Abstract
Background
Individuals with cardiovascular disease (CVD) report psychological distress and poor physical functioning and may benefit from mindfulness training.
Purpose
To examine the effects of mindfulness-based interventions (MBIs) on psychological and physiological measures in adults with CVD using meta-analysis.
Methods
Comprehensive searches identified studies that (a) evaluated MBIs in adults with CVD or who had experienced a cardiac event, (b) included a comparison condition, and (c) assessed psychological (e.g., anxiety and depression) or physiological (e.g., systolic or diastolic blood pressure [BP]) outcomes. Independent raters coded methodological (e.g., design and quality) and intervention features (e.g., intervention content) as potential moderators. Weighted mean effect sizes (d+), using full information maximum likelihood estimation, were calculated.
Results
Of the 1,507 records reviewed, 16 studies met inclusion criteria (N = 1,476; M age = 56 years; 40% women). Compared to controls, participants who received an MBI reported greater improvements in psychological outcomes (i.e., anxiety, depression, distress, and perceived stress: d+s = 0.49 to 0.64). MBI recipients also reduced their systolic (d+ = 0.89, 95% confidence interval [CI] = 0.26, 1.51; k = 7) but not diastolic (d+ = 0.07, 95% CI = −0.47, 0.60; k = 6) BP relative to controls.
Conclusions
MBIs demonstrated favorable effects on psychological and physiological outcomes among adults with CVD. Future research should investigate if such benefits lead to improvements in disease outcomes in studies with longer follow-ups.
Keywords: Mindfulness, Cardiovascular disease, Stress, Adults, Meta-analysis
Mindfulness-based interventions improved psychological and physiological outcomes among adults with cardiovascular disease but the long-term benefits could not be established.
Introduction
Psychological distress is prevalent among individuals with established cardiovascular disease (CVD) and contributes to poor outcomes such as greater risk of cardiovascular events and mortality [1]. Stress management interventions (SMIs) are often recommended to reduce psychological distress and improve coping among patients with CVD [2]. SMIs aim to reduce distress associated with the physical symptoms of CVD and to assist with the adjustment process after a cardiac event. Prior reviews show that SMIs have a small to moderate effect on psychological outcomes among patients with CVD [3, 4] but the effects on physiological markers are unclear. The broad range of SMIs included in these reviews (e.g., cognitive behavioral therapy, yoga, and mindful meditation) also makes it difficult to know which approaches are helpful [5].
There has been growing interest in the use of SMIs, including mindfulness-based interventions (MBIs), to alleviate psychological distress and improve physical health outcomes. The overarching goal of MBIs is to increase mindfulness—that is, an individual’s attention and awareness to his or her present moment experiences nonjudgmentally [6]. Mindfulness training strengthens metacognitive awareness (i.e., the self-reflective capacity to monitor mental experience), allowing participants to shift their perspective (“reperceiving”) and reduce emotional reactivity [7–10]. Thus, mindfulness can mitigate stress appraisals reducing the stress-reactivity response [11]. From a psychoneuroimmunological perspective, strategies to manage stress—including mindfulness—can improve coping skills and psychological functioning complemented by a normalization of the autonomic nervous system leading to hormonal changes, improved immune functioning, and slower disease progression (cf. [12]). Therefore, mindfulness is one stress management strategy that may be beneficial for patients with CVD.
Meta-analyses confirm the psychological benefits of MBIs in both healthy and unhealthy populations [13–15]. Pooled evidence indicates small to medium effects on psychological-related outcomes (anxiety and depression) in clinical populations [13]. A meta-analysis of MBIs among individuals with vascular diseases, including cardiovascular conditions, demonstrated equivalent effects on psychological outcomes, including anxiety, depression, and stress, but the effects of MBIs on physiological markers, such as blood pressure (BP), were mixed [16]. Finally, a meta-analysis evaluating the efficacy of a broad range of mind-body practices, including two (out of 11) randomized controlled trials (RCTs) delivering an MBI, showed improvements in both psychological outcomes (anxiety and depression) and physiological markers (systolic and diastolic BP) [17].
Given the role that heightened sympathetic activation plays in the physiopathology of CVD [18], the relaxation response induced by MBIs [19] may be beneficial for CVD patients. Others have hypothesized that MBIs may not only improve coping processes and psychological outcomes but may improve other CVD risk factors [6, 9]. Yet, the effects of MBIs exclusively among CVD patients have been relatively unexplored. The current meta-analysis was designed to examine the effects of MBIs on psychological outcomes and physiological markers in adults with CVD.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [20] guidelines (see Supplementary Material 1). Included were studies that (a) evaluated a MBI in adults with CVD or who had experienced a cardiac event, (b) included a comparison group, and (c) assessed psychological outcomes (anxiety, depression, perceived stress, and distress) or physiological markers (systolic or diastolic BP).
Studies were identified by searching 10 electronic bibliographic databases using a Boolean search strategy (see Supplementary Material 2 for the search string used for each database searched). Searches were conducted in December 2016 and updated in January 2018. No restrictions (e.g., language and geographical location) were applied. We also reviewed reference lists, databases of funded research and clinical trials (NIH RePORTER, clinicaltrials.gov), and relevant journals (e.g., Journal of the American Medical Association). All bibliographic records were screened based on title and abstract. Full-text manuscripts of potentially relevant records were retrieved and reviewed for inclusion. Research reported in multiple records were linked in the database and represented as a single unit.
Relevant study information (e.g., publication year), sample characteristics (e.g., age and heart condition), design (e.g., RCT), intervention details (e.g., sessions and delivery method), and intervention components (e.g., relaxation exercises) were extracted by two independent coders (MLD, BB, MMF, JD) using detailed coding forms. Coders also assessed the methodological quality (MQ) of each study using 17 items (total score 25) adapted from validated measures [21–24]. Four authors were contacted for additional information; only one author provided the information requested. Interrater reliability was assessed: for categorical variables, raters agreed on 93% of the judgments (M Cohen’s κ = 0.86), and reliability for continuous variables yielded an average intraclass correlation coefficient of 0.96 across categories (Mdn = 1.00). Discrepancies were resolved by coders, and the final data were verified by the principal investigator (PI) (LAJSS).
The study outcomes of interest included psychological outcomes (anxiety, depression, distress, and perceived stress) and physiological markers (systolic and diastolic BP). Psychological outcomes were assessed using validated self-report measures (e.g., Hospital Anxiety and Depression Scale [25]); physiological markers were assessed using objective measures (e.g., sphygmomanometer). Summary effect sizes (ESs) were calculated as the standardized mean difference between the MBI and comparison groups, controlling for baseline [26, 27]. All ES estimates were corrected for sample size bias [28]. Positive ESs indicated that psychological outcomes (e.g., fewer depressive symptoms and less distress) or physiological markers (e.g., lower systolic BP) were improved in the MBIs compared to controls. Two independent coders calculated ESs for each study; discrepancies between coders were resolved through discussion, corrected, and finalized.
Weighted mean ESs (and corresponding 95% confidence intervals [CIs]) were calculated using random-effects procedures following full information maximum likelihood methods to estimate the between-study variance [29, 30]. Heterogeneity was assessed by computing Q; a significant Q indicates a lack of homogeneity and an inference of heterogeneity. To assess outcome consistency across studies, we calculated the I2 index and its corresponding 95% CIs [31, 32]. The I2 values of 25%, 50%, and 75% are considered to be low, medium, and high heterogeneity, respectively [33]. All analyses were conducted in Stata 15.1 [34] using published macros [29].
Results
Of the 1,507 records reviewed, 16 studies and 14 supplemental manuscripts providing additional study details or data met inclusion criteria (details of the study selection can be found in Supplementary Fig. 1).
Study, Sample, Intervention, and Design Characteristics
Study, sample, intervention, and design characteristics can be found in Supplementary Table 1. Studies were published (or available) between 1995 and 2017 (M = 2,012, SD = 5.67) and conducted in North America (five in United States and one in Canada), Europe (two in The Netherlands, one in Ireland, and one in Spain), and Asia (two in Iran, two in India, one in China, and one in South Korea). Participants were typically recruited from clinics or hospitals (k =13); two studies used multiple recruitment methods (clinical contact, posted flyers, and newspaper advertisements) and one study recruited teachers from postsecondary schools. Samples included 1,476 adults (Mage = 56 years; 40% women; 76% White; 71% married) who consented to participate in the studies with an average retention rate of 81% (SD = 0.15) at follow-up. Studies most often included patients diagnosed with coronary heart disease (k = 10); two studies sampled patients with hypertension, one study involved heart failure patients, and the remaining three studies sampled patients with multiple CVD conditions. Of the 10 studies describing patients’ ongoing treatment, most reported pharmacological therapy (k = 5), cardiac rehabilitation (k =2), and diet/exercise modifications (k =2). One study reported that none of the participants were using pharmacological therapy.
All MBIs were designed for adults living with a CVD condition, and three interventions were targeted to patients who had undergone percutaneous coronary intervention or who had an implantable cardioverter defibrillator. MBIs were also targeted to women or men and adults meeting criteria for a current major depressive episode. The types of MBIs included mindfulness-based stress reduction (MBSR; k =10), mindfulness meditation (k = 3), mindfulness-based cognitive therapy (MBCT; k = 1), or a combined MBSR/MBCT (k = 2). MBIs were typically delivered over a median of nine sessions (range = 3–24) with a median total dose of 16 hr (range = 4–60). MBIs were typically delivered in groups except for three studies which delivered the MBI individually in-person, online, or via telephone. Home practice was emphasized for most of the MBIs (k = 13); participants were expected to practice at home for a median of 56 days (M = 53, SD = 27; range = 3–108) with a median of 30 min of practice per day (M = 32, SD = 9; range = 19–45).
All studies used an independent control group design with pretest–post-test assessments (14 RCTs; 2 non-RCTs). The control conditions reported were wait-list or assessment-only (38% of studies), treatment as usual (31%), or an active comparison group (e.g., support group; brief education; 31%). The active comparison conditions were delivered over a median of nine sessions, each lasting 60 min. The median number of post-intervention follow-up assessments was 1 (range = 1–3). Assessments were conducted at immediate post-test through 44 weeks post-intervention; only five studies measured outcomes at a delayed post-intervention assessment. Therefore, the first post-intervention assessment was used in analyses (Mdn = 0 weeks; range = 0–4). Studies satisfied an average of 62% (SD = 9%) of the MQ criteria, with scores ranging from 10 to 19 (M = 16, SD = 2) out of 25. There were no differences for any of the outcomes based on the proportion of MQ criteria satisfied, ps ≥ .38.
Synthesis of Results
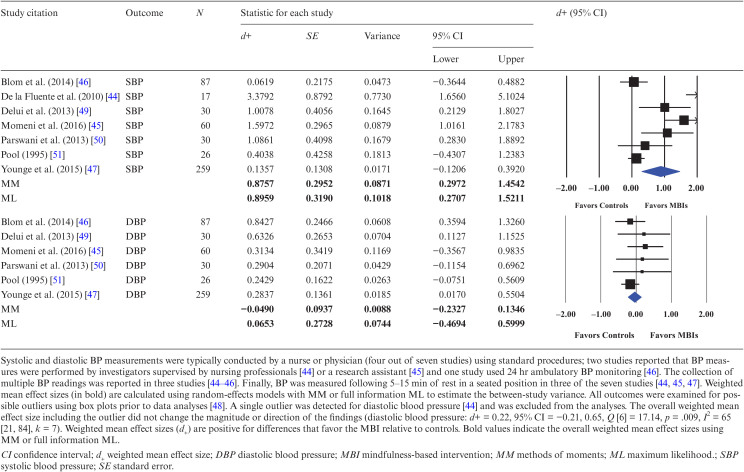
Participants who received the MBI reported significantly greater reductions in psychological outcomes (i.e., anxiety, depression, distress, and perceived stress) relative to controls (d+s = 0.49–0.64). The hypothesis of homogeneity was partially supported for distress (Q [4] = 5.68, p = .224; I2 = 30) but not for anxiety (Q [8] = 25.96, p < .001; I2 = 69), depression (Q [8] = 17.20, p = .028; I2 = 53), or perceived stress (Q [5] = 16.83, p = .005; I2 = 70), and uncertainty limits for the I2 test were wide for all psychological outcomes and exceeded the 50% threshold. Forest plots for systolic and diastolic BP appear in Table 1. Participants who received the MBI had greater improvements in systolic (but not diastolic) BP, d+ = 0.89 (95% CI = 0.26, 1.51). The hypothesis of homogeneity was not supported (Q [6] = 40.15, p < .001; I2 = 85, 95% CI = 71, 92), indicating significant heterogeneity across studies (diastolic BP: d+ = 0.07, 95% CI = −0.47, 0.60; Q [5] = 3.65, p = .601; I2 = 0, 95% CI = 0, 83). We found no between-group difference on any of the psychological or physiological outcomes among the five studies assessing outcomes at a delayed postintervention assessment (data not shown). Because few ESs (<10) were available for each outcome, we could not conduct formal tests (i.e., funnel plot asymmetry and trim and fill) to examine publication bias [35].
Table 1.
Weighted mean effect sizes comparing MBIs to controls on systolic and diastolic blood pressure
Discussion
This meta-analysis examined the effects of MBIs on psychological and physiological measures in adults with CVD. Sixteen studies with 1,476 adults comparing MBIs to a control group were evaluated. Participants receiving the MBI reported greater improvements in psychological outcomes (i.e., anxiety, depression, distress, and perceived stress) relative to controls. MBIs also significantly improved systolic BP. The magnitude of ESs ranged from medium to large (d+ = 0.49 to 0.89). These findings were observed, however, only at the immediate postintervention assessment and were not maintained over time, as there were no significant differences in psychological or physiological outcomes between MBIs and controls in the five studies reporting follow-up assessments. These findings were consistent with prior meta-analyses showing the short-term psychological and physiological benefits of MBIs across a broad range of chronic diseases [16, 36]. Therefore, our meta-analysis shows that mindfulness training has psychological and physiological benefits, specifically in adults with CVD, and is reasonable to be considered as a complementary treatment in routine clinical care.
Mindfulness is hypothesized to improve CVD by enhancing participants’ attention control, self-awareness, and emotion self-regulation but studies evaluating the benefits of MBIs among individuals with CVD have been limited [9]. Prior meta-analyses evaluated the efficacy of (a) MBIs in a broad range of clinical populations, including patients with CVD [16, 36], or (b) mind-body practices, including MBIs, among patients with CVD [17]. This is the first meta-analysis, to our knowledge, that evaluated MBIs specifically for people living with CVD. In this meta-analysis, we show that MBIs improved short-term psychological outcomes. Teaching patients to manage psychological distress is critical given the known impact of chronic stress on CVD progression [37]. For example, prior research shows that patients with CVD and co-occurring depression are more likely to experience worse outcomes than patients who are not depressed, and reducing depressive symptoms may be associated with reductions in mortality risk [38]. Results from this meta-analysis shows that MBIs are a promising approach to alleviate short-term psychological distress (i.e., anxiety and depression) associated with the management of CVD.
Mindfulness meditation can also lead to autonomic nervous system changes by lowering sympathetic activity and activating parasympathetic activity, which may explain the observed effect of MBIs on systolic BP [39]. Our meta-analysis showed that participants provided with a MBI reduced systolic BP relative to controls. We found that patients in the MBIs had a 14 mm Hg mean reduction in systolic BP, whereas controls had a 5 mm Hg mean reduction (QB [1] = 4.06, p = .044). This finding is consistent with prior research showing that mindfulness meditation produces statistical and clinically significant reductions in systolic BP [40]. We did not find significant between-group changes in diastolic BP, which is consistent with the prevalence of isolated systolic hypertension in adults aged 50 or older [41]. In fact, none of the samples had diastolic hypertension defined as diastolic BP ≥ 90 mm Hg at baseline (range = 72–87 mm Hg for MBIs; 68–86 mm Hg for controls). Therefore, MBIs are a promising adjuvant approach to lowering systolic BP among adults living with CVD. Future research evaluating studies measuring BP with longer follow-ups will be necessary to determine whether changes in systolic BP are sustained over time.
Several limitations should be considered when interpreting these findings. First, this meta-analysis was limited by amount of the information reported in the studies. We intended to evaluate the efficacy of MBIs on stress processes (e.g., problem- and emotion-focused coping), other physiological markers (e.g., heart rate variability), and clinical outcomes (e.g., rehospitalizations and deaths) associated with cardiovascular disease, but we were unable to do so due to the limited number of studies assessing these outcomes. For example, only four studies measured (or reported) resting heart rate or heart rate variability even though heart rate that is low and variable is associated with lower cardiovascular risk [42]. Second, we were unable to statistically assess publication bias given the limited number of studies available per outcome (i.e., <10) [35]. Finally, we were unable to assess the long-term impact of MBIs on psychological outcomes and physiological markers because most studies (k = 11) included only an immediate postintervention assessment. Longer duration of follow-up would allow for more time to determine the potential long-term benefits of MBIs in this population.
Conclusions
MBIs are a promising approach for the management of psychological distress and physical health outcomes among adults with CVD. Findings from this meta-analysis revealed short-term psychological and physiological benefits of MBIs but the long-term effects of MBIs on these outcomes has not yet been established. Based on this meta-analytic review, a Class IIB, Level of Evidence, a recommendation for MBIs among CVD patients is supported (cf. [43]). Future research using an RCT design should investigate whether such benefits lead to improvements in disease outcomes in studies with longer follow-ups.
Supplementary Material
Acknowledgements
Funding: The research reported in this paper was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under award number 5R01AT008815 to Drs. L. A. J. Scott-Sheldon and M. P. Carey (multiple PIs). Dr. E. C. Gathright was supported by the Cardiovascular Behavioral Medicine Training Grant (5T32HL076134; Dr. R. R. Wing, PI) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions The authors contributed to the manuscript in the following manner: Study concept and design: Lori A. J. Scott-Sheldon, Dean G. Cruess, Rena R. Wing, Michael P. Carey, and Elena Salmoirago-Blotcher. Acquisition of data: Lori A. J. Scott-Sheldon, Emily C. Gathright, Marissa L. Donahue, Brittany Balletto, Melissa M. Feulner, and Julie DeCosta. Analysis and interpretation of data: Lori A. J. Scott-Sheldon, Emily C. Gathright, and Elena Salmoirago-Blotcher. Drafting of the manuscript: Lori A. J. Scott-Sheldon, Emily C. Gathright, and Elena Salmoirago-Blotcher. Critical revision of the manuscript for important intellectual content: Lori A. J. Scott-Sheldon, Emily C. Gathright, Marissa L. Donahue, Brittany Balletto, Melissa M. Feulner, Julie DeCosta, Dean G. Cruess, Rena R. Wing, Michael P. Carey, and Elena Salmoirago-Blotcher. Statistical analysis: Lori A. J. Scott-Sheldon. Obtaining funding: Lori A. J. Scott-Sheldon and Michael P. Carey. Administrative, technical, or material support: Marissa L. Donahue, Brittany Balletto, Melissa M. Feulner, and Julie DeCosta. Study supervision: Lori A. J. Scott-Sheldon.
Ethical Approval This article does not contain any studies with human participants performed by any of the authors.
Informed Consent was not obtained by the authors as this study involved secondary data analysis of published (or available) studies.
References
- 1. Tawakol A, Ishai A, Takx RA, et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet. 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blumenthal JA, Sherwood A, Smith PJ, et al. Enhancing cardiac rehabilitation with stress management training: A randomized, clinical efficacy trial. Circulation. 2016;133:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2011;9: CD008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev. 2011;8:CD002902. [DOI] [PubMed] [Google Scholar]
- 5. Tan MP, Morgan K. Psychological interventions in cardiovascular disease: An update. Curr Opin Psychiatry. 2015;28:371–377. [DOI] [PubMed] [Google Scholar]
- 6. Creswell JD. Mindfulness interventions. Annu Rev Psychol. 2017;68:491–516. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. J Clin Psychol. 2006;62:373–386. [DOI] [PubMed] [Google Scholar]
- 8. Teasdale JD. Metacognition, mindfulness and the modification of mood disorders. Clin Psychol Psychot. 1999;6:146–155. [Google Scholar]
- 9. Loucks EB, Schuman-Olivier Z, Britton WB, et al. Mindfulness and cardiovascular disease risk: State of the evidence, plausible mechanisms, and theoretical framework. Curr Cardiol Rep. 2015;17:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garland EL, Farb NA, Goldin P, Fredrickson BL. Mindfulness broadens awareness and builds eudaimonic meaning: A process model of mindful positive emotion regulation. Psychol Inq. 2015;26:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Creswell JD, Lindsay EK. How does mindfulness training affect health? a mindfulness stress buffering account. Curr Dir Psychol Sci. 2014;23:401–407. [Google Scholar]
- 12. Scott-Sheldon LA, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: A meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychol. 2008;27:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang HP, He M, Wang HY, Zhou M. A meta-analysis of the benefits of mindfulness-based stress reduction (MBSR) on psychological function among breast cancer (BC) survivors. Breast Cancer. 2016;23:568–576. [DOI] [PubMed] [Google Scholar]
- 15. Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- 16. Abbott RA, Whear R, Rodgers LR, et al. Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. J Psychosom Res. 2014;76:341–351. [DOI] [PubMed] [Google Scholar]
- 17. Younge JO, Gotink RA, Baena CP, Roos-Hesselink JW, Hunink MG. Mind-body practices for patients with cardiac disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:1385–1398. [DOI] [PubMed] [Google Scholar]
- 18. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. [DOI] [PubMed] [Google Scholar]
- 19. Wallace RK, Benson H, Wilson AF. A wakeful hypometabolic physiologic state. Am J Physiol. 1971;221:795–799. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 22. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller WR, Brown JM, Simpson TL, et al. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, eds. Handbook of Alcohilsm Treatment Approaches: Effective Alternatives. Boston, MA: Allyn & Bacon; 1995:12–44. [Google Scholar]
- 24. Fowkes FG, Fulton PM. Critical appraisal of published research: Introductory guidelines. BMJ. 1991;302:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York, NY: Erlbaum; 1998. [Google Scholar]
- 27. Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. [DOI] [PubMed] [Google Scholar]
- 28. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat. 1981;6:107–128. [Google Scholar]
- 29. Lipsey MW, Wilson DB.. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 30. Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. StataCorp. Stata Statistical Software. College Station, TX: StataCorp LP; 2017. [Google Scholar]
- 35. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: A meta-analysis. J Psychosom Res. 2010;68:539–544. [DOI] [PubMed] [Google Scholar]
- 37. Cohen BE, Edmondson D, Kronish IM. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: A clinical review. Eur Heart J. 2014;35:1365–1372. [DOI] [PubMed] [Google Scholar]
- 39. Amihai I, Kozhevnikov M. The influence of buddhist meditation traditions on the autonomic system and attention. Biomed Res Int. 2015;2015:731579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldstein CM, Josephson R, Xie S, Hughes JW. Current perspectives on the use of meditation to reduce blood pressure. Int J Hypertens. 2012;2012:578397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menown IB, Davies S, Gupta S, et al. Resting heart rate and outcomes in patients with cardiovascular disease: Where do we currently stand? Cardiovasc Ther. 2013;31:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brook RD, Appel LJ, Rubenfire M, et al. ; American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity Beyond medications and diet: Alternative approaches to lowering blood pressure: A scientific statement from the American Heart Association. Hypertension. 2013;61:1360–1383. [DOI] [PubMed] [Google Scholar]
- 44. de la Fuente M, Franco C, Salvador M. Reduction of blood pressure in a group of hypertensive teachers through a program of mindfulness meditation. Psicol Conductual. 2010;18:533–552 [Google Scholar]
- 45. Momeni J, Omidi A, Raygan F, Akbari H. The effects of mindfulness-based stress reduction on cardiac patients’ blood pressure, perceived stress, and anger: A single-blind randomized controlled trial. J Am Soc Hypertens. 2016;10:763–771. [DOI] [PubMed] [Google Scholar]
- 46. Blom K, Baker B, How M, et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: Results from the HARMONY randomized controlled trial. Am J Hypertens. 2014;27:122–129. [DOI] [PubMed] [Google Scholar]
- 47. Younge JO, Wery MF, Gotink RA, et al. Web-Based mindfulness intervention in heart disease: A randomized controlled trial. PLoS One. 2015;10:e0143843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Emerson JD, Strenio J. Boxplots and batch comparison. In: Hoaglin DC, Mosteller F, Tukey JW, eds. Understanding Robust and Exploratory Data Analysis. New York, NY: John Wiley & Sons; 1983:58–96. [Google Scholar]
- 49.Delui MH, Yari M, Khouyinezhad G, Amini M, Bayazi MH. Comparison of cardiac rehabilitation programs combined with relaxation and meditation techniques on reduction of depression and anxiety of cardiovascular patients. Open Cardiovasc Med J. 2013;7(1):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parswani MJ, Sharma MP, Iyengar S. Mindfulness-based stress reduction program in coronary heart disease: A randomized control trial. Int J Yoga. 2013;6(2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pool JI. Cognitive restructuring and meditation training as stress management intervention in post-cardiac adjustment. US: ProQuest Information & Learning; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.