1167/iovs.08-2716 [DOI] [PubMed] [Google Scholar]
- 11. Lambiase A, Micera A, Sacchetti M, et al. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol 2011;129:981–6. 10.1001/archophthalmol.2011.200 [DOI] [PubMed] [Google Scholar]
- 12. Management and therapy of dry eye disease: report of the management and therapy Subcommittee of the International dry eye workshop (2007). Ocul Surf 2007;5:163–78. 10.1016/S1542-0124(12)70085-X [DOI] [PubMed] [Google Scholar]
- 13. Ferrari MP, Mantelli F, Sacchetti M, et al. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs 2014;28:275–83. 10.1007/s40259-013-0079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The definition and classification of dry eye disease: report of the definition and classification Subcommittee of the International dry eye workshop (2007). Ocul Surf 2007;5:75–92. 10.1016/S1542-0124(12)70081-2 [DOI] [PubMed] [Google Scholar]
- 15. Bonini S, Lambiase A, Rama P, et al. Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology 2018;125:1468–71. 10.1016/j.ophtha.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 16. Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, Vehicle-Controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology 2018;125:1332–43. 10.1016/j.ophtha.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 17. Sacchetti M, Bruscolini A, Lambiase A. Cenegermin for the treatment of neurotrophic keratitis. Drugs Today 2017;53:585–95. 10.1358/dot.2017.53.11.2722395 [DOI] [PubMed] [Google Scholar]
- 18. You L, Kruse FE, Völcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci 2000;41:692–702. [PubMed] [Google Scholar]
- 19. Lee HK, Ryu IH, Seo KY, et al. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 2006;113:198–205. 10.1016/j.ophtha.2005.09.033 [DOI] [PubMed] [Google Scholar]
- 20. Chang E-J, Im YS, Kay EP, et al. The role of nerve growth factor in hyperosmolar stress induced apoptosis. J Cell Physiol 2008;216:69–77. 10.1002/jcp.21377 [DOI] [PubMed] [Google Scholar]
- 21. Sacchetti M, Lambiase A. Neurotrophic factors and corneal nerve regeneration. Neural Regen Res 2017;12:1220–4. 10.4103/1673-5374.213534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mastropasqua L, Massaro-Giordano G, Nubile M, et al. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol 2017;232:717–24. 10.1002/jcp.25623 [DOI] [PubMed] [Google Scholar]
- 23. Baer AN, Walitt B. Sjögren syndrome and other causes of sicca in older adults. Clin Geriatr Med 2017;33:87–103. 10.1016/j.cger.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Exp Eye Res 2011;92:408–13. 10.1016/j.exer.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 25. Design and conduct of clinical trials: report of the clinical Trials Subcommittee of the International dry eye workshop (2007). Ocul Surf 2007;5:153–62. 10.1016/S1542-0124(12)70084-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2018-312470supp001.pdf (114.7KB, pdf)
bjophthalmol-2018-312470supp002.pdf (37.7KB, pdf)
bjophthalmol-2018-312470supp003.pdf (22.9KB, pdf)