Abstract
Background: There is variability in the reported Δ9-tetrahydrocannabinol (THC) and 11-hydroxy-tetrahydrocannabinol (11-OH-THC) pharmacokinetic (PK) and pharmacodynamic (PD) parameters between studies and there is limited investigation into how the presence of food or sex affect these parameters. In this study, we examined the PK and PD parameters of an encapsulated THC extract and its major active metabolite, 11-OH-THC, under different fed states.
Methods: The study was a single-dose, randomized, double-blinded, four-way crossover investigation. THC capsules (1 or 2×5 mg) were administered to 28 healthy adults (13 females: 15 males) under a fasted condition or after a high-fat meal. Blood samples were collected and PK parameters were determined through noncompartmental analysis. Adverse events (AEs), cognitive function (through completion of digit symbol substitution tests), blood pressure, and heart rate were also recorded.
Results: The presence of high-fat food significantly enhanced time to peak plasma concentration (Tmax) and area under the curve (AUC0–24) for both THC and 11-OH-THC and reduced THC's apparent volume of distribution (Vz/F) and apparent clearance (Cl/F). Females had a significantly greater peak plasma concentration (Cmax) compared with males after 5 mg THC in a fasted state. No cardiovascular or cognitive effects and only mild AEs (somnolence, fatigue, and euphoric mood) were reported.
Conclusion: These findings may help to inform the guidelines provided by governing health bodies on the effects of cannabis, such as time to onset and duration of action, and aid health care practitioners in their prescribing practices. Furthermore, the doses used in this study are safe to consider for future interventional studies in disease conditions where THC has been shown to have therapeutic efficacy.
Keywords: 11-OH-THC, fasted, fed, pharmacokinetic, sex, THC
Introduction
Cannabis contains over 100 cannabinoids and up to 200 terpenes, depending on the cultivar, with Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) thought to be responsible for the majority of the physiological effects induced by cannabis.1 THC and its active metabolite, 11-hydroxy-tetrahydrocannabinol (11-OH-THC) are primarily identified and responsible for causing intoxication.2–4 However, THC is much more than just an intoxicating compound, with many studies demonstrating its therapeutic potential in various medical conditions.1,5,6
An analysis of data collected from medical cannabis consumers found that THC levels in cannabis products, but not CBD levels, are independently associated with the degree of symptom relief across 27 different health symptom categories,7 whereas another observational study found patients reported high THC cultivars improved their symptoms from conditions such as arthritis, pain, and post-traumatic stress disorder.8
Although, Whiting et al. reported there was no clear evidence for efficacy based on route of administration,5 in contrast, a recent report of patient survey data found inhalation of dried flower was the most commonly used and therapeutically effective route of cannabis administration followed by ingestion of concentrates.7 These findings are not entirely surprising, as inhalation of cannabis results in a higher bioavailability of THC than orally administered cannabis extracts due to the avoidance of first pass metabolism. Oral bioavailability of THC is 6%±3% following oral consumption in a food product9,10 and 10–20% after consumption of a cannabis extract.11 This is in contrast to the bioavailability following inhalation, which ranges from 10% to 37%.9,10,12 However, while orally administered THC has poor bioavailability, capsules taken orally can be formulated with precise concentrations of THC allowing greater control over dosing compared with other administration methods. In addition, oral administration of THC not only results in minor increases in THC levels in the blood, but also leads to higher plasma levels of 11-OH-THC, THC's active metabolite,2,4 due to first pass hepatic metabolism. In fact, plasma levels of 11-OH-THC:THC after inhalation have been reported to be as low as 1:2013 while after oral administration, this ratio is increased to a range of 0.5:1–1:1.11 Since 11-OH-THC has shown physiological effects itself, a higher ratio of 11-OH-THC:THC from orally ingesting THC may have different therapeutic effects than a lower 11-OH-THC:THC ratio from inhalation of THC.
Despite the advantages associated with oral consumption of cannabis extracts compared with inhalation, the reported pharmacokinetic (PK) values for orally ingested THC and 11-OH-THC are limited and variable.4,9,11,14–16 There are several possible reasons for this variability. First, the polymorphic nature of cytochrome P450 (CYP450) genes17 may contribute to the wide-ranging PK parameters reported between and within studies. Second, there appears to be differences in cannabinoid absorption between males and females,16 although limited studies exist. Lastly, there is evidence that a fed over-fasted state may delay and/or increase absorption of cannabinoids.18,19 Thus, differences in fed states could explain the variation in reported values between trials.
Based on the complex interactions of THC in patients and the aforementioned variables, the goal of this study was to investigate the PK and pharmacodynamic (PD) parameters of oral THC capsules consumed by males and females during a fast or after a high-fat meal (Table 1).
Table 1.
The High-Fat, High-Calorie Breakfast Provided to Participants in Treatment B and D Before Them Consuming Their Δ9-Tetrahydrocannabinol Hard Gelatin Capsules
| Meal content | Calories | Carbohydrates (g) | Protein (g) | Fat (g) |
|---|---|---|---|---|
| Two eggs | 180 | 2 | 16 | 12 |
| Two slices of toast | 180 | 35 | 6 | 1.5 |
| Sixty gram hash browns | 150 | 20 | 1 | 7 |
| 240 mL of whole milk | 144 | 11 | 7.7 | 7.7 |
| Three slices of bacon | 120 | 0 | 9 | 9 |
| Maximum two tablespoons of butter | 180 | 0 | 0.2 | 20 |
| Total g | 68 | 39.9 | 57.2 | |
| Total calories | 954 | 272 | 160 | 515 |
Methods
Study procedures
The study designed was a single-dose, randomized, double-blinded, four-way crossover investigation of the PK and PD parameters of orally administered THC hard gelatin capsules and the PK parameters of its major metabolite, 11-OH-THC. The Clinical Trial Application (CTA) was reviewed and approved by Health Canada and ethics approval was granted by the Institutional Review Board Services before being conducted by a contract research organization, BioPharma Services, Inc., in Ontario, Canada. The study was carried out in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
For detailed summary of the methods, please see the Supplementary Data. In short, healthy adult volunteers completed the following treatments: 5 mg THC fasted (Treatment A), 5 mg THC and high-fat meal (Treatment B), 10 mg THC fasted (Treatment C), and 10 mg THC and high-fat meal (Treatment D) (Table 2). Blood samples were collected before the THC dose and then at multiple time points postdose in each study period. There was a 7-day washout period between treatments.
Table 2.
The Target Δ9-tetrahydrocannabinol Dosages and Fed States of Each of the Four Treatment Groups
| THC dose (mg) | Fed state | |
|---|---|---|
| Treatment A | 5 | Fasted |
| Treatment B | 5 | High-fat meal |
| Treatment C | 10 | Fasted |
| Treatment D | 10 | High-fat meal |
THC, Δ9-tetrahydrocannabinol.
Systolic blood pressure, diastolic blood pressure, heart rate, and cognitive function [through the digit symbol substitution test (DSST), adapted from the Wechsler Adult Intelligence Scale (WAIS)20], and adverse events (AEs) were monitored during each period.
Statistical analysis
Noncompartmental analysis and statistical analysis were done external to MedReleaf® and Aurora Cannabis, Inc.® with results stated as mean±SD. One-way ANOVA for PK parameter analysis, with a post hoc Bonferroni Test if the F was significant and no significant variance in homogeneity, and Student's t-test for demographic characteristics were carried out. Kruskal–Wallis H test was done for analysis of the DSST data. Statistical significance was determined as p<0.05.
Materials
THC hard gelatin capsules were obtained from MedReleaf®, a Canadian Licensed Producer of medical cannabis. Whole Cannabis sativa plant extract was dissolved in sunflower oil and encapsulated in hard gelatin capsules. Each capsule contained 5.4 mg THC, 0.2 mg THCA, <0.1% wt·wt−1 terpenes and no detectable CBD or cannabidiolic acid.
Results
Patient demographics
This study sought to establish the PK profile of different THC doses in healthy male and female volunteers. The PK and PD parameters of THC were examined after the consumption of a high-fat meal or after participants had fasted. PK parameters were also determined for 11-OH-THC.
There were 28 (13 female and 15 male; 20–54 years old) subjects enrolled into the study, of which 27 completed all 4 arms. One participant withdrew from the study before completing Treatment D due to an AE (see Adverse events section) and thus, the PK parameters for this arm were calculated from one less participant. Of the participants who completed the study, 56% were White, 26% were Black, 11% were Hispanic/Latino, and 7% were Asian. The age, height, body weight, and body mass index of the study population are reported in Table 3. As expected, the mean height and weight of male participants were found to be significantly greater than the female participants.
Table 3.
The Demographic Characteristics of the Study Population
| Male (15) | Female (12) | |
|---|---|---|
| Age (years) | 35±9.6 (20–51) | 35±9 (23–54) |
| Height (cm) | 182.2±8.3 (164.5–200) | 160.5±5.1 (152.2–170.3)* |
| Body weight (kg) | 86.5±10.6 (74–110.3) | 61.9±9.0 (50.2–75.7)* |
| BMI (kg/m2) | 26.0±2.3 (22.4–29.6) | 24.0±3.2 (19.4–29.4) |
Data are represented as mean±SD with the range in brackets and * denotes statistical significance.
BMI, body mass index.
Adverse events
In total, 43 AEs were reported during this study, with all AEs except one assessed as minor. Somnolence, fatigue, and euphoric mood were the most common mild events (Table 4). The one moderate AE of fainting (duration of 2 minutes) was reported 4.32 hours after Treatment C. No serious AEs were reported.
Table 4.
The Most Common Adverse Events Reported for All Treatment Arms
| Event | Treatment A | Treatment B | Treatment C | Treatment D |
|---|---|---|---|---|
| Somnolence | 3 | 1 | 4 | 1 |
| Fatigue | 2 | 1 | 1 | 2 |
| Euphoric mood | 2 | 0 | 3 | 1 |
| Headache | 1 | 3 | 1 | 0 |
| Dizziness | 1 | 1 | 2 | 1 |
| Other | 0 | 3 | 8 | 1 |
| Total | 9 | 9 | 19 | 6 |
Where Treatment A is 5 mg THC fasted, Treatment B is 5 mg THC and a high-fat meal, Treatment C is 10 mg THC fasted and Treatment D is 10 mg THC and a high-fat meal.
The majority (67.9%) of AEs were reported after administration of Treatment C. There was a decrease in the total number of AEs (32.1%) in both Treatment A and B compared with Treatment C. Treatment D showed the least number of AEs (22.2%).
One AE of note is that one study participant experienced a depressive mood 22.64 hours after Treatment D that resolved ∼96 hours after onset with no actions taken. However, the participant chose to withdraw from the study due to this AE and was the only one to do so.
PK parameters
Using noncompartmental analysis, the following parameters were calculated for both THC and 11-OH-THC: peak plasma concentration (Cmax), time to peak plasma concentration (Tmax), t1/2, and AUC0–24. Apparent volume of distribution (Vz/F) and apparent clearance rate (Cl/F) were determined for THC, whereas lag time (Tlag) and last measurable concentration (Clast) were calculated for 11-OH-THC. These values were compared within dosages (Treatment A vs. Treatment B and Treatment C vs. Treatment D) and between males and females under the different dietary conditions.
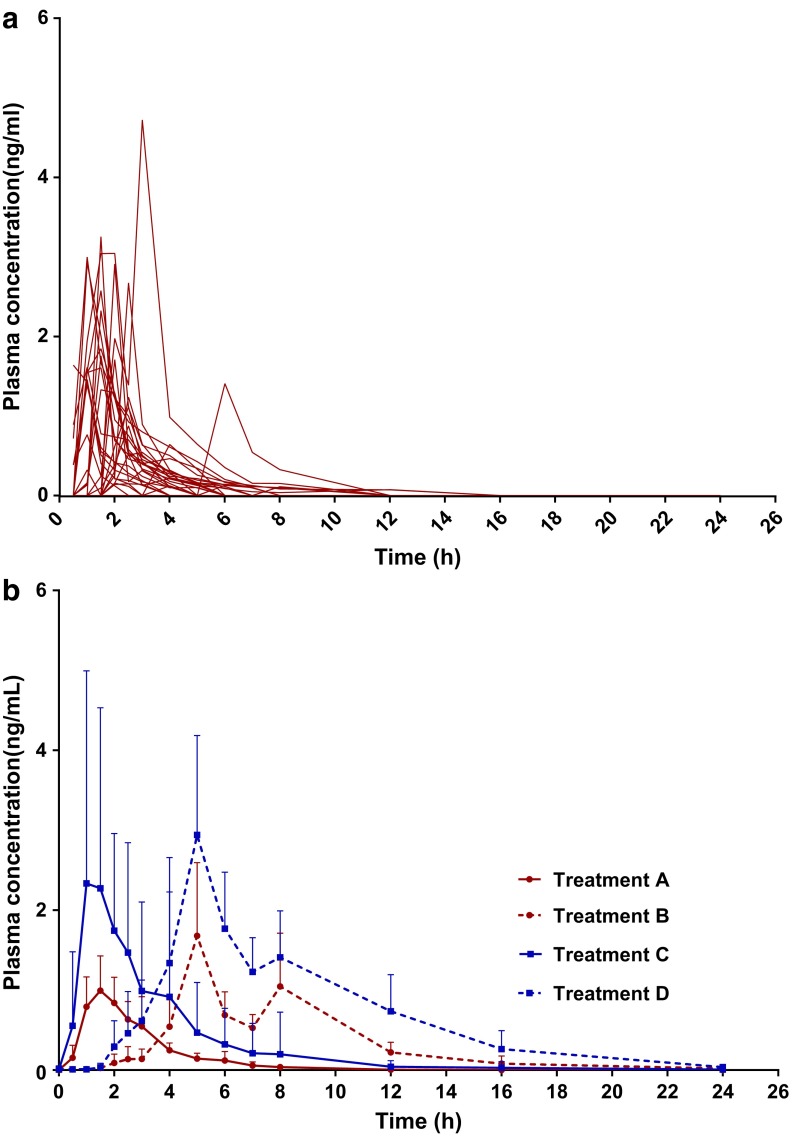
For THC, there was no difference in Cmax or t1/2 between consumption of the capsules under fed or fasted states. However, dosing immediately after a high-fat meal was found to significantly increase Tmax by about 3.5-fold in comparison to the fasted state for both the 5 and 10 mg THC doses. AUC0–24 was also significantly augmented by the presence of food upon dosing; AUC0–24 increased by about 2.7- and 2-fold for the 5 and 10 mg doses, respectively. Alternatively, both Vz/F and Cl/F were reduced by about 1.6-fold when the oral doses were consumed after a high-fat meal versus after fasting (Fig. 1 and Table 5). For Treatment A, males had a significantly smaller Cmax than females (Cmax for males was 1.39±0.69 ng·mL−1 and for females it was 2.36±1.08 ng·mL−1). No other differences between the sexes were found.
FIG. 1.
THC's plasma concentration over time (a) for each individual after Treatment A (n=28) and (b) expressed as a mean (+SD) for Treatments A–D (n=28 for Treatments A–C; n=27 for Treatment D). Where Treatment A is 5 mg THC fasted, Treatment B is 5 mg THC and a high-fat meal, Treatment C is 10 mg THC fasted, and Treatment D is 10 mg THC and a high-fat meal. THC, Δ9-tetrahydrocannabinol.
Table 5.
Δ9-Tetrahydrocannabinol Pharmacokinetic Parameters of Cmax, Tmax, t1/2, AUC0–24, Vz/F and Cl/F Were Determined from Noncompartmental Analysis
| Treatment | Cmax (ng·mL−1) | Tmax (hours) | t1/2 (hours) | AUC0–24 (ng·h·mL−1) | VZ/F (L) | Cl/F (L·h−1) |
|---|---|---|---|---|---|---|
| A | 1.86±1.01 | 1.9±1.1 | 1.6±1.3 | 3.13±1.89 | 66.1±27.5 | 66.4±27.4 |
| B | 2.60±2.33 | 6.6±2.6* | 2.8±2.0 | 8.75±7.38* | 41.0±34.7* | 41.1±34.8* |
| C | 4.24±2.53 | 1.8±1.4 | 2.6±2.1 | 8.71±5.95 | 70.5±26.2 | 70.4±26.2 |
| D | 4.51±2.54 | 6.6±2.8* | 2.6±1.9 | 17.60±9.22* | 44.3±28.8* | 44.1±28.7* |
Data are represented as mean±SD and * denotes statistical significance between the same THC dosages (Treatment A compared with Treatment B and Treatment C compared with Treatment D). Where Treatment A is 5 mg THC fasted, Treatment B is 5 mg THC and a high-fat meal, Treatment C is 10 mg THC fasted, and Treatment D is 10 mg THC and a high-fat meal.
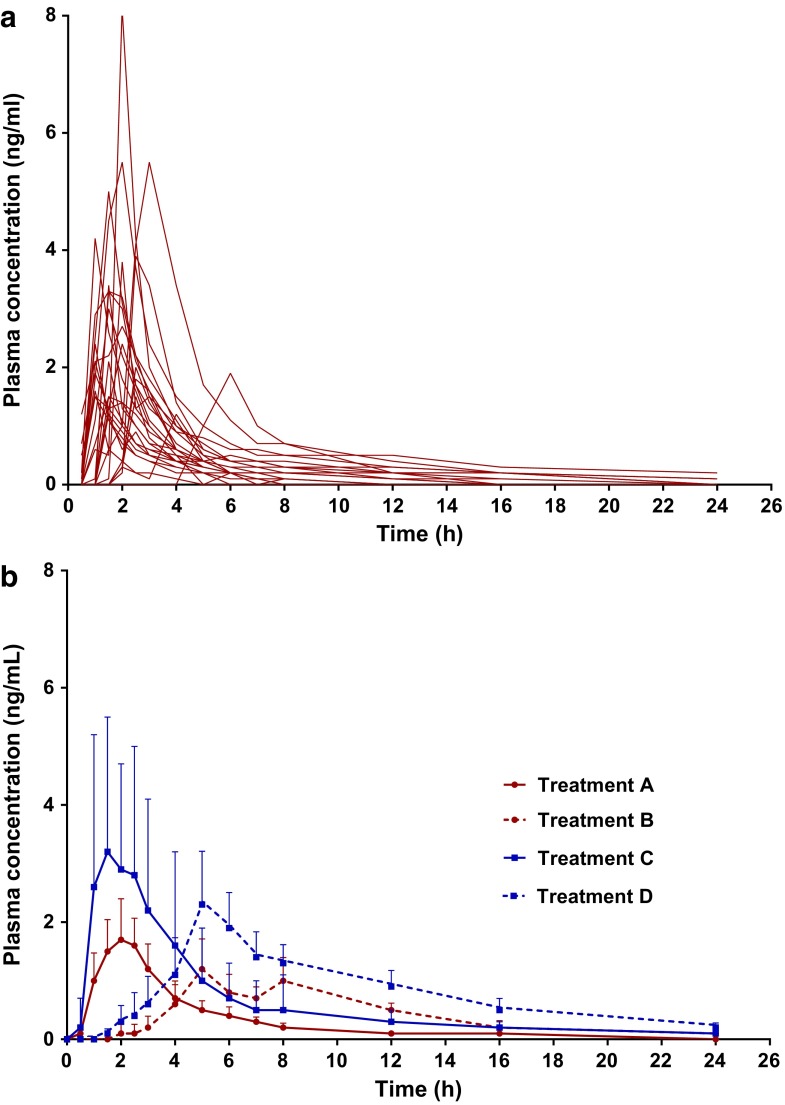
The results for 11-OH-THC were similar. For both dosages, Tmax increased by ∼3.5-fold when dosing occurred with food present versus when food was absent. Interestingly, there did not appear to be any delay between Tmax for THC and Tmax for 11-OH-THC between treatments of the same fed state, thus, Tmax was consistent across parent compound, metabolite, and dosages when the fed state was kept constant (Fig. 2 and Table 6).
FIG. 2.
11-OH-THC's plasma concentration over time (a) for each individual after Treatment A (n=28) and (b) expressed as a mean (+SD) for Treatments A–D (n=28 for Treatments A–C; n=27 for Treatment D). Where Treatment A is 5 mg THC fasted, Treatment B is 5 mg THC and a high-fat meal, Treatment C is 10 mg THC fasted, and Treatment D is 10 mg THC and a high-fat meal. 11-OH-THC, 11-hydroxy-tetrahydrocannabinol.
Table 6.
11-Hydroxy-Tetrahydrocannabinol Pharmacokinetic Parameters of Cmax, Tmax, tlag, Clast, t1/2 and AUC0–24 Were Determined from Noncompartmental Analysis
| Treatment | Cmax (ng·mL−1) | Tmax (hours) | Tlag (hours) | Clast (ng·mL−1) | t1/2 (hours) | AUC0–24 (ng·h·mL−1) |
|---|---|---|---|---|---|---|
| A | 2.86±1.64 | 1.9±1.1 | 1.2±1.6 | 0.12±0.02 | 4.3±2.6 | 7.16±4.70 |
| B | 1.91±1.38 | 6.8±2.6* | 4.0±2.4* | 0.18±0.13 | 4.2±1.9 | 9.40±5.70* |
| C | 5.10±2.44 | 2.0±1.4 | 0.9±1.5 | 0.14±0.048 | 5.8±3.0 | 14.72±7.33 |
| D | 3.40±1.69 | 6.7±2.6* | 3.3±1.5* | 0.23±0.15 | 6.3±2.4 | 17.74±6.64* |
Data are represented as mean±SD and * denotes statistical significance between the same THC dosages (Treatment A compared with Treatment B and Treatment C compared with Treatment D). Where Treatment A is 5 mg THC fasted, Treatment B is 5 mg THC and a high-fat meal, Treatment C is 10 mg THC fasted and Treatment D is 10 mg THC and a high-fat meal.
Tlag for 11-OH-THC was significantly increased by the consumption of the high-fat meal for both the 5 and 10 mg doses; tripling for the 5 mg THC dose and almost doubling for the 10 mg THC dose. Clast, Cmax, and t1/2 were unchanged by the consumption of a high-fat meal before dosing, whereas AUC0–24 was significantly enhanced; AUC0–24 increased by 1.3- and 1.2-fold for the 5 and 10 mg doses, respectively (Fig. 2 and Table 6).
PD effects
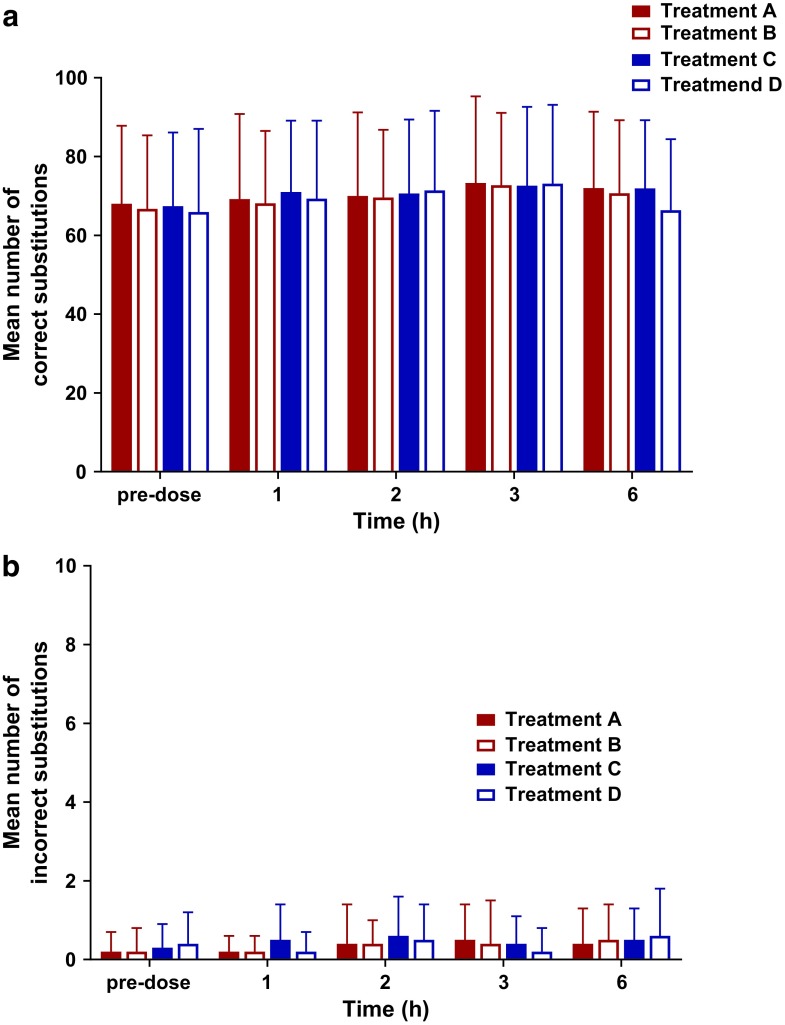
No treatment altered systolic blood pressure, diastolic blood pressure, or heart rate (data not shown). Furthermore, the DSST results showed no changes in cognitive function (Fig. 3).
FIG. 3.
The mean number of completed (a) correct and (b) incorrect symbol substitutions from the completion of the DSST at different time points for Treatments A–D (n=28 for Treatments A–C; n=27 for Treatment D). Where Treatment A is 5 mg THC fasted, Treatment B is 5 mg THC and a high-fat meal, Treatment C is 10 mg THC fasted, and Treatment D is 10 mg THC and a high-fat meal. DSST, digit symbol substitution test.
Discussion
With the recent legalization of cannabis in Canada and growing interest in both medicinal and recreational cannabis worldwide, there is a need to determine the efficacy of different cannabis formulations at specific doses and to establish the possible AEs surrounding these dosages. Thus, further PK and PD studies are crucial to gather data on the primary cannabinoids, such as THC, from different routes of administration to enhance comfort with cannabis-based medicines and inform safe and effective prescribing practices within the health care community. This study aimed to fill the need for PK and PD data for orally administered THC under fasting and fed conditions and to compare the PK parameters between males and females. To our knowledge, this is the first time such a study focusing on orally administered THC extract in a hard gelatin capsule form has been conducted in humans.
Our results show orally consumed 5 and 10 mg THC capsules are well tolerated in healthy adults under fed or fasted states. No cardiovascular effects or impaired cognition were reported. Furthermore, most AEs were classified as mild and there was a low withdrawal rate of 4%. The most commonly reported AEs were somnolence, fatigue, and euphoric mood, with no serious AEs occurring. These findings align with those reported in the wider literature in terms of AE severity (mainly mild–moderate), specific symptoms (i.e., somnolence, fatigue, euphoria, dizziness),5 and a lack of impaired cognition.21–23 Thus, our data suggest that THC extract in hard gelatin capsules at 5 and 10 mg could be explored for therapeutic benefits in different conditions. Further investigation into how differences in PK parameters affect the therapeutic efficacy of oral THC as well as the long-term safety of oral THC consumption should be examined in the relevant medical conditions in future studies.
Cmax, Tmax, and AUC0–24 were determined through noncompartmental analysis for both THC and 11-OH-THC. Vz/F and Cl/F and Tlag and Clast were also calculated for THC and 11-OH-THC, respectively. It should be noted however, only relative bioavailability can be determined from this study, not absolute bioavailability. We found that consuming a high-fat meal before administration of a THC capsule significantly delayed the time to peak plasma concentration for both THC and 11-OH-THC and enhanced the overall levels of both compounds, although peak plasma concentrations were unaffected by either a fed or fasted state. Furthermore, both Vz/F and Cl/F of THC were significantly diminished after a high-fat meal was consumed. Altogether, these findings suggest that the presence of a high-fat meal before administrating an oral dose of THC increases the levels of both THC and 11-OH-THC, but the rate at which this occurs is slower. These findings may help to inform the overarching guidelines provided to the public from governing health bodies on the effects of cannabis, such as expected time to onset and duration of effect. Furthermore, these data may aid health care practitioners in their prescribing practices. For instance, the variability in Cmax values could have significant impacts on efficacy and safety in patients, highlighting the importance of tailoring THC doses to each patient through careful titration. A summary of these findings can be found in Table 7.
Table 7.
A Summary of How an Orally Ingested Encapsulated Δ9-Tetrahydrocannabinol Extract's Pharmacokinetic Parameters for Δ9-Tetrahydrocannabinol and 11-Hydroxy-Tetrahydrocannabinol Were Altered by the Presence of a High-Fat Meal in Comparison to Consumption under a Fasting State
| PK parameters | High-fat meal vs. fasted state | |
|---|---|---|
| THC | Cmax (ng·mL−1) | No change |
| Tmax (hours) | ↑ | |
| t1/2 (hours) | No change | |
| AUC0–24 (ng·h·mL−1) | ↑ | |
| VZ/F (L) | ↓ | |
| Cl/F (L·h−1) | ↓ | |
| 11-OH-THC | Cmax (ng·mL−1) | No change |
| Tmax (hours) | ↑ | |
| Tlag (hours) | ↑ | |
| Clast (ng·mL−1) | No change | |
| t1/2 (hours) | No change | |
| AUC0–24 (ng·h·mL−1) | ↑ |
11-OH-THC, 11-hydroxy-tetrahydrocannabinol.
We also found that although there was a significant difference between male and female body weight and height, these differences only affected one parameter, the THC Cmax after Treatment A (females had a significantly greater Cmax than males). Our findings are supported by a previous study that showed females had significantly higher THC and 11-OH-THC Cmax than males after oral THC consumption.16 Alternatively, this difference in Cmax could be partly attributed to the significant weight difference between our male and female participants and not solely the result of sex differences. Therefore, our data add to the body of literature describing sex differences in cannabinoid PK parameters while also indicating further studies that utilize milligram per kilogram dosages may be helpful in investigating sex differences in cannabinoid PK parameters.
While the dose/response relationship for THC and 11-OH-THC has yet to be fully elucidated, it could be extrapolated from our data that the time to peak concentration seen when the THC capsules were administered after the high-fat meal would correlate to a longer delay in experiencing the physiological effects of THC versus administration in a fasted state. As oral administration already results in delayed physiological responses compared with inhalation, it may be recommended that patients take THC capsules on an empty stomach to experience a quicker onset of effects compared with consuming with a meal. However, more AEs were reported after Treatment D, indicating the potential for less AEs when ingesting THC with food, which may be attractive to patients, caregivers, and health care practitioners. In addition, the significant delay in clearance associated with a high-fat meal being consumed before dosing may also be attractive to patients who are seeking a longer duration of action for THC-based medicine. Altogether, these data have the potential to help inform prescription guidelines for patients depending on their tolerance for mild AEs and desired time to onset and duration of action.
Data presented from our PK study on THC capsules support the wider literature that reports consumption of cannabinoids in the presence of food leads to altered PK parameters in comparison to administration under a fasting condition.18,19 Our data align with these previous findings showing that the mean absorption of THC was increased by 2- to 3-fold (depending on dose), whereas the mean levels of 11-OH-THC were enhanced ∼1.3-fold for both doses. The increased mean absorption of THC in the presence of food is possibly due to the slowed transit time through the gastrointestinal tract when fat is present. However, the exact mechanism of this enhanced absorption has yet to be investigated. In contrast to Stott et al.,18 we did not find a significant increase in Cmax fed and fasted conditions. As peak plasma concentration after oral administration of a drug is influenced by both absorption and metabolism, these differences could be the result of the polymorphic nature of the CYP450 enzymes and/or heterogeneity of the participant populations.17 Activity of CYP450 enzymes could also be altered by the presence or absence of different cannabinoids, as cannabinoids such as CBD have been shown to inhibit CYP450 enzymes responsible for the metabolism of THC.24,25 Our capsules had no detectable amounts of CBD, avoiding this potentially confounding variable for our measured THC and 11-OH-THC plasma levels. However, Stott et al. examined a THC:CBD spray18 and therefore, the presence of CBD could explain the differences between our findings and theirs. Thus, perhaps future PK studies should examine the genetic profile of the main CYP450 enzymes that metabolize THC (i.e., CYP2C926 and CYP3A427) of participants and report the other cannabinoids present in their extracts to aid in data interpretation between and within studies.
An interesting finding from our data was that 11-OH-THC reached peak plasma concentrations in a similar time frame as THC. As 11-OH-THC has been suggested to have physiological effects of its own,2,4 it is possible that some of the therapeutic benefits attributed to the consumption of THC could be caused by 11-OH-THC. Future studies examining THC's PD should perhaps also consider 11-OH-THC plasma levels to begin to tease out the complexities of the effects of THC and its major metabolite.
Our study does present some limitations, one such limitation was that all participants were healthy. CBD's PK parameters were significantly altered by moderate and severe hepatic impairment28 subsequently, the PKs of THC and 11-OH-THC may also differ across patient groups in comparison to healthy participants. Furthermore, while participants ranged from 20 to 54 years of age, we did not examine if age influenced the PK of the THC extract capsule. As well, we did not investigate if chronic dosing significantly alters any PK parameters.
Conclusions
This study examined the PK parameters of THC and its major active metabolite, 11-OH-THC, following oral administration of a THC capsule2–4 during a fasted or fed condition. We found that the presence of high-fat food significantly delayed Tmax while also enhancing mean levels of both THC and 11-OH-THC. Furthermore, we found no cardiovascular or cognitive effects and primarily mild AEs, suggesting that the doses used in this study are safe to consider for future interventional studies in medical conditions where THC has been shown to have therapeutic efficacy.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Carolina Koutras, Connor Batchelor, Dr. Barry Waisglass, and Meital Matalon for their comments and input to the article.
Abbreviations Used
- 11-OH-THC
11-hydroxy-tetrahydrocannabinol
- BMI
body mass index
- Cl/F
apparent clearance rate
- Clast
last measurable concentration
- Cmax
peak plasma concentration
- CBD
cannabidiol
- CTA
clinical trial application
- CYP450
cytochrome P450
- DSST
digital symbol substitution test
- PD
pharmacodynamic
- PK
pharmacokinetic
- Tlag
lag time
- Tmax
time to peak plasma concentration
- THCA
tetrahydrocannabolic acid
- THC
Δ9-tetrahydrocannabinol
- Vz/F
apparent volume of distribution
- WAIS
Wechsler Adult Intelligence Scale
Author Contributions
All authors have made substantial contributions to the conception and design of the study or drafting of the article. S.L.: Drafting of the article; P.D.: Drafting of the article; S.O.: Conception and design of the study; S.C.: Drafting of the article; A.B.: Conception and design of the study; K.N.: Drafting of the article; JRBD.: Drafting of the article.
Author Disclosure Statement
All authors are employees of Aurora Cannabis, Inc.® or MedReleaf®, a wholly owned subsidiary of Aurora Cannabis, Inc®.
Funding Information
This work was sponsored and supported by MedReleaf®, a wholly owned subsidiary of Aurora Cannabis Inc®.
Supplementary Material
Cite this article as: Lunn S, Diaz P, O'Hearn S, Cahill SP, Blake A, Narine K, Dyck JRB (2019) Human pharmacokinetic parameters of orally administered Δ9-tetrahydrocannabinol capsules are altered by fed versus fasted conditions and sex differences, Cannabis and Cannabinoid Research 4:4, 255–264, DOI: 10.1089/can.2019.0037.
References
- 1. Baron EP. Medicinal Properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, headache, and pain: an update on current evidence and cannabis science. Headache. 2018;58:1139–1186 [DOI] [PubMed] [Google Scholar]
- 2. Lemberger L, Crabtree RE, Rowe HM. 11-Hydroxy-A′-tetrahydrocannabinol:-Pharmacology. Science (80-). 1972;177:62–64 [DOI] [PubMed] [Google Scholar]
- 3. Turkanis SA, Karler R, Partlow LM. Differential effects of delta-9-tetrahydrocannabinol and its 11-hydroxy metabolite on sodium current in neuroblastoma cells. Brain Res. 1991;560:245–250 [DOI] [PubMed] [Google Scholar]
- 4. Vandrey R, Herrmann ES, Mitchell JM, et al. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol. 2017;41:83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whiting PFP, Wolff RRFR, Deshpande S, et al. Cannabinoids for medical use A systematic review and meta-analysis. JAMA. 2015;313:2456–2473 [DOI] [PubMed] [Google Scholar]
- 6. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: the current state of evidence and recommendations for research. The National Academies Press: Washington, DC, 2017 [PubMed] [Google Scholar]
- 7. Stith SS, Vigil JM, Brockelman F, et al. The association between cannabis product characteristics and symptom relief. Sci Rep. 2019;9:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angela WB, Diaz P, Blake A, et al. Efficacy of different varieties of medical cannabis in relieving symptoms. J Pain Manag. 2017;10:375–383 [Google Scholar]
- 9. Ohlsson A, Lindgren JE, Wahlen A, et al. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416 [DOI] [PubMed] [Google Scholar]
- 10. Lindgren J-E, Ohlsson A, Agurell S, et al. Clinical effects and plasma levels of A 9-tetrahydrocannabinol (A 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl). 1981;74:208–212 [DOI] [PubMed] [Google Scholar]
- 11. Wall M, Sadler B, Brine D, et al. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–363 [DOI] [PubMed] [Google Scholar]
- 12. Ohlsson A, Lindgren J-E, Wahlén A, et al. Single dose kinetics of deuterium labelled Δ1-tetrahydrocannabinol in heavy and light cannabis users. Biol Mass Spectrom. 1982;9:6–10 [DOI] [PubMed] [Google Scholar]
- 13. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282 [DOI] [PubMed] [Google Scholar]
- 14. Pellesi L, Licata M, Verri P, et al. Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH)—a pilot study. Eur J Clin Pharmacol. 2018;74:1427–1436 [DOI] [PubMed] [Google Scholar]
- 15. De Vries M, van Rijckevorsel DCM, Vissers KCP, et al. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol. 2017;15:1079–1086.e4. [DOI] [PubMed] [Google Scholar]
- 16. Nadulski T, Pragst F, Weinberg G, et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810 [DOI] [PubMed] [Google Scholar]
- 17. Zhou S-F, Liu J-P, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295 [DOI] [PubMed] [Google Scholar]
- 18. Stott CG, White L, Wright S, et al. A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur J Clin Pharmacol. 2013;69:825–834 [DOI] [PubMed] [Google Scholar]
- 19. Łebkowska-Wieruszewska B, Stefanelli F, Chericoni S, et al. Pharmacokinetics of Bedrocan®, a cannabis oil extract, in fasting and fed dogs: an explorative study. Res Vet Sci. 2018;123:26–28 [DOI] [PubMed] [Google Scholar]
- 20. Matarazzo JD, Herman DO. Base rate data for the WAIS-R: test-retest stability and VIQ-PIQ differences. J Clin Neuropsychol. 1984;6:351–366 [DOI] [PubMed] [Google Scholar]
- 21. Naftali T, Schleider LB-L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276–1289 [DOI] [PubMed] [Google Scholar]
- 22. Walther S, Mahlberg R, Eichmann U, et al. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl). 2006;185:524–528 [DOI] [PubMed] [Google Scholar]
- 23. Ahmed AIA, van den Elsen GAH, Colbers A, et al. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24:1475–1482 [DOI] [PubMed] [Google Scholar]
- 24. Yamaori S, Ebisawa J, Okushima Y, et al. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88:730–736 [DOI] [PubMed] [Google Scholar]
- 25. Yamaori S, Okamoto Y, Yamamoto I, et al. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos. 2011;39:2049–2056 [DOI] [PubMed] [Google Scholar]
- 26. Watanabe K, Yamaori S, Funahashi T, et al. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–1419 [DOI] [PubMed] [Google Scholar]
- 27. Stott C, White L, Wright S, et al. A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus. 2013;2:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor L, Crockett J, Tayo B, et al. A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol. 2019;59:1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.