Abstract
Introduction: Since endocannabinoids have been implicated in migraine pathophysiology, we conducted a randomized, controlled clinical trial to test the effects of a 12-week aerobic exercise intervention on plasma anandamide (AEA) and its relation with clinical, psychological, and cardiorespiratory outcomes.
Materials and Methods: Episodic migraine patients taking no preventive drugs and nonheadache individuals were recruited from Hospital São Paulo and a tertiary headache clinic between March 2012 and March 2015. Participants were randomly assigned to receive aerobic exercise or enter the waitlist. Primary outcome was changes in plasma AEA; secondary outcome was number of days with migraine/month; and other clinical variables, mood scores, and cardiorespiratory fitness were chosen as tertiary outcomes. Measurements were taken on headache-free days. Data were analyzed by generalized linear models.
Discussion: Fifty participants concluded the study (mean±SD age=36.2±10.9, and BMI=26.5±4.5). The plasma AEA reduced in migraine exercise (p<0.05) and control exercise groups (p<0.01). The number of days with migraine (p<0.01), migraine attacks (p<0.05), and abortive medication used (p<0.05) reduced in the migraine exercise group, whereas cardiorespiratory fitness increased in migraine exercise and control exercise groups (both p<0.05). Anxiety, depression, anger, and fatigue scores improved in the migraine exercise group (p<0.05 for all). Significant correlations between reduction in abortive medication used and cardiorespiratory fitness (r=−0.81 p<0.001), and reduced AEA (r=0.68 p<0.05) were found.
Conclusions: This study suggests that peripheral AEA metabolism may be partly linked to the clinical and cardiorespiratory benefits of regular aerobic exercise in migraine patients. Trials registration: #NCT01972607.
Keywords: cardiorespiratory fitness, endocannabinoids, exercise, headache disorders, mood, physical activity
Introduction
Migraine constitutes a very disabling neurological disorder, affecting around 10% of the population worldwide.1,2 Aerobic exercise training has been consistently shown effective as a nonpharmacological option to reduce migraine attacks and improve psychological outcomes related to perceived stress.3–8 Improvement in cardiorespiratory fitness has also been observed together with migraine improvement,5,8 and poor cardiorespiratory fitness is associated with increased risk for migraine.9 However, the mechanisms underlying these therapeutic effects of aerobic exercise remain poorly explored.
There have been speculations on the participation of endocannabinoids in anti-migraine mechanisms through aerobic exercise.6,10 N-arachidonoyl-ethanolamine, or anandamide (AEA), is a major endocannabinoid that targets at both cannabinoid type-1 (CB1) and type-2 (CB2) receptors, but it is also considered an endovanilloid as it binds to transient receptor potential vanilloid type-1 receptors (TRPV1).11–13 The AEA has a prominent role in several physiological and neurobehavioral processes, in particular pain perception,11 emotional behavior,12 and energy metabolism,13 all of which are affected by aerobic exercise.14–16
Indeed, these multiple neurobiological actions of AEA have made this compound a target of therapeutic exploitation, by either pharmacological11,17 or lifestyle changes approaches, as through increased physical activity.18,19
In migraine patients, evidence from biochemical studies suggests increased AEA degradation, which has been interpreted as an impaired endocannabinoid signaling.20–23 In animal models of migraine, exogenous AEA diminishes hyperalgesic behavior, coupled with reduced c-Fos expression in brain areas related to pain.17 However, there is limited information regarding the effects of aerobic exercise training on resting, steady-state plasma AEA concentration, and no study so far has prospectively investigated its clinical implications in migraine patients.
Data on changes in AEA in response to aerobic exercise indicate a distinct effect for acute (single bout) versus chronic exercise (training). The AEA has been found to be increased in peripheral circulation and pain-related brain areas after acute aerobic exercise in rodents, and this response has been ascribed for exercise-induced analgesia and mood improvement found in humans.14–16 On the other hand, chronic exercise (e.g., aerobic exercise training) seems to reduce circulating AEA levels in obese men, a response implicated in improved metabolic regulation.18,24 These distinct responses regarding acute versus chronic exercise on AEA, its affinity with multiple receptors, and its pleiotropic, tissue-specific actions render not obvious the relationship between resting plasma AEA after chronic exercise and clinical/psychophysiological outcomes in migraine.
Therefore, to investigate whether AEA participates in the therapeutic effects of aerobic exercise for migraine, this study aimed at evaluating the effect of an aerobic exercise program on resting plasma AEA concentration in humans with or without migraine, and whether there would be correlations between changes in AEA, clinical, psychological, and cardiorespiratory outcomes.
Materials and Methods
Study design and participants
This is a prospective, open-label, randomized, and controlled clinical trial designed to test the effects of a 12-week aerobic exercise program on plasma AEA levels, and to explore its correlation with clinical and psychophysiological outcomes. Participants were recruited from the Headache Unit of Hospital São Paulo and a headache tertiary clinic, through printed and electronic media advertisements, between March 2012 and March 2015. All participants were screened and evaluated by two headache-trained neurologists (R.T.R. and M.F.P.P.).
Inclusion criteria were: individuals of both sex, aged between 18 and 60 years; nonheadache individuals (defined as controls) or patients with episodic migraine with/without aura (2–14 attacks/month), or presenting both subtypes, according to the second version of the International Classification of Headache Disorders25; and patients taking no migraine preventive drugs (with the exception for abortive medication during attacks), or any other prescribed drugs or dietary supplement.
Individuals had to be physically inactive (≤1 day/week of leisure-time physical activity during the previous 12 months). Exclusion criteria were: having any other neurological disorder, including another primary or secondary headache; clinical history of cardiovascular, metabolic, musculoskeletal, or rheumatic disease; and participation on any body–mind practice or beginning any other treatment during the study.
Participants were randomly assigned to receive intervention with aerobic exercise training or enter a waitlist. The study's protocol duration was 20 weeks, comprising a 4-week baseline period, followed by another 4-week period wherein the blood sampling, psychometric interview, and cardiorespiratory fitness test were scheduled, and the 12-week intervention period.
The study's protocol complied with the Helsinki declaration on human research and was approved by the UNIFESP's Research Ethical Committee, registered under No. 081511. This study is registered in the National Institute of Health (www.ClinicalTrials.gov) under No. NCT01972607, and it complies with the CONSORT's Statement for nonpharmacological trials.26 All participants gave written informed consent.
Measures and procedures
After screening, participants were evaluated every 4 weeks (clinical visits) for neurological examination and checking of headache diaries. The clinical data filled in the diaries in the last 4 weeks of the intervention period were set as “post-intervention” period data.
Blood sampling and psychometric interviews were scheduled on the same visit, and the cardiorespiratory fitness test was conducted ∼1 week later. Test–retest visits for blood collection, filling of psychometric questionnaire, and cardiorespiratory fitness test were scheduled in the same order. All women were at the late follicular phase of the menstrual cycle at the blood sampling visit. Retest visits for blood sampling were performed between 2 and 5 days after the last exercise session or 48 h after the last exercise session within the late follicular phase of the menstrual cycle. For all test–retest visits, participants were instructed to breakfast regularly, but to abstain from coffee, teas, and foods containing cocoa derivatives, the latter shown to increase circulating AEA concentrations.27 All patients were within the interictal period (headache-free days) during all test–retest measurements.
Headache diary
Patients were instructed to fill a paper-based headache diary, wherein the number of days with migraine, frequency of attacks, migraine pain intensity (0=“no pain”; 1=“mild”; 2=“moderate”; 3=“severe”), and the number of abortive medications consumed were retrieved.
Psychometric questionnaire
The profile of mood state (POMS) questionnaire was used in this study. The POMS is a questionnaire amply used in the exercise research field and captures psychometrical data from six mood domains, namely, Depression, Tension-Anxiety, Anger-Hostility, Vigor, Fatigue, and Confusion.28 The POMS questionnaire was filled at the Psychobiology Department between 9:00 and 11:00 AM before blood sampling.
AEA analysis
After questionnaire filling, blood samples were collected by venepuncture of the antecubital vein in cooled EDTA vacutainers (BD Vacutainer®, Franking Lakes, NY), and they were immediately centrifuged for 10 min at a 3,400 g at 4°C. Plasma was separated, aliquoted in 1-mL vials, and stored at −80°C until analysis. The vials were labeled through random numbering, so that the laboratory staff had no information of participant's allocation.
The AEA extraction and quantification was performed at the Psychobiology Department by a laboratory staff coordinated by S.T., following the recommendations of Zoerner et al.29 For extraction, 100 μL of internal standard stable isotope-labeled AEA-d8, >95% purity (Cayman, Ann Harbor, MI) was added to 500 μL of plasma and 4 mL of methyl tertiary-butyl ether (MTBE; Sigma-Aldrich, San Louis, MO).
The mixture was vortexed for 30 sec, and centrifuged for 6 min at 12,000 g. The supernatant was transferred to a 5-mL glass tube and dried by a stream of N2 at 37°C. The dry organic mobile phase was reconstituted in 100-μL acetonitrile/formic acid (0.1%), vortexed for 30 sec, and the clear solution was transferred to an HPLC/MS-MS system. Separation and quantification were performed by an LC-10AD system (binary pump and auto sampler; Shimadzu®, Columbia, MD) tandem to a triple quadrupole mass spectrometer (Waters® Micromass Quattro Micro, Milford, MA), equipped with an electrospray ionization source operated in positive mode. The Mass Lynx (Version 4.0) software was used for the data processing. A Kinetex® (50×2.10 mm, 2.6 μm particle size) analytical column was used for chromatographic separation. Mobile phase was eluted isocratically with acetonitrile:water/ammonium formate 2 nM (90:10, v/v) at a flow rate of 0.15 mL/min−1, with source temperature at 100°C and gas flow source of 350 psi (N2 as drying gas). The injection volumes were 20 μL. Selected reaction monitoring m/z transitions for AEA was 348.2→62.1. Pure solution of AEA (Sigma-Aldrich) and blank plasma (Golden West Biologicals, Inc.®, Tamecula, CA) were used to build the calibration curve. We tested seven points of AEA concentrations (from 0.05 to 50 ng/mL) in sextuplicate, and the curve was linear within this range (r=0.998). The retention and total analysis time were 2.7 and 4 min, respectively. The lower detection limit of this method (defined as signal/noise ratio=4:1) was 0.025 ng/mL.
Cardiorespiratory fitness test
Cardiorespiratory fitness tests were conducted in the morning by two cardiologists and two exercise physiologists independent of the study, at the Center for Studies in Psychobiology and Exercise. The peak oxygen uptake (VO2Peak) was obtained during the maximal exercise test on treadmill with ramp protocol.30 Determination criteria are detailed elsewhere.30
VO2Peak represents a gold-standard measure of cardiorespiratory fitness, comprising the product of cardiac output and musculoskeletal oxygen extraction/utilization.31 Respiratory gas exchange measurements were obtained breath-by-breath by an open-circuit computerized spirometry system (Quark CPET; COSMED, Rome, Italy), and 30-sec averages were calculated for analysis.
The ventilatory threshold (VO2VT), a standardized cardiometabolic parameter of submaximal exercise intensity, was determined by the ventilatory equivalent method, defined as the stage where the first rise in the ventilatory equivalent of O2 (VE/VO2) occurs without a concurrent rise in the ventilatory equivalent of CO2 (VE/VCO2).31 The gas analyzer and spirometer were calibrated before each test by using known gas concentrations and a 3-L syringe. An integrated 12-lead digital electrocardiogram and software (ERGO PC 13; MICROMED, Brazil) were used for heart rate recording and monitoring.
Aerobic exercise training protocol
The 12-week aerobic exercise training program was conducted at the Center for Studies in Psychobiology and Exercise, São Paulo, Brazil. It consisted of 40-min sessions of walking or jogging on treadmill (5 min of warm-up, 30 min inside the preconized heart rate, and another 5-min cool down period), performed three times per week at the work rate (m·min−1), heart rate, and the rate of perceived effort corresponding to the participant's ventilatory threshold. The heart rate was monitored by a cardiac monitor (model F5; Polar Electro®, Finland), and the rate of perceived effort was assessed by the Borg's scale. All exercise sessions were supervised by exercise physiologists (A.B.O. and M.T.M.).
Outcome variables
Changes in plasma AEA concentration was the primary outcome variable; secondary outcome variables were days with migraine, pain intensity, and the number of abortive medication used during attacks; and mood scores and cardiorespiratory fitness were tertiary variables.
Randomization and masking
Simple randomization (1:1) was performed at blood sampling/psychometric interview visit by researcher A.B.O. Random numbers were generated by an online software, and even and odd numbers were previously attributed to exercise training (exercise groups) or waiting list (waitlist groups) allocations, respectively.
Sample size and statistics
To detect a medium effect size (f)=0.25 of interaction for the primary outcome variable in a 4×2 study design (i.e., four groups with pre–postintervention measures), and accepting a type I error rate limit of 5% and type II error rate limit of 20%, the necessity for enrolling 48 participants in the total sample was predicted (G*Power software; Version 3.1.7.). Estimating a drop-out rate of around 20%, we aimed at including 58 participants in the intention-to-treat analyses. We included two groups of nonheadache individuals besides two migraine groups, as we intended to control for disease as well.
Continuous variables with normal distribution, which did not violate the assumptions of homogeneity (Levene's test) and sphericity (Mauchly's test), were analyzed by repeated-measure ANOVA. Variables with non-normal distribution were tested by generalizing estimating equations (GEE). In this modeling, several structures tested for the correlation matrix. The model with the lowest Quasi-Akaike Information Criterion and the best goodness-of-fit was adopted as the final model. Pairwise comparisons were performed for both ANOVA and GEE models to identify where between-/within-groups differences occurred. Correlational hypotheses were tested by Spearman's bivariate correlations. A p-value <0.05 was considered statistically significant. Calculations were computed by SPSS software (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY).
Results
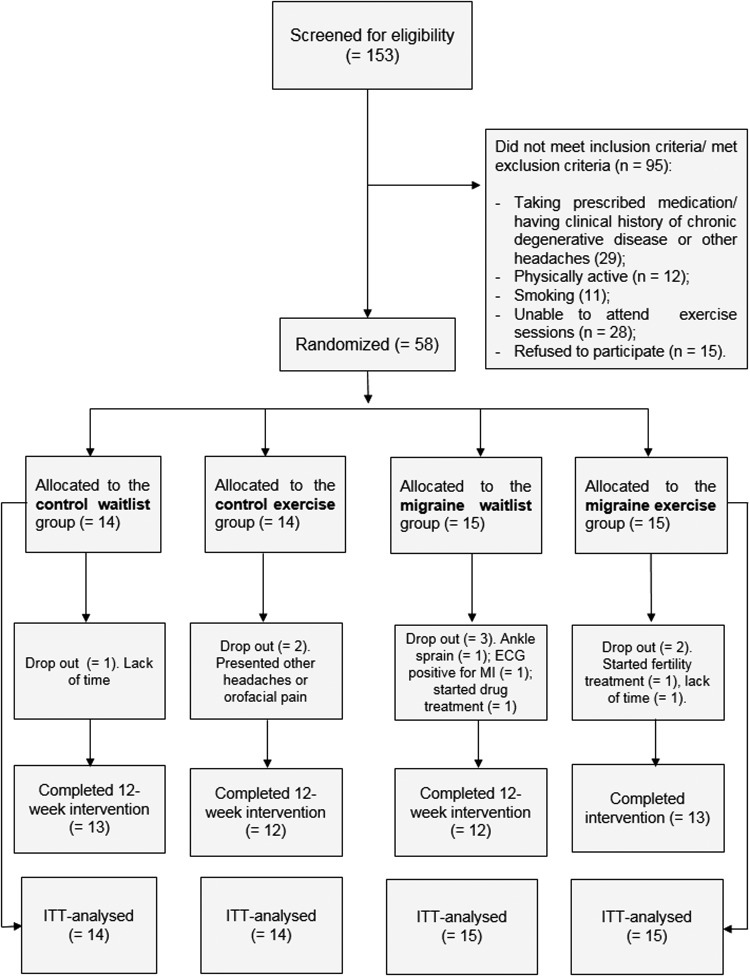
Fifty-eight participants (mean±SD age=36.2±10.9, and BMI=26.5±4.5) were included in the intention-to-treat analysis. Figure 1 shows participants' flow in the study. Attrition rates for control waitlist, control exercise, migraine waitlist, and migraine exercise groups were 7.1%, 14.2%, 20%, and 23.5%, respectively.
FIG. 1.
Participants' flow in the study.
Participants reported no adverse event with the exercise training protocol. There were no differences in sociodemographic and anthropometric characteristics between migraine and control groups (Table 1), nor in clinical characteristics between migraine patients of the exercise and waitlist groups (Table 2). There was no statistically significant difference between control exercise and migraine exercise groups regarding the adherence to the exercise program (66.4%±13.0% vs. 55.9%±18.4%, p=0.11, respectively).
Table 1.
Participants’ Anthropometric Characteristics and Sociodemographic Data
| Variables | Control waitlist | Control exercise | Migraine waitlist | Migraine exercise |
|---|---|---|---|---|
| Age (years) | 38.0±8.8 | 34.7±12.0 | 34.2±9.0 | 37.4±13.8 |
| Body mass (kg) | 72.3±13.7 | 67.6±9.4 | 69.6±18.9 | 72.9±15.7 |
| Height (m) | 1.62±0.1 | 1.63±0.1 | 1.63±0.1 | 1.64±0.1 |
| BMI (kg/m2) | 27.6±4.2 | 25.5±3.1 | 25.9±6.0 | 27.0±4.5 |
| Sex, n (%) | ||||
| Male | 2 (15.4) | 2 (16.6) | 3 (25.0) | 2 (15.4) |
| Female | 11 (84.6) | 10 (83.4) | 9 (75.0) | 11 (84.6) |
| Ethnicity, n (%) | ||||
| White | 12 (92.3) | 11 (91.7) | 9 (75) | 12 (92.3) |
| Black | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) |
| Asian | 1 (7.7) | 1 (8.3) | 1 (8.3) | 1 (7.7) |
| Education, n (%) | ||||
| Superior | 11 (84.6) | 10 (83.3) | 9 (75.0) | 9 (69.2) |
| Secondary | 1 (7.7) | 2 (16.7) | 2 (16.7) | 4 (30.8) |
| Elementary | 1 (7.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
Data are shown as mean±SD, or informed otherwise.
Table 2.
Intention-to-Treat Analyses of Secondary and Tertiary Outcome Variables
| Variables [mean, (95% CI)] | Control waitlist | Control exercise | Migraine waitlist | Migraine exercise |
|---|---|---|---|---|
| Days w/migraine (n/month) | ||||
| Baseline | — | — | 7.6 (5.2–10.0) | 8.9 (7–10.9) |
| Post | — | — | 8.2 (5.2–11.2) | 5.6 (3.9–7.4)a |
| Attacks' frequency (n/month) | ||||
| Baseline | — | — | 5.1 (3.7–6.3) | 6.3 (4.7–8) |
| Post | — | — | 4.3 (2.7–5.9) | 3.8 (2.4–5.2)a |
| Pain intensity (0–3) | ||||
| Baseline | — | — | 1.9 (1.6–2.1) | 1.7 (1.5–2) |
| Post | — | — | 2.1 (1.8–2.4) | 1.9 (1.5–2.2) |
| Acute medication (n/month) | ||||
| Baseline | — | — | 6.0 (2.9–9.2) | 6.5 (4.0–8.9) |
| Post | — | — | 5.8 (3.3–8.2) | 4.1 (2.6–5.6)b |
| VO2Peak (mL·kg−1·min−1) | ||||
| Baseline | 32.8 (28.6–36.9) | 31.2 (27.5–34.9) | 32.8 (28.2–37.4) | 30.6 (27.0–34.1) |
| Post | 32.6 (27.8–36.7) | 33.6 (29.7–37.6)c | 34.5 (29.7–39.5) | 32.3 (28.5–36.1)b |
| VO2VT (mL·kg−1·min−1) | ||||
| Baseline | 18.9 (16.3–21.5) | 17.9 (15.5–20.3) | 17.3 (14.7–19.9) | 16.0 (13.6–18.4) |
| Post | 19.3 (16.1–22.5) | 20.2 (17.2–23.2)c | 16.8 (13.6–20) | 17.7 (14.8–20.7)b |
| Tension | ||||
| Baseline | 8.6 (4.9–12.3) | 8.9 (5.2–12.7) | 16.4 (12.4–20.3)d,e | 12.5 (9.1–15.9) |
| Post | 7.8 (4.4–11.1) | 8.4 (5–11.8) | 14.1 (10.5–17.3) | 8.4 (5.3–11.5)f |
| Depression | ||||
| Baseline | 7.3 (3.3–16.3) | 3.5 (1.7–7.3) | 14.5 (9.1–23.1)d.e | 8.4 (4.7–15.1) |
| Post | 5.8 (2.7–12.1) | 4.1 (2.1–8.1) | 12.8 (6.4–25.6) | 1.6 (0.96–2.7)f |
| Anger | ||||
| Baseline | 5.4 (2.7–10.7) | 4.3 (2.4–7.7) | 11.0 (7.4–16.4)e | 7.3 (4.3–12.6) |
| Post | 5.8 (3.1–10.7) | 3.9 (1.7–8.6) | 9.1 (5.8–14) | 4.0 (2.2–7.3)f |
| Vigor | ||||
| Baseline | 16.2 (13.7–19.2) | 18.6 (16.2–21.4) | 15.3 (12.3–19.1) | 16.0 (13.4–19) |
| Post | 16.3 (13.9–19.1) | 19.0 (16.6–21.6) | 14.9 (11.2–19.7) | 17.4 (14.8–20.4) |
| Fatigue | ||||
| Baseline | 5.4 (3.2–9.1) | 5.3 (3.6–7.7) | 11.3 (8.9–14.3)d,g | 8.0 (5.3–12) |
| Post | 4.9 (2.5–9.5) | 4.7 (2.6–8.4) | 10.8 (7.5–15.4) | 5.1 (3.4–7.7)f |
| Confusion | ||||
| Baseline | 6.1 (4.3–8.7) | 4.4 (3.1–6.2) | 8.0 (5.2–12.3) | 7.0 (5.0–9.7) |
| Post | 4.9 (3.3–7.2) | 5.3 (3.0–9.3) | 8.0 (5.5–11.4) | 4.9 (3.6–6.6) |
p<0.01 versus baseline.
p<0.05 versus baseline.
p<0.01 versus baseline; pairwise comparisons of repeated-measure ANOVAs main effects.
p<0.05 versus control waitlist.
p<0.05 versus control exercise.
p<0.05 versus baseline; pairwise comparisons of generalizing estimating equations main effects.
p<0.01 versus control exercise; pairwise comparisons of repeated-measure ANOVAs main effects.
VO2Peak, peak oxygen uptake; VO2VT, oxygen uptake elicited at the ventilatory threshold.
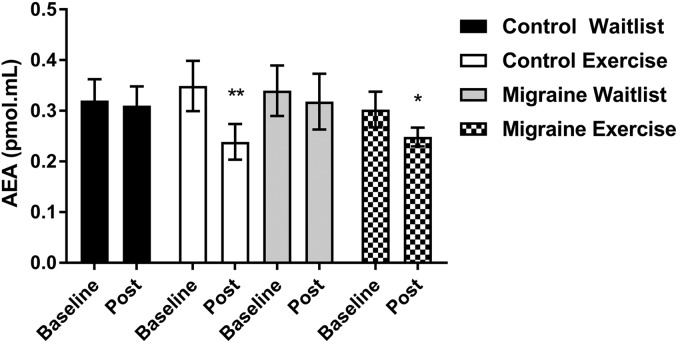
Repeated-measure ANOVA showed a significant interaction for plasma AEA [F(3, 47)=5.4, p=0.024, f=0.4]. Pairwise comparisons showed no significant differences for baseline AEA between groups (Fig. 2). Pairwise comparisons of mean differences (95% CI) between baseline-postintervention periods showed statistically significant reductions in the control exercise [−0.1 (−0.1 to −0.02), p<0.01] and migraine exercise [−0.06 (−0.1 to −0.001), p=0.04] groups, but no significant changes in control waitlist [−0.03 (−0.1 to 0.04), p=0.85] and migraine waitlist [0.003 (−0.07 to 0.08), p=0.6] groups (Fig. 2).
FIG. 2.
Primary outcome variable plasma AEA concentrations. Data are shown as mean±SEM. *p<0.05; **p<0.01, pairwise comparisons of repeated-measure ANOVA. AEA, anandamide.
The responder rates for the clinical outcome days with migraine (i.e., ≥50% improvement) in waitlist and exercise groups were 16.6% (2/12) and 53.8% (7/13), respectively. Multiple pairwise comparisons of GEE and ANOVA models for the tertiary outcome variables (clinical variables, cardiorespiratory fitness, and mood profile) are summarized in Table 2. Analyses of GEE effect models for days with migraine showed a significant main effect of interaction between time×allocation [5.4(1), p=0.02, β=3.8]. There were main effects of time for frequency of attacks [8.1(1), p<0.01, β=−2.5] and acute medication [4.4(1), p=0.03, β=−0.45]. Repeated-measure ANOVA showed a significant main effect of time for VO2Peak [F(3, 47)=15.2, p<0.01, f=0.97] and VO2VT [F(3, 47)=4.6, p=0.03, f=0.36]. Pairwise comparisons showed a significant increase in VO2Peak and VO2VT only for the exercise groups after the intervention period (Table 2).
For mood variables, GEE effect models for Tension showed a significant main effect of time [4.2(1), p=0.03, β=1.1]. For Depression, there were significant main effects of time [4.8(1), p=0.02, β=1.6] and allocation [4.8(1), p=0.02, β=−6.3]. There were main effects of allocation for Fatigue [14.3(1), p<0.01, β=0.74] and Anger [4.6(1), p=0.03, β=0.8]. There were no significant main effects for Vigor or Confusion. Pairwise comparisons showed significant reductions of Tension, Depression, Anger, and Fatigue in the migraine exercise group after the intervention period; whereas this group showed increased baseline scores for these negative mood domains compared with control groups (Table 2).
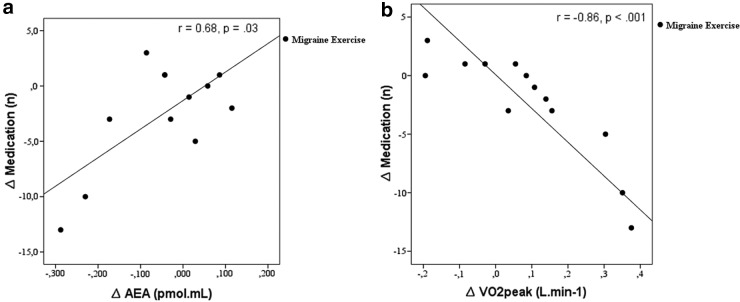
Exploratory correlational analyses using the values of the difference between baseline and postintervention period, that is, the delta (“Δ”) values, showed no correlation between clinical data, VO2Peak, VOVT, AEA, or mood variables. One exception was the amount of abortive medication consumed, which was inversely correlated with VO2Peak (r=−0.47, p=0.04) and positively correlated with changes in plasma AEA concentrations (r=0.41, p=0.03). Figure 3 shows the split group analyses, wherein these correlations were even stronger in the migraine exercise group.
FIG. 3.
Correlations between changes in medication consumed and AEA (a), and between medication consumed and VO2Peak (b). VO2Peak, peak oxygen uptake.
Analysis including both control exercise and migraine exercise groups showed a significant inverse correlation between adherence to the exercise program and reductions in AEA concentrations (r=−0.35, p=0.02). There was an even stronger inverse correlation between changes in AEA postintervention and adherence to exercise in the migraine exercise group (Fig. 4). Adherence to the exercise program did not correlate with the other clinical outcome variables. No other correlations between clinical, psychometric, and AEA were found.
FIG. 4.
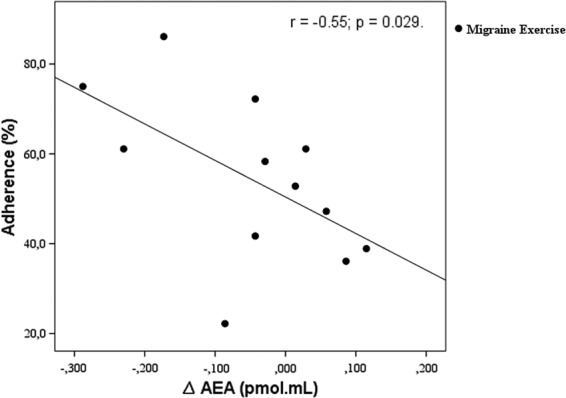
Correlations between changes in AEA and adherence to the exercise training program.
Discussion
The main finding of this study is that aerobic exercise reduces plasma AEA levels, with possible implication in the correlation between migraine and cardiorespiratory fitness in migraine patients.8,9 This study also confirms previous studies showing reductions in migraine attacks4–8 and mood-enhancing effects3,5 through aerobic exercise training.
The AEA exerts complex and pleiotropic actions on psychological and physiological processes,11–13,17–19 thus the interpretations of this study must take into account its distinct effects on peripheral and central mechanisms. At the peripheral level, AEA-CB1 receptor signaling is shown to affect oxidative metabolic function through impaired mitochondrial biogenesis/function in adipocytes, liver, and skeletal muscle,13 whereas lower circulating AEA levels after aerobic training have been implicated in improved energy metabolism homeostasis in obese humans.18
Likewise, systemic administration of CB1 receptors antagonists increases resting energy expenditure and oxygen uptake in rodents32 and humans,33 implicating improved oxidative metabolic function. Here, in the exercise groups, but not in waitlist groups, both peak and submaximal oxygen uptake (i.e., VO2Peak and VO2VT, respectively) increased after the intervention period (Table 2). Our data, together with the aforementioned animal and human studies,13,18,32,33 suggest the participation of lower tonic peripheral AEA signaling in the improvement of oxidative function, which would presumably culminate in increased cardiorespiratory fitness. Importantly, the link between exercise, AEA, and mitochondrial function may be particularly relevant for migraine patients, since mitochondrial dysfunction has been also implicated in the pathogenesis of the disease.34,35
Correspondingly, a cross-sectional populational study showed an up to fourfold increased risk for having migraine among individuals in the lower quintile of cardiorespiratory fitness (i.e., the lowest VO2Peak values).9 Therefore, this study set forth evidence for an adaptive mechanism by which patients with migraine may benefit from regular exercise involving the interactions between reduced resting AEA and increased cardiorespiratory fitness.
In addition, although we did not find correlations between changes in AEA and mood scores, decrease in tension-anxiety/depression scores and lower abortive medication in the migraine exercise group may suggest the participation of exercise training-mediated effects on patients' ability to cope with the migraine-related pain and psychiatric comorbidities (e.g., irritability, fear, anxiety, and depression).36 It is recognized that some patients may excessively consume these medications due to anxiety/fear of pain, or addictive/dependence behavior, rather than pain per se.37 Migraine, anxiety, and depression are, indeed, the most important risk factors for medication-overuse headaches.25,37,38 Thus, in agreement with the data showing lower risk for medication-overuse headaches in physically active people,37,39 our data strengthen the idea that exercise-induced neuropsychological mechanisms would be operant in this protective effect. Whether the reduction in plasma AEA concentration is mechanistically related or not to these neurobehavioral processes, as well as to the aforementioned possible clinical and physiological mechanisms cannot be concluded from this study.
Studies have shown that increased CB1 receptors signaling in pain-processing brain areas mediates analgesia in rodents, including exercise-induced analgesia,16 elicits antidepressant/anxiolytic behavior,12 whereas higher plasma AEA concentration is strongly correlated with mood improvement after acute aerobic exercise in humans.14 At this point, it is worth mentioning that we did not provide direct evidence on the source of circulating AEA measured, or whether the peripheral AEA would reflect its metabolism in the central nervous system. Thus, one cannot rule out the possibility of even an increased brain AEA after aerobic exercise training despite reductions in plasma levels. Furthermore, AEA exerts dual actions on pain perception11 and mood regulation,12 depending on whether it targets CB1 receptors or TRPV1 receptors. Central CB1 receptors signaling is believed to promote analgesia and anti-hyperalgesic effects,11,17 at primary sensory neurons,40 as well as sensory or spinal trigeminal neurons; whereas AEA has excitatory activity via sensitization of TRPV1 receptors, and it stimulates the release of calcitonin gene-related peptide,41 a nociceptive and vasodilator neuropeptide believed to play a critical role in the etiology of migraines.42 At the neurobehavioral level, low doses of AEA elicit anxiolysis via CB1 receptors, whereas at higher doses it promotes anxiogenic behavior by activating TRPV1 receptors within the periaqueductal gray matter.43 In this context, one could speculate that reductions in peripheral AEA concentration through regular exercise might hinder activation of pro-nociceptive/anxiogenic TRPV1-mediated pathways.
Lastly, we did not find baseline differences for plasma AEA levels between healthy individuals and migraine patients, as suggested by some,20–23 but not all44 studies. A possible explanation for these discrepant data may lie on the effects of acute abortive medications used during attacks on enzymatic pathways of AEA metabolism. For example, common abortive medications used by patients, as the case in this study (i.e., acetaminophen, R-profens, or metamizole), are known to inhibit cyclooxygenases, which are enzymes that contribute to AEA degradation.11,45 Although patients were at headache-free days during blood sampling, one cannot rule out possible interferences from repeated consumption of these medications over time, and its proximity with regard the blood sampling.
In this sense, further studies are needed to investigate whether reduced AEA observed here was not related to reduced abortive medications use per se. The reduction in plasma AEA levels observed in the control exercise group, the inverse correlation between change in AEA and adherence to the exercise program (Fig. 3a), indeed, indicate an exercise-mediated effect.
This study has limitations and flaws that weaken interpretations and the generalizability of its findings. First, there was selection bias, as participants were spontaneously interested in exercise training rather than other prophylactic treatments. Second, the exclusion criteria were very restrictive, making this sample not representative of the general migraine population. Third, regarding the assessors, although they were not informed about allocation of patients and visits were scheduled without previous knowledge of their work shift scales, there was no randomization of assessors. Fourth, the exercise supervisor was not blind. The two latter aforementioned factors may have caused performance bias and unbalanced care and attention delivered to participants. Finally, we did not perform follow-up analyses; therefore, long-term effects of aerobic exercise on the variables assessed here are still speculative.
The strengths of this study were the factorial design with parallel groups controlling for disease and intervention, the use of gold-standard measure of cardiorespiratory fitness, and the use of the ventilatory threshold as a standardized parameter of exercise intensity, to our knowledge the first time prospectively assessed in migraine patients. Our rigor on scheduling the blood sampling with regard to the menstrual cycle to minimize the influence of sex hormones on AEA metabolism46 is worth mentioning as well.
Conclusions
Regular moderate aerobic exercise, and hence improved cardiorespiratory fitness, is effective in the management of migraine and may attenuate psychiatric comorbidities-related symptoms of anxiety and depression.4–6 This study suggests a possible participation of AEA in these clinical and cardiorespiratory outcomes. As a molecule with flexible actions in pain, energy metabolism, and neurobehavioral processes, AEA merits further investigation to better understand its possible participation in the therapeutic effects of regular aerobic exercise, and in migraine pathophysiology.
Acknowledgments
The authors are grateful to the Coordenadoria de Aperfeiçoamento de Pessoal de Nivel Superior for granting this study. The authors appreciate the whole staff of the Center for Studies in Psychobiology and Exercise, particularly Giscard de Lima and Paulo Minalli for their interpretation of cardiorespiratory fitness tests. We also appreciate José Rocha and Eduardo Sugawara for their critical help in the spectrometric analyses.
Abbreviations Used
- AEA
anandamide
- CB1
cannabinoid type-1 receptors
- CB2
cannabinoid type-2 receptors
- GEE
generalizing estimating equations
- POMS
Profile of mood state
- TRPV1
transient receptor potential vanilloid type-1 receptors
- VE/VO2
ventilatory equivalent of O2
- VE/VCO2
ventilatory equivalent of CO2
- VO2Peak
peak oxygen uptake
- VO2VT
oxygen uptake elicited at the ventilatory threshold
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Oliveira AB, Ribeiro RT, Mello MT, Tufik S, and Peres MFP (2019) Anandamide is related to clinical and cardiorespiratory benefits of aerobic exercise training in migraine patients: A randomized controlled clinical trial, Cannabis and Cannabinoid Research 4:4, 275–284, DOI: 10.1089/can.2018.0057.
References
- 1. Woldeamanuel Y, Cowan R. Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. 2017;372:307–315 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Neurological Disorders Collaborator Group. Global Health Metrics Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;11:877–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santiago MDS, Carvalho D de S, Gabbai AA, et al. . Amitriptyline and aerobic exercise or amitriptyline alone in the treatment of chronic migraine: a randomized comparative study. Arq Neuropsiquiatr. 2014;72:851–855 [DOI] [PubMed] [Google Scholar]
- 4. Varkey E, Cider A, Carlsson J, et al. . Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darabaneanu S, Overath CH, Rubin D, et al. . Aerobic exercise as a therapy option for migraine: a pilot study. Int J Sports Med. 2011;32:455–460 [DOI] [PubMed] [Google Scholar]
- 6. Overath CH, Darabaneanu S, Evers MC, et al. . Does an aerobic endurance programme have an influence on information processing in migraineurs? J Headache Pain. 2014;15:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woldeamanuel Y, Cowan R. The impact of regular lifestyle behavior in migraine: a prevalence case-referent study. J Neurol. 2016;263:669–676 [DOI] [PubMed] [Google Scholar]
- 8. Varkey E, Cider A, Carlsson J, et al. . A study to evaluate the feasibility of an aerobic exercise program in patients with migraine. Headache. 2009;49:563–570 [DOI] [PubMed] [Google Scholar]
- 9. Hagen K, Wisløff U, Ellingsen Ø, et al. Headache and peak oxygen uptake: the HUNT3 study. Cephalalgia. 2016;36:437–444 [DOI] [PubMed] [Google Scholar]
- 10. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep. 2013;17:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piscitelli F, Di Marzo V. “Redundancy” of endocannabinoid inactivation: new challenges and opportunities for pain control. ACS Chem Neurosci. 2012;3:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Micale V, Di Marzo V, Sulcova A, et al. . Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther. 2013;138:18–37 [DOI] [PubMed] [Google Scholar]
- 13. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490 [DOI] [PubMed] [Google Scholar]
- 14. Raichlen DA, Foster A, Gerdeman G, et al. . Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner's high.” J Exp Biol. 2012;215:1331–1336 [DOI] [PubMed] [Google Scholar]
- 15. Heyman E, Gameli F-X, Goekint M, et al. . Intense exercise increases circulating endocannabinoid and BDNF levels in humans-Possible implications for reward and depression. Psychoneuroendocrinology. 2011;37:844–851 [DOI] [PubMed] [Google Scholar]
- 16. Galdino G, Romero TRL, Silva JFP, et al. . The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology. 2014;77:313–324 [DOI] [PubMed] [Google Scholar]
- 17. Greco R, Mangione AS, Sandrini G et al. . Effects of anandamide in migraine: data from an animal model. J Headache Pain. 2011;12:177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Marzo V, Côté M, Matias I, et al. . Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217 [DOI] [PubMed] [Google Scholar]
- 19. McPartland JM, Guy GW, Di Marzo V. Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One. 2014;9:e89566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cupini L, Bari M, Battista N, et al. . Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia. 2006;26:277–281 [DOI] [PubMed] [Google Scholar]
- 21. Rossi C, Pini LA, Cupini ML, et al. . Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: relation with serotonin levels. Eur J Clin Pharmacol. 2008;64:1–8 [DOI] [PubMed] [Google Scholar]
- 22. Sarchielli P, Pini LA, Coppola F, et al. . Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32:1384–1390 [DOI] [PubMed] [Google Scholar]
- 23. Cupini LM, Costa C, Sarchielli P, et al. . Degradation of endocannabinoids in chronic migraine and medication overuse headache. Neurobiol Dis. 2008;30:186–189 [DOI] [PubMed] [Google Scholar]
- 24. Heyman E, Gameli F-X, Aucouturier J, et al. . The role of the endocannabinoid system in skeletal muscle and metabolic adaptations to exercise: potential implications for the treatment of obesity. Obes Rev. 2012;13:1110–1124 [DOI] [PubMed] [Google Scholar]
- 25. Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160 [DOI] [PubMed] [Google Scholar]
- 26. Boutron I, Moher D, Altman DG et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309 [DOI] [PubMed] [Google Scholar]
- 27. Di Tomaso E, Beltramo M, Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382:677–678 [DOI] [PubMed] [Google Scholar]
- 28. Peluso M. Alterações de humor associadas à atividade física intensa. [Thesis]. São Paulo: Universidade de São Paulo, 2003 [Google Scholar]
- 29. Zoerner A, Gutzki F, Batkai S, et al. . Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochim Biophys Acta. 2011;1811:706–723 [DOI] [PubMed] [Google Scholar]
- 30. Oliveira AB, Bachi ALL, Ribeiro RT, et al. . Unbalanced plasma TNF-α and IL-12/IL-10 profile in women with migraine is associated with psychological and physiological outcomes. J Neuroimmunol. 2017;313:138–144 [DOI] [PubMed] [Google Scholar]
- 31. Balady GJ, Arena R, Sietsema K, et al. . Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. 2010;122:191–225 [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Connoley I, Wilson C, et al. . Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep ob/Lep ob mice. Int J Obes (Lond). 2005;29:183–187 [DOI] [PubMed] [Google Scholar]
- 33. Addy C, Wright H, Laere K Van, et al. . The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78 [DOI] [PubMed] [Google Scholar]
- 34. Sparaco M, Feleppa M, Lipton R, et al. . Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia. 2006;26:361–372 [DOI] [PubMed] [Google Scholar]
- 35. Guo S, Esserlind AL, Andersson Z, et al. . Prevalence of migraine in persons with the 3243A>G mutation in mitochondrial DNA. Eur J Neurol. 2016;23:175–181 [DOI] [PubMed] [Google Scholar]
- 36. Peres M, Mercante J, Tobo P, et al. . Anxiety and depression symptoms and migraine: a symptom-based approach research. J Headache Pain. 2017;18:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kristoffensen E, Lundqvist C. Medication-overuse headache: a review. J Pain Res. 2014;7:367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radat F, Lanteri-Minet M. What is the role of dependence-related behavior in medication-overuse headache? Headache. 2010;50:1597–1611 [DOI] [PubMed] [Google Scholar]
- 39. Le H, Tfelt-hansen P, Skytthe A et al. . Association between migraine, lifestyle and socioeconomic factors: a population-based cross-sectional study. J Headache Pain. 2011;12:157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Varga A, Jenes A, Marczylo TH, et al. . Anandamide produced by Ca(2+)-insensitive enzymes induces excitation in primary sensory neurons. Pflugers Arch. 2014;466:1421–1435 [DOI] [PubMed] [Google Scholar]
- 41. Sousa-Valente J, Varga A, Ananthan K, et al. . Anandamide in primary sensory neurons: too much of a good thing? Eur J Neurosci. 2014;39:409–418 [DOI] [PubMed] [Google Scholar]
- 42. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158:543–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moreira FA, Aguiar DC, Terzian ALB et al. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety—two sides of one coin? Neuroscience. 2012;204:186–192 [DOI] [PubMed] [Google Scholar]
- 44. Gouveia-Figueira S, Goldin K, Hashemian S, et al. . Plasma levels of the endocannabinoid anandamide, related N-acylethanolamines and linoleic acid-derived oxylipins in patients with migraine. Prostaglandins Leukot Essent Fat Acids. 2017;120:15–24 [DOI] [PubMed] [Google Scholar]
- 45. Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karasu T, Marczylo TH, Maccarrone M et al. . The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Reprod Update. 2011;17:347–361 [DOI] [PubMed] [Google Scholar]