Abstract
Objective:
To measure changes in glucose, lipid, and inflammation parameters after transitioning from a baseline diet (BD) to an isocaloric ketogenic diet (KD).
Methods:
Glucose and lipid homeostasis and inflammation were studied in 17 men (BMI 25–35 kg/m2) during 4 weeks of a BD (15% protein, 50% carbohydrate, 35% fat) followed by 4 weeks of an isocaloric KD (15% protein, 5% carbohydrate, 80% fat). Postprandial responses were assessed following mixed meal tests (MMT) matched to compositions of the BD (control meal, CM) and KD (ketogenic meal, KM).
Results:
Fasting ketones, glycerol, FFA, glucagon, adiponectin, GIP, total and LDL cholesterol, and CRP were significantly increased on the KD. Fasting insulin, C-peptide, triglycerides, and FGF-21 were significantly decreased. During the KD, glucose area under the curve (AUC) was significantly higher with both test meals and insulin AUC was significantly higher only for the CM. Analyses of glucose homeostasis suggested that the KD insulin sensitivity was decreased during the CM but increased during the KM. Insulin-mediated anti-lipolysis was decreased on the KD regardless of meal type.
Conclusions:
Switching to the KD was associated with increased cholesterol and inflammatory markers, decreased triglycerides and decreased insulin-mediated anti-lipolysis. Glucose homeostasis parameters were diet- and test meal-dependent.
Keywords: ketosis, insulin, glucose, lipids, inflammation, diet
Introduction
Very low carbohydrate, high fat, ketogenic diets (KD) have become increasingly popular for the treatment of obesity and type 2 diabetes (1). However, little is known about the effects of transitioning people from a high carbohydrate to an isocaloric KD on glucose and lipid homeostasis or inflammation. Such very low-carbohydrate diets have been reported in some studies to result in decreased energy intake (2) and improved glucose and lipid homeostasis and decreased inflammatory biomarkers (3). However, most of these studies have been in outpatients in whom it is difficult to disassociate physiological dietary effects from those related to dietary adherence (4, 5), even in controlled-feeding studies where all food is provided (6). Free-living diet studies do not investigate the effects of actually consuming diets, but rather the effects of the instructions to change diet in the prescribed way. To understand the metabolic effects of an isocaloric KD, an inpatient controlled feeding study is required.
We have previously reported the effects of transitioning from 4 weeks of a 15% protein, 50% carbohydrate, 35% fat (baseline diet, BD) followed immediately by 4 weeks of an isocaloric 15% protein, 5% carbohydrate, 80% fat ketogenic diet (KD) (7) on energy expenditure in 17 men with overweight or class I obesity. We also explored the changes in glucose and lipid homeostasis and in inflammatory markers in this population to test the hypothesis that these co-morbidity risk factors were significantly affected by dietary macronutrient content.
Methodology
Subjects and Study Protocol
As previously reported (7), 17 men without diabetes and with BMI between 25–35 kg/m2 were inpatients at four study sites (Table 1) where they resided on metabolic wards without access to food other than that provided within this study. Subjects spent 23 hours/day for 2 consecutive days each week in a respiratory chamber. An initial weight maintenance energy requirement estimate was made as 1.5x resting energy expenditure obtained by indirect calorimetry during the screening process. Subjects initially received the baseline diet (BD; 15% protein, 50% carbohydrate, 35% fat) and caloric intake was further adjusted to achieve energy balance to within 5% of the average daily energy expenditure measured by the respiratory chamber based on the average of the two 23-hour chamber stays during that week on the BD (range to completion 2–3 weeks). Subjects underwent two consecutive 23-hour chamber stays on the same days each week throughout the study. After 4 weeks of EI stability on the BD, subjects were switched to an isocaloric ketogenic diet (KD, 15% protein, 5% carbohydrate, 80%) for 4 weeks. The last two weeks of each diet were designated as the “test period” to allow subjects time to demonstrate persistent agreement between dietary intake and chamber calorimetry during the BD and to allow accommodation to the switch to the KD.
Table 1.
Subject characteristics and weight changes during the study. Subjects consistently lost weight during the study indicating that they were in negative energy balance. Previous studies of body composition and energy expenditure during the study indicate that the magnitude of this negative energy balance was approximately 60 kcal/day (43)
| Table 1. | Age (years) | Enrollment Weight (kg) | Week 2 Weight (kg) | Week 4 Weight (kg) |
|---|---|---|---|---|
| Baseline Diet | 33.6 (7.3)* | 89.1 (15.9) | 88.1 (15.6)†‡ | 87.4 (15.4)‡ |
| Ketogenic Diet | 85.7 (14.9) # | 85.1 (14.6) |
P<0.001 vs. all other time points
P<0.001 vs. BD week 4
P<0.001 vs. KD week 2 or week 4
P<0.001 vs. KD week 4.
Data are mean (SD).
The study protocol was approved by the Institutional Review Boards of the National Institute of Diabetes and Digestive and Kidney Diseases (ClinicalTrials.gov Identifier: NCT01967563), the Pennington Biomedical Research Center (2013–3-PBRC), Columbia University Medical Center (IRB-AAAL7113) and the Translational Research Institute for Metabolism and Diabetes (FH IRB-493675) and are consistent with guiding principles for research involving humans (8). Written informed consent was obtained from all subjects.
Diets
Diets consisted of 7-day rotating menus using NUTRITIONIST PRO software (Version 1.3, First Databank Inc., The Hearst Corporation, San Bruno, CA). The energy and macronutrient composition for each day were verified by chemical analysis (Covance Laboratories, Madison, WI). Food was prepared at the PBRC metabolic kitchen, frozen, and shipped to the study sites where meals were prepared for consumption according to standardized procedures and addition of fresh produce. Both menus contained minimal quantities of processed food and, despite the large differences in macronutrient composition, the ratio of refined to unrefined sugars were similar between the diets. This control of macronutrient quality permitted examination of the effects of the relative macronutrient contents without confounding due to differences in the types of nutrients (refined vs. unrefined sugars, simple vs. complex carbohydrates, saturated vs. mono- or poly- unsaturated fatty acids) utilized. Quality of protein was monitored by utilizing similar protein sources on corresponding BD and KD menu days – e.g., white meat chicken on the BD and dark meat chicken on the KD. Sample menus have been reported previously (7).
Laboratory
Subjects underwent weekly fasting blood draws for assessment of ketosis, lipids, glucose and fatty acid homeostasis, and inflammation (see Table 1). During the second week of energy intake (EI) stability on the BD (defined as week 4), subjects underwent two isocaloric mixed meal tests (MMT) at ~ 9 AM, at least 12 hours after their last meal. The test meal consisted of 20% of total daily prescribed energy with macronutrient distribution identical to the BD (designated as a “control meal”, CM); and at least 3 days later, subjects received a meal of equal calories providing the same macronutrient distribution as the KD (designated as a “ketogenic meal”, KM). Plasma and serum samples for glucose, insulin, free fatty acids, beta-hydroxybutyrate, and triglycerides were obtained at −10, −5, 0, 5, 10, 15, 30, 45, 60, 90, and 120 minutes relative to completion of meal consumption. Subjects were then switched to an isocaloric KD (15% protein, 5% carbohydrate, 80% fat) for 4 weeks with testing as described above during the last 2 weeks. During week 4 on the KD subjects underwent MMT testing. The order MMT testing on the KD was KM first, followed at least 3 days later by the CM. Assays are described in Supplement 1.
Calculations and Statistical Analyses
Data are presented as mean (SD). The primary outcome variables of these biochemical studies were those relevant to glucose homeostasis and circulating lipids in contrast to earlier studies which focused primarily on energy expenditure (7)
In order to justify the examination of multiple variables relevant to glucose homeostasis without necessitating post hoc adjustments for multiple comparisons (9), assessments of areas under the curve (AUC) relative to their fasting pre-meal concentrations for insulin (relAUCins) and glucose (relAUCglucose) during MMTs were assessed prior to analysis of other variables relevant to glucose homeostasis. Since there were significant dietary macronutrient effects on insulin and glucose during MMTs (see Results), further, more detailed, analyses of glucose homeostasis were performed. Molecules subsequently studied were those that might mechanistically affect or reflect gluconeogenesis (e.g. glucagon) (10), insulin sensitivity [cortisol, FFA) (11, 12) and fibroblast growth factor 21 (FGF21) (13)], insulinogenesis [gastric inhibitory peptide (GIP) (14), glucagon-like peptide 1 (GLP-1) (15), and peptide YY (PYY) (16)], and inflammation [C-reactive protein (CRP) and interleukin-6 (IL-6)] as well as AUC’s relative to their fasting pre-meal concentrations for triglycerides, FFA, and beta-hydroxy butyrate (relAUCtriglyceride, relAUCFFA. and relAUCβOHB). Similarly, analyses of cholesterol sub-fractions (HDL and LDL) and triglycerides were not made until a significant diet effect on total cholesterol was noted.
There are few studies of the effects of isocaloric ketogenic diets on β-cell function and insulin sensitivity, or fasting vs. post-prandial assessments of these variables. To address these issues, fasting measurements were used to calculate HOMA-IR (primarily hepatic insulin sensitivity), HOMA-β (insulin release), and ADIPO-IR (adipocyte insulin sensitivity) (17) along with the fasting Belfiore Index (BelfioreFASTING) (18). Glucose and insulin responses to MMT’s were determined using post-prandial AUCs relative to pre-meal concentrations (relAUC). Absolute AUCglucose and AUCinsulin were used in the calculation of the MMT Belfiore Index (BelfioreMMTglucose) (18) normalized using the mean absolute values of AUCglucose and AUCinsulin during the CM on the BD. The Matusuda Index - previously validated against clamp studies and in both OGTT and MMT (19) - was also calculated as a post-prandial index of insulin sensitivity. The Insulinogenic Index (20) and the Insulin Secretion-Sensitivity Secretion Index 2 (ISSI-2) were chosen to assesses, respectively, β-cell function using isolated time points (0 and 30 minutes) and the absolute AUC data, as well as uncorrected and corrected for variations in insulin sensitivity (20) from MMT results.
Adipose tissue sensitivity to insulin-mediated changes in circulating FFA (integrating simultaneous effects on lipolysis and esterification) was calculated from the Belfiore Index (BelfioreMMTFFA) (18) and compared with results from the ADIPO-IR which is based on fasting data (see above). Equations and additional calculations are presented in Table 2. Within-subjects comparisons were made by ANOVA with repeated measures in which either diet (BD or KD) or MMT test meal (Control Meal - CM or Ketogenic Meal-KM) were used as covariates. To increase the likelihood that all biochemical values were “stable” within study periods, regression equations were calculated comparing values for fasting blood concentrations during the last 2 weeks of each diet. To determine whether measures obtained during the BD were predictive of those observed on the KD, regression equations were calculated comparing the mean values of fasting concentrations over the last 2 weeks of the BD with those during the last 2 weeks ingesting the KD. Analyses of the relationship of background diet and MMT type on the relationship of fasting measures of insulin sensitivity to measures derived from the MMT were made by multiple linear regression analysis in which the dynamic (MMT-derived) measure was the dependent variable and the fasting measures, diet type and meal type were each treated as dichotomous variables.
Table 2.
Equations used to calculate indices of insulin secretion, glycemic insulin sensitivity, and FFA-flux insulin sensitivity based on fasting data and mixed meal tolerance test data.
| Table 2.* | Calculation | |
|---|---|---|
| Name | Fasting/during MMT | |
| Measures of Insulin Secretion in Response to Glucose | ||
| HOMA-β (20) | Fasting | (360 × FPI)/(FPG − 63) |
| Insulinogenic Index (20) | MMT | (InS30−FPI/GlU30) |
| ISSI-2 (20) | MMT | AUCinsulin/AUCglucose × Matsuda Index |
| Measures of Glycemic Insulin Sensitivity or Resistance | ||
| HOMA-IR (20) | Fasting | FPG × FPI/405 |
| BelfioreFASTING | Fasting | 2/[(nFPG × nFPI) + 1] |
| Matsuda (19) | MMT | 10,000/√(FPG × FPI) × × |
| BelfioreMMTglucose (18) | MMT | 2/[(nAUCinsulin × nAUCglucose) + 1] |
| Measures of Free Fatty Acid (FFA) Flux Insulin Sensitivity† | ||
| Adipo-IR (17) | Fasting | FPI × FPFFA |
| BelfioreMMTFFA (18) | MMT | 2/[(nAUCinsulin × nAUCFFA) + 1] |
FPG - Fasting plasma glucose (mg/dl);FPI - Fasting plasma insulin (microU/ml); - Mean plasma insulin during testing; - Mean plasma glucose during testing); FPFFA - Fasting plasma free fatty acids; G30 - Plasma glucose 30 minutes following a glucose load G120 - Plasma glucose 120 minutes following a glucose load; I30 - Plasma insulin 30 minutes following a glucose load; I120 - Plasma insulin 120 minutes following a glucose load; VD = volume of distribution calculated as 150 ml/kg of body weight; AUC - Absolute area under the curve during the mixed meal tests; nFPG and nFPI are the normalized fasting plasma glucose and insulin concentrations, respectively, for use in the BelfioreFASTING index where the fasting concentrations are divided by the mean fasting concentrations during the BD; nAUC is the normalized area under the curve used for the Belfiore indices where the absolute AUC for the test meal is divided by the mean absolute AUC for the baseline MMT on the BD.
FFA flux inssulin sensitivity refers to the magnitude of suppression of FFA circulating concentrations of FFA in response to insulin.
Statistical significance was prospectively defined as Pα<0.05. Values differing from the respective means by more than 3SD were prospectively excluded.
Results
Subjects (Table 1)
Subjects were in a state of negative energy balance due to inadvertent underfeeding throughout the study. Weight decreased by 0.8 (0.2) kg (P=0.002) during the last 15 days of the BD and 0.2 (0.1) kg (N.S.) during the last 15 days on the KD (7). EE by chamber calorimetry was the primary outcome variable of this study and was approximately 60 kcal/day higher on the KD due predominantly to early changes in EE (7).
Fasting Ketones, FFA, and Glycerol (Table 3)
Table 3.
Comparisons of fasting plasma concentrations of morning ketones and free fatty acids (FFA) in 17 subjects during isocaloric KD and BD diets.
| Table 3. | BD | KD | Correlations between BD and KD | Diet effect | ||||
|---|---|---|---|---|---|---|---|---|
| Week 3 | Week 4 | Week 3 | Week 4 | RBD Weeks 3 and 4 | RKD Weeks 3 and 4 | Rmean BD vs KD* | Mean Diff. KD-BD† | |
| Acetoacetate (mMol/L) | 0.10 (0.03) | 0.10 (0.03) | 0.81 (0.48) | 0.83 (0.40) | 0.56, P=0.026 | 0.86, P<0.001 | 0.22, N.S. | 0.70 (0.44), P<0.001 |
| β-hydroxy butyrate(mMol/L) | 0.09 (0.02) | 0.11 (0.02) | 0.77 (0.49) | 0.77 (0.45) | 0.56, P=0.024 | 0.92, P<0.001 | 0.24, N.S. | 0.71 (0.44), P<0.001 |
| Free Fatty Acids (mMol/L) | 0.44 (0.12) | 0.52 (0.10)* | 0.76 (0.17) | 0.84 (0.18) | 0.28, N.S. | 0.44, P=0.07 | −0.03, N.S. | 0.32 (0.18), P<0.001 |
| Glycerol (mg/L) | 6.6 (1.7) | 6.2 (1.5) | 10.2 (2.8) | 10.4 (3.1) | 0.50, P=0.014 | 0.48, P=0.05 | 0.13, N.S. | 3.7 (2.8), P<0.001 |
*P<0.05 vs. week 3 on the same diet. †P<0.05 vs. the CM meal on the same diet (meal effect), **P<0.001 vs. the CM meal during the same diet (meal effect), ‡P<0.001 vs the same meal during the BD (background diet effect).
Correlations between the mean values for weeks 3 and 4 on the BD with the mean values for weeks 3 and 4 on the KD.
Comparisons between the KD and BD were made using the mean of values obtained at last 2 weeks (designated as weeks 3 and 4).
Data are mean (SD).
Acetoacetate, β-hydroxybutyrate, and glycerol concentrations were stable and significantly correlated between weeks 3 and 4 within each diet indicating stable rates of ketosis. FFA values were significantly higher on week 4 of the BD compared to week 3 without significant inter-week correlation on either diet. Plasma ketones, FFA, and glycerol were all significantly higher during the last two weeks of the KD compared with the BD. Fasting plasma ketones, FFA, and glycerol measured during the BD were not significantly correlated with those measured during the KD.
Glucose and FFA Homeostasis (Tables 4–6, Figure 1)
Table 4.
Measures relevant to glucose homeostasis in subjects on isocaloric ketogenic and baseline diets.
| Table 4. | BD | KD | Correlationsa | Diet effectb | ||||
|---|---|---|---|---|---|---|---|---|
| Week 3 | Week 4 | Week 3 | Week 4 | RBD Weeks 3 and 4 | RKD Weeks 3 and 4 | Rmean BD vs KD | Mean Diff. KD-BD | |
| Glucose (mg/dl) | 82 (7) | 81 (4) | 81 (7) | 81 (6) | 0.63, P=0.006 | 0.73, P<0.001 | 0.68, P=0.003 | 0 (5), N.S. |
| Insulin (mIU/ml)a | 7.8 (4.5) | 7.1 (4.6) | 6.1 (3.7) | 6.1 (3.7) | 0.81, P<0.001 | 0.73, P<0.001 | 0.90, P<0.001 | −1.6 (1.9), P=0.006 |
| C-Peptide (ng/ml)a | 1.51 (0.52) | 1.43 (0.59) | 1.13 (0.49) | 1.13 (0.45) | 0.82, P<0.001 | 0.79, P<0.001 | 0.94, P<0.001 | −0.34 (0.21), P<0.001 |
Glucose homeostasis based on fasting plasma concentrations during weeks 3 and 4 of the BD and KD in 16 subjects. In one subject during the last week of the KD, insulin and glucose concentrations were more than 3 SD above the mean suggesting that the subject was not fasting. Glucose and insulin values from time zero on the morning of the mixed meal tolerance test (Table 5) in the same week were substituted for this one data set in this one subject. Exclusion of the subject did not affect the significance of the data presented in this table. One subject was excluded because one set of fasting plasma insulin concentrations were more than 3 SD above the mean.
Data are reported in only 16 of the 17 subjects due to an outlier whose fasting insulin was 40 mIU/ml and whose C-peptide was 4.4 nMol/L in week 4. Because these values were more than 3 SD above the mean, they were not included in the analysis. If they were included then the differences between fasting insulin and C-peptide on the KD and BD were not statistically significant.
Correlations between the mean values for weeks 3 and 4 on the BD with the mean values for weeks 3 and 4 on the KD.
Comparisons between the KD and BD were made using the mean of values obtained at last 2 weeks (designated as weeks 3 and 4).
Data are mean (SD).
Table 6.
Relative areas under the curve (relAUC) for plasma concentrations of glucose, insulin, FFA, glycerol, triglycerides, and beta-hydroxybuturate during a morning meal tolerance test in 17 subjects using a within-subjects fixed intake of 20% of total daily caloric intake of a CM or KM caloric load while subjects were ingesting the BD or KD diet and calculations of insulin sensitivity and islet cell function based on fasting values and those derived from MMT data.
| Table 6. | BD | KD | Correlations† | Diet effect‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CM test | KM test | CM test | KM test | R meal on BD | Rmeal on KD | Rdiet on CM | Rdiet on KM | CM test | KM test | |
| relAUCGluc (mg/dl × min) | 685 (687) | −102 (595)† | 2004 (1112) | 713 (1113)† | 0.69, P=0.002 | 0.36, N.S. | 0.03, N.S. | 0.52, P=0.03 | 1319 (1280), P<0.001 | 815 (951), P=0.003 |
| relAUCIns (mIU/ml × min) | 2525 (1386) | 1401 (1037)† | 3570 (2277) | 1348 (728)† | 0.67, P=0.003 | 0.65, P=0.004 | 0.59, P=0.012 | 0.62, P=0.008 | 1045 (1761), P=0.026 | 53 (845), N.S. |
| relAUCC-peptide (ng/ml × min) | 242 (87) | 131 (73)† | 377 (191) | 136 (66)† | 0.79, P<0.001 | 0.55, P=0.022 | 0.55, P=0.021 | 0.44, N.S. | 135 (160), P=0.003 | 5 (74), N.S. |
| relAUCFFA (mMol/L × min) | −16.0 (8.9) | 3.3 (10.2)‡ | −14.3 (19.0) | 1.9 (17.4)‡ | −0.03, N.S. | 0.18, N.S. | −0.06, N.S. | 0.46, N.S. | −1.7 (20.6), N.S. | 1.4 (15.7), N.S. |
| relAUCTG (mg/dl × min) | 2454 (1355) | 5768 (3052)‡ | 4346 (3801) | 7201 (3334)‡ | 0.67, P=0.003 | 0.60, P=0.011 | 0.22, N.S. | 0.65, P=0.005 | 451 (1776), N.S. | 1433 (2690) P=0.043 |
| relAUCglycerol (mg/L × min)a | 298 (272) | 651 (419)‡ | 849 (950) | 1374 (804)‡ | 0.24, N.S. | 0.77, P<0.001 | 0.33, N.S. | 0.68, P<0.005 | 406 (754), N.S. | 636 (469), P<0.001 |
| relAUCFFA/glycerol | −0.010 (0.20) | −0.001 (0.031) | −0.021 (0.19) | −0.019 (0.072) | −0.20, N.S. | 0.10, N.S. | 0.28, N.S. | 0.65, P=0.009 | 0.127 (0.251), N.S. | −0.019 (0.059), N.S. |
| Calculated Indices of β-cell function | ||||||||||
| HOMA- β (fasting)b | 128 (50) | 130 (54) | 137 (161) | 103 (155) | 0.63, P=0.007 | 00.37, N.S. | 0.11, N.S. | 0.59, P=0.013 | 9 (163), N.S. | −27 (131), N.S. |
| Insulinogenic Index | 0.33 (0.22) | 0.18 (0.13)‡ | 0.40 (0.18) | 0.17 (0.10)† | 0.48, P=0.053 | 0.39, N.S. | 0.49, P=0.045 | 0.48, P=0.051 | −0.07 (0.21), N.S. | −0.01 (0.12), N.S. |
| ISSI-2 | −21.7 (243.0) | 14.4 (91.2) | 22.8 (27.3) | 1.8 (134.1) | 0.02, N.S. | −0.04 (N.S.) | 0.26, N.S. | 0.08, N.S. | −44.4, (300.3), N.S. | −12.6 (159.7), N.S. |
| Calculated Indices of Glycemic Insulin Sensitivity | ||||||||||
| HOMA-IR (fasting)b | 1.4 (0.8) | 1.5 (0.9) | 1.3 (0.7) | 1.3 (1.5) | 0.75, P<0.001 | 0.78, P<0.001 | 0.71, P=0.0014 | 0.70, P=0.0016 | −0.1 (0.6), N.S. | −0.2 (1.1), N.S. |
| BelfioreFASTING Indexb | 1.06 (0.07) | 1.09 (0.07) | 1.09 (0.07) | 1.21 (0.09)* | 0.81, P<0.001 | 0.82, P<0.001 | 0.74, P<0.001 | 0.83, P<0.001 | 0.03 (0.05), N.S. | 0.13 (0.05), P=0.031 |
| Matsuda Index | 11.4 (6.4) | 14.4 (8.7)‡ | 10.0 (6.0) | 18.6 (10.4)† | 0.86, P<0.001 | 0.81, P<0.001 | 0.84, P<0.001 | 0.75, P<0.001 | −1.4 (3.3), N.S. | 4.2 (6.9), P=0.022 |
| BelfioreMMTglucose Index | 1.05 (0.07) | 1.27 (0.07)** | 0.90 (0.07) | 1.30 (0.06)** | 0.84, P<0.001 | 0.62, P=0.008 | 0.72, P=0.001 | 0.77, P<0.001 | −0.16 (0.04), P=0.002 | 0.03 (0.05), N.S. |
| Calculated Indices of FFA Flux Insulin Sensitivity | ||||||||||
| ADIPO-IR | 25.8 (13.9) | 26.2 (17.4) | 38.5 (25.9) | 30.6 (26.4) | 0.73, P=0.001 | 0.55, P=0.021 | 0.45, P=0.067 | 0.79, P<0.001 | 12.7 (23.1), P=0.037 | 4.5 (16.6), N.S. |
| BelfioreMMTFFA | 1.06 (0.24) | 1.14 (0.30) | 0.73 (0.29) | 1.00 (0.25) | 0.77, P<0.001 | 0.71, P=0.0017 | 0.65, P=0.005 | 0.71, P=0.0013 | −0.33 (0.22), P<0.001 | −0.14 (0.21), P=0.015 |
P<0.001 vs. baseline meal study
P<0.001 vs. baseline meal study.
Correlations between the mean values for weeks 3 and 4 on the BD with the mean values for weeks 3 and 4 on the KD.
Comparisons between the KD and BD were made using the mean of values obtained at last 2 weeks (designated as weeks 3 and 4)
n=16 due to incomplete data set in 1 subject
n=16 due to an outlier whose fasting insulin was 40 mIU/ml and whose C-peptide was 4.4 nMol/L in week 4.
Because these values were more than 3 SD above the mean, they were not included in the analysis. If data from this subject was included then the differences between groups in the Belfiorefasting index were no longer significant.Data are mean (SD).
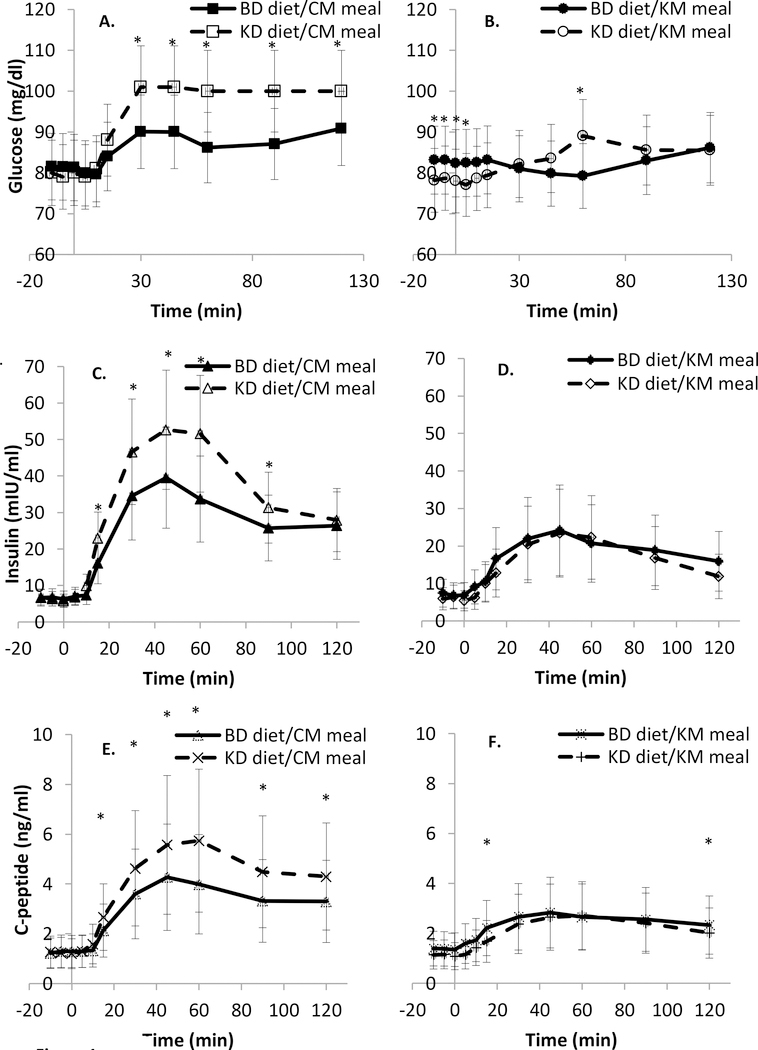
Figure 1.
Results of morning mixed meal testing. Glucose, insulin, and C-peptide excursions during the control meal (CM) with composition matched to the baseline diet (BD) (solid lines, solid markers) were significantly greater during the Ketogenic diet (KD) than the BD. After an isocaloric ketogenic meal (KM) (dashed lines, open markers), only the glucose excursion was significantly increased during the KD and C-peptide levels were significantly lower on the KD at 15 minutes and 120 minutes. *Diet difference P<0.05. See Methods for compositions of BD and KD. Data are mean (SD).
A. Glucose excursion during morning meal testing with a CM meal.
B. Glucose excursion during morning meal testing with a KM meal.
C. Insulin excursion during morning meal testing with a CM meal.
D. Insulin excursion during morning meal testing with a KM meal.
E. C-peptide excursion during morning meal testing with a CM meal.
F. C-peptide excursion during morning meal testing with a KM meal.
Fasting insulin, glucose, and C-peptide values were stable and significantly correlated between weeks 3 and 4 and within each diet. We have reported previously (7), that absolute insulin secretion, as estimated by 24hr urinary C-peptide excretion was significantly decreased on the KD. Figure 1 and Table 6 illustrate that the absolute and relative AUCglucose were significantly higher during the CM and KM on the KD (p=0.0001) that the absolute and relative AUCinsulin, but not AUCglucose, were significantly higher during the CM on the KD (p=0.0002), suggesting that insulin sensitivity was decreased during the CM (higher glucose and higher insulin) and probably decreased (higher glucose despite no significant decline in insulin) during the KM in subjects during the KD.
Absolute (Figure 1) and relative (Table 6) AUCC-pep were significantly higher during the CM on the KD (both p<0.0001), suggesting that the insulin differences during the CM between KD and BD were the result of increased insulin secretion rather than reduced clearance. Table 6 indicates that the AUCglucose relative to pre-meal values was significantly higher during both MMTs on the KD and the relative AUCinsulin was higher after the CM on the KD. The BelfioreMMTglucose index indicated that the KD resulted in impaired insulin sensitivity during the CM test, whereas the Matsuda index reflected improved insulin sensitivity during the KM test. Fasting tests of insulin sensitivity were not significantly different between diets. There were no significant effects of diet on any indices of insulinogenesis.
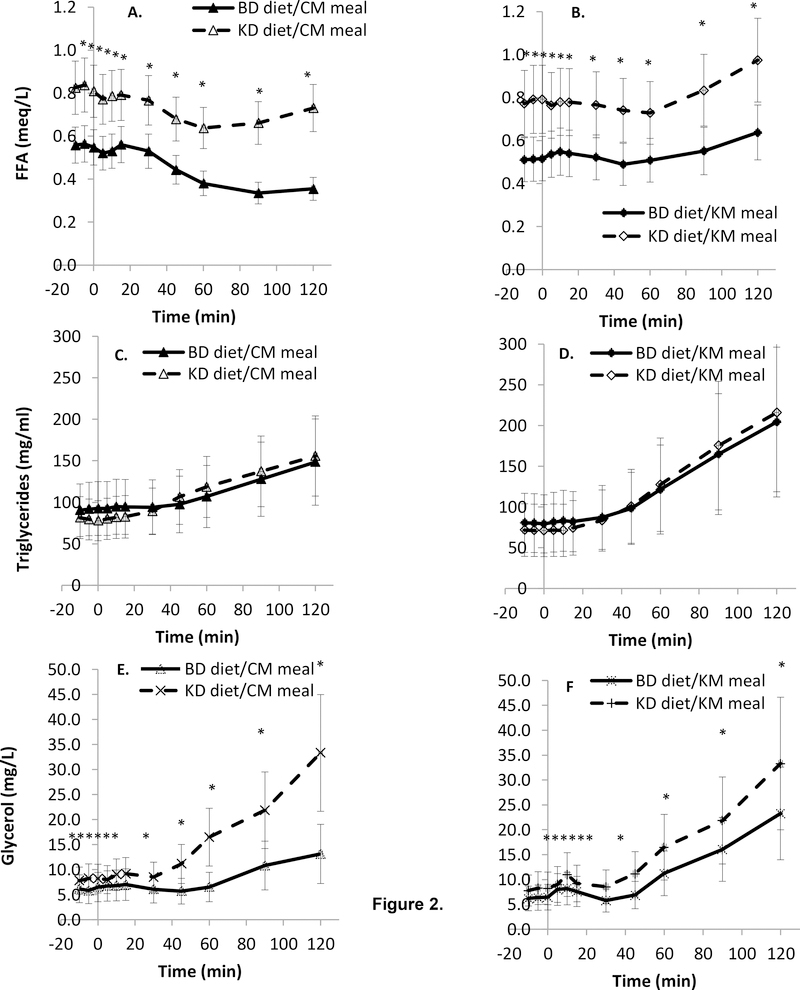
During the KM, the relative AUC for both glycerol and triglycerides was higher during the KD. Insulin-mediated anti-lipolysis was reduced during the KD whether calculated from fasting data (ADIPO-IR) or based on the MMT (BelfioreMMTFFA).
With the exception of relative AUCglucose during MMT on the BD, measurements of glucose and insulin concentrations and other molecules relevant to glucose and FFA homeostasis were highly correlated between BD and KD phases of the study.
Cytokines, Inflammatory Markers, and Other Molecules (Table 5)
Table 5.
Molecules relevant to insulin and glucose homeostasis. GIP - Gastric Inhibitory Polypeptide; GLP-1 - Glucagon- Like Peptide; FGF21 - Fibroblast Growth Factor 21.
| Table 5. | BD | KD | Correlationsa | Diet effectb | ||||
|---|---|---|---|---|---|---|---|---|
| Week 3 | Week 4 | Week 3 | Week 4 | RBD Weeks 3 and 4 | RKD Weeks 3 and 4 | Rmean BD vs KD | Mean Diff. KD-BD | |
| Glucagon (pg/ml) | 101(34) | 85 (33)* | 127 (39) | 119 (33) | 0.71, P=0.002 | 0.73, P=0.001 | 0.60, P=0.015 | 25 (29), P=0.003 |
| Adiponectin (ug/ml) | 3.74 (1.70) | 3.47 (1.67) | 6.31(2.98) | 6.59 (3.06) | 0.93, P<0.001 | 0.96, P<0.001 | 0.81, P<0.001 | 2.84 (1.91), P<0.001 |
| GIP (pg/ml) | 48.7 (21.2) | 47.6 (21.3) | 59.1(25.7) | 59.5 (28.0) | 0.85, P<0.001 | 0.73, P<0.001 | 0.73, P=0.001 | 11.1 (17.3), P=0.017 |
| GLP-1 (pM) | 4.97 (4.83) | 4.70 (4.82) | 5.83 (6.36) | 5.54 (6.72) | 0.99, P<0.001 | 0.97, P<0.001 | 0.98, P<0.001 | 0.85, N.S. |
| FGF 21 (pg/ml)a | 82 (27) | 75 (29) | 58 (17) | 58 (21) | 0.57, P=0.044 | 0.91, P<0.001 | −0.04, N.S. | −23 (30), P=0.022 |
| Peptide YY (pg/ml) | 71.8 (17.9) | 67.6 (17.3) | 72.0 (15.4) | 67.9 (19.9) | 0.58, P=0.014 | 0.47, P=0.060 | 0.64, P=0.006 | 0.2, N.S. |
| Cortisol (units) | 11.4 (2.9) | 10.2 (2.6) | 12.1(3.0) | 11.1 (2.1) | 0.64, P=0.006 | 0.35, N.S. | 0.80, P<0.001 | 0.8, N.S. |
| C-Reactive Protein (mg/L) | 1.17 (0.93) | 1.37 (1.01) | 1.70 (0.96) | 1.55 (0.89) | 0.95, P<0.001 | 0.84, P<0.001 | 0.75, P<0.001 | 0.45 (0.72), P=0.021 |
| Interleukin -6 (pg/ml) | 1.20 (0.50) | 1.21 (0.58) | 1.41 (0.90) | 1.13 (0.59) | 0.67, P=0.004 | 0.65, P=0.005 | 0.86, P<0.001 | 0.10, N.S. |
FGF21 values reported for only 12 subjects as the values for the remaining subjects were below the limits of detection for the assay (50 pg/ml).
n=12 due to the fact that at least one value for 5 of the subjects was below the detectable limit for the assay
Correlations between the mean values for weeks 3 and 4 on the BD with the mean values for weeks 3 and 4 on the KD.
Comparisons between the KD and BD were made using the mean of values obtained at last 2 weeks (designated as weeks 3 and 4). Data are mean (SD).
Data are mean (SD).
The inflammatory cytokines (CRP and IL-6), adiponectin, PYY, the insulinogenic and LPL-activating GIP, and the insulinogenic GLP-1 and FGF 21 were stable and significantly correlated between weeks 3 and 4 and within each diet. Circulating concentrations of CRP, adiponectin, glucagon, and GIP were significantly higher, while FGF21 were significantly decreased on the KD. PYY was not significantly changed. In all cases except FGF21, values during the BD were highly correlated with those measured during the KD.
Lipids (Table 7, Figure 2)
Table 7.
Comparisons of lipid values in 17 subjects ingesting isocaloric KD and BD. Total cholesterol, triglycerides, HDLcholesterol, and LDL-cholesterol were stable within each of the two testing periods during the BD and KD.
| Table 7. | BD | KD | Correlationsa | Diet effectb | ||||
|---|---|---|---|---|---|---|---|---|
| Week 3 | Week 4 | Week 3 | Week 4 | RBD Weeks 3–4 and 4 | RKD Weeks 3–4 and 4 | Rmean BD and KD | Mean Diff. KD-BD | |
| Total Plasma Cholesterol (mg/dl) | 190 (28) | 184 (28) | 213 (46) | 208 (36) | 0.87, P<0.001 | 0.93, P<0.001 | 0.93, P<0.001 | 9 (12), P=0.009 |
| Triglycerides (mg/dl) | 106 (28) | 102 (26) | 85 (33) | 82 (29) | 0.96, P<0.001 | 0.88, P<0.001 | 0.26 (N.S.) | −21 (35), P=0.026 |
| HDL Cholesterol (mg/dl) | 44 (11) | 43 (10) | 46 (15) | 44 (10) | 0.96, P<0.001 | 0.93, P<0.001 | 0.92, P<0.001 | 2 (5), N.S. |
| LDL Cholesterol (mg/dl) | 125 (27) | 122 (29) | 150 (38) | 148 (33) | 0.91, P<0.001 | 0.95, P<0.001 | 0.84, P<0.001 | 26 (19), P<0.001 |
Correlations between the mean values for weeks 3 and 4 on the BD with the mean values for weeks 3 and 4 on the KD.
Comparisons between the KD and BD were made using the mean of values obtained at last 2 weeks (designated as weeks 3 and 4).
Data are mean (SD).
Figure 2.
Lipids during the CM and KM mixed meal tolerance tests. FFA and glycerol were significantly greater at all time points on the KD. Data are mean (SD).
A. FFA excursion during morning meal testing with the CM.
B. FFA excursion during morning meal testing with the KM.
C. Triglyceride excursion during morning meal testing with the CM.
D. Triglyceride excursion during morning meal testing with the KM.
E. Glycerol excursion during morning meal testing with the CM.
F. Glycerol excursion during morning meal testing with the KM.
Fasting total, HDL-, and LDL- cholesterol and triglycerides were all stable and significantly correlated between weeks 3 and 4 and within each diet. Fasting total- and HDL- cholesterol were significantly higher, and triglyceride was significantly lower, on the KD. The KM led to increased relative AUCTG regardless of the prevailing diet and relative AUCTG during the KM was increased on the KD. Fasting concentrations of total-, HDL-, and LDL-cholesterol (but not triglycerides) on the BD were highly correlated with values during the KD.
Correlations between different measures of fuel utilization
Correlational analyses between fasting and MMT-derived indices of fuel utilization were examined for diet and meal effects (see Supplement 2). We found highly significant correlations of fasting and dynamic indices of insulin-sensitivity and FFA-flux, but not insulin secretion suggesting that extrapolations of fasting to post-prandial measures of insulin-mediated glucose disposal are dependent on both current diet and acute meal type.
Discussion
We examined biochemical parameters relevant to insulin sensitivity, inflammation, and dyslipidemia during consumption of a BD whose relative composition of fat, carbohydrate, and protein was similar to the average American diet, and following the transition to an isocaloric KD as part of a study of the effects of diet composition on energy homeostasis (7). We found that switching from the BD to the KD resulted in significantly increased circulating concentrations of fasting ketones, glycerol, FFA, glucagon, adiponectin, GIP, total and LDL cholesterol, CRP, and decreased fasting insulin, C-peptide, triglycerides, and FGF-21. MMTs indicated that the AUC for glucose was significantly higher on the KD regardless of whether the test meal was CM or KM, and the AUC for insulin and C-peptide on the KD were significantly higher during the CM. Measures of glucose homeostasis derived from the MMT suggested that the KD impaired insulin sensitivity in response to the CM, and improved sensitivity in response to the KM. Insulin-mediated anti-lipolysis on the KD was decreased following both meal tests.
Similar to the present study, Jebb et al (21) found no effects of isocaloric low vs. high glycemic index diets on insulin sensitivity over a 4-week period. Partsalaki et al (22) examined the effects of an ad libitum ketogenic diet vs. a low calorie diet (30–35% fat, 50–55% carbohydrate; 500 kcal/day deficit) in children with obesity and found that while there was greater weight loss on the KD, there were no significant differences in insulin sensitivity beyond those attributable to weight loss. Some studies have reported significant adverse effects of overfeeding of high vs. low carbohydrate diets on insulin sensitivity during weight gain (23) and on hepatic insulin sensitivity during weight regain (24), suggesting that both weight change and diet composition significantly alter carbohydrate effects on glucose homeostasis. Other studies, some recent (25) and others dating back over 75 years (26), have reported that low-carbohydrate, but not necessarily ketogenic diets, may result in impaired glucose tolerance in some individuals. The overall lack of clear generalizable health benefit of one diet over the other is similar to that recently reported by Gardner et al in subjects studied over 12 months on low-carbohydrate vs. low-fat weight loss diets (27).
The physiological significance of the apparent increased insulin sensitivity in response to a ketogenic MMT vs. the decreased insulin sensitivity to a baseline diet MMT on the KD (both relative to the baseline diet) cannot be determined from these data. The health consequences of improved insulin sensitivity as long as one remains on a ketogenic diet may be small because of the consistently low circulating insulin concentrations on said diet. Similarly, the duration of the implied decreased insulin sensitivity in someone transitioning from a low carbohydrate or ketogenic diet to a higher carbohydrate diet and so the long-term health effects cannot be assessed at present.
Blunting of insulin-mediated anti-lipolysis on the KD should favor loss of body fat. However, the physiological significance of this finding is unclear because it seems unlikely that the additional effects of the KD to diminish insulin-mediated anti-lipolysis would add significantly to the high levels of lipolysis already occurring as a result of low circulating insulin concentrations. In fact, loss of body fat was not increased during the KD period as compared to the BD period as previously reported (7).
The increases in plasma adiponectin during the KD would presumably promote insulin sensitivity (28), but may reflect the state of negative energy balance and weight loss rather than an effect of reduced carbohydrate ingestion per se. Other studies of moderate carbohydrate restriction (<30% total calories) without weight loss, or with caloric restriction (−500 kcal/day for 4–6 weeks, a greater negative energy balance and weight loss than in the present study) have not found a significant carbohydrate effect on circulating adiponectin (29). There are, to our knowledge, no studies of the effects of a weight maintenance KD on adiponectin in humans other than a study of 10 children with refractory epilepsy due to GLUT1 deficiency which found no significant changes in circulating adiponectin after 3 months of a ketogenic diet (30).
Low protein and diets in humans and mice have been shown to result in increased FGF21 (31) while calorie restricted ketogenic diets have been reported to decrease FGF 21 (32). In this isocaloric study, since the protein content of the BD and KD remained constant, the observed decrease in FGF21 on the KD most likely reflected the ketogenic diet (33). Reduced FGF21 could be associated with decreased glucose uptake by adipocytes during carbohydrate restriction, as has been reported in mice (13) and may be ameliorated by ketosis (34). Circulating concentrations of FGF21 on either diet were not significantly correlated with any fasting or MMT-derived indices of insulin secretion or sensitivity.
A recent meta-analysis (35) suggested that a ketogenic diet during weight loss results in a small but significant decrease in appetite. Studies during weight maintenance or using a low carbohydrate diet have yielded varied results (36, 37). The lack of differences between diets in in circulating concentrations of PYYand GLP-1 suggests that any effects of a ketogenic diet on appetite are not mediated by these molecules. Circulating concentrations of CRP, but not the inflammatory cytokine IL-6, were significantly higher on the KD. Ketosis in mice has been reported to increase tissue-specific (liver and white adipose tissue) expression of inflammatory cytokines [e.g., tumor necrosis factor-alpha (TNF-α), IL-6 and macrophage markers (38)] and to down regulate the FGF receptor (39). Outpatient studies of low carbohydrate diets in humans have found significant increases in plasma CRP but not IL-6 (5, 40) In subjects with type 2 diabetes, advice to follow a lower carbohydrate diet decreased IL-6, but not CRP concentrations (5, 41).
During the KD our subjects had higher fasting levels of plasma total and LDL cholesterol but lower triglycerides. Interestingly, the AUCs for triglycerides were higher during the KD regardless of the composition of the mixed meal test, but the KM led to significantly greater postprandial triglycerides excursion (peak – baseline) on both diets. Furthermore, the AUC for triglycerides after the KM was further increased during the KD period; this result differs from previous studies showing that adaptation to a high fat/low carbohydrate diet decreases postprandial triglycerides following a high fat meal (42). Perhaps the limited duration of the meal tests in the current study was not sufficient to reflect improvements in triglyceride clearance associated with adaptation to a high fat diet.
With the exception of triglycerides and FGF 21, all BD measures of insulin sensitivity, molecules affecting glucose homeostasis, and plasma lipids were highly predictive of values obtained during the KD. These correlations suggest that while the rank order of propensity towards adiposity-related co-morbidities within a given population on a similar diet remains stable, the absolute risk of adiposity-related co-morbidities can be modified, but not eliminated, by diet modification.
A strength of this study is that it was conducted in an inpatient environment with assured dietary and behavioral compliance as evident in biochemical/endocrine test stability. This investigation is limited by its examination of an extremely low carbohydrate (ketogenic) diet that differed only in relative macronutrient proportions, not macronutrient quality. The results cannot be extrapolated to low carbohydrate, non-ketogenic diets or diets with specific restrictions on types of sugars or fats or to the effects of dietary macronutrient content on co-morbidities over longer periods of time. The lack of a randomized crossover design fails to control for possible significant effects of testing order (5). The unintentional weight loss may have biased the results towards improved insulin sensitivity, lipids, and inflammatory markers, in subjects on the KD. (5)
In summary, this study shows that switching from a BD to an isocaloric KD is associated with: increased plasma LDL cholesterol but decreased triglycerides; increased plasma CRP but decreased FGF 21; reduced glycemic insulin sensitivity to a meal with normal carbohydrate content; improved insulin sensitivity to a ketogenic meal; and impaired anti-lipolysis insulin sensitivity regardless of meal type. Importantly, even drastic changes in diet macronutrient composition did not affect the rank order of an individual’s risk factors for metabolic disease. Those subjects with the highest and lowest risk reflected by glucose homeostasis, inflammation, and circulating lipid concentrations maintained the same relative rank order regardless of diet.
Supplementary Material
What is already known about this subject?
The use of low carbohydrate and very low carbohydrate diets to reduce weight and promote better glucose homeostasis, lipid homeostasis, and reduce inflammatory markers is increasing.
Outpatient clinical trials to test the hypothesis that reduction in dietary carbohydrate intake reduces obesity and its associated co-morbidities are often confounded by variable subject compliance, lack of control of physical activity, or difficulties separating diet quality (types of carbohydrate, fat, and protein) from macronutrient composition.
What does this study add?
This is a highly controlled in-patient study of the effects of switching from a baseline diet (15% protein, 50% carbohydrate, 35% fat) to an isocaloric ketogenic diet (15% protein, 5% carbohydrate, 80% fat)on glucose and lipid metabolism and inflammation in men with obesity.
This study demonstrates that that switching to a ketogenic diet is associated with increased cholesterol and inflammatory markers, decreased triglycerides and decreased insulin-mediated anti-lipolysis.
This study also demonstrates that the relationship between fasting and meal-related indices of glucose homeostasis may be significantly affected by the background diet.
Acknowledgements
The primary funder of this study, Nutrition Sciences Initiative (NuSI), convened the research team, helped formulate the hypotheses, and provided partial funding. NuSI and its scientific advisors were given the opportunity to comment on the study design and this manuscript, but the investigators retained full editorial control. Jeff Volek and Brittanie Volk from Beyond Nutrition Inc. helped design the study diets in collaboration with the investigators and the study dietitians: Courtney Brock, Amber Courville, Wahida Karmally, Pamela Legowski, and Renee Puyau. Serge Cremers, Mary Walter, and Peter Walter assayed the blood samples. Crystal Brown, Emma Crayner, Lilian Howard, Karen Jones, Kalle Liimatta, Elinor Naor, Stacy Shankleton, Monica Skarulis, Celeste Waguespack, and Laura Yannai were study coordinators and research support staff. Dympna Gallgher (CUIMC) was responsible for the detailed body composition analyses. We are most grateful to the study subjects who volunteered to participate in this demanding protocol.
Grant Support: The Nutrition Science Initiative (NuSI) was the primary funder for this study. This work was also supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (KDH, KYC, MLR), NIH UL1 TR00040 [Columbia CTSA, ( MR and RL)], and partially by a NORC Center Grant # P30DK072476 (ER).
Footnotes
Disclosures: None
Literature Cited
- 1.Abbasi J Interest in the Ketogenic Diet Grows for Weight Loss and Type 2 Diabetes. JAMA 2018;319: 215–217. [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Sargard K, Homko C, Mozzoli M, Stein T. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142: 403–411. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien K, Brehm B, Seeley R, Bean J, Wener M, Daniels S, et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and c-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab 2005;90: 2244–2249. [DOI] [PubMed] [Google Scholar]
- 4.Hall K A review of the carbohydrate-insulin model of obesity. Eur J Clin Nutr 2017;71: 323–326. [DOI] [PubMed] [Google Scholar]
- 5.Ebbeling C, Swain J, Feldman H, Wong W, Hachey D, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight loss maintenance. JAMA 2012;307: 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, GIlhooly C, Golden J, Pittas A, Fuss P, Cheatham R, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 2007;85: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 7.Hall K, Chen K, Guo J, Lam Y, Leibel R, Mayer L, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Amer J Clin Nutr 2016;104: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 2002;283: R281–R283. [DOI] [PubMed] [Google Scholar]
- 9.Neuhauser M How to deal with multiple endpoints in clinical trials. Fundam Clin Pharmacol 2006;20: 515–523. [DOI] [PubMed] [Google Scholar]
- 10.Unger R, Cherrington A. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham S, Rubino D, Sinali N, Ramsey S, Nieman L. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obes 2013;21: E105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szendroedi J, Frossard M, Klein N, Beiglmayer C, Wagner O, Pacini G, et al. Lipid-induced insulin resistance is not mediated by impaired transcapillary transport of insulin and glucose in humans. Diabetes 2012;61: 3176–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata Y, Nishio K, Mochiyama T, Konshi M, Shimada M, Ohta H, et al. Fgf21 impairs adipocyte insulin sensitivity in mice fed a low-carbohydrate, high-fat ketogenic diet. PLoS One 2013;8: e69330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck B Gastric inhibitory polypeptide: a gut hormone with anabolic function. J Mol Endocrinol 1989;2: 169–174. [DOI] [PubMed] [Google Scholar]
- 15.D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-dependent glucose disposal. J Clin Invest 1994;93: 2263–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boey D, Sainsbury A, Herzog H. The role of peptide YY in regulating glucose homeostasis. Peptides 2007;28: 390–395. [DOI] [PubMed] [Google Scholar]
- 17.Søndergaard E, De Ycaza A, Morgan-Bathke M, Jensen M. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2017;102: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belfiore F, Iannello S, Volpicelli G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Mol Gen Metab 1998;63: 134–141. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo C, Haffner S, Kuusisto J, Laakso M. Fasting and OGTT-derived measures of insulin resistance as compared with the euglycemic-hyperinsulinemic clamp in nondiabetic Finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab 2015;100: 544–550. [DOI] [PubMed] [Google Scholar]
- 20.Patarrao R, Lautt W, Macedo M. Assessment of methods and indexes of insulin sensitivity. Rev Port Endocrinol Diabetes Metab 2014;9: 65–73. [Google Scholar]
- 21.Jebb S, Lovegrove J, Griffin B, Frost G, moore C, Chatfield M, et al. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Amer J Clin Nutr 2010;92: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partsalaki I, Karvela A, Spiliotis B. Metabolic impact of ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocr Met 2012;24: 697–704. [DOI] [PubMed] [Google Scholar]
- 23.Lagerpusch M, Enderle J, Eggeling B, Braun W, Johannsen M, Paper D, et al. Carbohydrate quality and quantity affect glucose and lipid metabolims during weight regain in healthy men. J Nutr 2013;143: 1593–1601. [DOI] [PubMed] [Google Scholar]
- 24.Hron B, Ebbeling C, Feldman H, Ludwig D. Hepatic, adipocyte, enteric and pancreatic hormones: response to dietary macronutrient composition and relationship with metabolism. Nutr Metab (London) 2017;14: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Numao S, Kawano H, Endo N, Yamada Y, Konishi M, Takahashi M, et al. Short-term low carbohydrate/high-fat diet intake increaed postprandial glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test. Eur J Clin Nutr 2012;66: 926–931. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney J Dietary factors that influence the dextrose tolerance test: A preliminary study. Arch Int Med 1927;40: 818–830. [Google Scholar]
- 27.Gardner C, Trepanowski J, Del Gobbo L, Hauser M, RIgdon J, Ioannidis J, et al. Effects of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018;319: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuhouser M, Schwartz Y, Wang C, Breymeyer K, Coronado G, Wang C, et al. A low-glycemic load diet reduces derum C-Reactive protein and modestly increases adiponectin in overweight andobese Adults. J Nutr 2012;142: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajaie S, Azadbakhy L, Saneei P, Khazaei M, Esmaillzadeh A. Comparative effects of carbohydrate versus fat restriction on serum levels of adipocytokines, markers of inflammation, and endothelial function among women with the metabolic syndrome: a randomized cross-over clinical tria. Ann Nutr Metab 2013;63: 159–167. [DOI] [PubMed] [Google Scholar]
- 30.Bertoli S, Neri I, Trentani C, Ferraris C, De Amicis R, Battezzati A, et al. Short-term effects of ketogenic diet on anthropometric parameters, body fat distribution, and inflammatory cytokine production in GLUT1 deficiency syndrome. Nutr 2015;31: 981–997. [DOI] [PubMed] [Google Scholar]
- 31.Laeger T, Henagan T, Albarado D, Redman L, Bray G, Noland R, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014;124: 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circualting fibroblast growth fact 21 is induced by peroxisome proigerator-activiated receptor agonists but not ketosis in man. J Clin Endocrinol Metab 2009;94: 3594–3601. [DOI] [PubMed] [Google Scholar]
- 33.Hill C, Berthoud H, Munzberg H, Morrison C. Homeostastic sensing of dietary protein restriction: A case for FGF21. Front Neuroendocrinol 2018: doi: 10.1016/j.yfrne.2018.1006.1002. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazeli P, Lun M, Kim S, Bredella M, Wright S, Zhang Y, et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest 2015;125: 4601–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GIbson A, Seimon R, Lee C, Ayre J, Franklin J, Markovic T, et al. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev 2015;16: 64–76. [DOI] [PubMed] [Google Scholar]
- 36.Veldhorst M, Westerterp K, van Vught AMW-P. Presence or absence of carbohydrates and the proportion of fat in a high-protein diet affect appetite suppression but not energy expenditure in normal-weight human subjects fed in energy balance. Br J Nutr 2010;104: 1395–1405. [DOI] [PubMed] [Google Scholar]
- 37.Stubbs R, Mazian N, Whybrow S. Carbohydrates, appetite and feeding behavior in huamns. J Nutr 2001;131: 2775S–2781S. [DOI] [PubMed] [Google Scholar]
- 38.Garbow J, Doherty J, Schugar R, Travers S, Weber M, Wentz A, et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Phyiol 2011;300: G956–G967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asrih M, Altirriba J, Rohner-Jeanrenaud F, Jornavyaz F. Ketogenic diet impairs FGF21 signaling and promotes differential inflammatory responses in the liver and white adipose tissue. PLoS One 2015;10: e0126364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song X, Kestin M, Schwarz Y, Yang P, Hu X, Lampe J, et al. A low-fat high-carbohydrate diet reduces plasma total adiponectin concentrations compared to a moderate-fat diet with no impact on biomarkers of systemic inflammation in a randomized controlled feeding study. Eur J Nutr (Epub Ahead of Print) 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonasson L, Guldbrand H, Lundberg A, Nystrom F. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med 2014;46: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volek J, Phinney S, Forsythe C, Quann E, Woods R, Puglisi M, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009;44: 297–309. [DOI] [PubMed] [Google Scholar]
- 43.Hall K, Bemis T, Brychata R, Chen K, Courville A, Crayner E, et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab 2015;22: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.