Supplemental Digital Content is available in the text.
Key Words: metastatic renal cell carcinoma, stereotactic body radiotherapy, tyrosine kinase inhibitor, survival
Objective:
Long-lasting control is rarely achieved with tyrosine kinase inhibitors (TKI) alone in metastatic renal cell carcinoma (mRCC). Our study aimed to investigate the survival outcomes of adding stereotactic body radiotherapy (SBRT) to TKI in mRCC.
Materials and Methods:
From September 2015 to September 2018, 56 patients treated with TKI received SBRT for 103 unresectable lesions. A total of 24 and 32 patients were irradiated before and after TKI failure, respectively. Overall survival (OS) was calculated from metastases. Progression-free survival (PFS) was calculated from SBRT.
Results:
Overall, 10, 32, and 12 patients had International Metastatic Renal Cell Carcinoma Database Consortium favorable, intermediate, and poor risk. Median follow-up was 21.7 months (range, 5.1 to 110.6 mo). Median OS was 61.2 months. The median PFS was 11.5 months, while the 2-year LC rate was 94%. Sixteen (34%) lesions achieved complete response (CR) in patients irradiated before TKI failure, whereas only 4 (7%) lesions yielded CR in those irradiated after TKI failure (P=0.001). The median PFS in CR group was significantly longer than that of non-CR group (18.9 vs. 7.1 mo; P=0.003). The 5-year OS in CR group was 86%, compared with 48% in non-CR group (P=0.010). Four (7%) patients experienced Grade 3 toxicity.
Conclusions:
Adding SBRT to TKI is safe and seems to improve survival in mRCC. Patients irradiated before TKI failure have higher CR rate, and the favorable local response might turn into survival benefit.
Although targeted therapy has substantially improved the prognosis of patients with metastatic renal cell carcinoma (mRCC), survival rates seem to plateau, despite the development of targeted agents. The objective response rate (ORR) after targeted monotherapy ranges from 10% to 30%, with complete and durable responses being rarely observed.1–3 The combined use of immunotherapy and targeted therapy has improved survival time and doubled the ORR; however, complete response (CR) remains rare, accounting for about 3% cases.4
Definitive local therapy directed against metastatic sites represents an important component of multidisciplinary treatment. Local therapy is not only an indispensable symptom palliation approach, but also an effective way to eradicate metastatic tumor deposits. Complete metastasectomy combined with targeted therapy could prolong overall survival (OS); however, perioperative targeted therapy is associated with an increasing incidence of wound-related complications.5 In addition, metastasectomy is only feasible in carefully selected patients, and those with unfavorable tumor size, site, local extent, comorbidity, or functional status lose their potential chance to benefit. By contrast, radiotherapy is a less selective form of local therapy.
Radiotherapy was reserved for symptom relief because of the dogma that renal cell carcinoma is radioresistant. Stereotactic body radiotherapy (SBRT) could overcome radioresistance by intensified dose delivery, emphasizing long-term local control (LC) rather than short-term palliation. Previous studies suggested that SBRT could yield a noninferior LC compared with surgical resection (1-year LC, 80% to 90%), and is preferred when patients present with risk factors.6–9 SBRT coupled with targeted therapy might provide additional benefit given their synergetic action. A previous study demonstrated that concurrent targeted therapy and SBRT is safe and offers superior LC compared with SBRT or targeted therapy alone.10
Previous studies have primarily focused on LC after SBRT; therefore, there is a lack of data regarding the survival benefits and the timing of SBRT when combined with targeted therapy. Therefore, we conducted this study to investigate the survival outcomes and the potential efficacy of the combination strategy.
METHODS AND MATERIALS
Patient Eligibility
The medical records of 350 consecutive patients with mRCC treated with tyrosine kinase inhibitor (TKI) between 2013 and 2018 were reviewed retrospectively. Eligible patients were adults (≥18 y of age) who underwent SBRT for at least 1 unresectable metastatic lesion at the discretion of the genitourinary multidisciplinary team. SBRT was indicated in patients with oligometastases or oligoprogression, or in patients with multiple metastases who required local therapy for symptom relief or tumor burden reduction. Those with spinal instability or rapidly progressive neurological deficit who received SBRT after surgical intervention were also included. Patients were excluded if they were followed up for <2 months, received TKI after SBRT for systemic disease progression or were treated with conventionally fractionated radiotherapy. Finally, 56 patients were included in the analysis.
TKI Administration
All patients were treated systemically with TKI for mRCC. Sunitinib, sorafenib, and axitinib were the most frequently chosen TKI. Suntinib was administered orally in a 6-week cycle at 50 mg daily for 4 weeks. Sorafenib was given orally at a dose of 400 mg twice daily, and axitinib was given at 5 mg twice daily. The TKI dose was not withheld or reduced during SBRT.
SBRT Procedure
SBRT was indicated for curative intent, major tumor burden, or symptom relief. For patients treated with curative intent, all metastatic sites at the time of SBRT were irradiated. Tumor burden was defined as the sum of the longest unidimensional diameter of target lesions according to Response Evaluation and Criteria in Solid Tumors (RECIST) version 1.1. Major tumor burden was defined as the largest lesion accounting for at least 50% of the tumor burden in each patient. Symptom relief was performed in frail patients with disseminated metastases to relieve pain, spinal cord compression, or bleeding. A total of 103 unresectable lesions were irradiated.
SBRT was performed using Volumetric Intensity Modulated Arc Therapy planning. All patients underwent 3 mm slice thickness contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging simulation scanning with site-specific immobilization. Four-dimensional CT simulation scans were performed in lung metastatic lesions, allowing for visualization of tumor motion. Biologically effective dose (BED) was calculated using linear-quadratic model with α/β=5.9 The most common fractionation scheme was 35 to 45 Gy delivered in 5 fractions, which was prescribed in 84% patients (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/AJCO/A296). Normal tissue dose constraints were in accordance with the United Kingdom consensus since 2018. Before that, BED was calculated for dose constraints. Prescription dose was required to cover >95% of the target; however, normal tissue protection had priority over target coverage. Cone beam CT was mandatory for daily image-guided radiation therapy during SBRT.
Outcomes and Statistical Analysis
Clinical examination and follow-up scans were recommended every 3 months for the first 2 years; however, early scans might be ordered for patients’ complaints. OS was defined as the time from the diagnosis of metastatic disease until last follow-up or death from any cause. Progression-free survival (PFS) was measured from the initiation of SBRT until disease progression or death. LC and distant PFS were defined as freedom from in-field and out-field progression after SBRT, respectively. Irradiated sites were independently assessed for treatment response. Bone metastases were evaluated using The University of Texas MD Anderson Cancer Center (MDA) criteria,11 and the rest were evaluated using Response Evaluation and Criteria in Solid Tumors version 1.1. Toxicities were graded according to Common Terminology Criteria for Adverse Events rating scale (CTCAE 4.0). Categorical data were compared using a χ2 test. Survival rates were estimated using the Kaplan-Meier method and compared using the log-rank test. A hazard ratio with a 95% confidence interval for univariate and multivariate analyses was calculated using the Cox proportional hazard model. A P-value <0.05 was considered significant. SPSS version 23 (IBM Corp., Armonk, NY) was used for the statistical analyses.
RESULTS
Patient Characteristics
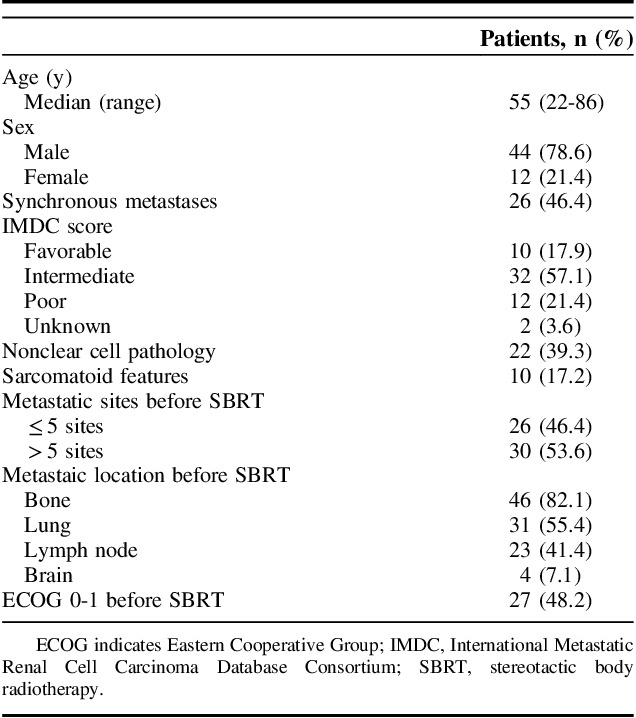
The clinical features of the patients are summarized in Table 1. Among the 56 patients, 26 (46%) had synchronous metastases. Ten (18%), 32 (57%), and 12 (21%) patients were categorized as International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) favorable, intermediate, and poor risk, respectively at the diagnosis of mRCC. Patients had predominantly clear-cell type disease, with >5 metastatic lesions before SBRT. Bone was the most common metastatic site, followed by the lungs and lymph nodes. Only 4 patients presented with brain metastases.
TABLE 1.
Patient Characteristics (N=56)
Treatment Characteristics
The treatment characteristics of the patients were summarized in Table 2. First-line targeted therapy comprised sunitinib, axitinib, or sorafenib in 91% of the patients. Thirty-eight (68%) patients switched to second-line therapy after systemic disease progression. Two patients changed to second-line TKI because of drug intolerance, and 2 patients discontinued systemic treatment because of intolerable toxicity caused by the first-line TKI. All of these patients were treated with first-line sunitinib. Nephrectomy was performed in 84% of the patients, and ~38% of the patients underwent incomplete metastasectomy. The sequence of systemic and local therapy for each patient was presented in Figure 1.
TABLE 2.
Therapeutic Characteristics (N=56)

FIGURE 1.
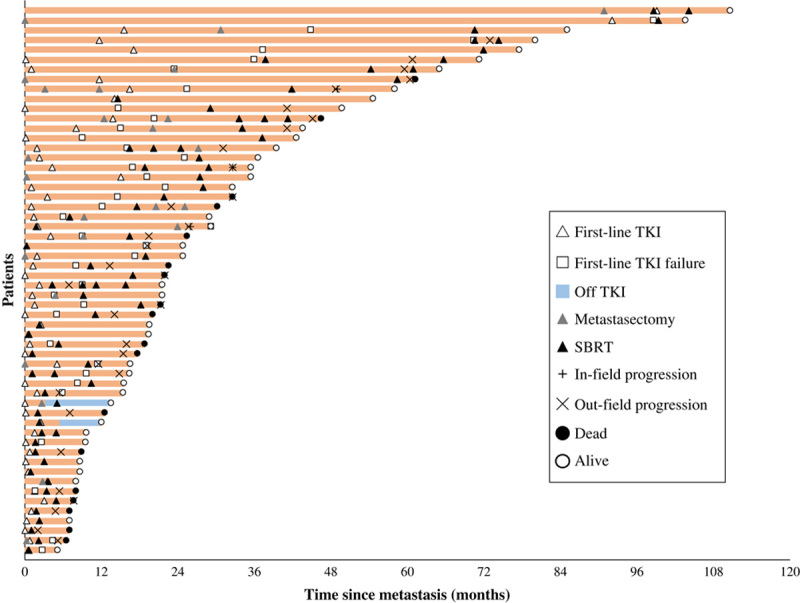
Swimmer plot of time since metastases (N=56). Individual patients are depicted as lines. Two patients who discontinued TKI due to toxicity are demonstrated as blue lines. SBRT indicates stereotactic body radiotherapy; TKI, tyrosine kinase inhibitor.
The median number of irradiated metastases was 1 (range, 1 to 6), and the median BED was 104 Gy (range, 50 to 165 Gy) (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/AJCO/A296). The majority of irradiated lesions were located in bones, accounting for 71% of cases. The median time between the initiation of first-line TKI and SBRT was 5.3 months (range, 0 to 58.9 mo). Twenty-four (43%) patients received SBRT before TKI failure, whereas 32 (57%) patients received SBRT after TKI failure.
Response and Disease Control
CR, partial response, stable disease, and progressive disease were observed in 20 (19%), 67 (65%), 13 (13%), and 3 (3%) lesions after SBRT, leading to an ORR of 84%. In patients irradiated before TKI failure, 16 (34%) lesions achieved CR, whereas only 4 (7%) lesions yielded CR in patients irradiated after TKI failure (P=0.001). At last follow-up, 3 lesions (located on the vertebra, pleura, and adrenal gland, respectively) developed progressive disease. The 1-year and 2-year LC rates were 98% and 94%, respectively. The median BED was not different between the CR (104 Gy; range, 50 to 165 Gy) and the non-CR (104 Gy; range, 59 to 134 Gy; P=0.099) lesions.
Survival and Prognostic Factors
At a median follow-up of 21.7 months (range, 5.1 to 110.6 mo), 18 patients died. All deaths were tumor-related. The median OS was 61.2 months, with 2-year and 5-year OS rates of 71% and 58%, respectively. The median PFS was 11.5 months, mainly for out-field progression. Thirty-two patients had out-field progression after SBRT, and the median distant PFS was 5.0 months (Fig. 2). The 2-year OS of patients treated for curative intent, major tumor burden, and symptom relief were 93%, 68%, and 58%, respectively (P=0.032). Patients who achieved CR at irradiated sites after SBRT had a more favorable prognosis. The median PFS in CR group was significantly longer than that of non-CR group (18.9 vs. 7.1 mo; P=0.003). The 5-year OS in CR group was 86%, compared with 48% in non-CR group (P=0.010; Fig. 2).
FIGURE 2.
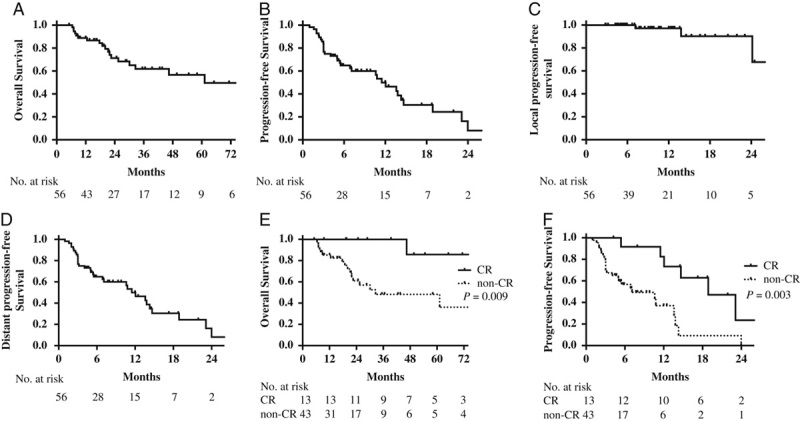
Survival outcomes of metastatic renal cell carcinoma patients treated with SBRT combined with TKI (N=56). A, Overall survival. B, Progression-free survival. C, Local progression-free survival. D, Distant progression-free survival of all patients. Overall survival (E) and progression-free survival of patients who achieved local CR and non-CR after SBRT (F). CR indicates complete response; SBRT, stereotactic body radiotherapy; TKI, tyrosine kinase inhibitors.
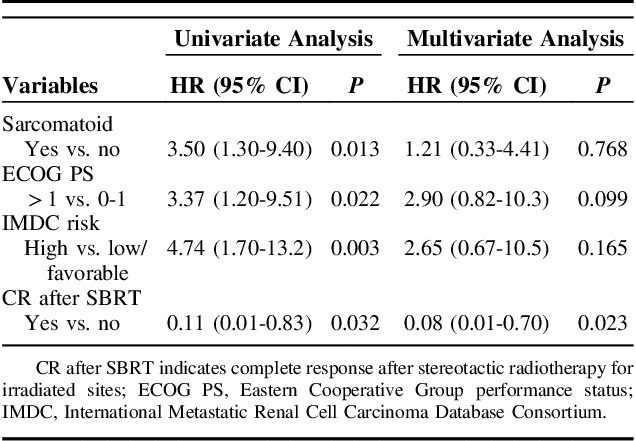
In univariate analysis, IMDC high-risk group, sarcomatoid features, CR after SBRT, ECOG >1 were significant prognostic factors for OS. No difference in OS was found between the concurrent first-line TKI and the concurrent second-line TKI group (P=0.249). In multivariate analysis, only CR after SBRT was associated with OS (hazard ration 0.08; 95% confidence interval, 0.01-0.70; P=0.023; Table 3).
TABLE 3.
Prognostic Factors of Overall Survival
Toxicity
Treatment-related toxicities are presented in Table 4. Toxicity occurred in 30 (54%) patients. There were 5 cases of Grade 3 toxicity in 4 patients, and 60% were Grade 3 anemia. One patient with neural invasion before radiotherapy suffered from late radiation-related neuropathy. One patient with pretreatment skin invasion developed skin perforation in addition to Grade 3 anemia, and received skin flap surgery after radiotherapy. One patient with a prior history of multiple pulmonary bullae developed bronchopleural fistula 7 months after radiotherapy. Among the patients with Grade 3 toxicity, half were treated concurrently with axitinib, and the others received concurrent sunitinib.
TABLE 4.
Treatment-related Toxicity

DISCUSSION
Local treatment has become increasingly popular to treat patients with oligometastatic mRCC; however, its role in patients with nonoligometastatic disease remains controversial.12 Our study focused exclusively on the survival benefit of patients treated with combined TKI and SBRT in both the oligometastatic setting and nonoligometastatic setting. SBRT combined with TKI was safe and there was no need to pause the targeted therapy several weeks before SBRT. We also observed superior local treatment response in patients irradiated before TKI failure, and better local treatment response was associated with improved OS. Thus, we suggest the adoption of SBRT early in the course of systemic treatment, preferably before TKI failure.
Acquired resistance will inevitably occur at a median of 6 to 12 months in patients treated with TKI.1–3,13,14 Sequential targeted therapy has prolonged the median OS of patients with mRCC to about 30 months; however, cross-resistance after first-line TKI failure creates a bottleneck to further improve survival outcomes.15–17 Thus, systemic therapy alone is insufficient for optimal mRCC management. When assessing the survival outcomes, the median OS of our patients (61.2 mo) was unexpectedly high compared with patients treated with targeted agents alone. Previous studies in patients treated with TKI combined with complete metastasectomy or SBRT demonstrated similar survival benefit (median OS 51 to 66 mo).9,18,19 Although the result is only exploratory, we believe that the clear survival improvement was realized by the incorporation of local treatment. Complete metastasectomy, a well-recognized local therapy, could reduce the risk of mortality by 51%, even in the era of targeted therapy.19 However, up to 25% of patients receiving metastasectomy suffered from major postoperative complications, and perioperative discontinuation of targeted agents would lead to a rapid increase in angiogenesis.20,21 SBRT has been shown to be noninferior to metastasectomy, especially in patients with risk factors.9 In addition, there was no need no pause TKI during SBRT, ensuring uninterrupted benefit from the combined therapy. Therefore, with improved systemic control and advanced treatment techniques, SBRT might be considered in patients with less favorable risk profiles.
Our study considered sites with major tumor burden as target for SBRT, based on the evidence that tumor burden is a significant prognostic factor.22–24 Heterogenous responses of different metastatic sites within individual patients are frequently observed during TKI treatment, among which larger lesions exhibit inferior response rates.25 Furthermore, the development of local resistance is the initial step for systemic progression in most cases, and bulky lesions have a higher proportion of clonal and subclonal alterations that might drive TKI resistance.26,27 Theoretically, irradiation to the major tumor burden could reduce the chance of developing resistant clones or subclones and could maintain TKI sensitivity. A previous study suggested that a 1 cm increase in the tumor burden elevates the risk of progression by 4.5% and death by 5%.23 A greater percentage of tumor burden removal is associated with improved PFS.28 Indeed, our study suggests that those who yielded CR at irradiated sites had more favorable survival outcomes. Thus, cytoreductive radiotherapy for nonoligometastatic patients is promising, and the site and extent of tumor burden removal is worthy of further investigation.
For patients treated with the combined therapy, SBRT delivered before TKI failure resulted in superior PFS, OS, and CR rates. Multivariate analyses revealed that SBRT timing was an independent prognostic factor for survival outcome. Consideration of the use of SBRT early in the course of treatment is encouraged29; however, the use SBRT before TKI requires further discussion. mRCC has a variable spectrum of biological behavior, and patients with certain driver events show rapid systemic progression and extremely short survival.27 On the one hand, TKI could sift out resistant lesions that may benefit from local intervention in patients with multiple metastases. On the other hand, initial TKI could identify patients with rapid progression, who require intensive systemic treatment, rather than local treatment. As observed in the SURTIME and CARMENA trials, cytoreductive nephrectomy (CN) before TKI had a significantly inferior survival outcome compared with sunitinib followed by CN (32.4 vs. 15 mo); however, delaying CN for symptom control could only achieve a median OS of 18.4 months.13,30 Thus, TKI followed by SBRT before systemic progression is likely to be the ideal temporal combination.
All of the patients in the present study received concurrent TKI during SBRT, and treatment was well tolerated, despite common concerns about severe toxicity. Only 5 Grade 3 events were observed, 4 of which were myelosuppression. Previous studies suggested that Grade 3 toxicity after SBRT is 0% to 7% in mRCC, and the few studies focusing on combined modality therapy in mRCC found 0% to 7% Grade 3 toxicities following concurrent treatment.6,9,29,31 Meta-analyses showed that the vast majority of evaluated TKI do not alter the adverse effect profile of SBRT when administered concurrently; however, liver SBRT in conjunction with sorafenib should be administered with caution.32
Our study is limited by its retrospective nature and the relatively small sample size. However, in the absence of prospective randomized trials, our study contributes useful information regarding combined modality therapy for mRCC. In addition, patients with oligometastatic mRCC comprised a relatively small proportion of the series, which might represent a more real-world clinical situation. Thirdly, our patients were treated at a large academic center that has more access to investigational drugs. Thus, results might be difficult to replicate in small centers.
CONCLUSIONS
The present study evaluated survival outcomes and toxicity profiles after combining SBRT with TKI. Our findings suggest that adding SBRT to TKI is safe and seems to improve survival in patients with mRCC. Patients irradiated before TKI failure have higher CR rate, and the favorable local treatment response might turn info survival benefit.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.
Footnotes
L.H. and Y.L. contributed equally to the work.
The authors declare no conflicts of interest.
REFERENCES
- 1.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 2.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14:1287–1294. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. [DOI] [PubMed] [Google Scholar]
- 4.Buchler T, Bortlicek Z, Poprach A, et al. Outcomes for patients with metastatic renal cell carcinoma achieving a complete response on targeted therapy: a registry-based analysis. Eur Urol. 2016;70:469–475. [DOI] [PubMed] [Google Scholar]
- 5.Chapin BF, Delacroix SE, Jr, Culp SH, et al. Safety of presurgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol. 2011;60:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15:e170–e177. [DOI] [PubMed] [Google Scholar]
- 7.Altoos B, Amini A, Yacoub M, et al. Local control rates of metastatic renal cell carcinoma (RCC) to thoracic, abdominal, and soft tissue lesions using stereotactic body radiotherapy (SBRT). Radiat Oncol. 2015;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzese C, Franceschini D, Di Brina L, et al. Role of Stereotactic body radiation therapy for the management of oligometastatic renal cell carcinoma. J Urol. 2019;201:70–75. [DOI] [PubMed] [Google Scholar]
- 9.Stenman M, Sinclair G, Paavola P, et al. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005-2014. Radiother Oncol. 2018;127:501–506. [DOI] [PubMed] [Google Scholar]
- 10.Kao J, Chen CT, Tong CC, et al. Concurrent sunitinib and stereotactic body radiotherapy for patients with oligometastases: final report of a prospective clinical trial. Target Oncol. 2014;9:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costelloe CM, Chuang HH, Madewell JE, et al. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 2014;15:e549–e561. [DOI] [PubMed] [Google Scholar]
- 13.Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379:417–427. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 15.Eichelberg C, Vervenne WL, De Santis M, et al. SWITCH: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the treatment of metastatic renal cell cancer. Eur Urol. 2015;68:837–847. [DOI] [PubMed] [Google Scholar]
- 16.Knox JJ, Barrios CH, Kim TM, et al. Final overall survival analysis for the phase II RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC. Ann Oncol. 2017;28:1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juengel E, Kim D, Makarevic J, et al. Molecular analysis of sunitinib resistant renal cell carcinoma cells after sequential treatment with RAD001 (everolimus) or sorafenib. J Cell Mol Med. 2015;19:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rausch S, Kruck S, Walter K, et al. Metastasectomy for metastatic renal cell carcinoma in the era of modern systemic treatment: C-reactive protein is an independent predictor of overall survival. Int J Urol. 2016;23:916–921. [DOI] [PubMed] [Google Scholar]
- 19.Yu X, Wang B, Li X, et al. The significance of metastasectomy in patients with metastatic renal cell carcinoma in the era of targeted therapy. Biomed Res Int. 2015;2015:176373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffioen AW, Mans LA, de Graaf AMA, et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res. 2012;18:3961–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer CP, Sun M, Karam JA, et al. Complications after metastasectomy for renal cell carcinoma: a population-based assessment. Eur Urol. 2017;72:171–174. [DOI] [PubMed] [Google Scholar]
- 22.Basappa NS, Elson P, Golshayan AR, et al. The impact of tumor burden characteristics in patients with metastatic renal cell carcinoma treated with sunitinib. Cancer. 2011;117:1183–1189. [DOI] [PubMed] [Google Scholar]
- 23.Iacovelli R, Lanoy E, Albiges L, et al. Tumour burden is an independent prognostic factor in metastatic renal cell carcinoma. BJU Int. 2012;110:1747–1753. [DOI] [PubMed] [Google Scholar]
- 24.Stein A, Bellmunt J, Escudier B, et al. Survival prediction in everolimus-treated patients with metastatic renal cell carcinoma incorporating tumor burden response in the RECORD-1 trial. Eur Urol. 2013;64:994–1002. [DOI] [PubMed] [Google Scholar]
- 25.Crusz SM, Tang YZ, Sarker SJ, et al. Heterogeneous response and progression patterns reveal phenotypic heterogeneity of tyrosine kinase inhibitor response in metastatic renal cell carcinoma. BMC Med. 2016;14:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basler L, Kroeze SG, Guckenberger M. SBRT for oligoprogressive oncogene addicted NSCLC. Lung Cancer. 2017;106:50–57. [DOI] [PubMed] [Google Scholar]
- 27.Turajlic S, Xu H, Litchfield K, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell. 2018;173:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbastefano J, Garcia JA, Elson P, et al. Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU Int. 2010;106:1266–1269. [DOI] [PubMed] [Google Scholar]
- 29.Miller JA, Balagamwala EH, Angelov L, et al. Spine stereotactic radiosurgery with concurrent tyrosine kinase inhibitors for metastatic renal cell carcinoma. J Neurosurg Spine. 2016;25:766–774. [DOI] [PubMed] [Google Scholar]
- 30.Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol. 2019;5:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int. 2011;108:673–678. [DOI] [PubMed] [Google Scholar]
- 32.Kroeze SG, Fritz C, Hoyer M, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev. 2017;53:25–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.


