Abstract
Singapore is one of the fastest-aging populations due to increased life expectancy and lowered fertility. Lifestyle changes increase the burden of chronic diseases and disability. These have important implications for social protection systems. The goal of this paper is to model future functional disability and healthcare expenditures based on current trends.
To project the health, disability and hospitalization spending of future elders, we adapted the Future Elderly Model (FEM) to Singapore. The FEM is a dynamic Markov microsimulation model developed in the US. Our main source of population data was the Singapore Chinese Health Study (SCHS) consisting of 63,000 respondents followed up over three waves from 1993 to 2010. The FEM model enables us to investigate the effects of disability compounded over the lifecycle and hospitalization spending, while adjusting for competing risk of multi-comorbidities.
Results indicate that by 2050, 1 in 6 elders in Singapore will have at least one ADL disability and 1 in 3 elders will have at least one IADL disability, an increase from 1 in 12 elders and 1 in 5 elders respectively in 2014. The highest prevalence of functional disability will be in those aged 85 years and above. Lifetime hospitalization spending of elders aged 55 and above is US$24,400 (30.2%) higher among people with functional disability compared to those without disability.
Policies that successfully tackle diabetes and promote healthy living may reduce or delay the onset of disability, leading to potential saving. In addition, further technological improvements may reduce the financial burden of disability.
Keywords: Disability, Ageing, Microsimulation, Hospitalization, Spending
1. Introduction
Population aging is expected to be one of the most disruptive social transformations of the 21st century.[1] Globally, changes in age composition have important implications for functional disability and healthcare financing. With the world’s population aging rapidly, many elderly persons are at risk of disability, resulting in increased demand for healthcare services and higher medical spending.[2–4] Healthcare expenditure among the elderly is rising, with a significant driver being the economic burden of disability and long-term care.[5–8] In Taiwan, for instance the government reported a 7% increase in the prevalence of disabilities among older adults (from 29.6% in 1997 to 36.6% in 2010).[9] In addition, the rate of hospitalization for disabled elders was found to be 3.5 times of the general population in Taiwan during the year 2005.[10] In Japan, medical spending were 4 times higher in older men and 3 times more in older women with disabilities in performing self-care, compared to their respective peers with no functional limitations.[11] In contrast, in the United States, the spending of medical coverage for disability declined from 1982 to 2004, due to the trend of transferring severely impaired individuals to palliative institutions.[12, 13].
Sound policy planning for aging is critical because of the severe social risks and fiscal strain it can otherwise impose on nations.[1] Modelling is a critical tool to support such planning, allowing policymakers to forecast better and potentially manage the consequences of changes in population demography, including future comorbidities and disability, and healthcare spending and appropriately adjusting for multiple risk factors. While many studies have associated population aging with increasing chronic disease burden and functional disability with a higher incidence of mortality,[14–17] few studies have accounted for population and health dynamics which include demographic change, competing risks, and background mortality.[18–21] In addition, while many studies have found an association between disability and spending, few have examined the long-term hospitalization spending of disability. Retrospective studies detail how disabled elders incur substantially higher spending and utilize more healthcare resources. [8, 22–24] For example, Picco et al illustrated how functional disability would cost an individual more in social care notwithstanding comorbidities. [5, 8, 22–24].
A robust approach to policy analysis related to aging and disability and its associated financial burden is particularly important in Asia, where over the next 15 years, the number of elderly persons is expected to grow by 66%.[1] Singapore has one of the world’s highest life expectancies and has aged ahead of many societies. Singapore has been projected to take only 27 years to transition from an ‘ageing society’ in 1999 (7% seniors) to a ‘super-aged society’ (20% seniors) in 2026, beating Japan, China, Germany and the United States, which took or will take 36, 32, 76 and 86 years to make that transition respectively.[25, 26] Healthcare expenditure has already quadrupled within ten years from S$2 billion (US$1.5 billion) in 2006 to S$8.5 billion (US$6.4 billion) in 2015,[27] and is expected to rise by at least another S$3 billion (US$2.3 billion) in the next three to five years. [28]
This paper reports a dynamic Markov microsimulation model with Singapore-specific disease prevalence, sociodemographic covariates and transition probabilities, which we use to forecast disability prevalence in Singapore. Our model deviates from previous work in forecasting disability prevalence as we account for individual level heterogeneity. Preexisting microsimulation models may not have accounted for health status as a predictor of disability. [29] They may also have utilized cell-based microsimulation approaches which lose individual-level heterogeneity in functional disability transitions.[30] Our results suggest that disability prevalence is expected to rise in the future, and disabled individuals incur a far larger lifetime inpatient hospitalization spending compared to individuals who are not disabled. These results are put into the context of Singapore and used as a tool to aid policymaking in terms of healthcare delivery and healthcare financing.
2. Methods
2.1. Data
The Singapore Chinese Health Study (SCHS) is a prospective cohort study of ethnic Chinese men and women aged 45–74 years at baseline who were followed up for a mean duration of 12 years. Inclusion criteria were either citizens or permanent residents, residing in public housing estates and belonging to either of the two major dialect groups of Chinese, namely Hokkien and Cantonese. The baseline study (n=63,257) was conducted between 1993 and 1999, follow-up 1 (n=52,325) was collected between 1999 and 2004 and follow-up 2 (n=39,528) was collected between 2006 and 2010. At baseline, each participant completed an in-person interview at their home using a structured questionnaire that requested information about demographic characteristics, self-reported height and weight, smoking status, current physical activity, occupational exposure, medical history and family history of cancer. A follow-up telephone interview asked participants for an update on their tobacco and alcohol use as well as medical history. SCHS data was used to determine the transition probabilities from positive health status to a disease state.
The Mediclaims dataset contains records of individual-level acute care inpatient hospitalization and day surgery expenditures before any government subsidy within both public and private hospitals. This information includes patients’ gender, ethnicity, birthdate, date of admission, date of discharge, diagnosed diseases, medical spending and insurance claims. Reported medical spending is based on total resources used and is not broken down by contributions of other payers, including Medisave, MediShield, Medifund and private insurers. The actual share of individual out-of-pocket expenditure will be lower after accounting for payments by these different payers. The SCHS was linked with hospitalization spending from Mediclaims through participants’ ID and harmonized across both data sets.
The Singapore Longitudinal Aging Study (SLAS) is a smaller cohort that consists of 2,804 subjects aged 55 or above interviewed in 2004/2005, 2007/2008 and 2010/2011. Older adults who were citizens or permanent residents aged 55 years or above were identified by door-to-door census and invited to participate voluntarily in the study. Another cohort, the Panel on Health and Ageing of Singaporean Elderly (PHASE) consisted of 4,990 elders aged 60 or above who were interviewed in 2009 and 2011/2012. All respondents consisted of citizens chosen through single-stage stratified random sampling from a national database of dwellings and were interviewed face-to-face at their residences. SLAS and PHASE were incorporated into our projections as they provide additional information on other dimensions of health and well-being not covered by the SCHS, such as psychosocial metrics, questions on functional disability that can be used to construct indices of activities of daily living (ADL) and instrumental activities of daily living (IADL) disability prevalence among the elderly, quality of life measures and other diseases.
The Singapore National Health Survey (NHS) provides information on the prevalence of major non-communicable diseases such as diabetes mellitus, hypertension and related risk factors like obesity and smoking in four periods from 1992 to 2010. [31] It is conducted once every six years on a cross-section of individuals aged 18 to 74 years.
2.2. Microsimulation model
The Future Elderly Model (FEM) microsimulation model includes several components and the structure of the model is almost entirely based on population dynamics. We adapted the FEM ─ a dynamic Markov microsimulation model that predicts future health status and hospitalization spending for Singapore Chinese, which constitute the majority of residents at 74.3% (Malays were 14%, Indians were 9%, Others were 3%). [32] It was first developed by RAND Corporation to support decision-making related to Medicare and Medicaid, the public health insurance and welfare programs for the elderly/needy in the United States. Since then, the FEM has been used for studies exploring health and social issues in ageing societies in several countries including the United States, European Union and, Japan.[33–35] Its ongoing development is supported by the National Institute on Aging through the USC Roybal Center for Health Policy Simulation. Key features of this microsimulation model are: (a) projecting individual level cohorts from observed data with diverse characteristics and behaviours; (b) projecting those individuals over time to estimate variation in outcomes within populations; and (c) updating populations which enter the microsimulation to reflect future socio-demographic characteristics and health status. We further developed this model to project IADL and ADL disability prevalence and the acute care inpatient hospitalization spending disparity between elders with and without ADL disability in Singapore.
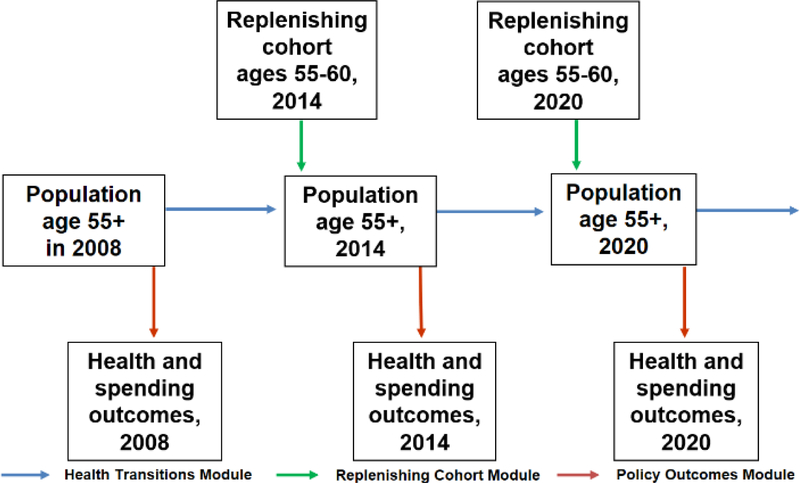
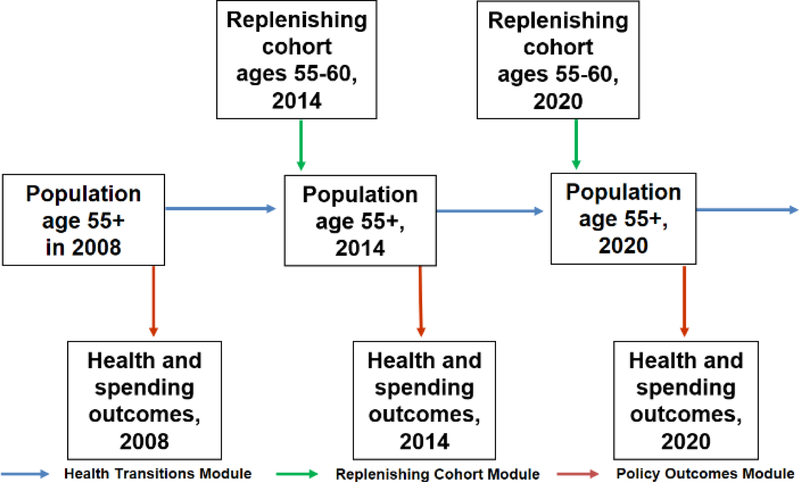
The model tracks elders characterized by socioeconomic drivers and health states who transition from one period to another (Figure 1). In each period, new elders enter the simulation at the default starting age, which we set as 55 to 60 years old with their composition adjusted using sociodemographic and disease trends in NHS. Population projections were based on the demographic epidemiological model of Singapore. [36] In each simulation cycle, the population moves forward in time and we can observe their health status as well as their healthcare utilization and spending. It loses some elders due to mortality and gains some because there is renewal, i.e., a new cohort of individuals aged 55 to 60 years old enters the model as mentioned above. We assume that there is no gain due to migration in the elderly. The model then integrates data from the SCHS with hospitalization spending data from Ministry of Health to project future healthcare spending.
Figure 1. FEM simulation.
2.3. Health transition and spending model
To project health transitions, a discrete piecewise linear hazard model was estimated from the SCHS based on six-year transitions. The hazard of transitioning to an absorbing disease state (hypertension, diabetes, heart disease, stroke) and dying depends on risk factors (gender, highest attained education, obesity, smoking status); other conditions if medically warranted; functional status; and age. We used probit regression to estimate the probability of transition to each health condition, controlling for demographic variables and comorbidities at the previous period. We treated all diseases as absorbing in reflection of the question asked: “Have you ever been told by a doctor…”. The unit of observation for modelling transition probabilities was the interview-pair. All independent variables were measured with a six-year lag in the SCHS, and represent the respondent’s characteristics from 1993 to 2010.[37] As diseases were treated as absorbing state, we assumed that elders with the chronic condition (e.g. diabetes) in the previous wave will continue to have diabetes in the current wave. As such, transition probabilities were estimated on elders who did not suffer from a specific condition at the previous survey wave.
Covariates such as BMI were log transformed to adjust for high peaks in BMI measurements, while age was splined into the 55 to 70, 70 to 84 and above 85 groups to account for the differential effect that the age groups have on comorbidities. Education was modelled according to the Singaporean schooling system, with low attainment defined as having primary education or less (less than 6 years of education), middle attainment as having secondary school education or technical education (6 to 12 years of education) and high attainment as having at least a diploma or college education (at least 13 years of education).
2.4. Disability model
The disability model is a cross-sectional model which uses current chronic diseases and socio-demographic covariates to model the burden of disability using data from SLAS and PHASE. We defined disability as having any ADL disability such as washing, dressing, feeding, toileting, mobility and transferring [38] or having any IADL disability such as taking transportation, shopping, managing money, making phone calls, doing household chores and meal preparation. The former measures an individual’s physical ability to perform basic tasks whereas the latter are higher order tasks which measure an individual’s engagement and management of resources. We projected future functional disability through two models with ADL and IADL disability as the dependent variable using probit regression. The covariates included were age, gender, education attainment, BMI, marital status and chronic diseases.
2.5. Spending model
We linked SCHS data with elders’ demographic information, current reported health status, risk factors and functional status with annualized hospitalization spending based on Mediclaims data, and predicted healthcare spending using ordinary least squares (OLS) regression. Nominal expenditure (in Singapore dollars) was converted to real expenditure using the medical component of the consumer price index (with base year of 2014) from Singapore Department of Statistics (DOS). The nominal expenditure was the total healthcare expenditure before government subsidies. In addition, the regression adjusted for gender, educational attainment, obesity, smoking status and self-reported conditions. A robustness check was conducted using a two-part model, which first predicts who will likely incur spending, and subsequently predicts the spending in a regression conditional on the earlier likelihood. Given that the estimates of the models were similar, we chose to run OLS on the grounds of parsimony. We then apply the FEM on the initial cohort of individuals, whom were born before 1953, to estimate the longitudinal inpatient hospitalization spending due to disability conditional on other comorbidities. We report the lifetime hospitalization spending of elders by tracking our initial cohort of individuals incurs from 2014 to 2060 using the model above. All spending is expressed in 2014 US Dollars.
3. Results
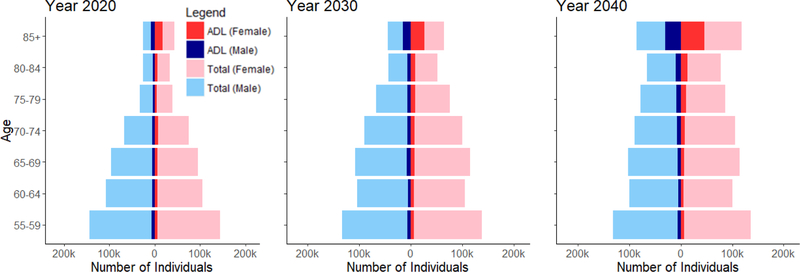
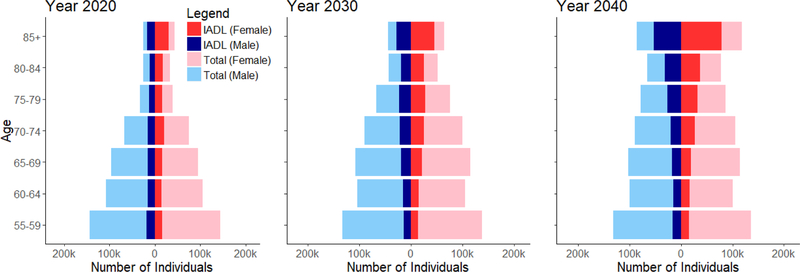
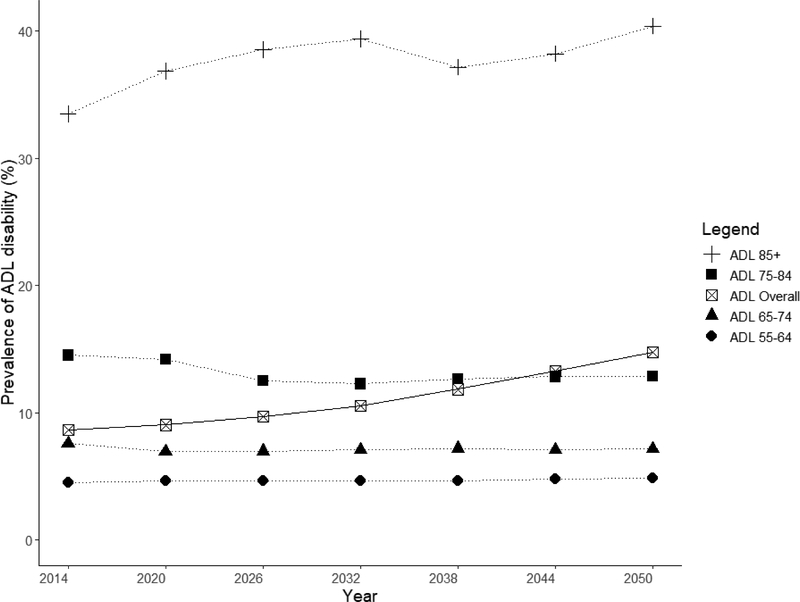
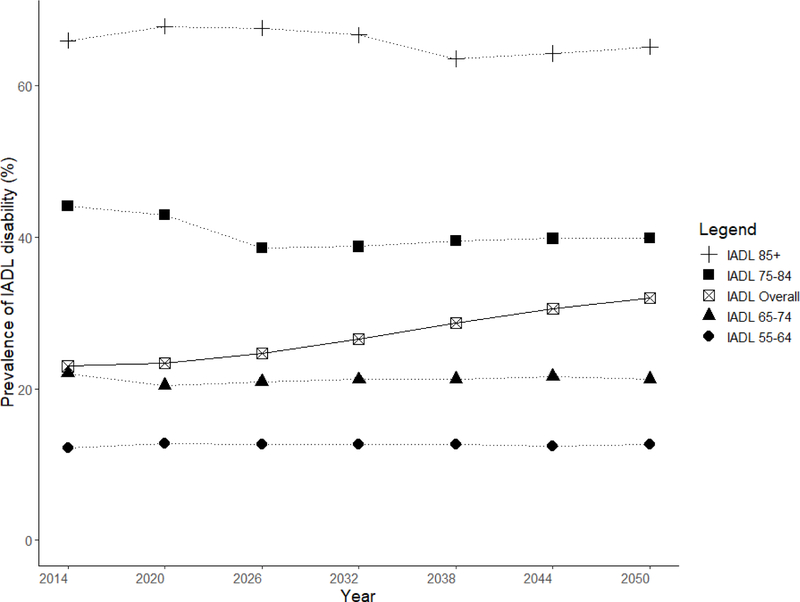
Overall, Singapore is projected to be a top-heavy society with increasing numbers of ADL and IADL disability, especially in the oldest elders, aged 85 years and above. Projected IADL disabilities were more prevalent than ADL disabilities from 2020 to 2050. Both ADL and IADL disabilities were more prevalent in females than males. This difference is accentuated in the oldest-old group, where the projected prevalence of functional disability was much higher in females than males (Figure 2a and 2b).
Figure 2a. ADL disability breakdowns by gender and year.
Figure 2b. IADL disability breakdowns by gender and year.
ADL: Activities of daily living
IADL: Instrumental activities of daily living
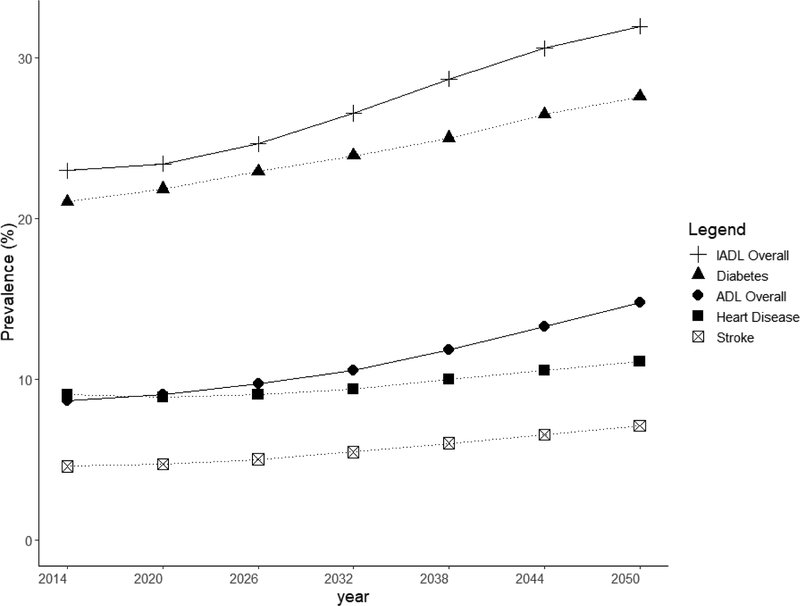
We projected an increasing prevalence in chronic disease burden from 2014 to 2050. Heart disease prevalence increased from 9 to 11%, diabetes prevalence increased from 21% to 28%, and stroke prevalence increased from 5% to 7%. ADL disability prevalence was projected to increase from 9% to 15% and IADL disability prevalence was projected to increase from 23% and 32% respectively (Figure 3).
Figure 3. Projections of disease prevalence in general population aged more than 55 years old.
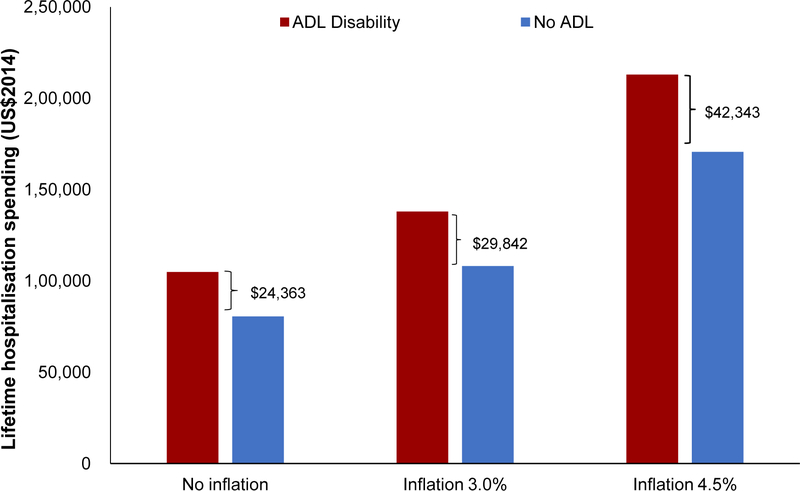
The proportion of elders having at least one ADL disabilities was 8.6% in 2014 and this was projected to double to 14.7% in 2050. The steepest rise in ADL disability was among the oldest age group of elders aged 85 and above, among whom prevalence was projected to increase from 33.5% in 2014 to 40.4% in 2050. There was little projected increase in ADL disability from 2014 to 2050 in younger age groups, with disability prevalence being around 4.5% for age 55 to 64, 7% for age 65 to 74 and 13% for age 75 to 84 years (Figure 4). In 2014, prevalence of any IADL disabilities was 22.9%; this was projected to increase to 31.9% in 2050. There was no significant projected increase in disability among the age groups of 55–64, 65–74, 75–84 and above 85 years old, with a prevalence of 12%, 21%, 41% and 65%, respectively in 2050 (Figure 5). The decline in prevalence of ADL and IADL after year 2026 among elders aged 75 to 84 was due better education of the baby boomer generation compared to earlier cohorts. Thus, they were less likely to develop chronic diseases and disability. Although the disability prevalence within each individual age group did not reflect increasing trends, the increase in overall disability and chronic diseases prevalence in Figure 3 were driven mainly due to an aging population. Lifetime hospitalization spending is higher among elders with disability. We projected the elderly lifetime hospitalization spending for elders aged 55 and above with ADL disability was US$24,363 (30.3%) higher compared to those without disability (US$104,883 for those with disability and US$80,520 for elders without disability), assuming no medical inflation. Next, we assumed two sensitivity scenarios: (a) an average annualized inflation rate of 3.0% based on CPI for medical treatment in the last five years [39] with 2% discounting and (b) a high inflation rate of 4.5% with 2% discounting. We projected a difference in hospitalization spending of US$29,842 and US$42,343 respectively, comparing elders with and without ADL disability. (Figure 6) The increased hospitalization spending for ADL disability was due to the underlying manifestation of chronic diseases such as stroke, diabetes and heart disease. We were unable to model the lifetime hospitalization spending from IADL disability as our spending model focuses on physical disability from the underlying manifestation of chronic diseases.
Figure 4. Prevalence of one or more ADL disability in elderly by age.
Figure 5. Prevalence of one or more IADL disability in elderly by age.
Figure 6. Lifetime hospitalization spending1 of elders aged 55 and above, by ADL disability.
1Lifetime hospitalization spending is computed by tracking the hospitalization spending the initial cohort of individuals incur from 2014 to 2060.
Females were projected to have a higher prevalence of both ADL and IADL disability than males. For ADL disability, females had 2.0% (9.6% for females and 7.6% for males) higher prevalence than males in 2014, a gap that was projected to increase to 3.0% (16.1% for females and 13.1% for males) in 2050. Our projection of IADL disability prevalence by gender shows similar disparities. In 2014, we observed prevalence rates of 20.8% and 25.1% for males and females respectively; these were projected to increase to 30.3% and 33.3% in 2050.
4. Discussion
We estimate that by 2050, 1 in 6 elders in Singapore will have at least one ADL disability and 1 in 3 elders will have at least one IADL disability, an increase from 1 in 12 elders with ADL disability and 1 in 5 elders with IADL disability in 2014. The projected prevalence of ADL and IADL disability by 2050 among the oldest-old are 46% and 68% respectively, an increase in disability prevalence due to Singapore’s aging demography and related comorbidities. These projected trends are consistent with other studies that have found functional disability to be associated with comorbidities [40–42], and an increased prevalence of disability in the oldest-old. They also utilize more healthcare resources such as ambulatory healthcare services, outpatient, emergency and inpatient services and have longer inpatient stays. [4, 43, 44] For example, in China, IADL prevalence was consistently higher than ADL prevalence from 1998 to 2008. The prevalence of having any IADL disability was 30.1% while having any ADL disability was 14.9% in 2008.[45] In Spain, the prevalence for having any IADL disability was 53.5% and any ADL disability was 34.6%. [46] In the U.S, the percentage of elders aged 75 and above with any IADL disability was 19.2% versus any ADL disability was 10.0%. In addition, people in each age group were approximately twice as likely to require help with IADLs as with ADLs. [47] The overall findings echo existing international microsimulation work from Singapore, Japan and Australia that also predict sharp increases in disability prevalence driven by population aging. [29, 48–50]
While women are having longer life expectancy compared to men, they continue to experience inferior health outcomes. This increased longevity of women has significant implications for women living alone as they have outlived their spouses, with potentially less resources and support. Frailty, falls, fracture and disability are likely to be more prevalent among older women.[51] This is further compounded by the fact that women are physiologically more likely to suffer from chronic conditions. The probability of being diagnosed with breast cancer, the most common cancer in women, also increases by 40% in the elderly. Moreover, the osteoporosis is more likely to develop in women after menopause.[52] These result in women facing a diminished quality of life as compared to men. It is a general phenomenon that ADL is more prevalent in females than in males. In the U.S., the total risk of incident disability rates was higher for females compared to males in the following activities: walking, bathing, transferring, dressing, toileting and feeding disabilities.[53] In Japan, a comparative study of ADL dependence in residential care home and community-dwelling elderly showed that the female gender was statistically significant predictor for ADL dependence with an odds ratio (OR)=2.55 (p-value = 0.004) for females compared to males in residential care and OR=1.67 (p-value = 0.001) in the community.[54] The higher reported prevalence of ADL in females can be attributed to physical risk factors more prevalent in women (chronic conditions, less exercise, less outside activity etc.)[55], as well as psychological factors: women may be more likely to report their disability as compared to men (overall 52% vs 37% (in the U.S.), p-value<0.001).[56] It benefits the society both economically and socially, when older women are functionally independent, productive and healthy – as these disabilities create an additional burden of expenses for assistive devices, hospitalization and long-term care.[57] It is also possible that women and men age differently, and may have different health care needs and utilization, making a gendered perspective towards aging appropriate for future research.
The Singapore government has been relatively successful at controlling healthcare spending. As part of their commitment to universal health coverage but sensitive to the changing demand and supply of healthcare, the government has recently introduced lifetime catastrophic insurance coverage under Medishield as well as an expanded long-term care insurance scheme, while also introducing new initiatives to address concerns with over-consumption, over-servicing and over-charging. These include the elimination of private insurance full-rider policies, which previously covered individual co-payments,[58] in order to reduce moral hazard as well as potential supplier-induced demand, which may in turn curb medical inflation. In addition to the review of private insurance plans, the Ministry of Health has also published fee benchmarks for professional fees to guide private sector healthcare providers in charging appropriately and is working to educate patients on the use of these benchmarks to make better informed decisions.[59, 60]
The implications are especially important for policymakers both in Singapore and globally. It reinforces the need to plan for the future burden due to aging, which is the strongest driver of disability and comorbidities. Healthcare policies that may alter the trajectory of disability and comorbidities are crucial. In Singapore, the government has shifted from secondary to primary prevention, focusing on health promotion and health programs. Concerted screening of chronic conditions like hypertension within clinical institutions, progressive taxation to restrict smoking uptake and purchase [61], prohibition of smoking in designated public areas, [62] as well as a recent policy on combating diabetes have been enacted in hopes of preventing or delay the onset of chronic conditions highly associated with disability. [63] Technological improvements such as assistive devices may reduce self-reported prevalence and burden, by allowing elders who would previously experience disabilities to lead normal lives [12]. Finally, factors such as higher education, social support networks and engagement in leisure time activities can promote the maintenance of function [64] and alleviate mortality for disabled elders [65]. This suggests that policies that address social disadvantage and isolation directly, or target more socially-vulnerable groups also have a role to play. [66] The policy scenarios explored above, as well as other potential longer-term changes in financing and service delivery models, will be further investigated with our Singapore Future Elderly Model in future research, as more data on specific financing mechanisms become available. Our current work however provides the first concrete step towards a policy discussion on the fiscal sustainability of healthcare financing in Singapore and provides a novel contribution to aging policy and research in the broader region.
In our study, we also projected a 30.2% higher elderly lifetime hospitalization spending for individuals with disabilities. Our spending projection is an underestimate compared to other studies as long-term care spending were not considered. Coupled with the expected increase in ADL/IADL disability prevalence in elderly and life expectancy, the need for long-term healthcare is very likely to increase steeply in the future. In light of the increasing demand for healthcare, Singapore has plans to extend current disability insurance from six years to lifetime coverage to protect individuals with severe disability against the financial risk of ADL disabilities. There are proposals for the implementation of universal disability insurance (“CareShield Life”) [67] and subsidies for hired help to facilitate elderly caregiving. [68]
4.1. Limitations
Modeling in this complex environment necessarily reflects only best available information, and is subject to limitations. There are several limitations regarding our projections of functional disability and hospitalization spending incurred by these groups. In 2010 to 2015, Singapore has an ethnic composition of around 74% Chinese, 14% Malays, 9% Indians and 3% of other races. [32] The SCHS contains data only for the Chinese, thus medical spending for ethnic minorities such as the Malays and Indians are not included. As a result, our disability projections may be an underestimate as these minority groups have been shown to bear a greater chronic disease burden. [69–72]
The model also assumes that the current health care delivery model continues into the future. In addition, current treatments will also continue and FEM does not take into account technological innovations or care transformation, and does not account for improvements in future health states due to screening, which may shift the demand to primary care and induce cost-savings in inpatient hospitalization spending. We did not model migration as current migration policy emphasizes on temporary rather than long-term migration. We were also unable to model shorter disease dynamics as our transitional probabilities were estimated based on the survey with six-year median follow-up. Due to small numbers, we were also unable to model severity of disability after adjusting for individual heterogeneity.
Studies also have documented the large indirect burden of disability on productivity loss in work, as well as changes in standard of living due to spending required to alleviate disability itself. [73–75] Our analysis omits these, and hence, our spending estimates are likely to be an underestimate of the true burden of disability on the Singaporean Chinese elderly population as we have only considered hospitalization spending projections in the FEM simulations. Further work on spending projections should account for these latent dynamics which underlie the burden of disability. In addition, we aim to evaluate the long-term impact of health policies when preliminary evidence is available. Regular future updates to the model and subsequent policy reviews are an important part of ensuring the continued value and relevance of the model and its application.
4.2. Conclusion
This is the first study in Singapore that simultaneously models three dimensions (i.e. economic, health and demographic) to provides a comprehensive understanding of disability and hospitalization spending. The FEM’s approach allows for this multi-dimensional characterization of health status using a life course dynamic microsimulation model to project the elderly disability prevalence and lifetime hospitalization spending. To the best of the authors’ knowledge, no lifetime hospitalization spending projections for disabled elders has been done in Asia, and this provides both a foundation for policies to improve the fiscal sustainability of healthcare financing in Singapore and a novel contribution to aging policy and research in the broader region.
Acknowledgements
We thank Kenwin Maung, Sucitro Sidharta and Chia Jia Hui for the helpful discussions and statistical support that have contributed to this work.
Funding
The research was supported by the National Medical Research Council HSRG-0077/2017 and the National Institute On Aging of the National Institutes of Health under Award Numbers P30AG024968, R03AG054120 and R01AG055401. The Singapore Chinese Health Study was supported by the US NIH R01CA144034 and UM1CA182876. Koh WP was supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013). Waves 1 and 2 of the Panel on Health and Ageing among Singaporean Elderly (PHASE) were funded or supported by the following sources: Ministry of Social and Family Development, Singapore; and Singapore Ministry of Health’s National Medical Research Council under its Singapore Translational Research Investigator Award “Establishing a Practical and Theoretical Foundation for Comprehensive and Integrated Community, Policy and Academic Efforts to Improve Dementia Care in Singapore” (NMRC-STAR-0005–2009). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- ADL
Activities of Daily Living
- DOS
Singapore Department of Statistics
- FEM
Future Elderly Model
- IADL
Instrumental Activities of Daily Living
- NHS
National Health Survey
- PHASE
Panel on Health and Ageing of Singaporean Elderly
- SCHS
Singapore Chinese Health Study
- SLAS
Singapore Longitudinal Aging Study
Appendix: Singapore FEM Technical Documentation
1. Functioning of the dynamic model
1.1. Background
The Future Elderly Model (FEM) is a microsimulation model originally developed to examine health and healthcare spending among the elderly Medicare population. The model structure almost entirely based on the population dynamics. The FEM was first developed to support decision making related to Medicare and Medicaid, the public health insurance and welfare programs for the elderly in the United States and now it has been successfully implemented internationally. The original development of FEM is led by the USC Roybal Center for Health Policy Simulation, with collaborators from Harvard University, Stanford University, RAND Corporation, University of Michigan, and University of Pennsylvania. A description of the original FEM model is available in Goldman et al. (2004).
We adapted the FEM, a dynamic Markov microsimulation model, to predict future health status and medical spending for the Singaporean population. Data from the Singapore Chinese Health Study (SCHS), the Singapore Longitudinal Ageing Study (SLAS), the National Health Survey (NHS), the Demographic Epidemiological Model of Singapore (DEMOS), the Singapore Multi-Ethnic Cohort Study (MEC) as well as Mediclaims data were integrated into FEM framework. The model then simulates individual health states such as the chronic conditions hypertension, diabetes, heart attack and stroke, and associated expenditures for the elderly population that evolves over time. It also accounts for Singapore-specific trends in covariates which drive health status such as ageing, obesity, diseases and disability.
The model tracks individuals characterized by socioeconomic drivers and health states which exhibit transitions from one period to another. In each period, new individuals enter the simulation at a prespecified starting age, which we set as 55 to 60 years old. Their life in the simulation model ends at their time of death. In each replication of the simulation, the population moves forward in time with their health status, healthcare utilization and spending tracked. It loses individuals due to mortality and gains some because of incoming individuals, i.e., when a new cohort of individuals with the age of 55 years (or 55 to 60 years when using 6-year simulation cycles) enters the model. FEM-Singapore assumed that there are no incoming individuals due to migration in the elderly. The technical documentation provides an overview for the data used for the FEM-Singapore, as well as modifications and new development efforts to adapt FEM into the Singapore context.
1.2. Overview
The defining characteristic of the model is the modeling of real rather than synthetic cohorts, all of whom are followed at the individual level. This allows for more heterogeneity in behavior than otherwise would be allowed by a cell-based approach. The omission of the population younger than age 55 sacrifices little generality, since the bulk of expenditure on the public programs we consider occurs after age 55.
The model has three core components for the simulation cycles:
The initial cohort module predicts the economic and health outcomes of base cohorts utilized in the simulation. This module takes the input from the data of Singapore Chinese Health Study (SCHS) and Singapore Multi-Ethnic Cohort Study (MEC) with financial spending data from Mediclaims, along with other sources detailed in section 2. It allows us to generate cohorts as the simulation proceeds This facilitates measurements of outcomes for the population with age 55 and above during the projection period.
The transition module calculates the probabilities of transiting across various health states and financial outcomes. The module takes different risk factors such as smoking, weight, BMI values, age, education levels and lagged health status. This allows for a great deal of heterogeneity and fairly general feedback effects. The transition probabilities are estimated from the longitudinal data from the SCHS and the healthcare utilization data from Mediclaims.
The policy outcomes module aggregates projections of individual-level outcomes into policy-related outcomes such as healthcare spending, pension benefits paid, third party private insurance benefits and disability benefits. This component takes account of public and private program rules to the extent allowed by the available outcomes. In addition, different levels of reduction to disease prevalence are empirically applied to reflect the effectiveness of government intervention in FEM simulations. The corresponding outcomes which include healthcare utilization and hospitalization spending were then compared among different scenarios to investigate the effectiveness in terms of policy outcomes.
Appendix Figure 1 provides a schematic overview of the model. The FEM-Singapore simulation starts in 2008 with an initial population aged 55 and above obtained from the SCHS. We then predict outcomes using our estimation for the initial cohort (see section 4.1) to initialize the simulation. Individuals who survive are defined as those who are still living at the end of the corresponding year. Projections on policy outcomes are then made. Thereafter, the FEM moves to the following time period within the simulation cycle, when a new cohort of 55 to 60 years old enters. The current cohort and the replenishing cohort form the new population with age of 55 and above, which then proceeds through the transition module as before. The replenishing cohort enters the simulation every six years. This process of replenishing new cohorts and transiting across various health states is repeated until we reach the final year of the simulation. Each individual module is explained in more detail in Estimation, within section 4.
Appendix Figure 1. Schematic overview of FEM simulation model.
2. Data sources used for estimation
The Singapore Chinese Health Study (SCHS) is the main data source used for FEM-Singapore. In order to facilitate the running of FEM-Singapore simulations, we augment it with additional data on health trends, healthcare utilization, inpatient spending from major health surveys in Singapore. We describe these health surveys below and the samples selected for the analysis.
2.1. Singapore Chinese Health Study (SCHS)
The data requirement of the initial cohort and transition module is best met by the Singapore Chinese Health Study (SCHS) which provides a detailed characterization on the evolution of disease and risk factors in a large age-appropriate sample over a sufficiently long period of time.
The SCHS is a population-based prospective cohort study with a large array of questions about demographics, health status and behaviors and medical history drawn from ethnically Chinese citizens or permanent residents of Singapore (77% of the population) residing in public housing estates where 85% of the Singaporean population reside. 63257 subjects with the age between 45 and 74 were recruited between February 1993 and January 1999, and they were re-visited and followed up in two waves for the periods of 1999 to 2004 and 2006 to 2010, respectively. Recruitment to the study entailed an initial letter that informed potential participants of the study and an invitation for them to participate. Approximately 5–7 days later, a door-to-door invitation was given; around 85% of eligible subjects who were invited responded positively. At recruitment, a face-to-face interview was conducted in the subject’s home by a trained interviewer with the use of a structured, scanner-readable questionnaire that collected information on demographics, height, weight, use of tobacco, usual physical activity, menstrual and reproductive history (women only), medical history, and family history of cancer and a 165-item food-frequency section that assessed usual dietary intake. Disease status is measured by responses to survey questions. For instance, the question about the history of diabetes is included as “Have you ever been told by a doctor that you are diagnosed with diabetes before?”. The follow-up interviews took place between 1999 and 2004 for 52322 cohort members and between 2006 and 2010 for 39528 cohort members, respectively, and questions were asked to update tobacco and alcohol use, medical history, and menopausal status of women.
2.2. Mediclaims
The Mediclaims data contain records such as gender, ethnicity, birthdate, date of admission, date of discharge, diagnosed diseases, medical spending and insurance claims from multiple hospitals for Singaporean residents. The information of Singapore Burden of Disease (SBoD) categories and International Statistical Classification of Diseases (ICD) codes are retrievable from Mediclaims data so that the medical history for respondents recruited in the survey of SCHS could be updated to improve the accuracy on healthcare service utilization. The Mediclaims data consist of multiple groups of data including hospitalization, polyclinic visits, general practitioner visits, and emergency department utilization.
In particular, the data coverage for hospitalization spending contains information such as the date of admission, total spending, MediSave benefits, MediShield benefits and reimbursement from private insurance. The data on medical spending from Mediclaims are analyzed in the spending model to project healthcare spending in the FEM-Singapore simulation.
2.3. Singapore Longitudinal Ageing Studies (SLAS)
We draw on the Singapore Longitudinal Ageing Studies (SLAS), a health survey of 2808 subjects with the age of 55 and above in Singapore interviewed in 2004/2005, 2007/2008 and 2010/2011. All elderly adults who were citizens or permanent residents aged 55 years or above were identified by door-to-door census and invited to participate voluntarily in the study. A total of 2808 eligible residents participated, representing a response rate of 78.5%. However, only 2716 participants provided complete information on productive and social activities and mental well-being at the baseline in 2004/2005. By the end of the 2-year observation period, 45 elderly adults had died and 917 provided no response (15 were unhealthy to be interviewed, 457 refused and 445 could not be contacted).
While the SLAS is smaller and less geographically representative than the SCHS, it supplements our main dataset by providing additional information on other dimensions of health and well-being. These include the respondents’ ability to conduct activities of daily living (ADLs) and instrumental activities of daily living (IADLs), various quality of life measures and different diseases not covered in SCHS as well as psychosocial metrics which enable the study of conditions such as Alzheimer’s disease and dementia. The study was approved by National University of Singapore Institutional Review Board.
2.4. National Health Survey (NHS)
The Singapore National Health Survey (NHS) provides information on the prevalence of major noncommunicable diseases such as diabetes mellitus, hypertension and related risk factors such as age, smoking status, alcohol intake, BMI values and education levels. It also includes information on the practice of chronic disease screening, utilization of primary healthcare services, mental health and self-rated overall health, as well as relevant data on caregiving, hearing loss and renal impairment among Singaporean residents. The NHS is conducted once in every six years, and the four cohorts utilized in our study are in the years of 1992, 1998, 2004, 2010. The number of participants in the NHS are 3568, 4723, 4168 and 4337, respectively.
More specifically, demographic data and data on lifestyle factors were collected using an interviewer-administered questionnaire. Smoking status was defined as either ever smoking or never smoking. Alcohol intake was assessed using a questionnaire based on the Behavioral Risk Factor Surveillance System questionnaire of the Center for Disease Control and Prevention. Educational level was ascertained according to the following classification: no formal education, Primary School Leaving Examination (PSLE, reflecting the completion of 6 years of formal education), General Certificate of Education, ordinary level (O-level) and General Certificate of Education, advanced level (A-level).
The NHS was used to correct for potential selection issues in the MEC and to allocate trends of chronic diseases and associated risk factors across individuals in FEM-Singapore simulations.
3. Data sources used for trends and baseline scenario
3.1. Data for trends in entering cohorts
Chronic disease trends are computed using the data of National Health Survey (NHS). This is possible as the NHS contains a census data set representative of the Singapore population. The FEM-Singapore projects the development of chronic diseases in younger cohorts into the future until they reach an age of 55 using the NHS data set. This is done by studying the disease patterns of sampled NHS individuals aged younger than 55 while taking their covariates into account.
With the utilization of the NHS, we project the prevalence of chronic diseases and covariates of younger cohorts into the future. The projection method is tailored to the synthetic cohorts observed in NHS. After observing a representative sample of individuals, we follow disease patterns while accounting for their status of chronic disease, education, mortality and other covariates. Disease trends were then applied to the entire population as the NHS is census data.
3.2. Data for other projections
The Mediclaims data contain the information of medical spending of individuals admitted to the Singapore healthcare system. The selected portion of Mediclaims data are of Chinese linked to the SCHS database through matching unique Mediclaims and SCHS identifiers. Due to excess real growth in medical costs, we adjusted medical spending to real values according to the Consumer Price Index (CPI) on medical spending retrieved from the Department of Statistics, Singapore (SingStat). Both two parts model and linear regression were applied to study the relationship between hospitalization spending and explanatory factors such as demographic information, medical history, current status of comorbidities and risk factors of the patients. The linear regression was applied on the grounds of parsimony, due to the similarity in their coefficient estimates. The estimates generated from the regression analysis form the basis for projecting future medical spending in the FEM-Singapore microsimulation.
3.3. Demographic adjustments
Two demographic adjustments were applied to FEM-Singapore simulations. These allow our cohorts of individuals from SCHS to match population statistics. First, the SCHS is post-stratified by age group, gender and education. It is then rebalanced with corresponding population weights using population projections obtained from the UN Population Division. This adjustment is made for both the initial cohort as well as the replenishing cohorts over the simulation cycles. Second, the SCHS samples are post-stratified by year-age group, gender and education levels and reweighted based on their time to mortality. This is done to ensure both our incoming and replenishing cohorts reflect baseline mortality consistent with the Singapore population.
4. Estimation
In this section we describe the core part of the FEM-Singapore simulations. This includes the approaches used to estimate the transition model, setting up the initial cohort prior to the implementation of the transition process, and the procedures to deal with incoming cohorts and mortalities during the simulation cycles.
4.1. Setting up for initial cohort
The transition model is the core part of the FEM-Singapore. The transition model describes the health status of individuals and how it evolves into the next simulation cycle. Health status is defined by 7 variables based on self-reported health conditions in SCHS. The survey in SCHS asks respondents about a multiplicity of health conditions. Self-reported measures of health conditions are based on a positive response for current or past treatment for a medical condition, or through communication by a physician that the respondent has that specific health condition.
The model’s main variables consist of socio-demographic and behavioural variables denoted as AGEit, GENDERi, EDUCATIONi, MARITIAL_STATUSi, SMOKE_CURRENTit, SMOKE_EVERit, BMIit, as well as a set of 7 indicator variables for diseases: DISEASEit = {DISEASE1it,…,DISEASE7it} with reference to the 7 chronic conditions, where refers to the individual and refers to the time. In addition, a morality indicator variable denoted DIEDit was created and is 1 if the simulated individual i dies at the time t during the simulation or 0 otherwise. The baseline cohort is defined at the initial time period (t=1). The variables of AGEit, GENDERi, EDUCATIONi, MARITIAL_STATUSi, SMOKE_CURRENTit, SMOKE_EVERit, BMIit and DISEASEit in the baseline cohort are retrievable from the SCHS data set. All of these samples will be allocated with a specific weight such that the number of individuals in each age, sex and education level category are consistent to that in Singapore population distribution in 2008 (Statistics Bureau 2010).
4.2. Transition Model
After establishing the baseline cohort, the microsimulation iterates to the next time period (t=6) by projecting the values of each variable for the next six years. Since the individuals of 49 to 54 years old in the initial cohort grow to 55 to 60 years old at t=6, respectively, new individuals aged 55 and above are added to the simulation to replenish the youngest age group. Likewise, some individuals are moved out of the model due to mortality. The simulation iterates further to the next six years, until the simulation reaches the final year specified.
As inputs for the transition model, we used coefficients estimated with probit regression to ascertain the incidence rate of each health state.
where pi,t is probability of chronic disease (such as hypertension, diabetes, stroke, and heart attack) of individual i at year t, agei,t-1 measures age at previous wave, edui is education status of the individual, BMIi,t-1 is BMI at previous wave, ChronicDisi,t-1 is other chronic diseases at the previous wave. It is estimated as the probability of transitioning into one of the 7 mutually exclusive health states in the follow up wave of SCHS, conditional on not having that health condition during the previous wave, controlling for demographic and comorbidity risk factors. Health states in the transition model include diabetes, hypertension, heart disease, stroke etc. The independent variables include health status, risk factors and basic demographic characteristics such as age, gender, marital status, smoking status, BMI values and educational levels (Appendix Table 1).
The disability model is a cross-sectional model which uses current chronic diseases and socio-demographic covariates to model the burden of disability. Several modifications were made to our regressions and covariates, such as age and functional disability. First, several studies have found accumulative effects of age on health status and mortality. We put splines on age to model the accumulative influence of age on mortality. Second, functional disability was defined as having any ADLs (Activities of Daily Living) or IADLs (Instrumental Activities of Daily Living). ADLs measure an individual’s physical ability to perform basic tasks whereas the latter are higher order tasks which measure an individual’s engagement and management of resources. To have a better understanding of the dynamics surrounding functional disability, we treated ADL and IADL as separate states in our simulation.
Lastly, within the FEM-Singapore simulation cycles, we treat all diagnosed diseases as absorbing states. There was little evidence that individuals in our sample recover from the listed medical conditions after separately estimating the likelihood of transitioning out of the disease states. Consequently, only transitions into these absorbing states were modelled.
Appendix Table 1:
Regression coefficients for chronic conditions, disability, cost and mortality
| probit diabe | probit hibpe | probit stroke | |||
| l6age | .0034858 | l6age | .019739 | l6age | .0157595 |
| male | .0326885 | male | .0548319 | male | .1196691 |
| eduPSLE | −.0260528 | eduPSLE | −.0127345 | eduPSLE | −.0403922 |
| eduOlevel | −.0479019 | eduOlevel | .022141 | eduOlevel | −.0751498 |
| eduAlevel | −.0314348 | eduAlevel | −.0201606 | eduAlevel | −.0064129 |
| eduUni | −.2339131 | eduUni | −.0073572 | eduUni | −.13166 |
| mseparate | .0006047 | mseparate | −.0098071 | mseparate | .1172925 |
| mwidowed | .0161423 | mwidowed | −.021261 | mwidowed | .0196596 |
| msingle | −.0967496 | msingle | −.0914226 | msingle | −.0713426 |
| l6smokev | .0367244 | l6smokev | −.0102226 | l6smokev | .0502368 |
| l6smoken | .0782178 | l6smoken | −.1709904 | l6smoken | .2464291 |
| logdeltaage | .4874033 | logdeltaage | .5705445 | logdeltaage | .4911405 |
| l6logbmi | 1.773442 | l6logbmi | 1.396057 | l6logbmi | .3670865 |
| l6hibpe | .368159 | l6diabe | .2601623 | l6diabe | .2644677 |
| _cons | −8.21138 | _cons | −7.227644 | l6hibpe | .3753358 |
| l6hearte | .1157185 | ||||
| _cons | −5.203308 | ||||
| probit hearte | probit anyadl | probit anyiadl | |||
| l6age | .0131706 | age5570 | .0171245 | age5570 | .032131 |
| male | .2854268 | age7080 | .0341249 | age7080 | .0711864 |
| eduPSLE | −.033627 | age80p | .081621 | age80p | .0538257 |
| eduOlevel | −.0142003 | smokev | .0309689 | smokev | .2191181 |
| eduAlevel | .0653275 | mseparate | −.3232565 | mseparate | .0507498 |
| eduUni | −.1068054 | mwidowed | .0313146 | mwidowed | −.1452246 |
| mseparate | .0673106 | msingle | .2676817 | msingle | .2121902 |
| mwidowed | .0349808 | eduPSLE | −.0171268 | eduPSLE | −.413947 |
| msingle | −.1225263 | eduOlevelup | −.2006153 | eduOlevelup | −.6017016 |
| l6smokev | .0196041 | smoken | .0747181 | smoken | .1780275 |
| l6smoken | .193083 | bmil85l | −.1061127 | bmil85l | −.0347235 |
| logdeltaage | .1619877 | bmil85_230 | −.0012949 | bmil85_230 | −.0285497 |
| l6logbmi | .4065553 | bmi230_275 | −.0569831 | bmi230_275 | −.0035165 |
| l6diabe | .3296078 | bmi275u | .0793571 | bmi275u | .0698969 |
| l6hibpe | .3517479 | diabe | .1841014 | diabe | .215088 |
| l6stroke | .1497888 | hearte | .2004105 | hearte | −.1629356 |
| _cons | −4.479123 | stroke | .9747602 | stroke | .9599543 |
| male | −.0713153 | hibpe | .1145063 | ||
| _cons | −.6806777 | _cons | −2.089921 | ||
| regress hospcost | regress logbmi | probit died | |||
| age | 22.36592 | l6age | −.0012367 | l6age5570 | .037473 |
| male | − 25.7245 | male | .0007479 | l6age7080 | .0280594 |
| mseparate | 30.71168 | eduPSLE | −.0057334 | l6age80p | .0683679 |
| mwidowed | 38.52968 | eduOlevel | −.0072428 | male | .2947736 |
| msingle | − 34.57407 | eduAlevel | −.006934 | eduPSLE | −.0862872 |
| l6smokev | 53.98045 | eduUni | −.0033753 | eduOlevel | −.1675543 |
| l6smoken | 0.7733787 | mseparate | −.003405 | eduAlevel | −.2415354 |
| bmil85_230 | −162.385 | mwidowed | .0033073 | eduUni | −.3942799 |
| bmi230_275 | −198.7417 | msingle | −.0151492 | mseparate | −.0002536 |
| bmi275u | −109.9613 | l6smokev | .0025044 | mwidowed | .0919796 |
| diabe | 569.7108 | l6smoken | −.0035611 | msingle | .1268473 |
| hibpe | 283.9964 | logdeltaage | −.0077814 | l6smokev | .0734612 |
| hearte | 1802.6805 | l6logbmi | .6664802 | l6smoken | .3057811 |
| stroke | 788.2797 | l6diabe | −.0044335 | l6bmil85l | −.0490057 |
| cancre | 2130.7601 | l6hibpe | .0143948 | l6bmil85_230 | −.0008677 |
| _cons | 1.14325 | l6hearte | .0017852 | l6bmi230_275 | −.0093904 |
| l6stroke | .0019446 | l6bmi275u | .0274951 | ||
| l6cancre | −.0073043 | l6diabe | .3156914 | ||
| _cons | 1.14325 | l6hibpe | .0630988 | ||
| l6hearte | .1947352 | ||||
| l6stroke | .085338 | ||||
| l6cancre | .3513282 | ||||
| anyadl | .1647161 | ||||
| _cons | −2.548998 |
4.3. Mortality
At the end of each simulation cycle, individuals will move out of the model due to mortality. We used logistic regressions to estimate the probability of death for samples in the second and third wave of SCHS study (in 1999 or 2006) if individuals were still alive during the previous wave of study, controlling for socio-demographic status and comorbidities in the previous wave. The estimated probability is then implemented in the FEM-Singapore simulation to project future mortality.
5. Model for new cohorts
The baseline cohort in the FEM-Singapore simulations is based on the second follow-up wave in the SCHS. In addition, the model for new cohorts integrates trends of health status among younger cohorts with the joint distribution of outcomes in the population of respondents in the SCHS.
New cohorts will be added into the simulation module during each iteration. The incoming cohorts are generated based on the samples of individuals of 55 to 60 years old in the last wave of SCHS data. We sample the individuals of incoming cohorts with replacement from the SCHS data with adjustments such that chronic diseases in the incoming cohorts could abide by the pre-defined trends in prevalence. In addition, individuals in the incoming cohorts are reweighted by age, gender and education to adjust for changes in specific population groups. For example, the replenishing cohorts within the simulation are adjusted with higher education levels to model the increasing trend of attaining high education in future. The trends of both chronic disease prevalence and demographic change in age, gender and education are derived from official projections (National Health Survey and UN population projection). For detailed information on how the trends of chronic disease prevalence are computed and how the trends are adjusted in the replenishing cohorts, one may refer to Goldman et al (2015).
6. Medical spending
One objective is to make projections on medical spending for Singaporean elderly population to ascertain the burden future individuals place on healthcare utilization. In this section, first we describe how databases are integrated to ascertain the dynamics of medical spending, healthcare and sociodemographic status. Second, how we model our covariates to medical spending. Lastly, how medical spending is integrated into the FEM-Singapore structure to obtain projections for medical spending.
First, socio-demographic factors such as medical history and risk factors obtained from SCHS and NHS data are used as explanatory variables in the regression analysis for medical spending. We consider gender, age and marital status of the respondents to be included in the simulation. While socioeconomic status such as respondent income or occupation are not directly attainable from the data sources, proxies such as respondent education levels are available in the SCHS to estimate the corresponding impact on medical spending. The risk factors include the smoking status of individuals and the body mass index (BMI) as a measurement of obesity. These were then linked to corresponding medical spending on diseases such as cancer, hypertension, heart disease, stroke, diagnosed diabetes and undiagnosed diabetes in Mediclaims.
Next, coefficients based on the regression analysis for total medical spending on relevant explanatory variables from the merged data of SCHS and Mediclaims are utilized for the projections in the FEM-Singapore simulation. In the process of merging the data, we segregate the different periods of medical spending according to the cohorts (the initial wave and the two follow-up waves) of SCHS data at the corresponding interview dates.
Lastly, in the FEM-Singapore simulation, the spending module links the current state of individuals on demographics, socioeconomic status, medical history and risk factors to the medical spending and makes the projections over the simulation cycles.
This approach allows us to delineate the relationship between the medical spending and the information of individual samples by considering the different interview dates among the participants of SCHS and their underlying health status and risk factors in the different waves. For patients who suffer from multiple chronic conditions, the additional ADL disability would have higher marginal cost compared to those with no ADL disability (Appendix Table 2).
Appendix Table 2:
Average hospitalization cost by ADL and comorbidities ($2014)
| None* | Diabetes | Hypertension | Stroke | |
|---|---|---|---|---|
| ADL disability | 378 | 2147 | 1764 | 2453 |
| NoADL | 251 | 1502 | 108 | 2065 |
None: no comorbidities (DM, HPT, Stroke)
7. Scenarios and Robustness
In addition to making projections on the prevalence and medical spending in the baseline scenario, the FEM-Singapore simulations are used to estimate the effects of health policy changes on health status in the future. Below, we discuss an example of simulating policy changes with the FEM-Singapore.
One form of policy simulation involves changing the trends in risk factors for chronic conditions. This is implemented by altering the characteristics of individuals in the incoming cohorts. One may change the distribution of BMI values among the individuals for replenishing cohorts and obtain the change in prevalence of hypertension and heart attack in the incoming cohorts. Incorporating these changes of trends through the modification of incoming cohorts will provide the new projections on the prevalence of diseases and medical spending for the Singaporean elderly population. This allows us to create informed which can be utilized as the foundation for promoting public awareness and making policy recommendation based on relevant interventions in chronic disease risk factors.
8. Implementation
We now delineate the entire procedure to implement FEM-Singapore in multiple stages. First, the estimation of the transition and cross-sectional models detailed in section 4.1 and 4.2 is performed in Stata. Second, the incoming cohort model detailed in section 5 is estimated in Stata using the CMP package (Roodman, 2011). Third, the simulation, integrating the transition, cross-sectional and incoming cohort model is implemented in C++ to achieve computational efficiency, which we describe next.
First, to match the six-year structure used to estimate the transition models, the FEM-Singapore simulation proceeds in the cycle of every six years. The end of each six-year step is designed to occur on July 1st to allow for the ease of matching to population forecasts. Second, a simulation of the FEM-Singapore proceeds by first loading a population representative of Singaporeans age 55 and above in the year of 2008, generated from the reweighted last wave of SCHS. Third, in increments of six years, the FEM-Singapore applies the transition models for health status and mortality with Monte Carlo decisions to determine the new state of individuals. When the incoming cohorts are included during simulation cycles, the new individuals age between 55 to 60 years are added to the simulation. The number of new individuals age 55 to 60 years are made to be consistent with the estimates obtained from the Census data. Lastly, once the new states have been determined and new individuals of 55 to 60 years old are added, the cross-sectional model for medical spending is applied to make projections on medical spending in each simulation cycle.
Summary variables are then computed accordingly based on demographics such as gender, age or educational level. Computation of medical spending includes the individuals that died to account for the end of life spending. To relieve the issue of uncertainty originated from stochastic Monte Carlo decision rules, the simulations are performed in multiple iterations (typically in the multiples of 100), and the summary statistics are generated across iterations. The FEM-Singapore simulation takes the inputs the assumptions regarding real medical cost growth and the consumer price index. Medical spending is reported in Singapore dollars with the base year of 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United Nations, World Population Ageing 2017 Report. 2017.
- 2.Broe G, et al. , Determinants of service use among the elderly: the Sydney older persons study. Australasian Journal on Ageing, 2002. 21(2): p. 61–66. [Google Scholar]
- 3.Edelbrock D, et al. , The relation between unpaid support and the use of formal health services: the Sydney Older Persons Study. Australasian Journal on Ageing, 2003. 22(1): p. 2–8. [Google Scholar]
- 4.Wu C-Y, et al. , The association between functional disability and acute care utilization among the elderly in Taiwan. Archives of Gerontology and Geriatrics, 2013. 57(2): p. 177–183. [DOI] [PubMed] [Google Scholar]
- 5.Picco L, et al. , Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Services Research, 2016. 16(1): p. 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James Lubitz LC, Ellen Kramarow, and Harold Lentzner., Health, Life Expectancy, and Health Care Spending among the Elderly. New England Journal of Medicine, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Fried TR, et al. , Functional disability and health care expenditures for older persons. Archives of Internal Medicine, 2001. 161(21): p. 2602–2607. [DOI] [PubMed] [Google Scholar]
- 8.Spillman BC, Changes in elderly disability rates and the implications for health care utilization and cost. The Milbank Quarterly, 2004. 82(1): p. 157–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of the Interior, Statistical Yearbook of the Interior, 2010, 04–18 The disabled population by age and grade. Republic of China: Department of statistics; {MOI, 2010 #44} 2010. [Google Scholar]
- 10.Lin L-P, et al. , Disability and hospital care expenses among national health insurance beneficiaries: Analyses of population-based data in Taiwan. Research in Developmental Disabilities, 2011. 32(5): p. 1589–1595. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji I, et al. , Medical cost for disability: a longitudinal observation of national health insurance beneficiaries in Japan. Journal of the American Geriatrics Society, 1999. 47(4): p. 470–476. [DOI] [PubMed] [Google Scholar]
- 12.Manton KG, Lamb VL, and Gu X, Medicare cost effects of recent US disability trends in the elderly: future implications. Journal of Aging and Health, 2007. 19(3): p. 359–381. [DOI] [PubMed] [Google Scholar]
- 13.Manton KG, Gu X, and Lamb VL, Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the US elderly population. Proceedings of the National Academy of Sciences, 2006. 103(48): p. 18374–18379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C-M, et al. , Health-related services use and the onset of functional disability: 10 year follow-up study. Archives of gerontology and geriatrics, 2014. 58(3): p. 356–363. [DOI] [PubMed] [Google Scholar]
- 15.Hajek A and König H-H, Longitudinal predictors of functional impairment in older adults in Europe–evidence from the survey of health, ageing and retirement in Europe. PloS one, 2016. 11(1): p. e0146967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moe JO and Hagen TP, Trends and variation in mild disability and functional limitations among older adults in Norway, 1986–2008. European Journal of Ageing, 2011. 8(1): p. 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Alarcón M and Perelman J, Ageing under unequal circumstances: a cross-sectional analysis of the gender and socioeconomic patterning of functional limitations among the Southern European elderly. International Journal for Equity in Health, 2017. 16(1): p. 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, et al. , The synergistic effect of functional status and comorbidity burden on mortality: a 16-year survival analysis. PloS one, 2014. 9(8): p. e106248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caskie GI, Sutton MC, and Margrett JA, The relation of hypertension to changes in ADL/IADL limitations of Mexican American older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 2010. 65(3): p. 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodge HH, et al. , Cognitive impairment as a strong predictor of incident disability in specific ADL–IADL tasks among community-dwelling elders: the Azuchi Study. The Gerontologist, 2005. 45(2): p. 222–230. [DOI] [PubMed] [Google Scholar]
- 21.Solfrizzi V, et al. , Reversible cognitive frailty, dementia, and all-cause mortality. The Italian Longitudinal Study on Aging. Journal of the American Medical Directors Association, 2017. 18(1): p. 89.e1–89.e8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson Wayne L., P., Armour Brian S. PhD, Finkelstein Eric A. PhD, Wiener Joshua M. PhD, Estimates of state-level health-care expenditures associated with disability. Public Health Reports, 2010. 125(1): p. 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetzel RZ, et al. , Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting US employers. Journal of Occupational and Environmental Medicine, 2004. 46(4): p. 398–412. [DOI] [PubMed] [Google Scholar]
- 24.Hsu HC, Effects of physical function trajectories on later long-term care utilization among the Taiwanese elderly. Geriatrics & gerontology international, 2013. 13(3): p. 751–758. [DOI] [PubMed] [Google Scholar]
- 25.East Asia Forum. Can a Rapidly Aging Singapore Stave Off Economic Disaster? 2015. [cited 2018 21 Jan]; Available from: http://www.economywatch.com/features/Can-a-Rapidly-Aging-Singapore-Stave-Off-Economic-Disaster1005.html.
- 26.Tan Teck Boon. A super-aged Singapore: Policy implications for a Smart Nation 2015. [cited 2018 21 Jan]; Available from: https://www.todayonline.com/singapore/super-aged-singapore-policy-implications-smart-nation.
- 27.MInistry of Health, Healthcare Financing. 2018.
- 28.Business Times Singapore, Singapore Budget 2018: Govt expenditure on healthcare expected to ‘rise quite sharply’: Heng Swee Keat. 2018.
- 29.Ansah JP, et al. , Projection of young-old and old-old with functional disability: does accounting for the changing educational composition of the elderly population make a difference? PLoS One, 2015. 10(5): p. e0126471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly S, et al. , Modelling the cost of ill health in Health&WealthMOD (Version II): Lost labour force participation, income and taxation, and the impact of disease prevention. Vol. 4 2011. 32–36. [Google Scholar]
- 31.MInistry of Health, National Health Survey 1998. 1999.
- 32.Department of Statistics, Population Trends 2012 Archived 13 November 2012 at the Wayback Machine.
- 33.Chen BK, et al. , Forecasting Trends in Disability in a Super-Aging Society: Adapting the Future Elderly Model to Japan. J Econ Ageing, 2016. 8: p. 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce GF, et al. , The lifetime burden of chronic disease among the elderly. Health Aff (Millwood), 2005. 24 Suppl 2: p. W5R18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaud PC, et al. , Differences in health between Americans and Western Europeans: Effects on longevity and public finance. Soc Sci Med, 2011. 73(2): p. 254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan TP, et al. , Forecasting the burden of type 2 diabetes in Singapore using a demographic epidemiological model of Singapore. BMJ Open Diabetes Research and Care, 2014. 2(1): p. e000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National University of Singapore, Introduction to SCHS. 2018.
- 38.Ministry of Health, About Eldershield. 2018.
- 39.Department of Statistics. Consumer Price Index. 2018. [cited 2018 12 Dec]; Available from: https://www.singstat.gov.sg/modules/infographics/consumer-price-index.
- 40.Lin S-F, Beck AN, and Finch BK, The Dynamic contribution of chronic conditions to temporal trends in disability among US adults. Disability and health journal, 2016. 9(2): p. 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman VA, et al. , Declines in late-life disability: the role of early-and mid-life factors. Social science & medicine, 2008. 66(7): p. 1588–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokota R, et al. , Contribution of Chronic Conditions to the Disability Burden across Smoking Categories in Middle-Aged Adults, Belgium. PloS one, 2016. 11(4): p. e0153726–e0153726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie JX, et al. , A population-based cohort study of ambulatory care service utilization among older adults. Journal of evaluation in clinical practice, 2010. 16(4): p. 825–831. [DOI] [PubMed] [Google Scholar]
- 44.Vilpert S, et al. , Emergency department use by oldest-old patients from 2005 to 2010 in a Swiss university hospital. BMC health services research, 2013. 13(1): p. 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Q, et al. , Trends in ADL and IADL disability in community-dwelling older adults in Shanghai, China, 1998–2008. J Gerontol B Psychol Sci Soc Sci, 2013. 68(3): p. 476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millan-Calenti JC, et al. , Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr, 2010. 50(3): p. 306–10. [DOI] [PubMed] [Google Scholar]
- 47.Services, D.o.H.a.H. QuickStats: Percentage of Adults with Activity Limitations, by Age Group and Type of Limitation --- National Health Interview Survey, United States, 2008*. 2009 [cited 2018 10 Dec]; Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5848a6.htm.
- 48.Nepal B, et al. , Projecting the need for formal and informal aged care in Australia: A dynamic microsimulation approach. National Centre for Social and Economic Modelling (NATSEM) Working Paper 11, 2011. 7. [Google Scholar]
- 49.Chen BK, et al. , Forecasting trends in disability in a super-aging society: Adapting the Future Elderly Model to Japan. The Journal of the Economics of Ageing, 2016. 8: p. 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson J, et al. , Projecting the Number of Older Singaporeans with Activity of Daily Living Limitations Requiring Human Assistance Through 2030. Ann Acad Med Singapore, 2014. 43: p. 51–6. [PubMed] [Google Scholar]
- 51.Davidson PM, Digiacomo M, and McGrath SJ, The feminization of aging: how will this impact on health outcomes and services? Health Care Women Int, 2011. 32(12): p. 1031–45. [DOI] [PubMed] [Google Scholar]
- 52.Owens GM, Gender differences in health care expenditures, resource utilization, and quality of care. J Manag Care Pharm, 2008. 14(3 Suppl): p. 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunlop DD, Hughes SL, and Manheim LM, Disability in activities of daily living: patterns of change and a hierarchy of disability. Am J Public Health, 1997. 87(3): p. 378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho HK, et al. , Factors associated with ADL dependence: A comparative study of residential care home and community-dwelling elderly in Japan. 2002. 2(2): p. 80–86. [Google Scholar]
- 55.Oman D, Reed D, and Ferrara A, Do Elderly Women Have More Physical Disability than Men Do? American Journal of Epidemiology, 1999. 150(8): p. 834–842. [DOI] [PubMed] [Google Scholar]
- 56.Murtagh KN and Hubert HB, Gender differences in physical disability among an elderly cohort. Am J Public Health, 2004. 94(8): p. 1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organisation, Global Health and Aging. 2011, National Institute on Aging, National Institutes of Health. [Google Scholar]
- 58.Khalik S, Parliament: Patients who buy new riders for Integrated Shield Plans will have to pay 5 per cent of hospital bills, in The Straits Times. 2018. [Google Scholar]
- 59.Ministry of Health, FEE BENCHMARKS ADVISORY COMMITTEE REPORT 2018.
- 60.Ministry of Health, Fee Benchmarks for Private Sector Surgeon Fees 2018.
- 61.Ministry of Finance, FY 2018 Budget Statement. 2018.
- 62.National Environment Agency, Smoking Prohibition Overview. 2018.
- 63.Ministry of Health, Diabetes: The War Continues. 2018.
- 64.Yu H-W, et al. , Disability trajectories and associated disablement process factors among older adults in Taiwan. Archives of gerontology and geriatrics, 2015. 60(2): p. 272–280. [DOI] [PubMed] [Google Scholar]
- 65.Liao C-C, et al. , Social support and mortality among the aged people with major diseases or ADL disabilities in Taiwan: A national study. Archives of gerontology and geriatrics, 2015. 60(2): p. 317–321. [DOI] [PubMed] [Google Scholar]
- 66.Calderón-Larrañaga A, et al. , Rapidly developing multimorbidity and disability in older adults: does social background matter? Journal of internal medicine, 2018. 283(5): p. 489–499. [DOI] [PubMed] [Google Scholar]
- 67.Ministry of Health, Eldershield Coverage. 2018.
- 68.Ministry of Manpower, Levy concession for a foreign domestic worker. 2018.
- 69.Williams DR and Mohammed SA, Discrimination and racial disparities in health: evidence and needed research. Journal of behavioral medicine, 2009. 32(1): p. 20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma VK, et al. , Stroke risk factors and outcomes among various Asian ethnic groups in Singapore. Journal of Stroke and Cerebrovascular Diseases, 2012. 21(4): p. 299–304. [DOI] [PubMed] [Google Scholar]
- 71.Lee R, et al. , Impact of race on morbidity and mortality in patients with congestive heart failure: a study of the multiracial population in Singapore. International journal of cardiology, 2009. 134(3): p. 422–425. [DOI] [PubMed] [Google Scholar]
- 72.Venketasubramanian N, et al. , Prevalence of stroke among Chinese, Malay, and Indian Singaporeans: a community-based tri-racial cross-sectional survey. Stroke, 2005. 36(3): p. 551–556. [DOI] [PubMed] [Google Scholar]
- 73.Yassin AS, Beckles GL, and Messonnier ML, Disability and its economic impact among adults with diabetes. Journal of occupational and environmental medicine, 2002. 44(2): p. 136–142. [DOI] [PubMed] [Google Scholar]
- 74.Loyalka P, et al. , The cost of disability in China. Demography, 2014. 51(1): p. 97–118. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson-Meyers L, et al. , Estimating the additional cost of disability: Beyond budget standards. Social science & medicine, 2010. 71(10): p. 1882–1889. [DOI] [PubMed] [Google Scholar]