Abstract
Operant behavior tasks are widely used in neuroscience research, but little is known about how variables such as housing and testing conditions affect rodent operant performance. We have previously observed differences in operant performance in male and female mice depending on whether mice were housed and tested in rooms containing only one sex versus rooms containing both sexes. Here, male and female mice in either single-sex or mixed sex housing rooms were trained on fixed ratio 1 (FR1) and progressive ratio (PR) tasks. For both sexes, animals in the mixed sex room had more accurate performance in FR1 and were more motivated in the PR task. We then moved the single sex housed animals to the mixed sex room and vice versa. Animals that started in mixed sex housing had no change to PR, but both sexes who started in single sex housing were more motivated after the switch. Additionally, the females that moved into single-sex housing performed less accurately in FR1. We conclude that housing and testing conditions can affect performance on FR1 and PR tasks. As these tasks are commonly used as training steps to more complex tasks, housing and testing conditions should be carefully considered during experiment design and reported in publications.
Keywords: Operant behavior, housing conditions, fixed ratio, progressive ratio, attention, motivation
1. Introduction
Operant behavior tasks are a staple of neuroscience research, and are used to study processes including attention, working memory, behavioral flexibility, and decision making (Chudasama, 2011). One of the most basic operant tasks is the fixed ratio schedule (FR), where the animal learns to make an operant response such as a lever-press or nose-poke, and is rewarded with a reinforcer, generally food, after every n responses. FR tasks require a response for a reward and can therefore be used to measure reward-related (e.g., drug-seeking) behavior (Weissenborn, Robbins, & Everitt, 1997), and are typically used as a training step for more complex operant tests, such as the 5-choice serial reaction time task (Grissom, Herdt, Desilets, Lidsky-Everson, & Reyes, 2015), delayed matching/nonmatching to sample (Auger & Floresco, 2017), and probability discounting (Nasrallah, Yang, & Bernstein, 2009). Presumably, factors that affect FR performance can influence future performance on any of these operant tasks.
The present experiments are based on differences we observed in the acquisition of the FR1 task across similar experiments, with one notable variable being whether animals were housed and tested in rooms containing one sex or both sexes (referred to hereafter as the ‘single sex’ and ‘mixed sex’ rooms). When the sexes were housed separately, the males acquired the task faster (Grissom et al., 2015), that is, they completed more trials in a day. When the sexes were housed together, the females acquired the task faster (McKee, Grissom, Herdt, & Reyes, 2017). Many operant behavior studies only use animals of one sex, and those that include both sexes rarely report if the males and females were housed and tested in the same room. As the National Institutes of Health now requires the consideration of sex as a biological variable, these experimental details will increase in importance as more labs use both males and females in rodent operant experiments.
Based on our previous observations, this experiment was designed to explicitly test whether housing conditions would affect performance on simple operant behavior tasks. The initial endpoints of interest were rate of acquisition, motivation and accuracy. We hypothesized that the males would acquire the FR1 task faster when single-sex housed, and the females would acquire the task faster when housed in a mixed sex room. We also expected that housing conditions would affect motivation as measured by the progressive ratio (PR) task, and these differences in motivation would drive the differences in performance accuracy on the FR1 task.
2. Methods
2.1. Mice
All animals were cared for according to the guidelines of the University of Cincinnati Institutional Animal Care and Use Committee. Mice were purchased from Jackson Laboratories (Bar Harbor, ME) and virgin female C57BL/6J mice were bred with DBA/2J males to create F1 hybrid offspring used for this study. Hybrids are used to allow for allele tracking in other experiments (not reported here). At weaning, animals were group housed with same sex siblings in the colony room (mixed-sex) used for breeding. At age 8 weeks, all animals were re-housed with non-siblings (2–4/cage depending on the availability of non-sibling mice), and moved to testing rooms (described below) that contained a single sex (N=12 females, 13 males) or a room that contained both sexes (N=11 females, 12 males). Mice were then mildly food restricted to 85–95% of their baseline bodyweight, and behavior training started one week later. Before training, animals were pre-exposed to the reinforcer (20% sucrose with 0.2% grape Kool Aid) in the home cage.
2.2. Housing Rooms
Three housing rooms were used: one contained only males, one contained only females, and one contained both sexes. The rooms were located along the same hallway and were exposed to the same level of environmental noise. Animals were housed and tested in the same room. The mixed sex room was occupied only by animals involved in the current experiment, while the single-sex rooms each contained a second set of operant chambers and a cohort of animals involved in a different experiment run concurrently. The same experimenters provided husbandry care in all rooms. All experimental rooms had a 12 hour reverse light/dark cycle (lights off 0900).
2.3. Behavior Apparatus
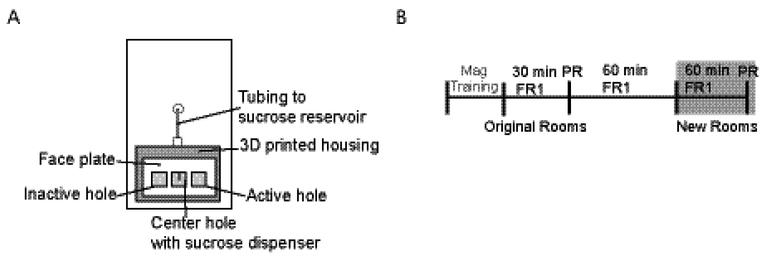
Animals were trained in ROBucket operant chambers (Devarakonda, Nguyen, & Kravitz, 2016) constructed in-house. The chamber consists of a 6.5”x 6.5”x 8” square plastic container outfitted with three nose poke holes containing photo interrupter sensors (Figure 1A). Liquid reinforcer was dispensed from the center hole. The right side hole was designated as active and pokes were rewarded. The left side hole was inactive: pokes to this hole had no programmed consequences. An Arduino Uno served as a processor and recorded the number of rewards dispensed and number of pokes to each hole.
Figure 1:
Equipment and experiment design. (A) Schematic of the ROBucket operant chamber interior, constructed according to 7. (B) Experimental timeline.
2.4. Behavior Training
All operant training took place during the dark phase of the light/dark cycle in the animal housing rooms. In the mixed sex room, individual operant chambers were used for only one sex, but males and females were run concurrently, meaning that mice could potentially smell each other through the plastic operant chambers. Additionally, the experimenter did not change gloves between handling the sexes in the coed room. The first day of training was exposure to the chambers (see Figure 1B for timeline). Animals were placed in the chambers for 30 minutes with the chambers turned off. The animals then underwent 4 days of magazine training, consisting of 30 minute sessions with automatic delivery of reinforcer once every 45 to 75 seconds.
After magazine training, the animals began training on a fixed-ratio 1 task (FR1). Reward was delivered after every poke in the active hole, with a 1 second time out between responses. The first 15 sessions lasted for 30 minutes. The 16th session and beyond were 1 hour, in an attempt to increase the number of trials performed. Outcome measures included number of reinforcers earned, number of pokes to each of the wells, and discrimination index, defined as percent of pokes to the active hole (discrimination index = 100*active/(active+inactive)).
Between the 30 and 60 minute FR1 sessions, the animals underwent sessions of a progressive ratio task (PR) in which the number of active pokes required to earn 1 reinforcer increased according to the equation r = 5e0.2n - 5 (rounded to the nearest integer) each trial (Richardson & Roberts, 1996). The session lasted 1 hour or timed out sooner if the animal made no responses for 5 minutes. Primary outcome measure was the number of reinforcers earned during the session.
2.5. Room switch
After FR1 and PR testing, the housing conditions of the animals was switched. Cages in the single sex rooms were moved to the mixed sex room, and cages in the mixed sex room were moved to the single sex rooms. Cagemate groups were kept constant during the switch. After the switch, the animals were left undisturbed for 4 days then testing continued as described above.
2.6. Statistics
Data were analyzed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). PR and discrimination index before the room switch were analyzed using a 2 way ANOVA (housing condition x sex) followed by Bonferroni post-hoc test. The discrimination index after the room switch was analyzed using a 3 way ANOVA (sex x original housing x switch) followed by Tukey’s multiple comparisons test. To measure change in PR score after the switch, ΔPR was calculated by subtracting the number of rewards earned before the switch from the number of rewards earned after the switch. One sample t-tests were used to compare each groups’ ΔPR to 0 (indicating no change). Data are presented as mean ± SEM, and p <0.05 was considered significant.
3. Results
3.1. FR1 training
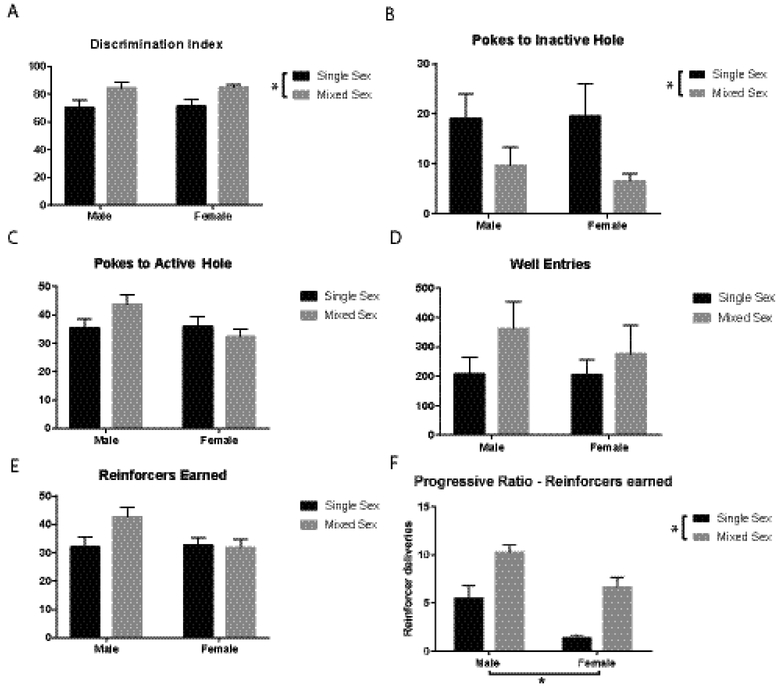
Animals were trained in 30-minute sessions of FR1 for 15 days, then, in an effort to increase the number of responses/session, in 60 minute sessions for an additional 21 days. Because two training protocols were required in the present experiment (30 min, then 60 min sessions), we were unable to utilize days-to-criterion to evaluate animals. Therefore, we used discrimination index (e.g., accuracy) as a measure of the animals’ performance. There was an effect of housing type on discrimination index during a 1-hour session on the last day of testing in the original housing rooms (F1,44=9.35, p=0.0038) (Figure 2A), such that animals housed in the mixed sex room performed significantly better (e.g., were more accurate). This was due to animals in the mixed sex room making fewer inactive pokes (F1,44=5.8, p=0.0203) (Figure 2B). Total number of active pokes, well entries, and rewards earned did not differ between any of the groups (Figure 2C–E).
Figure 2:
Effects of housing on FR1 and PR performance. (A) There was an effect of housing type on discrimination index during FR1. (B) There was a main effect of housing type on number of inactive pokes. (C-F) There were no effects on active pokes, well entries, or rewards earned. (F) There was a main effect of sex and a main effect of housing type on number of reinforcers earned during PR.
3.2. PR
Using PR to assess motivation, there was a main effect of housing type (F1,44=26.86, p<0.0003) on number of reinforcers earned (Figure 2F), with mixed sex room housed animals earning more reinforcers than single-sex housed animals. There was also a main effect of sex (F1,44=15.81, p=0.0003), with males earning more reinforcers than females.
3.3. Room switch
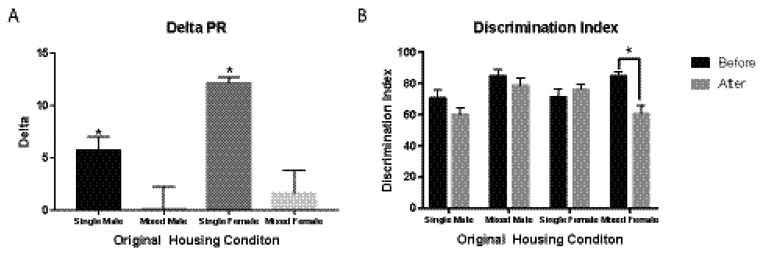
The room switch had no effect on PR performance for animals that started in mixed sex housing, but both sexes who started in single sex housing performed significantly more trials after the switch (male started single sex t12=4.337, p=0.001, females started single sex t11=22.19, p<0.0001, Figure 3A). For FR1 discrimination index, a 3 way interaction of sex x original housing x switch (F1,1=5.388, p=0.0228) was observed, with main effects of switch (F1,1=7.351, p=0.0082) and original housing (F1,1=5.318, p=0.0237). Posthoc testing revealed that females that started in the mixed sex room had a decreased discrimination index after the switch (p=0.011), while performance of the other groups was unchanged (Figure 3B).
Figure 3:
Effects of room switch. (A) Both sexes that started in the single sex rooms performed more PR trials after the switch. (B) Only the females who started in the mixed-sex room demonstrated a change in discrimination index.
4. Discussion
The goal of the present experiment was to determine whether housing environment, either in single sex or mixed sex rooms, would affect operant performance. We hypothesized that in the mixed sex room, females would be positively influenced by the presence of males, such that they would be motivated to perform more trials, while males would be negatively affected by the presence of females, and would perform fewer trials. These predictions were based on the idea that in a mixed sex environment, females may be more motivated to obtain calories (food reward) in an effort to support a potential pregnancy, while male performance would decrease, as motivation would shift away from the operant task, and instead focus on a potential mate. Prior work has shown that food restricted female Syrian hamsters prefer to eat chow than to associate with a male (Benton et al., 2017; Schneider et al., 2007), while hungry male rats (fasted for 6 hours) will copulate with a sexually receptive female and ignore a sucrose bottle (Kaplan, Bednar, & Södersten, 1992). Contrary to our original hypothesis, both sexes had a significantly higher motivation (reached a higher breakpoint) in the progressive ratio task when housed in the mixed sex room. We also found that PR breakpoint tracks housing such that when animals moved from single-sex to mixed-sex housing, they performed more PR trials.
Performance in the FR1 task was evaluated using the discrimination index, an indication of the animal’s ability to correctly identify the active versus inactive side. We had initially planned to analyze time-to-criterion, however 30 min training sessions (as used in our previous work) proved insufficient for mice to reach criterion performance (likely because the chambers used in the current experiments did not include a light cue, as was used in previous experiments). Once the session length was extended to 60 min, all animals reached criterion performance, but due to the two-step process (30 min, then 60 min), evaluating time to criterion was no longer valid as a performance metric. Surprisingly, mixed sex room housed animals of both sexes had significantly greater accuracy as compared to single sex housed animals. The increase in accuracy is likely independent from the increased motivation, as others have found a dissociation between motivation and accuracy (Bhandari, Daya, & Mishra, 2016; Dalley et al., 2005).
At the conclusion of this part of the experiment, housing type was switched and animals were once again evaluated, to determine whether their performance metrics would also track the housing. For all the males (regardless of the original housing condition) and for females who were originally housed in the single sex room, the room switch had no significant effect on their performance. Interestingly, females that were formerly mixed sex housed now made more inactive pokes after the switch, significantly decreasing their discrimination index. There was no effect on PR, indicating that the room switch did not affect motivation. For females, it appears that the mixed sex housing improves motivation and accuracy, and that moving from mixed sex to single sex housing decreases accuracy.
While estrus cycling was not monitored for the females in this experiment, we did not see any cyclic changes in FR1 performance that would indicate changes across the estrus cycle. Additionally, the variability in females’ performance in FR and PR is comparable to or even less than the variability of the males, suggesting that estrus cycle stage does not have a significant effect on these operant behaviors. Similarly, estrus cycle did not affect rate of lever-pressing during either phase of a multiple fixed interval extinction schedule in Wistar-Kyoto or spontaneously hypertensive rats (Berger & Sagvolden, 1998), and did not affect rat’s PR performance for nicotine reward (Donny et al., 2000).
In sum, we have demonstrated that housing and testing conditions can affect performance on an uncued nose-poke FR1 task. As FR1 is used as a training protocol for other tasks (including the 5CSRTT, delay discounting, and extinction) as well as a test in and of itself, these housing-based differences can affect data on a variety of cognitive functions. We have also shown that housing conditions can affect PR performance, indicating altered motivation for reward. Because our animals were housed and tested in the same room, this experiment cannot differentiate between housing effects and testing effects. We speculate that conditions during the task may be more important than conditions during housing because the presence of the opposite sex during the task introduces a competing motivation between seeking food (i.e. performing the task to earn a reward) and seeking a mate (Benton et al., 2017; Kaplan et al., 1992; Schneider et al., 2007). Housing conditions, both after weaning and during behavior experiments, should be considered during the design of the experiment and clearly reported in the results to increase reproducibility.
Mice perform Fixed Ratio 1 more accurately when both sexes are in the same room
Presence of the opposite sex increases motivation to work for food reward
Moving from single sex to mixed sex housing rooms increases motivation
Moving to mixed sex housing does not improve Fixed Ratio 1 performance
Acknowledgements
This work was supported by NIH grant MH106330 (TMR). Dana Buesing, Nicola Grissom, and Niusha Jahanpanah aided in the construction of the ROBuckets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auger ML, & Floresco SB (2017). Prefrontal cortical GABAergic and NMDA glutamatergic regulation of delayed responding. Neuropharmacology, 113, 10–20. 10.1016/j.neuropharm.2016.09.022 [DOI] [PubMed] [Google Scholar]
- Benton NA, Russo KA, Brozek JM, Andrews RJ, Kim VJ, Kriegsfeld LJ, & Schneider JE (2017). Food restriction-induced changes in motivation differ with stages of the estrous cycle and are closely linked to RFamide-related peptide-3 but not kisspeptin in Syrian hamsters. Physiology & Behavior. 10.1016/J.PHYSBEH.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger DF, & Sagvolden T (1998). Sex differences in operant discrimination behaviour in an animal model of attention-deficit hyperactivity disorder. Behavioural Brain Research, 94(1), 73–82. [DOI] [PubMed] [Google Scholar]
- Bhandari J, Daya R, & Mishra RK (2016). Improvements and important considerations for the 5-choice serial reaction time task—An effective measurement of visual attention in rats. Journal of Neuroscience Methods, 270, 17–29. 10.1016/j.jneumeth.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Chudasama Y (2011). Animal Models of Prefrontal-Executive Function. Behavioral Neuroscience, 125(3), 327–343. 10.1037/a0023766 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Pena Y, Theobald DEH, Everitt BJ, & Robbins TW (2005). Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology, 182(4), 579–87. 10.1007/s00213-005-0107-3 [DOI] [PubMed] [Google Scholar]
- Devarakonda K, Nguyen KP, & Kravitz AV (2016). ROBucket: A low cost operant chamber based on the Arduino microcontroller. Behavior Research Methods, 48(2), 503–509. 10.3758/s13428-015-0603-2 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, … McCallum S (2000). Nicotine self-administration in rats: Estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology, 151(4), 392–405. 10.1007/s002130000497 [DOI] [PubMed] [Google Scholar]
- Grissom NM, Herdt CT, Desilets J, Lidsky-Everson J, & Reyes TM (2015). Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacology, 40(6), 1353–1363. 10.1038/npp.2014.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Bednar I, & Södersten P (1992). Simultaneous Display of Sexual and Ingestive Behaviour by Rats. Journal of Neuroendocrinology, 4(4), 381–392. 10.1111/j.1365-2826.1992.tb00184.x [DOI] [PubMed] [Google Scholar]
- McKee SE, Grissom NM, Herdt CT, & Reyes TM (2017). Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet–fed dams. The FASEB Journal, 31 10.1096/fj.201601172R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TWH, & Bernstein IL (2009). Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proceedings of the National Academy of Sciences of the United States of America, 106(41), 17600–4. 10.1073/pnas.0906629106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, & Roberts DCS (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods, 66(1), 1–11. 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Casper JF, Barisich A, Schoengold C, Cherry S, Surico J, … Rabold E (2007). Food deprivation and leptin prioritize ingestive and sex behavior without affecting estrous cycles in Syrian hamsters. Hormones and Behavior, 51(3), 413–427. 10.1016/J.YHBEH.2006.12.010 [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, & Everitt BJ (1997). Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology, 134(3), 242–257. 10.1007/s002130050447 [DOI] [PubMed] [Google Scholar]