Abstract
Background
Primary immune-deficiency disease (PIDD) is a rare, debilitating disease of the immune system that predisposes the affected individual to infection, autoimmune conditions, and neoplasm. A major component of the cost of treating PIDD is the high price of immunoglobulin drugs, which can be administered via an intravenous (IV) or subcutaneous (SC) route.
Objective
To compare real-world costs for patients with PIDD who are receiving IV immunoglobulin (IVIG) or SC immunoglobulin (SCIG) treatment, from a US payer perspective, using a large claims database.
Methods
Based on 2011 to 2013 data from the PharMetrics Plus database, a large national healthcare claims database, patients who were newly diagnosed with PIDD were included in the study if they had ≥2 claims for PIDD that were ≥90 days apart, and if they were treatment-naïve for a minimum of 1 year before the study period. Patients who switched the route of immunoglobulin administration were excluded, with the exception of patients who received SCIG who could initially receive ≤2 IV-loading infusions, as directed by treatment guidelines. We used propensity score analysis to match the patients in the SCIG cohort to patients in the IVIG cohort based on age, sex, and all Elixhauser comorbidities. We compared the patient characteristics and direct medical costs (all-cause, PIDD-related, and pharmacy-related) before and after matching, using t-tests for continuous variables, chi-square test for categorical variables, and Wilcoxon rank-sum test for differences in medians.
Results
A total of 1639 patients with PIDD (986 who received IVIG and 653 who received SCIG) met all the study inclusion criteria. Compared with the patients who received IVIG, the patients who received SCIG were predominantly female (58% vs 63%, respectively) and significantly younger (mean age, 49.1 vs 40.3 years, respectively). Significantly fewer patients who received SCIG than those receiving IVIG had claims with International Classification of Diseases, Ninth Revision codes for Elixhauser comorbidities, including cardiovascular and pulmonary conditions, diabetes, renal failure, liver disease, cancers, weight loss, fluid and electrolyte disorders, and psychoses (P <.05 for all), and their Charlson Comorbidity Index scores were lower than those receiving IVIG (1.74 vs 3.01, respectively; P ≤.05 for all). After matching the 2 cohorts (N = 553 in each), the 1-year postindex median total PIDD-related costs were significantly lower in the IVIG group than in the SCIG group ($38,064 vs $43,266, respectively; P = .002).
Conclusions
In matched analyses, PIDD-related treatment costs were higher for patients who received SCIG than for those who received IVIG. Furthermore, patients who received SCIG were significantly younger and had significantly less comorbidities than their counterparts who received IVIG, suggesting that patient characteristics that reflect a desire and greater capacity for autonomy may affect physicians' choice of the route of administration for immunoglobulin.
Keywords: healthcare costs, immunoglobulin therapies, intravenous therapy, IVIG, primary immune-deficiency disease, SCIG, subcutaneous therapy
Primary immune-deficiency disease (PIDD) is a group of more than 350 heterogeneous genetic or hereditary disorders, characterized by a variety of clinical manifestations, most notably an increased susceptibility to infection.1 PIDD results from an inherited immune system defect that can present during childhood or in adults.2 Although an accurate estimate of the prevalence of PIDD has not been reported, it has been suggested that 1 of 1200 persons in the United States are diagnosed with PIDD.3 Further estimates suggest that 43% of patients with PIDD are not correctly diagnosed until adulthood, primarily because of the absence of family history regarding these disorders.4 The early diagnosis and treatment of PIDD are critical to reduce the associated morbidity and mortality, prevent serious disease-related complications and hospitalizations, and improve the patient's health-related quality of life.5–11
Recurrent or unusual infections are the hallmarks of primary immunodeficiency, and, if left untreated, these infections can result in a shortened life span.2,12 Long-term immunoglobulin replacement therapy is therefore prescribed for patients with PIDD to reduce the incidence and severity of infections and associated complications.13 Immunoglobulin replacement therapy may be administered via 2 primary routes of administration—intravenous (IV) or subcutaneous (SC) route.
Many studies have confirmed the safety and efficacy of IV immunoglobulin (IVIG) therapies and have established them as the standard of care in the areas of neurology and immunology.14–31 In addition, SC immunoglobulin (SCIG) therapies have emerged in the past decade as a safe and effective alternative route of administration.14–19,21,23,26–31 The primary difference between these 2 routes of administration in the United States is that SCIG is often self-administered, weekly, at home by patients or caregivers, whereas IVIG is most frequently administered, monthly, by an infusion nurse in the home setting or at a healthcare facility.32
Although both routes of immunoglobulin administration have been deemed safe and effective,14–31 the preferred route can be predicated on multiple factors, such as clinical circumstances, lifestyle, dose requirements, volume and infusion rates, target immunoglobulin level, the drug used, site of care, adverse events, adherence issues, support system, and the number of infusion sites, many of which can affect the cost of therapy.13
Jolles and colleagues proposed an algorithm to predict the selection of the administration route based on individual clinical outcomes and patient-related factors associated with immunoglobulin therapy.13 The algorithm predicts that if a patient begins treatment with an IVIG therapy and subsequently has poor tolerance, poor venous access, or inconvenience with treatment, switching to an SCIG therapy may be ideal. Conversely, if a patient begins treatment with SCIG therapy and then has poor compliance, inconvenience with treatment, or the inability to self-administer the treatment, it is suggested that they switch to IVIG therapy.13
There are positive and negative attributes associated with each route of administration for immunoglobulin, which are described in the published literature.13,33 The decision regarding which route of administration to use should ideally be made jointly by the physician and the patient, with full consideration of the patient's preferences.
Studies that evaluate the costs of the SC and IV routes of administration for patients with PIDD have frequently suggested that SCIG is economically advantageous versus IVIG, at least in part as a result of the avoidance of the costs of facility and healthcare professional administration.15,34–41 In a 2012 review and meta-analysis by Abolhassani and colleagues, moving patients from hospital-based IVIG treatment to home-based SCIG treatment resulted in a 25% to 33% savings in annual healthcare costs in Sweden; 50% in Germany; 25% to 50% in France; $2000 per patient annually in Canada; and $2000 to $5000 per patient annually in the United States.42 However, a review by Beauté and colleagues suggested that the major cost driver is the site of care, not the route of administration, and that home-based IVIG therapy was the least costly route, followed by home-based SCIG and then hospital-based IVIG.43
Often, the economic differences reported in past comparative studies were confounded by the institutional site of infusion, because the comparisons were conducted in European countries, where the home-based infusion of IV drugs was not an option to patients.43–45 In addition, IVIG and SCIG drugs are similarly priced in many European countries; however, this is not the case in the United States, where SCIG drugs are typically priced much higher per gram.46 In a cost-minimization analysis in which IVIG and SCIG were similarly priced, a sensitivity analysis demonstrated that the cost of the immunoglobulin drug was the major cost driver.40
Given the variation in treatment administration routes, population demographics, healthcare systems, and the associated costs of immunoglobulin therapy itself, it is not always accurate to assume that the cost-savings reported in the international literature predict the costs in the United States. Therefore, the objective of our study was to evaluate the all-cause and PIDD-related cost differences between IVIG and SCIG for the treatment of PIDD in a commercially insured population from a US payer perspective, using real-world data.
KEY POINTS
-
▸
Primary immune-deficiency disease (PIDD) is a rare condition that is managed with intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG).
-
▸
Using a large national healthcare claims database, this study compared the real-world costs of treating PIDD in commercially insured patients using IVIG or SCIG.
-
▸
Of 1639 patients who were newly diagnosed with PIDD, 986 received IVIG and 653 received SCIG and were divided into 2 cohorts based on the treatment route of administration.
-
▸
The initial SCIG cohort was younger, had more female patients, and was relatively healthier than the IVIG cohort (CCI score 1.74 vs 3.01, respectively), and had fewer Elixhauser comorbidities.
-
▸
After matching the 2 cohorts, the 1-year postindex median PIDD-related costs were lower in the IVIG cohort than in the SCIG cohort ($38,064 vs $43,266, respectively).
-
▸
Future studies are needed to determine the impact of physician and patient preferences, drug costs, delivery, and adherence to IVIG versus SCIG.
Methods
We conducted this study using claims data between 2011 and 2013 from the PharMetrics Plus database (QuintilesIMS, now IQVIA), a large US healthcare claims database that is frequently used for healthcare research. This database includes the administrative health insurance claims of approximately 50 million commercially insured patients and more than 250 insurance providers across the United States. The population in this database is generally representative of commercially insured patients in the United States younger than age 65 years, with less than 2% of patients covered by Medicaid and/or Medicare.41 Patients who were newly diagnosed with PIDD, based on International Classification of Diseases, Ninth Revision (ICD-9) code 279.XX, were selected from this database.
To decrease the potential for misclassification bias resulting from miscoding and to provide for the confirmation of diagnosis based on ICD-9 coding, only patients with 2 or more claims for PIDD at least 90 days apart were included in the study. The index date was a patient's first claim for PIDD. After the initial cohort selection, 1-year preindex and 1-year postindex date periods were used to evaluate the inclusion criteria. To reduce bias and to compare a more homogeneous population, we restricted our population to newly diagnosed patients. Patients who were immunoglobulin treatment–naïve were included in this study to evaluate the cost associated with a newly diagnosed patient in the first 2 years of treatment. Patients who did not meet the above criterion were excluded from the study.
Patients entered the study at their first drug exposure to IVIG or SCIG after receiving a diagnosis of PIDD. To create the 2 cohorts, the SCIG cohort included patients with a claim (Healthcare Common Procedure Coding System or National Drug Code) for the 20% concentration SC drug (ie, Hizentra), whereas the IVIG cohort included patients with a claim for any of the top 3 prescribed 10% concentration IVIG therapies (ie, Gammagard, Privigen, or Gamunex-C). These 3 drugs are among the highest-priced IVIG drugs in terms of average wholesale price and wholesale acquisition cost (WAC).46 Patients who did not meet either criterion were excluded from the study. Because the official prescribing information for the immunoglobulin drugs listed above suggest that patients who are receiving SCIG begin immunoglobulin therapy with IVIG and then switch to SCIG, patients in the study who received SCIG could have had up to 2 doses of IVIG before initiating SCIG therapy.
We collected the baseline demographic and clinical characteristics of all patients from the 1-year preperiod, including age, sex, Charlson Comorbidity Index (CCI) conditions, and Elixhauser comorbidity conditions. For the past several decades, the CCI has been the most widely used comorbidity assessment tool and includes 17 comorbidity measures. The Elixhauser method is a more comprehensive set of 31 comorbidity measures and is superior to the CCI for risk adjustment.47 Therefore, the use of both comorbidity measures provides a more complete comorbidity picture of the patient cohorts as a whole.
We evaluated the costs in the postindex period, and they reflect the amount paid by the health plan at the time of service, with no cost adjustments made to account for inflation. The postindex costs include the mean and median total all-cause, total PIDD-related, and PIDD-related pharmacy costs. We evaluated the all-cause costs and the PIDD-related costs, because these 2 comparators provide a holistic view of the overall economic impact of a therapy.
The total all-cause costs include the sum of the hospital, emergency department, physician, and pharmacy costs, with a primary or a secondary claim for PIDD. The total PIDD-related costs include the paid claims with a primary diagnosis of PIDD, and claims for immunoglobulin medications—Hizentra, Gammagard, Privigen, and Gamunex-C—that have an indication for the treatment of PIDD. The PIDD-related pharmacy costs include paid claims for these 4 immunoglobulin medications, as well as for the administration costs (ie, claims for the delivery of immunoglobulin).
To minimize confounding between the groups as a result of costly comorbidities, patients in the SCIG cohort were matched (1:1), using propensity score, to patients in the IVIG cohort based on age, sex, and each of the 31 Elixhauser conditions.
Statistical Analyses
The patients' characteristics and costs were compared before and after matching, using t-tests for continuous variables, chi-square for categorical variables, and Wilcoxon rank-sum tests for differences in median values, with significance reached at P <.05. For all mean values, standard deviations were used to quantify the amount of variation in demographic, clinical, and cost data.
Results
In the initial patient selection, we identified 1639 treatment-naïve patients with PIDD; of these, 986 patients were included in the IVIG cohort and 653 in the SCIG cohort. The patients in the IVIG group were, on average, significantly older than those in the SCIG cohort (49.1 years vs 40.3 years, respectively; P <.001) and included more male patients (41.7% vs 36.9%; P = .049). The CCI scores of the patients who received IVIG were significantly higher than those who received SCIG, with an average score of 3.0 ± 2.7 compared with 1.7 ± 1.9 (P <.001; Table 1).
Table 1.
Prematch Patient Demographics and Clinical Characteristics
| Demographics and clinical characteristics | IVIG cohort (N = 986) | SCIG cohort (N = 653) | P value |
|---|---|---|---|
| Age, mean,a yrs (SD) | 49.1 (± 20.15) | 40.3 (± 38.7) | <.001 |
| Age-groupa | |||
| 0–20, yrs (%) | 155 (15.72) | 178 (27.26) | <.001 |
| 21–30, yrs (%) | 26 (2.64) | 60 (9.19) | <.001 |
| 31–40, yrs (%) | 77 (7.81) | 58 (8.88) | <.001 |
| 41–50, yrs (%) | 134 (13.59) | 90 (13.78) | <.001 |
| 51–60, yrs (%) | 285 (28.90) | 134 (20.52) | <.001 |
| 61–70, yrs (%) | 224 (22.72) | 103 (15.77) | <.001 |
| ≥71, yrs (%) | 85 (8.62) | 30 (4.59) | <.001 |
| Sexa | |||
| Female, N (%) | 575 (58.32) | 412 (63.09) | .049 |
| Male, N (%) | 411 (41.68) | 241 (36.91) | .049 |
| Charlson Comorbidity Index score | |||
| Mean score,a N (SD) | 3.01 (± 2.73) | 1.74 (± 1.91) | <.001 |
| Elixhauser conditions (comorbidities) | |||
| Congestive heart failure,a N (%) | 68 (6.90) | 16 (2.45) | <.001 |
| Arrythmia,a N (%) | 167 (16.94) | 39 (5.97) | <.001 |
| Valvular disease,a N (%) | 84 (8.52) | 19 (2.91) | <.001 |
| Pulmonary circulation disorder,a N (%) | 44 (4.46) | 17 (2.60) | .052 |
| Peripheral vascular disorders,a N (%) | 46 (4.67) | 15 (2.30) | .013 |
| Hypertension, uncomplicated,a N (%) | 328 (33.27) | 133 (20.37) | <.001 |
| Hypertension, complicated,a N (%) | 44 (4.46) | 15 (2.30) | .021 |
| Paralysis, N (%) | 12 (1.22) | 10 (1.53) | .588 |
| Other neurologic disorders, N (%) | 79 (8.01) | 42 (6.43) | .231 |
| Chronic obstructive pulmonary disease, N (%) | 458 (46.45) | 320 (49.00) | .311 |
| Diabetes, uncomplicated,a N (%) | 144 (14.60) | 60 (9.19) | .001 |
| Diabetes, complicated,a N (%) | 36 (3.65) | 12 (1.84) | .003 |
| Hypothyroid, N (%) | 144 (14.60) | 88 (13.48) | .521 |
| Renal failure,a N (%) | 58 (5.88) | 17 (2.60) | .002 |
| Liver disease,a N (%) | 58 (5.88) | 13 (1.99) | <.001 |
| Peptic ulcer disease, excluding bleeding, N (%) | 6 (0.61) | 2 (0.31) | .390 |
| AIDS/HIV, N (%) | 3 (0.30) | 2 (0.31) | .994 |
| Lymphoma,a N (%) | 180 (18.26) | 21 (3.22) | <.001 |
| Metastatic cancer,a N (%) | 24 (2.43) | 3 (0.46) | .002 |
| Solid tumor without metastasis,a N (%) | 77 (7.81) | 17 (2.60) | <.001 |
| Rheumatoid arthritis/collagen, N (%) | 126 (12.78) | 73 (11.18) | .332 |
| Coagulopathy,a N (%) | 109 (11.05) | 23 (3.52) | <.001 |
| Obesity, N (%) | 48 (4.87) | 27 (4.13) | .487 |
| Weight loss,a N (%) | 70 (7.10) | 15 (2.30) | <.001 |
| Fluid and electrolyte disorders,a N (%) | 191 (19.37) | 57 (8.73) | <.001 |
| Blood-loss anemia, N (%) | 9 (0.91) | 5 (0.77) | .751 |
| Deficiency anemia, N (%) | 73 (7.40) | 42 (6.43) | .451 |
| Alcohol abuse, N (%) | 3 (0.30) | 3 (0.46) | .612 |
| Drug abuse, N (%) | 12 (1.22) | 7 (1.07) | .788 |
| Psychoses, N (%) | 24 (2.43) | 5 (0.77) | .121 |
| Depression, N (%) | 175 (17.75) | 94 (14.40) | .073 |
Significant difference.
IVIG indicates intravenous immunoglobulin; SCIG, subcutaneous immunoglobulin; SD, standard deviation.
When comparing individual Elixhauser conditions, the IVIG cohort had significantly more patients than those in the SCIG cohort who had congestive heart failure, arrhythmia, valvular disease, pulmonary circulation disorders, peripheral vascular disorder, complicated and uncomplicated hypertension, complicated and uncomplicated diabetes, renal failure, liver disease, lymphoma, metastatic cancer, solid tumors, coagulopathy, weight loss, fluid and electrolyte disorders, and psychoses (Table 1). However, there was no condition on the Elixhauser list for which a significantly greater proportion of patients received SCIG.
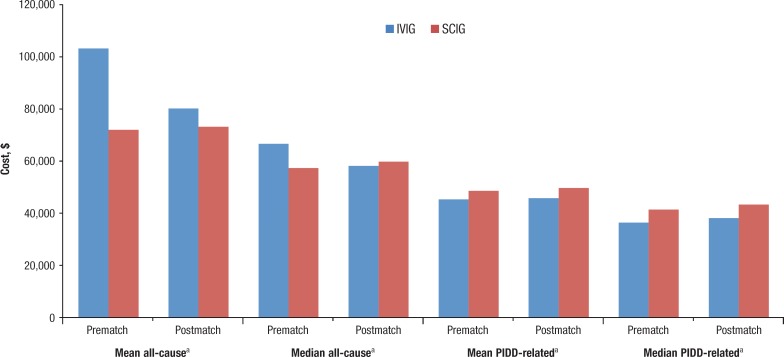
The cost comparisons in the initial unmatched patient pool resulted in conflicting trends between the total all-cause costs and PIDD-related costs. The mean total all-cause costs in the 1-year postperiod were significantly greater for the IVIG group ($103,177 ± $113,723) than for the SCIG group ($71,949 ± $59,209; P <.001). The median total all-cause costs were $66,568 for the IVIG group and $57,296 for the SCIG group (P <.001). In contrast, the unmatched mean total PIDD-related costs between the 2 cohorts were significantly lower for the IVIG group ($45,225 ± $44,863) than for the SCIG group ($48,517 ± $38,255; P = .042). The median total PIDD-related costs were also lower for the IVIG group than for the SCIG group ($36,277 vs $41,339; P <.001; Figure 1 and Table 2).
Figure 1. Total All-Cause and PIDD-Related Cost Differences Between IVIG and SCIG.
aSignificant difference.
IVIG indicates intravenous immunoglobulin; PIDD, primary-immune deficiency disorder; SCIG, subcutaneous immunoglobulin.
Table 2.
Cost Analysis: Prematch and Postmatch Cohorts
| Cost analysis |
Prematch cohorts |
Postmatch cohorts |
||||
|---|---|---|---|---|---|---|
| IVIG cohort (N = 986) | SCIG cohort (N = 653) | P value | IVIG cohort (N = 553) | SCIG cohort (N = 553) | P value | |
| Total all-cause costs | ||||||
| Mean (SD), $ | 103,177 (± 113,723) | 71,949 (± 59,209) | <.001a | 80,089 (± 81,702) | 73,108 (± 57,559) | .101 |
| Median, $ | 66,568 | 57,296 | <.001a | 58,095 | 59,726 | .711 |
| PIDD-related total costs | ||||||
| Mean (SD), $ | 45,225 (± 44,863) | 48,517 (± 38,255) | .042 | 45,713 (± 40,844) | 49,630 (± 38,714) | .102 |
| Median,a $ | 36,277 | 41,339 | .001 | 38,064 | 43,266 | .002 |
| PIDD-related pharmacy costs | ||||||
| Mean (SD), $ | 24,259 (± 24,575) | 25,584 (± 23,911) | .280 | 23,331 (± 20,206) | 26,565 (± 24,818) | .030 |
| Median,a $ | 19,037 | 22,073 | <.001 | 19,195 | 23,097 | .002 |
| Immunoglobulin drug costs | ||||||
| Mean (SD), $ | 23,271 (± 24,033) | 24,626 (± 23,870) | .313 | 22,475 (± 19,953) | 25,475 (± 24,775) | .035 |
| Median,a $ | 18,082 | 21,242 | <.001 | 18,449 | 21,773 | <.001 |
| Administration costs | ||||||
| Mean (SD), $ | 988 (± 1742) | 1094 (± 1627) | .207 | 856 (± 1026) | 1089 (± 1804) | .267 |
| Median, $ | 600 | 550 | .593 | 581 | 300 | <.001a |
Significant difference.
IVIG indicates intravenous immunoglobulin; PIDD, primary immune-deficiency disorder; SCIG, subcutaneous immunoglobulin; SD, standard deviation.
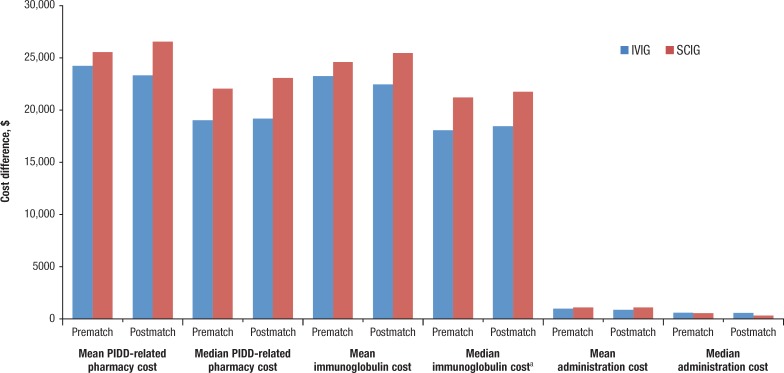
The mean pharmacy-related (ie, drug plus administration) costs were not significantly different between the unmatched IVIG ($24,259 ± $24,575) and SCIG ($25,584 ± $23,911; P = .028) groups, although the median pharmacy-related costs were significantly lower for IVIG ($19,037 vs $22,073 for SCIG; P <.001). Similarly, the mean immunoglobulin drug costs were not significantly different between the IVIG ($23,271 ± $24,033) and SCIG ($24,626 ± $23,870; P = .313) groups; however, the median immunoglobulin-related costs were significantly lower for the IVIG group ($18,082 vs $21,242 for SCIG; P <.001). There was no significant difference in the mean or median administration costs between the 2 cohorts (Figure 2).
Figure 2. PIDD-Related Pharmacy Cost Differences Between IVIG and SCIG.
aSignificant difference.
IVIG indicates intravenous immunoglobulin; PIDD, primary immune-deficiency disorder; SCIG, subcutaneous immunoglobulin.
Matching the 2 groups adjusted the demographic and clinical differences between the IVIG and SCIG cohorts (Table 3). Matching the groups also had an impact on the cost-comparison results. The mean total all-cause costs were no longer significantly different between the IVIG and SCIG cohorts ($80,089 ± $81,702 vs $73,108 ± $57,559, respectively; P = .101), nor were the median total all-cause costs ($58,095 vs $59,726, respectively; P = .711). The postmatched mean total PIDD-related costs were also no longer significantly different between the IVIG and SCIG groups ($45,713 ± $40,844 vs $49,631 ± $38,714; P = .102). However, the median total PIDD-related costs were still significantly lower for the IVIG group ($38,064) than for the SCIG cohort ($43,266; P = .002) as can be seen in Figure 1 and Table 2.
Table 3.
Postmatch Patient Demographics and Clinical Characteristics
| Demographics and clinical characteristics | IVIG cohort (N = 986) | SCIG cohort (N = 653) | P value |
|---|---|---|---|
| Age, mean, yrs (SD) | 44.3 (± 20.6) | 44.3 (± 20.5) | .994 |
| Age-group | |||
| 0–20 yrs (%) | 118 (21.34) | 115 (20.80) | .985 |
| 21–30 yrs (%) | 24 (4.34) | 29 (5.24) | .985 |
| 31–40 yrs (%) | 56 (10.13) | 52 (9.4) | .985 |
| 41–50 yrs (%) | 84 (15.19) | 90 (16.27) | .985 |
| 51–60 yrs (%) | 136 (24.59) | 134 (24.23) | .985 |
| 61–70 yrs (%) | 107 (19.35) | 103 (18.63) | .985 |
| ≥71 yrs (%) | 28 (5.06) | 30 (5.42) | .985 |
| Sex | |||
| Female, N (%) | 359 (64.92) | 372 (67.27) | .409 |
| Male, N (%) | 194 (35.08) | 181 (32.73) | .409 |
| Charlson Comorbidity Index score | |||
| Mean score, N (SD) | 2.02 (± 1.89) | 1.86 (± 1.96) | .161 |
| Elixhauser conditions (comorbidities) | |||
| Congestive heart failure, N (%) | 13 (2.33) | 14 (2.51) | .846 |
| Arrythmia, N (%) | 38 (6.82) | 38 (6.82) | 1.00 |
| Valvular disease, N (%) | 26 (4.67) | 18 (3.23) | .218 |
| Pulmonary circulation disorder, N (%) | 14 (2.51) | 16 (2.87) | .711 |
| Peripheral vascular disorders, N (%) | 13 (2.33) | 15 (2.69) | .702 |
| Hypertension, uncomplicated, N (%) | 139 (24.96) | 129 (23.16) | .483 |
| Hypertension, complicated, N (%) | 7 (1.26) | 15 (2.69) | .085 |
| Paralysis, N (%) | 5 (0.90) | 7 (1.26) | .562 |
| Other neurologic disorders, N (%) | 39 (7.00) | 30 (5.39) | .263 |
| Chronic obstructive pulmonary disease, N (%) | 259 (46.50) | 283 (50.81) | .149 |
| Diabetes, uncomplicated, N (%) | 64 (11.49) | 56 (10.05) | .439 |
| Diabetes, complicated, N (%) | 13 (2.33) | 12 (2.15) | .840 |
| Hypothyroid, N (%) | 63 (11.31) | 84 (15.08) | .063 |
| Renal failure, N (%) | 17 (3.05) | 17 (3.05) | 1.00 |
| Liver disease, N (%) | 16 (2.87) | 10 (1.80) | .234 |
| Peptic ulcer disease, excluding bleeding, N (%) | 0 (0.00) | 2 (0.36) | .157 |
| AIDS/HIV, N (%) | 2 (0.36) | 2 (0.36) | 1.00 |
| Lymphoma, N (%) | 20 (3.59) | 20 (3.59) | 1.00 |
| Metastatic cancer, N (%) | 1 (0.18) | 3 (0.54) | .316 |
| Solid tumor without metastasis, N (%) | 15 (2.69) | 15 (2.69) | 1.00 |
| Rheumatoid arthritis/collagen, N (%) | 73 (13.11) | 72 (12.93) | .929 |
| Coagulopathy, N (%) | 12 (2.15) | 12 (2.15) | 1.00 |
| Obesity, N (%) | 19 (3.41) | 26 (4.67) | .287 |
| Weight loss, N (%) | 24 (4.31) | 13 (2.33) | .066 |
| Fluid and electrolyte disorders, N (%) | 59 (10.59) | 48 (8.62) | .263 |
| Blood-loss anemia, N (%) | 1 (0.18) | 4 (0.72) | .179 |
| Deficiency anemia, N (%) | 26 (4.67) | 39 (7.00) | .097 |
| Alcohol abuse, N (%) | 1 (0.18) | 3 (0.54) | .316 |
| Drug abuse, N (%) | 3 (0.54) | 6 (1.08) | .315 |
| Psychoses, N (%) | 10 (1.80) | 5 (0.90) | .194 |
| Depression, N (%) | 79 (14.18) | 83 (14.90) | .734 |
IVIG indicates intravenous immunoglobulin; SCIG, subcutaneous immunoglobulin; SD, standard deviation.
The mean and median PIDD-related pharmacy (ie, immunoglobulin plus administration) costs for the patients receiving IVIG in the matched population were consistently lower than for those in the SCIG cohort. The mean PIDD-related pharmacy costs were $23,331 ± $20,206 versus $26,565 ± $24,818 for the IV and SC cohorts, respectively (P = .03), whereas the median PIDD-related pharmacy costs were $19,195 and $23,097 (P = .002). The mean and median immunoglobulin drug costs trended similarly: the means were $22,475 ± $19,953 for the IV cohort versus $25,475 ± $24,775 for the SC cohort (P = .035), whereas the medians were $18,449 for the IV cohort and $21,773 for the SC cohort, respectively. The mean administration costs were not significantly different between the cohorts ($1089 ± $1804 for the SC cohort vs $856 ± $1026 for the IV cohort; P = .267); however, the median administration costs trended in different directions, with IV drugs being significantly greater than SC drugs ($581 vs $300, respectively; P <.001; Figure 2 and Table 2).
Discussion
The SC and IV routes of administration for immunoglobulin therapy are safe and effective.14–31 Previous studies of the economics of IV and SC routes of immunoglobulin administration have reported SCIG to be less expensive than IVIG.15,34–37,39,40,43,44,48,49 These conclusions partially resulted from the projected lower costs of administering SCIG, which eliminates the need for healthcare provider and medical facility use. However, an important variable that has been omitted in previous economic analyses or minimized because of similar drug costs is the cost difference of SC versus IV immunoglobulin itself.15,37,39,43,44,48,50 This could be because during the period that these studies were conducted, immunoglobulin drugs for these 2 routes of administration were similarly priced. However, SCIG costs in the United States, as reflected by WAC, rose substantially over the 3-year span of 2012 to 2015.46,51
Based on WAC, as of August 2018, SCIG is 1.2 to 1.6 times more expensive per gram than IVIG, which equates to an average cost difference of more than $1800 per infusion for an 80-kg (176-lb) patient with PIDD; this far outweighs the average administration cost charged by a healthcare provider (mean, $216 per infusion).52 Given that several European economic analyses40,43,48 have identified the cost of immunoglobulins as a driver in the economics of PIDD treatment, not accounting for SCIG price increases in the United States could be misleading and can severely underestimate the total cost of this therapy to payers.
In our study, we found that patients with PIDD who received IVIG had more comorbidities, were older, and were more likely to be male than their counterparts who received SCIG. These demographic differences are important when making economic comparisons between SC and IV routes of administration, because older patients typically incur higher healthcare costs and have a higher incidence of comorbidities (particularly malignancies). In addition, because immunoglobulin treatment is weight-based, a larger number of males in a cohort would likely incur increased costs based on a greater number of grams of immunoglobulin needed for treatment. The patients' demographic and clinical differences could help explain much of the higher total all-cause costs for patients who received IVIG than those who received SCIG.
We performed propensity score matching to control for these differences in patient characteristics. Before matching, the postindex mean and median total all-cause costs were significantly higher in the IVIG group than in the SCIG group. However, after matching was performed, the median total all-cause costs were lower for patients who received IVIG than for those who received SCIG, whereas the mean total all-cause cost differences decreased for the IVIG cohort and were still slightly higher than for the SCIG cohort. Neither difference was significant. These reductions in the cost differences of IVIG versus SCIG support the hypothesis that the demographic and clinical differences between patients who receive IVIG and those who receive SCIG may be inflating the costs of IVIG treatment.
Before the propensity score matching, the postindex mean and median total PIDD-related costs were significantly lower for the IV cohort. However, only the median PIDD-related pharmacy costs, and specifically the immunoglobulin drug costs alone, were lower for IVIG, with no differences in administration costs between the unmatched cohorts. The postmatching results did not substantially change the mean and median total PIDD-related costs. However, after matching, the PIDD-related pharmacy costs (excluding administration costs) were all lower for the IVIG group, whereas in the unmatched groups only the median PIDD-related pharmacy and immunoglobulin drug costs were significantly lower for IVIG. Because these 2 matched cohorts were similar (except for more cases of nonmetastatic cancer in the IVIG group), these findings suggest that the total PIDD-related costs and PIDD-related pharmacy costs favor IVIG treatment, outweighing the lower administration costs associated with SCIG treatment.
This also suggests that the higher rate of comorbidities (particularly cancer), older age, and greater proportion of males in the IVIG unmatched population contributed to increased costs because, after matching, the PIDD-related pharmacy and immunoglobulin drug costs associated with IVIG were significantly lower. In our previous study that utilized the same data set (years 2012–2015), the annualized total costs per treated member per treated month (drug plus administration costs) were $4863 and $4678 for patients receiving SCIG and IVIG, respectively, for the year 2015.51 In another study based on data from PharMetrics Plus that assessed the cost of care for treatment-naïve patients with PIDD, the median total and pharmacy costs for the IVIG cohort ($19,195 and $18,449, respectively) were significantly lower than for the SCIG group ($23,097 and $21,773, respectively; P <.05), whereas the IVIG-related median administration costs were $281 higher than for the SCIG group (P <.05).53
The decision-making used by physicians to determine the route of administration for patients with PIDD are unknown. Because the baseline characteristics are different between the IV and SC cohorts, it is possible that physicians recommend SCIG for younger, healthier, more active patients because of the more flexible dosing regimen. Moreover, physicians may prefer IVIG treatment for patients who are frailer and less able to self-administer the drug. It is also unclear whether patients with lymphoma and other types of cancers have secondary immunodeficiency but their diagnosis is coded as PIDD. This may explain why patients who received IVIG in this study had more severe disease and higher total all-cause costs before matching. Ongoing and future studies of patient and physician preferences may give insight into how these decisions are made.
The IVIG group in our study was comprised of patients with J codes for 3 immunoglobulins (Gamunex-C, Gammagard, and Privigen), which are the most frequently prescribed immunoglobulins for PIDD, with a combined market share of nearly 80% of the US immunoglobulin market.54 These 3 medications, which are all at the higher end of the price range for IV drugs, were selected in this study as a more conservative cost comparator to SCIG treatment. Including the less expensive IVIG treatments in this analysis would theoretically lower the IVIG-related costs relative to SCIG even more.
During the inclusion years of this analysis, 3 SCIG drugs were approved for the treatment of PIDD in the United States—Hizentra, Gammagard, and Gamunex-C. Approximately 4% to 5% of Gamunex is administered subcutaneously.54 It was difficult to differentiate between patients receiving immunoglobulin drugs with dual routes of administration in any health plan claims database; therefore, the potential for misclassification exists, because any patients receiving SC Gamunex or Gammagard would be included in the IVIG cohort. Regardless of whether some patients who received SC Gamunex or SC Gammagard were mistakenly classified as receiving IV drugs, the impact would be minimal, because the cost per gram is the same for both routes of immunoglobulin administration.
Subsequent to the conclusion of this analysis, 2 additional SC immunoglobulin drugs have been approved—Hyqvia in 2014 and Cuvitru in 2016.55,56 Hyqvia and Cuvitru are currently the highest-priced immunoglobulin drugs available in the United States.46 Adding the cost data for these 2 drugs to our analysis would have resulted in even higher costs for SCIG compared with IVIG.46 As more data become available, future analyses will need to include Hyqvia and Cuvitru as a part of the PIDD treatment cost evaluations.
Limitations
Several potential limitations should be considered when reviewing this study. Claims data analysis is an effective but limited tool for understanding the costs associated with treating patients with PIDD. Claims data can provide the opportunity to assess a large patient population, as well as detailed information regarding medical claims that were submitted for each patient. However, clinical information, such as diagnostic test results and patient-reported feedback, is lacking. This limitation should affect SCIG and IVIG groups equally.
Another limitation is the potential for the misclassification of claims. As is often the case with claims analyses, a single diagnosis is not easily verified. Our analysis relied on multiple diagnoses over a 90-day period to identify a patient with PIDD. The effect of such a misclassification should again equally affect patients who received IVIG or SCIG. Another potential shortcoming regarding misclassification is the possibility that secondary immunodeficiency diseases were erroneously coded as PIDD to facilitate the initiation of treatment.
Using the PharMetrics Plus health plan claims database, it was not feasible to identify the indirect costs associated with immunoglobulin therapy, some of which include immunoglobulin therapy–related adverse events, missed hours from work or school, travel to and from physician office visits, and the impact of the route of administration on patients' quality of life. Past studies have suggested that SCIG is more cost-effective than IVIG; however, the majority of these studies were conducted when IV therapy was not available for home infusion, and when the payers' costs for IVIG and SCIG were similar.15,36,44,46,48,49,53 Assessing the treatment costs for PIDD provides insight into treatment strategies and major events, such as hospitalizations and unplanned physician visits, but detailed information regarding clinical outcomes is lacking.
Furthermore, this study utilized data from 2011 to 2013, which could be considered outdated. Given that 2 new SC treatment options (Hyqvia and Cuvitru) have entered the US market since 2014, this study should have been updated to include those treatments. However, because of the time lag associated with the availability of the claims data, the earliest this study could be replicated with the inclusion of Cuvitru would have been approximately late 2018, because the study design requires 1 year before and after the initiation of immunoglobulin.56
Finally, because the PharMetrics Plus database was used for this study, these findings may not apply to all populations, including noncommercially insured patients with Medicare fee-for-service insurance, patients with Medicaid, and others.
Conclusions
The safety and efficacy of SCIG and IVIG have been well-documented in the medical literature. Our study provides insight into 2 important aspects of care for patients with PIDD. First, patients with PIDD who started treatment with IVIG had significant demographic differences and more severe disease than patients who started treatment with SCIG. Second, after patient matching in the study, PIDD-related pharmacy and immunoglobulin drug costs incurred over the first year of treatment were significantly lower for patients who received IVIG than for those who received SCIG, which significantly outweighed the lower costs associated with the administration of SCIG.
Future studies should address what is contributing to the overall cost difference between IVIG and SCIG, such as clinical events and deterioration, as well as differences in drug costs, drug delivery, and medication adherence among various patient subpopulations, based on the insurance type—commercial, Medicare, or Medicaid.
In addition, research is needed to clarify what influence the choice of the route of immunoglobulin administration, physician and patient preferences, clinical outcomes, patients' out-of-pocket cost burden, payment methods that drive improved outcomes, and how the emerging home infusion market may change the cost dynamics.
Funding Source
Funding for this study was provided by Grifols SSNA.
Author Disclosure Statement
Dr Runken is an employee of Grifols SSNA; Dr Noone, Ms Zacherle, and Dr Howden are Consultants to Grifols SSNA; Dr Blanchette has no conflicts of interest to report.
Contributor Information
Michael C. Runken, Senior Director Global HEOR, Grifols SSNA, Research Triangle Park, NC.
Joshua M. Noone, Research Assistant Professor, University of North Carolina at Charlotte.
Christopher M. Blanchette, Research Professor, University of North Carolina at Charlotte.
Emily Zacherle, Research Associate, University of North Carolina at Charlotte.
Reuben Howden, Associate Professor, University of North Carolina at Charlotte.
References
- 1. Immune Deficiency Foundation. About primary immunodeficiencies. https://primaryimmune.org/about-primary-immunodeficiencies. Accessed September 21, 2017.
- 2. Cooper MA, Pommering TL, Korányi K. Primary immunodeficiencies. Am Fam Physician. 2003;68:2001–2008. [PubMed] [Google Scholar]
- 3. Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27:497–502. [DOI] [PubMed] [Google Scholar]
- 4. Immune Deficiency Foundation. Primary immune deficiency diseases in America: the first national survey of patients and specialists. 1995. https://primaryimmune.org/wp-content/uploads/2011/04/Primary-Immune-Deficiency-Diseases-in-America-The-First-National-Survey-of-Patients-and-Specialists-1995.pdf. Accessed September 19, 2017.
- 5. Jiang F, Torgerson TR, Ayars AG. Health-related quality of life in patients with primary immunodeficiency disease. Allergy Asthma Clin Immunol. 2015;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aghamohammadi A, Montazeri A, Abolhassani H, et al. Health-related quality of life in primary antibody deficiency. Iran J Allergy Asthma Immunol. 2011; 10:47–51. [PubMed] [Google Scholar]
- 7. Seymour B, Miles J, Haeney M. Primary antibody deficiency and diagnostic delay. J Clin Pathol. 2005;58:546–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eades-Perner AM, Gathmann B, Knerr V, et al; for the ESID Registry working party. The European internet-based patient and research database for primary immunodeficiencies: results 2004–06. Clin Exp Immunol. 2007;147:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roifman CM, Schroeder H, Berger M, et al. Comparison of the efficacy of IGIV-C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency: a randomized double-blind trial. Int Immunopharmacol. 2003;3:1325–1333. [DOI] [PubMed] [Google Scholar]
- 10. Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–2004. [DOI] [PubMed] [Google Scholar]
- 11. de Gracia J, Vendrell M, Álvarez A, et al. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. Int Immunopharmacol. 2004;4:745–753. [DOI] [PubMed] [Google Scholar]
- 12. Immune Deficiency Foundation. Diagnostic & clinical care guidelines for primary immunodeficiency diseases. 3rd edition. 2015. https://primaryimmune.org/wp-content/uploads/2015/03/2015-Diagnostic-and-Clinical-Care-Guidelines-for-PI.pdf. Accessed February 2, 2017.
- 13. Jolles S, Orange JS, Gardulf A, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desai SH, Chouksey A, Poll J, Berger M. A pilot study of equal doses of 10% IGIV given intravenously or subcutaneously. J Allergy Clin Immunol. 2009;124:854–856. [DOI] [PubMed] [Google Scholar]
- 15. Gardulf A, Andersen V, Björkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345:365–369. [DOI] [PubMed] [Google Scholar]
- 16. Chapel HM, Spickett GP, Ericson D, et al. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. [DOI] [PubMed] [Google Scholar]
- 17. Fasth A, Nyström J. Safety and efficacy of subcutaneous human immunoglobulin in children with primary immunodeficiency. Acta Paediatr. 2007;96:1474–1478. [DOI] [PubMed] [Google Scholar]
- 18. Hagan JB, Fasano MB, Spector S, et al. Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. J Clin Immunol. 2010;30:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borte M, Quinti I, Soresina A, et al. Efficacy and safety of subcutaneous Vivaglobin replacement therapy in previously untreated patients with primary immunodeficiency: a prospective, multicenter study. J Clin Immunol. 2011;31:952–961. [DOI] [PubMed] [Google Scholar]
- 20. Caress JB, Kennedy BL, Eickman KD. Safety of intravenous immunoglobulin treatment. Expert Opin Drug Saf. 2010;9:971–979. [DOI] [PubMed] [Google Scholar]
- 21. International Patient Organisation for Primary Immunodeficiencies. Safety and efficacy of immunoglobulin therapies for primary immunodeficiencies: a guide for users, assessors and funders. October 26, 2018. https://ipopi.org/wp-content/uploads/2011/03/IPOPI-Safety_and_Efficacy_of_Ig-_Print-version2018_Final.pdf. Accessed April 7, 2019.
- 22. Abolhassani H, Asgardoon MH, Rezaei N, et al. Different brands of intravenous immunoglobulin for primary immunodeficiencies: how to choose the best option for the patient? Expert Rev Clin Immunol. 2015;11:1229–1243. [DOI] [PubMed] [Google Scholar]
- 23. Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6:592–599. [DOI] [PubMed] [Google Scholar]
- 24. Stein M, Nemet A, Kumar S, et al. Efficacy, safety, and tolerability of Kedrion 10% IVIG in primary immunodeficiency. LymphoSign J. 2016;3:99–109. [Google Scholar]
- 25. Gelfand EW, Hanna K. Safety and tolerability of increased rate of infusion of intravenous immunoglobulin G, 10% in antibody-deficient patients. J Clin Immunol. 2006;26:284–290. [DOI] [PubMed] [Google Scholar]
- 26. Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abrahamsen TG, Sandersen H, Bustnes A. Home therapy with subcutaneous immunoglobulin infusions in children with congenital immunodeficiencies. Pediatrics. 1996;98:1127–1131. [PubMed] [Google Scholar]
- 28. Hansen S, Gustafson R, Smith CIE, Gardulf A. Express subcutaneous IgG infusions: decreased time of delivery with maintained safety. Clin Immunol. 2002;104:237–241. [DOI] [PubMed] [Google Scholar]
- 29. Gardulf A, Hammarström L, Smith CIE. Home treatment of hypogammaglobulinaemia with subcutaneous gammaglobulin by rapid infusion. Lancet. 1991;338:162–166. [DOI] [PubMed] [Google Scholar]
- 30. Welch MJ, Stiehm ER. Slow subcutaneous immunoglobulin therapy in a patient with reactions to intramuscular immunoglobulin. J Clin Immunol. 1983; 3:285–286. [DOI] [PubMed] [Google Scholar]
- 31. Church JA, Howard V, Sleasman JW, et al. Subcutaneous immunoglobulin replacement therapy in infants and children with primary immunodeficiencies. J Allergy Clin Immunol. 2011;127(suppl):AB213. [Google Scholar]
- 32. Immune Deficiency Foundation. Primary immunodeficiency diseases in America: 2007: the third national survey of patients. May 1, 2009. https://primaryimmune.org/wp-content/uploads/2011/04/Primary-Immunodeficiency-Diseases-in-America-2007The-Third-National-Survey-of-Patients.pdf. Accessed September 19, 2017.
- 33. Jin JF, Zhu LL, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gardulf A, Möller G, Jonsson E. A comparison of the patient-borne costs of therapy with gamma globulin given at the hospital or at home. Int J Technol Assess Health Care. 1995;11:345–353. [DOI] [PubMed] [Google Scholar]
- 35. Ducruet T, Levasseur MC, Des Roches A, et al. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol. 2013;131:585–587.e3. [DOI] [PubMed] [Google Scholar]
- 36. Martin A, Lavoie L, Goetghebeur M, Schellenberg R. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Radinsky S, Bonagura VR. Subcutaneous immunoglobulin infusion as an alternative to intravenous immunoglobulin. J Allergy Clin Immunol. 2003;112:630–633. [DOI] [PubMed] [Google Scholar]
- 38. Duff KA, Roy S, Poll J, Berger M. IgG replacement by the subcutaneous route using preparations licensed in the USA for administration by other routes. J Allergy Clin Immunol. 2004;113(suppl):S43. [Google Scholar]
- 39. Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. [DOI] [PubMed] [Google Scholar]
- 40. Lazzaro C, Lopiano L, Cocito D. Subcutaneous vs intravenous administration of immunoglobulin in chronic inflammatory demyelinating polyneuropathy: an Italian cost-minimization analysis. Neurol Sci. 2014;35:1023–1034. [DOI] [PubMed] [Google Scholar]
- 41. Cepeda MS, Fife D, Denarié M, et al. Quantification of missing prescriptions in commercial claims databases: results of a cohort study. Pharmacoepidemiol Drug Saf. 2017;26:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abolhassani H, Sahagian MS, Aghamohammadi A, et al. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta-analysis. J Clin Immunol. 2012;32:1180–1192. [DOI] [PubMed] [Google Scholar]
- 43. Beauté J, Levy P, Millet V, et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol. 2010;160:240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Högy B, Keinecke HO, Borte M. Pharmacoeconomic evaluation of immunoglobulin treatment in patients with antibody deficiencies from the perspective of the German statutory health insurance. Eur J Health Econ. 2005;6:24–29. Errata in: Eur J Health Econ. 2005;6: 243,; Eur J Health Econ. 2008;9: 203. [DOI] [PubMed] [Google Scholar]
- 45. Haddad L, Perrinet M, Parent D, et al. Economic evaluation of at home subcutaneous and intravenous immunoglobulin substitution. Rev Med Interne. 2006;27:924–926. [DOI] [PubMed] [Google Scholar]
- 46. Truven Health Analytics. RED BOOK. https://truvenhealth.com/products/micromedex/product-suites/clinical-knowledge/red-book. Accessed January 1, 2017. [Requires subscription to access.]
- 47. Chang HJ, Chen PC, Yang CC, et al. Comparison of Elixhauser and Charlson methods for predicting oral cancer survival. Medicine (Baltimore). 2016;95:e2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Membe SK, Ho C, Cimon K, et al. Economic assessment of different modalities of immunoglobulin replacement therapy. Immunol Allergy Clin North Am. 2008;28:861–874. [DOI] [PubMed] [Google Scholar]
- 49. Ho C, Membe S, Cimon K, et al. Overview of subcutaneous versus intravenous immunoglobulin for primary immunodeficiencies: systematic review and economic analysis. Technology overview number 36. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; January 2008. www.cadth.ca/indexphp/en/publication/785. Accessed November 14, 2011.
- 50. Perraudin C, Bourdun A, Spertini F, et al. Home-based subcutaneous immunoglobulin therapy versus hospital-based intravenous immunoglobulin therapy: a prospective economic analysis. Ann Allergy Asthma Immunol. 2018;120:195–199. [DOI] [PubMed] [Google Scholar]
- 51. Runken MC, Noone J, Zacherle E, et al. U.S. costs of intravenous and subcutaneous therapy for the treatment of primary immunodeficiency disease in the U.S. from 2012–2015. Presented at AMCP Nexus; October 3–6, 2016; National Harbor, MD. Poster 23.
- 52. Centers for Medicare & Medicaid Services. DMEPOS Fee Schedule. DME19-C July 2019 DMEPOS fee schedule. Updated June 28, 2019. www.cms.gov/medicare/medicare-fee-for-service-payment/DMEPOSFeeSched/DMEPOS-Fee-Schedule.html. Accessed September 19, 2019.
- 53. Runken MC, Noone J, Zacherle E, et al. Pharmacy and administration costs in primary immunodeficiency disease patients treated with intravenous and subcutaneous immunoglobulin. Presented at AMCP Nexus; October 3–6, 2016; National Harbor, MD. Poster 24.
- 54. Lexis-Nexis medical claims data. Data on file at Grifols SSNA.
- 55. Hyqvia (immune globulin infusion 10% [human] with recombinant human hyaluronidase) solution for subcutaneous administration [prescribing information]. Lexington, MA: Baxalta US; January 2019. [Google Scholar]
- 56. Cuvitru (immune globulin subcutaneous [human]), 20% solution [prescribing information]. Lexington, MA: Baxalta US; May 2019. [Google Scholar]