Abstract
Objective
To highlight changing trends of the clinical spectrum, and compare the management options and predictors of Fournier’s gangrene (FG) outcomes in a tertiary care referral center.
Material and methods
This study included patients with FG between August 2005 and July 2017. Patients were classified as “responders” and “nonresponders.” We compared the baseline characteristics, clinical spectrum, biochemical data, management modalities, outcomes, and FG severity index (FGSI) and age-adjusted Charlson Comorbidity Index (ACCI) between responders and nonresponders.
Results
We studied 72 patients and further divided them to responders (60 patients) and non-responders (12 patients). All were males; the mean age was 56.27+19.27 years (range, 47–85 years). The most common complaints were perineal discomfort (n=62; 86.1%) and fever (n=48; 66.7%). FG originated from the penoscrotal region in 64 patients (88.8%) and perineal region in 8 patients. Diabetes mellitus was the most common comorbidity (36%). The mean duration of the presentation was 10.19 days (range, 7–30 days). Sixteen patients underwent split skin grafting. The mortality rate was 8.3%. Nonresponders had distinct findings relative to responders: advanced age (71.5±7.17 vs. 53.23±19.85 years; p=0.00); high blood sugar (245.83±116.26 vs. 139.06±35.64 mg/dL; p<0.01); leukocytosis (27166.67±10295.75 vs. 10558.4±3130.64 cumm; p<0.01); elevated serum creatinine (3.78±1.43 vs. 1.38±1.00; p<0.01); hyponatremia (127.33±11.84 vs. 137.33±3.42 meq/l; p<0.01), elevated international normalized ratios (1.66±0.28 vs. 1.32±0.07; p<0.01); and high FGSI (9.83±1.11 vs. 6.46±1.68;p<0.01) and ACCI scores (6.33±0.49 vs. 5±0.82; p<0.01). On univariate and multivariate regression analysis, raised blood sugar and deranged international normalized ratios at presentation were significantly associated with decreased response to treatment (p<0.05).
Conclusion
An advanced age, diabetes mellitus, renal impairment, leukocytosis, altered sensorium, shock at presentation, deranged international normalized ratios, and high FGSI and ACCI scores can be used as predictors for poor response. FG risk scores adequately characterize the severity and prognosis of FG, but clinician’s judgement is vital. The management comprises of a multidisciplinary approach, including parenteral antibiotics, urgent surgical debridement, and comorbidities optimization.
Keywords: ACCI, antibiotics, debridement, FGSI, Fournier’s gangrene, mortality, outcome
Introduction
Fournier’s gangrene (FG) refers to a rapidly progressing infectious and necrotizing fasciitis, involving perineum, genitalia, or perianal regions with associated necrosis of overlying skin.[1] The initial description of FG was given by Nathan et al.[2] in 1764, while the entity was named in 1883 after Jean-Alfred Fournier, a French venereologist when he described a case of sudden-onset rapidly progressive genital gangrene in a young healthy man. FG is commonly associated with an advanced age, diabetes mellitus (DM), alcoholism, and conditions causing immunosuppression, such as malnutrition, obesity, chronic kidney disease (CKD), chronic liver disease, and malignancies.[3,4] FG can also arise secondary to untreated urogenital or anorectal diseases.[5] The etiology is mostly polymicrobial involving gram-positive, gram-negative, and anaerobic bacteria reflecting normal flora of urogenital or enteric region.[6–8] Despite various improvements in surgical practice and availability of new treatments, the reported mortality ranges from 4% to 80%, with an average mortality reported to be around 20%.[9,10] Various scoring systems have been proposed that predict the severity of FG.[11–13] Charlson et al.[11] proposed age-adjusted Charlson Comorbidity Index (ACCI) that includes different scores for each comorbid illnesses; they proposed that the ACCI score >5 was associated with higher mortality rates. FGSI is a severity score comprising of vital signs (heart rate, respiratory rate, body temperature) and metabolic variables (hematocrit, serum sodium, bicarbonate, potassium, creatinine, and leucocytes); it has been previously used to prognosticate and stratify patients with FG.[12] There is paucity of studies on outcomes of FG from developing countries, including the Indian subcontinent, so we wanted to review our experience in the management of this entity in past 12 years. Our study focused on the clinical spectrum, management, and outcomes of FG in responders and nonresponders.
Material and methods
In the present study, medical records of 91 patients from August 2005 to July 2017 were reviewed. Eight patients were lost to follow-up, and data of 11 patients were incomplete. Hence the final analysis included data of 72 patients, who were further categorized as responders (60 patients) and nonresponders (12 patients).
This study included a retrospective data analysis of patients, who were diagnosed with and received treatment for FG, in the Department of Urology, King George’s Medical University, Lucknow, Uttar Pradesh, India, between August 2005 and July 2017. Ethical approval was obtained from the Institutional Ethical Committee. The diagnosis of FG was based on clinical criteria comprising of the following features: local redness, edema, and tenderness arising initially from the urogenital or perianal area with or without further progression of the infection posteriorly and to other parts of the body (Figure 1).[14] If the appearance of these features initially occurred in other parts of the body and later on involved the urogenital and perineal regions, these cases were excluded. The parameters studied included baseline patient characteristics, clinical spectrum, basic laboratory parameters, given therapy, and the outcome. The baseline patient evaluation comprised of age, gender, body mass index (BMI), and history of comorbid illnesses. Clinical evaluation included duration from the onset of symptoms to access to medical care and diagnosis, hemodynamic status, and level of consciousness. Shock was defined as systolic blood pressure <90 mmHg. The level of consciousness was considered to be either confusion, delirium, stupor, and coma. Basic laboratory evaluation comprised complete blood counts, renal function tests, liver function tests, evaluation of blood sugar, urine examination, and microbial culture sensitivities from the wound. We also evaluated the surgical procedure done, the origin of infection, depth of infection, need of reoperation, postoperative complications, mortality, and hospital stay. Patients were managed according to the severity of infection and associated comorbidities. Patients were discharged when the wound was healthy and granulating, and when toxic symptoms resolved. In cases where the wound size was too large for healing by secondary intention, a split-thickness skin graft (SSG) was placed over the wound. We have classified our patients as “responders” and “nonresponders” to find the predictors of outcome. Responders were defined as those patients who were successfully treated or who showed the signs of improvement within one week. The nonresponder group consisted of the patients who died or had progressive worsening of symptoms 48 hours after aggressive medical/surgical treatment. The outcome parameters included the difference in baseline characteristics, clinical spectrum, biochemical data, management modalities, outcomes, and FG severity index (FGSI) and ACCI between responders and nonresponders.
Figure 1.
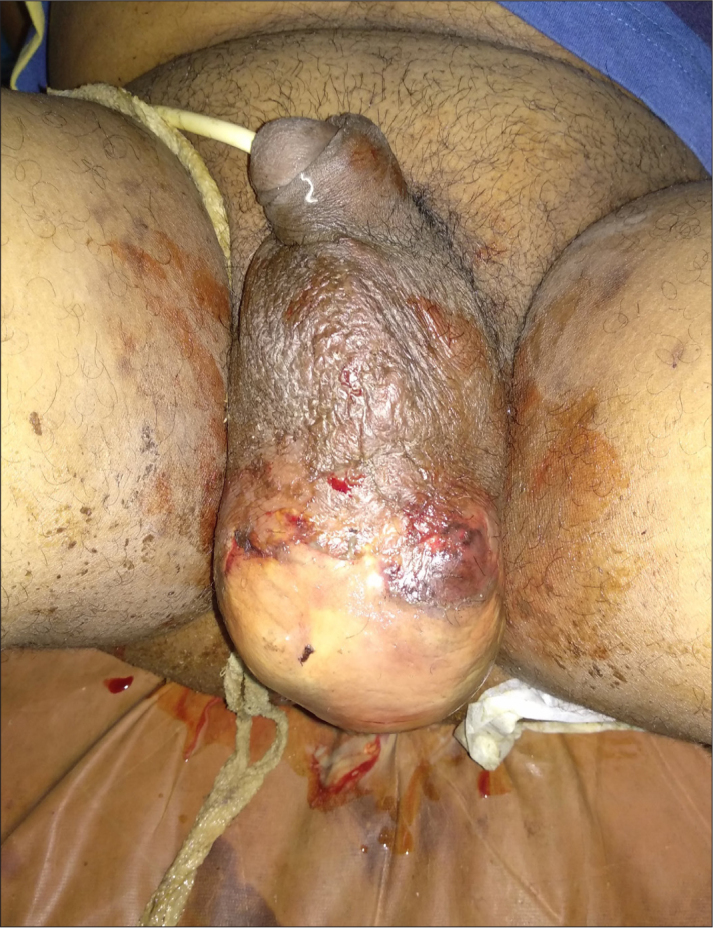
Clinical image depicting Fournier’s Gangrene
Statistical analysis
Data were recorded in the Microsoft Excel spreadsheet (Microsoft, Seattle, WA, USA) and analyzed using the IBM Statistical Package for the Social Sciences software package version 23.0 (IBM SPSS Corp.; Armonk, NY, USA). Fisher’s exact test was used for categorical data, and the unpaired t-test was used for continuous data. A univariate and multivariate regression analysis was used to test the correlation between nonresponders and several clinical variables. A p-value <0.05 was considered statistically significant.
Results
All affected patients were males with the mean age at presentation 56.27 years (range, 47–85 years). DM was the most common comorbidity in 26 patients (36.1%), followed by CKD in 24 patients (33.3%) and liver disorders in 15 patients (20.83%). Two patients (2.7%) had coexisting bladder neoplasms, while 18 subjects (25%) had preceding urethral stricture. The mean duration of presentation was 10.19 days (range, 7–30 days). The most common clinical complaints were perineal discomfort (n=62; 86.1%) and fever (n=48; 66.7%), and on clinical examination, most frequently, patients had local edema (n=60, 83.3%), erythema with induration (n=38, 52.7%), skin necrosis (n=66, 91.6%), and regional crepitation (n=20, 27.7%). Patients were managed according to the severity of infection with aggressive surgical debridement of necrotic tissue along with intravenous antibiotics (covering gram-positive and gram-negative microorganisms, and anaerobes). The antibiotics were later modified based on clinical response, laboratory parameters, and as per culture sensitivity reports. Tables 1 and 2 show clinical and laboratory features of responders and nonresponders. Microbial cultures revealed polymicrobial growth in cultures obtained from the wound in both the groups (Table 3). A significantly greater number of patients in the nonresponders group presented with advanced age, altered sensorium, shock, leucocytosis, raised blood sugars, serum creatinine, and FGSI and ACCI scores compared to responders (p<0.05). The outcome of FG patients is depicted in Table 4. Sixty patients responded to treatment. Six patients succumbed to death within 72 hours after the admission due to septic shock and multiorgan dysfunction syndrome resulting from necrotizing fasciitis. Urinary diversion in the form of suprapubic cystostomy was performed in 18 patients; however, none of the patients required bowel diversion. Thirty-seven (51.38 %) patients had to undergo re-debridement (single/multiple) due the progression of necrotic areas and presence of purulent material in the wound. Sixteen patients underwent split skin grafting (SSG) of the debrided areas by plastic surgeon (Figure 2). On the univariate and multivariate regression analysis, raised blood sugars and deranged international normalized ratios at presentation were significantly associated with a decreased treatment response (p<0.05). Medical complications were seen in 26 patients (36.1%): 17 lower respiratory tract infections, 7 urinary infections, and 2 central venous catheter infections. All the complications were successfully managed with conservative management and antibiotics.
Table 1.
Baseline characteristics and clinical features
| Parameter | Responders | Nonresponders | p |
|---|---|---|---|
| Number | 60 | 12 | |
|
| |||
| Age (years) | 53.23±19.85 | 71.5±7.17 | <0.01 |
|
| |||
| Body mass index (kg/m2) | 21.63±2.18 | 20.66±2.67 | 0.09 |
|
| |||
| Duration between presentation and admission (days) | 9.46±3.55 | 13.83±8.14 | 0.01 |
|
| |||
| Hemodynamic status* | Normal-60 | Normal -6 | 0.01 |
| Shock-6 | |||
|
| |||
| Level of consciousness& | Normal-50 | Normal-3 | 0.01 |
| Confusion-10 | Confusion-3 | ||
| Delirium-0 | Delirium-4 | ||
| Stupor-0 | Stupor-2 | ||
| Coma-0 | Coma-0 | ||
|
| |||
| Diabetes mellitus (DM) | 18 | 8 | 0.02 |
|
| |||
| Infection origin$ | Penoscrotal region-60 | Penoscrotal region-4 | 0.01 |
| Perineal region-0 | Perianal region-8 | ||
|
| |||
| Initial depth of involvement | Superficial-40 | Deep to fascia-6 | 0.01 |
| Deep to fascia-20 | Deep to muscle-6 | ||
Shock was defined as a systolic blood pressure <90 mmHg.
The level of consciousness was considered to be either normal, confused, delirium, stupor, and coma.
The infection origin was considered to be either penoscrotal or perineal region.
Initial depth of involvement was considered to be either superficial, deep to fascia, or deep to muscle.
Table 2.
Laboratory findings
| Parameter | Responders | Nonresponders | p |
|---|---|---|---|
| Hb (g/dL) | 10.74±0.83 | 10.53±1.26 | 0.23 |
| TLC (cu/mm) | 10558.4±3130.64 | 27166.67±10295.75 | <0.01 |
| S. creatinine (mg/dL) | 1.38±1.00 | 3.78±1.43 | <0.01 |
| S. sodium (meq/L) | 137.33±3.42 | 127.33±11.84 | <0.01 |
| RBS (mg/dL) | 139.06±35.64 | 245.83±116.26 | <0.01 |
| S. Bilirubin | 0.43±0.23 | 0.55±0.23 | 0.04 |
| SGOT (U/L) | 54.6±14.66 | 53.83±28.09 | 0.44 |
| SGPT (U/L) | 61.32±8.48 | 55.83±2.12 | 0.15 |
| PT-INR | 1.32±0.07 | 1.66±0.28 | <0.01 |
| FGSI | 6.46±1.68 | 9.83±1.11 | <0.01 |
| ACCI | 5±0.82 | 6.33±0.49 | <0.01 |
Hb: hemoglobin; TLC: total leucocyte count; RBS: random blood sugar; FGSI: Fournier’s gangrene severity index; ACCI: age adjusted Charlson Comorbidity Index
Table 3.
Bacteriology of isolates from wound cultures in patients with FG
| Parameter | Total Number | Responders | Nonresponders |
|---|---|---|---|
| Streptococcus pyogenes | 15 | 12 | 3 |
| Escherichia coli | 16 | 15 | 1 |
| Staphylococcus aureus | 7 | 6 | 1 |
| Pseudomonas aeruginosa | 3 | 2 | 1 |
| Klebsiella spp | 3 | 3 | 0 |
| Enterobacter spp | 2 | 2 | 0 |
| Bacteroides spp | 2 | 1 | 1 |
Table 4.
Outcome in patients with FG
| Parameter | Responders | Nonresponders | p |
|---|---|---|---|
| Intervention | |||
| Surgical debridement | 60 | 12 | 1.00 |
| Split skin grafts (SSG)/flaps | 20 | 6 | 0.33 |
|
| |||
| Re-operations | 1.23±0.76 | 2.67±1.15 | <0.01 |
|
| |||
| Hospital stay (days) | 11.53±2.71 | 18.33±19.04 | <0.01 |
|
| |||
| Mortality | 0 | 6 | <0.01 |
Figure 2.
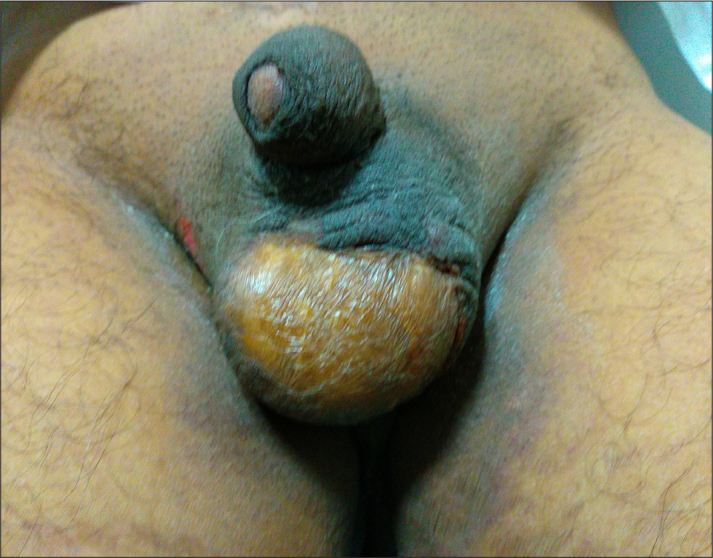
Clinical image depicting a healed scrotal wound with a split skin thickness graft
Discussion
Fournier’s gangrene is an uncommon disease accounting for <0.5% of annual hospital admissions globally.[15] The disease has a higher male predominance (male-to-female ratio, 10:1) with a median age at presentation being 50–79 years.[16] This was also evident in the present study as all the affected patients were males with an average age at presentation being 56.27 years. FG has been found to originate from the intentional or accidental trauma to the anorectal (30%–50%) or urogenital region (60%) that causes the entry of bacteria in the local tissue.[16] As depicted in the present study, DM is one of the most significant predisposing factors in patients affected by FG.[17] In our study, 36.1% patients were found to have coexisting uncontrolled DM, with all patients having the HbA1c levels greater than 9%. These patients were found to have higher ACCI scores (>8), a deep infection involving fascia and muscle compartment, multiple repeated operations required, and a prolonged hospital stay. Sustained hyperglycemia and microangiopathy in uncontrolled DM can decrease the neutrophil adhesion, chemotaxis, and cellular immunity.[17] The degree of diabetes control is considered to be an independent predictor of the extent of disease and prognosis in FG. Approximately one-third of patients in the present study had preexisting CKD. The presence of concomitant CKD predisposes these patients to dyselectrolytemia and metabolic acidosis, and increases the toxicity of antimicrobial drugs excreted through the renal route, ultimately resulting in increased FG morbidity.[18] Long-term CKD may cause immunosuppression and decreased wound healing.[18] FG is mostly recognized as a polymicrobial infection.[19] Most commonly identified organisms are streptococcus, staphylococcus, and Escherichia coli. Usually the wound cultures show evidence of more than one organism in the affected patient.[19] Various scoring systems are available to predict the severity of FG, such as FGSI, ACCI, the Uludag Fournier gangrene scoring index (UFGSI), Laboratory Risk Indicator for Necrotizing Fasciitis score (LRINEC), etc.[11–13] In a study done by Laor et al.[12], the authors predicted the FGSI score >9 to be associated with a 75% probability of death, while a score <9 was associated with a 78% chance of survival. All these scores adequately characterize the severity and prognosis of FG, but clinician’s judgment is vital. Certain scores such as ACCI are more useful than others, as these can be calculated easily in the primary care setting. Previous studies report mortality rates ranging from 20%–35% despite the aggressive medical and surgical management.[10,12] Such high mortality rates were described in the previous decade, but now the outcome of patients with FG has changed drastically. Various adjunctive treatments have come up in the last decade. The use of hyperbaric oxygen (HBO) therapy has been proposed by some authors to improve the immunological response, inhibit the growth of anaerobic bacteria, and prevent further extension of tissue necrosis in patients with FG.[20] However, the exact benefits of HBO therapy in FG management are yet to be defined.
An analysis of factors predicting the FG severity and its comparison with the present study is depicted in Table 5. There is a paradigm shift in FG outcomes with comparatively low mortality in the present study than previously reported.[21,22] The mortality rate in the present study was only 8%. Possible explanations for this low mortality rate include all the patients we admitted underwent urgent surgical debridement (within less than 8 hours from admission); we kept the threshold for re-debridement very low; we practice the step-down approach for the use of antimicrobials at our center, wherein parenteral administration of higher antibiotics (piperacillin-tazobactam/meropenem) is done empirically, which provides a broad-spectrum coverage, and later on, culture-sensitive specific antibiotics are given; the majority of patients admitted with us had lower severity of disease, as depicted by a low FGSI and ACCI. In conclusion, there is a paradigm shift in the FG clinical spectrum, and outcomes with comparatively low mortality are reported now when compared to previous decades. A thorough knowledge of the predictors of severity at the time of presentation may help clinicians decide an aggressive management strategy.
Table 5.
Comparison of factors predicting outcome of FG with present study
| Author | No. of patients studied | Mortality rate (%) | Parameters predicting outcome |
|---|---|---|---|
| Clayton et al.[4] | 57 | 17.5 | BUN>50 mg/dL |
| Laor et al.[12] | 30 | 43 | FGSI>9 |
| Ruiz-Tovar et al.[14] | 70 | 22.9 | Ethylism, coexistence of neoplasms, presence of skin necrosis, myonecrosis, abdominal wall affection, number of debrided areas, re-operations serum creatinine >1.4 mg/dL Hb<10 g/dL PLC<150×109/L |
| Kara et al.[16] | 15 | 20 | RBS>140 mg/dL, the existence of septic shock on admission, spread of gangrene to the perineum and abdominal wall, BSA>24 cm2, cutaneous source of infection FGSI>7 |
| Janane et al.[20] | 70 | 11.4 | Extent of BSA involved |
| Altarac et al.[21] | 41 | 36.6 | Elevated HR and RR, high serum creatinine, low serum bicarbonate, CKD, higher median BSA affected Severe sepsis on admission SBP<90 mmHg FGSI >11 |
| Tuncel et al.[22] | 50 | 14 | Low Hb, high BUN, low albumin levels |
| Present study | 72 | 8.3 | Advanced age, DM, CKD, raised TLC-altered sensorium, shock at presentation, high FGSI/ACCI scores, deranged INR |
BMI: body mass index; DM: diabetes mellitus; Hb: hemoglobin; TLC: total leukocyte count; RBS: random blood sugar; FGSI: Fournier’s gangrene severity index; ACCI: age-adjusted Charlson Comorbidity Index; INR: international normalized ratio; HR: heart rate; RR: respiratory rate; CKD: chronic kidney disease; BSA: body surface area; BUN: blood urea nitrogen; SBP: systolic blood pressure; PLC: platlet count
An advanced age, DM, renal impairment, leukocytosis, altered sensorium, shock at presentation, deranged international normalized ratios, and high FGSI and ACCI scores can be used as predictors of the poor response. FG risk scores adequately characterize the severity and prognosis of FG, but clinician’s judgement is of vital importance. Management comprises of a multidisciplinary approach including parenteral antibiotics, urgent surgical debridement, and comorbidities optimization.
Acknowledgements
We acknowledge the cooperation of the Urology Department residents of King George’s Medical University, who participated in data collection and evaluation of the patient. We also appreciate the commitment and compliance of the patient who reported the required data.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of King George’s Medical University.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.G., S.P., A.S., A.A.; Design - G.G.; Supervision - G.G., V.S., R.J.S., S.P., A.S., A.A.; Data Collection and/or Processing - G.G., V.S., R.J.S.; Writing Manuscript - G.G., V.S., R.J.S., S.P., A.S., A.A.; Critical Review - G.G., V.S., R.J.S., S.P., A.S., A.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Smith GL, Bunker CB, Dinneen MD. Fournier’s gangrene. Br J Urol. 1998;81:347–55. doi: 10.1046/j.1464-410x.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 2.Nathan B. Fournier’s gangrene: a historical vignette. Can J Surg. 1998;41:72. [PMC free article] [PubMed] [Google Scholar]
- 3.Morpurgo E, Galandiuk S. Fournier’s gangrene. Surg Clin North Am. 2002;82:1213–24. doi: 10.1016/S0039-6109(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 4.Clayton MD, Fowler JE, Jr, Sharifi R, Pearl RK. Causes, presentation and survival of fifty-seven patients with necrotizing fasciitis of the male genitalia. Surg Gynecol Obstet. 1990;170:49–55. [PubMed] [Google Scholar]
- 5.Eke N. Fournier’s gangrene: a review of 1726 cases. Br J Surg. 2000;87:718–28. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 6.Yaghan RJ, Al-Jaberi TM, Bani-Hani I. Fournier’s gangrene: changing face of the disease. Dis Colon Rectum. 2000;43:1300–8. doi: 10.1007/BF02237442. [DOI] [PubMed] [Google Scholar]
- 7.Rotstein OD, Pruett TL, Simmons RL. Mechanisms of microbial synergy in polymicrobial surgical infections. Rev Infect Dis. 1985;7:151–70. doi: 10.1093/clinids/7.2.151. [DOI] [PubMed] [Google Scholar]
- 8.Johnin K, Nakatoh M, Kadowaki T, Kushima M, Koizumi S, Okada Y. Fournier’s gangrene caused by Candida species as the primary organism. Urology. 2000;56:153. doi: 10.1016/S0090-4295(00)00527-6. [DOI] [PubMed] [Google Scholar]
- 9.Jeong HJ, Park SC, Seo IY, Rim JS. Prognostic factors in Fournier gangrene. Int J Urol. 2005;12:1041–4. doi: 10.1111/j.1442-2042.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 10.Stephens BJ, Lathrop JC, Rice WT, Gruenberg JC. Fournier’s gangrene: historic (1764–1978) versus contemporary (1979–1988) differences in etiology and clinical importance. Am Surg. 1993;59:149–54. [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier’s gangrene. J Urol. 1995;154:89–92. doi: 10.1016/S0022-5347(01)67236-7. [DOI] [PubMed] [Google Scholar]
- 13.Mallikarjuna MN, Vijayakumar A, Patil VS, Shivswamy BS. Fournier’s Gangrene: Current Practices. ISRN Surg. 2012;2012 doi: 10.5402/2012/942437. 942437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Tovar J, Cordoba L, Devesa JM. Prognostic factors in Fournier gangrene. Asian J Surg. 2012;35:37–41. doi: 10.1016/j.asjsur.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen MD, Krieger JN, Rivara FP, Broghammer JA, Klein MB, Mack CD, et al. Fournier’s Gangrene: population based epidemiology and outcomes. J Urol. 2009;181:2120–6. doi: 10.1016/j.juro.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kara E, Müezzinoğlu T, Temeltas G, Dinçer L, Kaya Y, Sakarya A, et al. Evaluation of risk factors and severity of a life threatening surgical emergency: Fournier’s gangrene (a report of 15 cases) Acta Chir Belg. 2009;109:191–7. doi: 10.1080/00015458.2009.11680404. [DOI] [PubMed] [Google Scholar]
- 17.Nisbet AA, Thompson IM. Impact of diabetes mellitus on the presentation and outcomes of Fournier’s gangrene. Urology. 2002;60:775–9. doi: 10.1016/S0090-4295(02)01951-9. [DOI] [PubMed] [Google Scholar]
- 18.Lin TY, Ou CH, Tzai TS, Tong YC, Chang CC, Cheng HL, et al. Validation and simplification of Fournier’s gangrene severity index. Int J Urol. 2014;21:696–701. doi: 10.1111/iju.12426. [DOI] [PubMed] [Google Scholar]
- 19.Uluğ M, Gedik E, Girgin S, Celen MK, Ayaz C. The evaluation of microbiology and Fournier’s gangrene severity index in 27 patients. Int J Infect Dis. 2009;13:e424–30. doi: 10.1016/j.ijid.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Janane A, Hajji F, Ismail TO, Chafiqui J, Ghadouane M, Ameur A, et al. Hyperbaric oxygen therapy adjunctive to surgical debridement in management of Fournier’s gangrene: usefulness of a severity index score in predicting disease gravity and patient survival. Actas Urol Esp. 2011;35:332–8. doi: 10.1016/j.acuro.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Altarac S, Katušin D, Crnica S, Papeš D, Rajković Z, Arslani N. Fournier’s gangrene: etiology and outcome analysis of 41 patients. Urol Int. 2012;88:289–93. doi: 10.1159/000335507. [DOI] [PubMed] [Google Scholar]
- 22.Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A. Fournier’s gangrene: Three years of experience with 20 patients and validity of the Fournier’s Gangrene Severity Index Score. Eur Urol. 2006;50:838–43. doi: 10.1016/j.eururo.2006.01.030. [DOI] [PubMed] [Google Scholar]


