Abstract
Background:
Numerous studies have illustrated the association between Helicobacter pylori (H pylori) infection and acute coronary syndrome (ACS). However, the results are contradictory. Therefore, we conducted the meta-analysis to identify the association between H pylori and ACS.
Methods:
We performed a systematic search through electronic databases (Excerpta Medica Database, PubMed, Cochrane Library, and Web of Science). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated with a random effect model. We also carried out the sensitivity analysis and publication bias.
Results:
Forty-four eligible studies involving 7522 cases and 8311 controls were included. The pooled result showed that H pylori infection was associated with an increase risk of ACS (OR = 2.03, 95% CI 1.66–2.47). In addition, similar results were obtained in subgroups of study quality, area, human development index, and H pylori detection method. The OR for developing countries was significantly higher than developed countries (OR = 2.58 vs OR = 1.69). Moreover, H pylori with cytotoxin-associated antigen A was also significantly associated with an increase risk of ACS (OR = 2.39, 95% CI 1.21–4.74).
Conclusion:
The meta-analysis suggested that H pylori infection was associated with an increased risk of ACS, especially in developing countries. H pylori is easily screened and can be treated with a wide range of drugs. Thus, more high-quality and well-designed studies are needed to confirm whether the treatment of H pylori is an effective way to reduce ACS risk.
Keywords: acute coronary syndrome, Helicobacter pylori, meta-analysis
1. Introduction
Acute coronary syndrome (ACS) is a common clinical syndrome of atherosclerotic progression in the coronary plaque, including unstable angina, non-ST-segment elevation myocardial infarction and ST-segment elevation myocardial infarction. Chronic inflammation is considered to be a possible causative agent in the development of ACS and local inflammation in the coronary artery wall may be involved in the pathogenesis of ACS.[1] Nowadays, chronic bacterial infection, such as Helicobacter pylori (H pylori) infection and the accompanying inflammation were considered to be associated with onset of ACS.
H pylori, a spiral gram-negative bacterium, may cause a persistent low-grade inflammation. Several studies have shown that H pylori may contribute to the progression of atherosclerosis through chronic low-grade inflammatory stimulation.[2,3] In addition, H pylori infection could increase risk of acute cardiovascular events by promoting atherosclerotic plaque instability or plaque disruption.[4] Up to now, numerous studies have illustrated the link between H pylori infection and ACS. However, the sample sizes of these studies were limited, and the results are conflicting. Theses debatable conclusions leave the H pylori – ACS association studies under debate for many years. Thus, we carried out a meta-analysis to identify the association between H pylori and ACS.
2. Materials and Methods
The meta-analysis was performed according to the preferred reporting items for systematic reviews and meta-analyses checklist and followed these guidelines.[5]
2.1. Search strategy
A systematic search was performed through PubMed, Cochrane, Excerpta Medica Database (Embase) and Web of Science. The systematic search was updated on October 18, 2019. The following search terms were combined: “(“acute coronary syndrome” or ACS or “myocardial infarction” or “unstable angina” or “ischemic heart disease” or “coronary disease” or “myocardial ischemia” or “coronary atherosclerosis” or “sudden cardia death”)” and “(“Helicobacter pylori” or Helicobacter or “Helicobacter infection” or “H. pylori” or HP).” Language and publication year are not restrictive in our search.
2.2. Inclusion and exclusion criteria
Eligible studies should meet the following inclusion criteria:
-
(1)
ACS as the outcome of study;
-
(2)
evaluated the association between ACS and H pylori;
-
(3)
presenting sufficient data to calculate odds ratios (ORs) and 95% confidence interval (CIs);
-
(4)
case-control or cohort studies for human.
Exclusion criteria included:
-
(1)
data deficiencies;
-
(2)
duplicate publications;
-
(3)
letter, comment, editorial, review, conference abstract;
-
(4)
case-only study;
-
(5)
no human study;
-
(6)
no full text.
Two authors separately screened the potential studies according to above criteria. Disagreement was decided by the third author.
2.3. Data extraction
Two authors separately extracted the information from all eligible studies. Disagreement was resolved by the third author until all authors were unanimous. The following data were collected: name of first author, year of publication, country, area, number of cases and controls, human development index (HDI), matched variables, cytotoxin-associated antigen A (CagA), and so on.
2.4. Quality score assessment
All included studies were scored by 2 authors separately according to the predetermined criteria (Table 1) which were adjusted and revised from the Newcastle–Ottawa scale. Any divergence was decided by the third author. A study with quality score ≥7 was regarded as “high-quality study,” while a study with quality score <7 indicated “low-quality study.”
Table 1.
Quality evaluation tabulation.

2.5. Statistical methods
The prevalence data of H pylori infection (cases/controls) in each study were obtained. ORs and 95% CIs were calculated to assess the strength of the association between H pylori and ACS risk. Heterogeneity was assessed by the Q statistic (significant value at P < .1) and the I2 statistic (I2 > 50% indicating a significant inconsistency). When heterogeneity existed, we used a random-effect model (the DerSimonian and Laird method) to evaluate the pooled ORs and 95% CIs, otherwise, a fixed-effect model (Mantel–Haenszel method) was used. Sensitivity analysis was performed to evaluate the effect of omitting any single study. Egger test and Begg funnel plot were used to examine the publication bias (P < .05 suggested a significant bias). All analyses were performed in the STATA software (version 12.0; StataCorp, College Station, TX), with 2-sided P-values.
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
3. Results
3.1. Characteristics of studies
After the systematic search, a total of 7992 relevant studies were acquired from the PubMed, Cochrane, Embase, and Web of Science databases. Figure 1 shows the studies selection process. We removed 2518 duplicate studies and 5416 irrelevant articles. Then 14 studies were finally excluded, for the following reasons:
Figure 1.
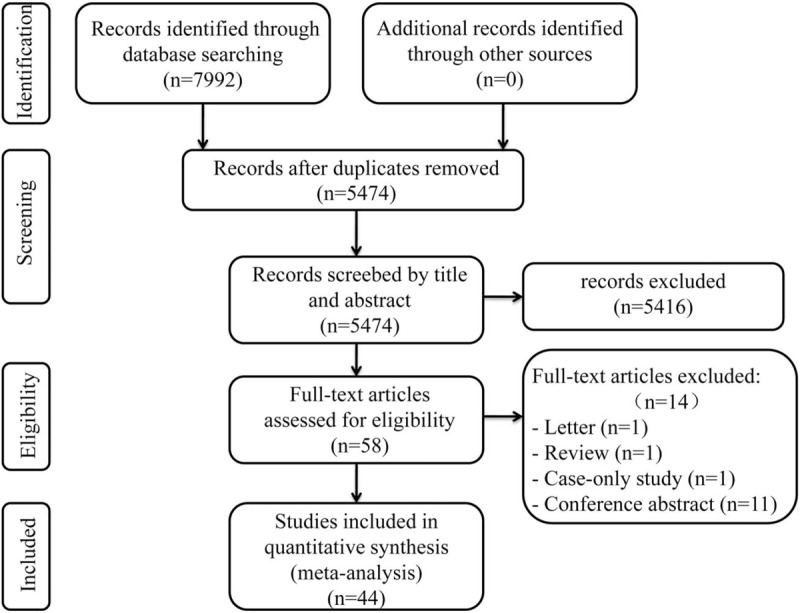
PRISMA flow diagram for study selection. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
-
(1)
1 study was letter and 1 study was review;
-
(2)
1 study was case-only study;
-
(3)
11 studies were conference abstracts.
Finally, 44 eligible studies, publishing between 1996 and 2017, were included in this meta-analysis.
The meta-analysis included 7522 cases and 8311 controls. ACS patients had a higher rate of H pylori infection (64.49%, 4851/7522) than controls (48.03%, 3992/8311). The main features of included studies[3,6–48] were shown in Table 2. Of these 44 studies, 27 studies indicated that H pylori infection was associated with an increased risk of ACS, while the others showed no association. Four of these studies were conducted in United Kingdom (UK), 1 in Croatia, 4 in Indian, 9 in Iran, 2 in Ireland, 9 in Italy, 4 in Japan, 1 in Macedonia, 1 in New Zealand, 2 in Pakistan, 1 in Spain, 1 in Sweden, 2 in Turkey, 2 in the United States, and 1 in China. UK contributed the most H pylori cases (9.95%) and the largest sample size (23.05%). The quality score for included 44 studies was ranged from 1 to 9, with 56.82% (25 of 44) of the studies being of high quality (score ≥7).
Table 2.
Characteristics of studies included in the meta-analysis.
3.2. Meta-analysis results
The pooled result showed that patients with H pylori infection had a significantly increased risk of ACS compared with individuals without H pylori infection (OR = 2.03, 95% CI 1.66–2.47, PH = .000) (Fig. 2). Moreover, studies with matched variables were chosen to perform another meta-analysis and the random effect pooled OR was 2.18 (95% CI 1.77–2.69, PH = .000). Furthermore, we also conducted another meta-analysis which was based on different countries. A significantly positive association between H pylori and ACS was found in UK (OR = 1.60, 95% CI 1.11–2.29, PH = .002), Iran (OR = 3.44, 95% CI 1.93–6.13, PH = .000), Indian (OR = 2.20 95% CI 1.75–2.76, PH = .168), Italy (OR = 2.80, 95% CI 2.03–3.86, PH = .036), Pakistan (OR = 3.95, 95% CI 2.40–6.52, PH = .532), and United States (OR = 1.47, 95% CI 1.03–2.10, PH = .311). However, no statistically significant association was observed in Japan (OR = 1.13, 95% CI 0.94–1.35, PH = .245), Ireland (OR = 1.27, 95% CI 0.98–1.64, PH = .140), and Turkey (OR = 2.07, 95% CI 0.38–11.34, PH = .005).
Figure 2.
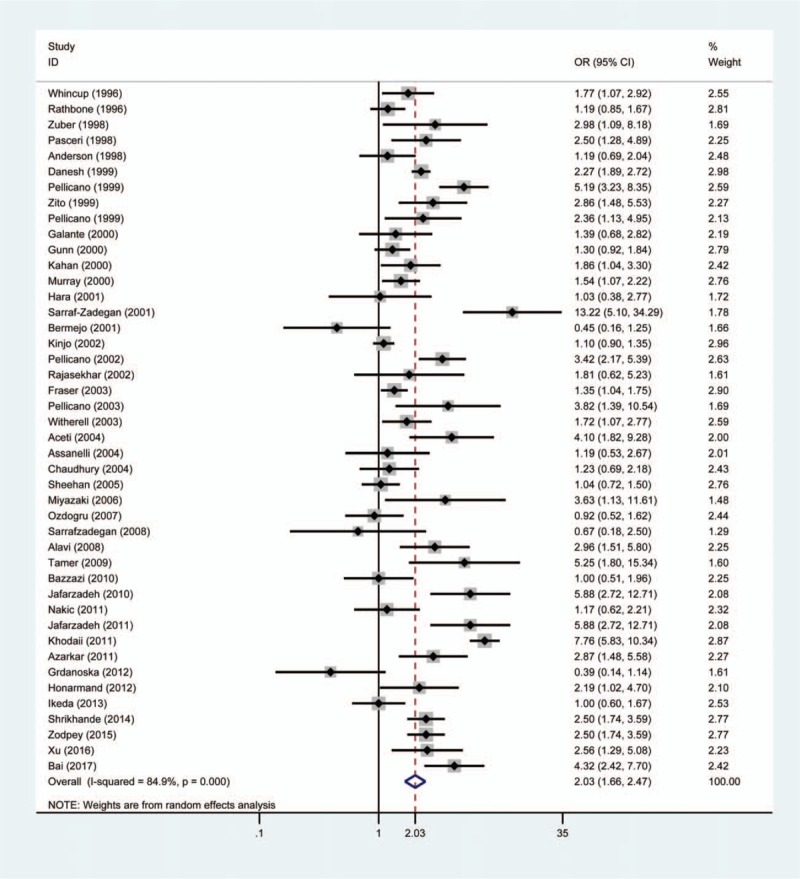
Forest plot of the association between Helicobacter pylori infection and acute coronary syndrome. The size of the black square represents the weight of the study in the meta-analysis. The rhombus represents the combined OR. OR = odds ratio.
3.3. Subgroup analysis
Subgroup analysis was carried out to investigate the effects of study quality, area, HDI and H pylori detection method. There was significant association between H pylori infection and ACS risk in high-quality studies (OR = 2.29, 95% CI 1.76–2.99, PH = .000) and low-quality studies (OR = 1.70, 95% CI 1.29–2.24, PH = .000) (Fig. 3). In addition, we found a significantly positive association between H pylori and ACS in studies from Europe (OR = 1.75, 95% CI 1.40–2.19, PH = .000), Asia (OR = 2.45, 95% CI 1.71–3.51, PH = .000), and America (OR = 1.46, 95% CI 1.02–2.10, PH = .311) (Fig. 4). A stronger association between ACS and H pylori was observed in developing countries than in developed countries (OR = 2.58, 95% CI 1.78–3.73 vs OR = 1.69, 95% CI 1.40–2.05) (Fig. 5). H pylori infection was assessed by the enzyme-linked immunosorbent assay (ELISA) in 95% included studies (42/44) and 2 other studies used 13C-urea breath test and H pylori stool antigen test to determine H pylori infection, respectively. A significant result was observed in the ELISA subgroup when stratifying findings by H pylori infection measure method (OR = 1.95, 95% CI 1.60–2.37, PH = .000).
Figure 3.
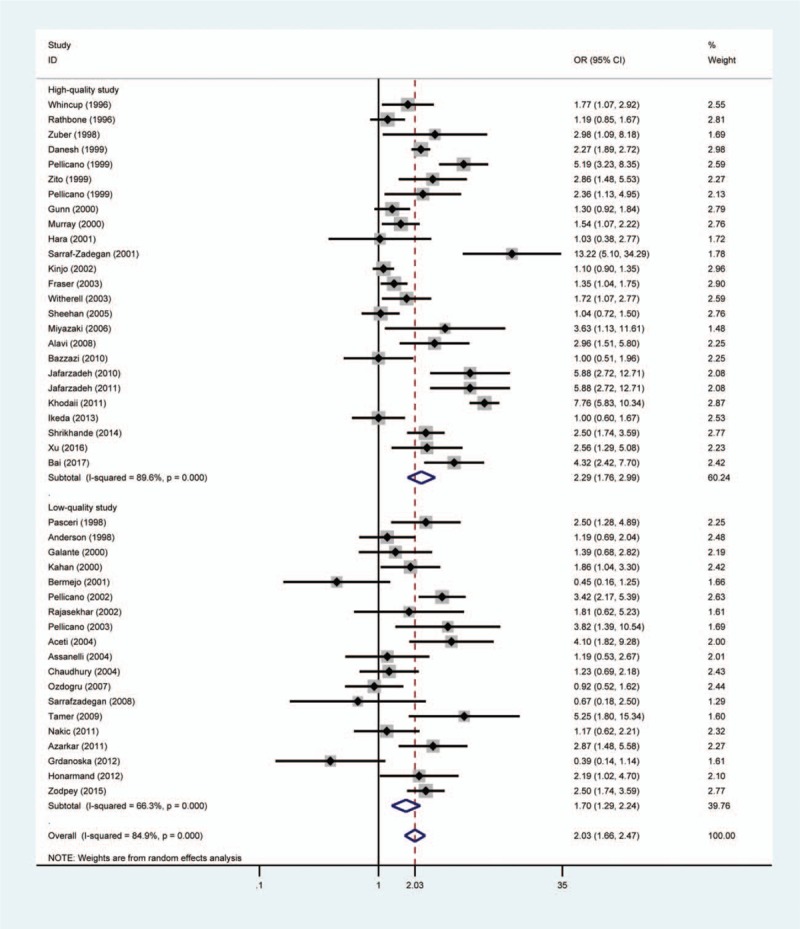
Forest plot of the association between Helicobacter pylori infection and acute coronary syndrome in the subgroup of study quality. The size of the black square represents the weight of the study in the meta-analysis. The rhombus represents the combined OR. OR = odds ratio.
Figure 4.
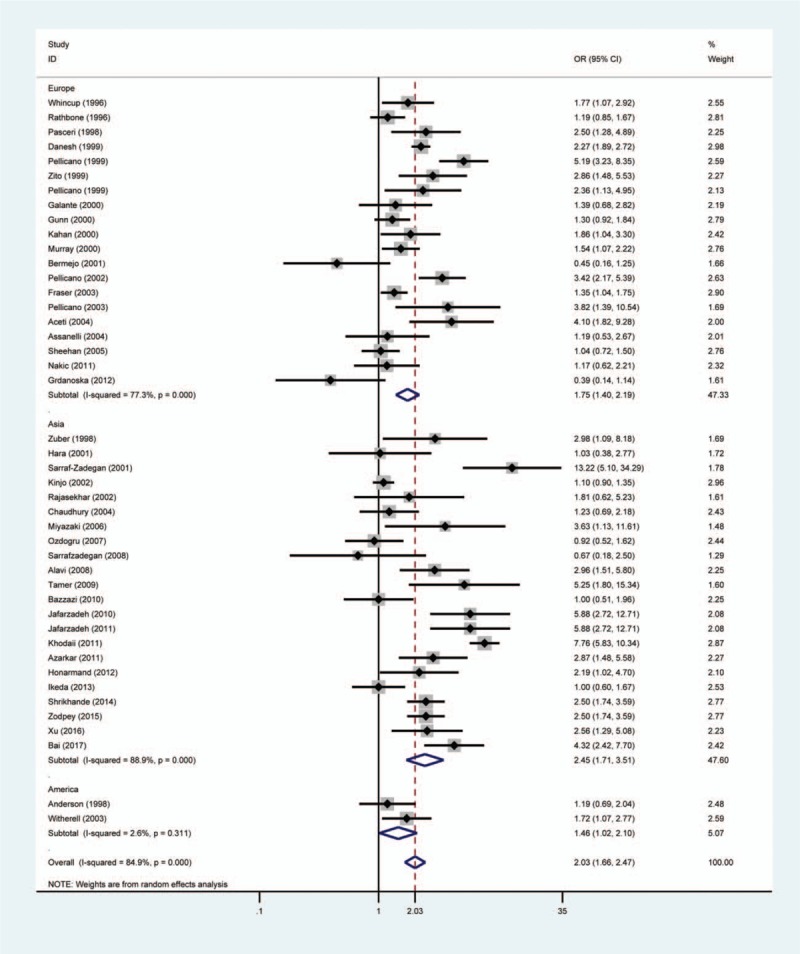
Forest plot of the association between Helicobacter pylori infection and acute coronary syndrome in the subgroup of geographical location. The size of the black square represents the weight of the study in the meta-analysis. The rhombus represents the combined OR. OR = odds ratio.
Figure 5.
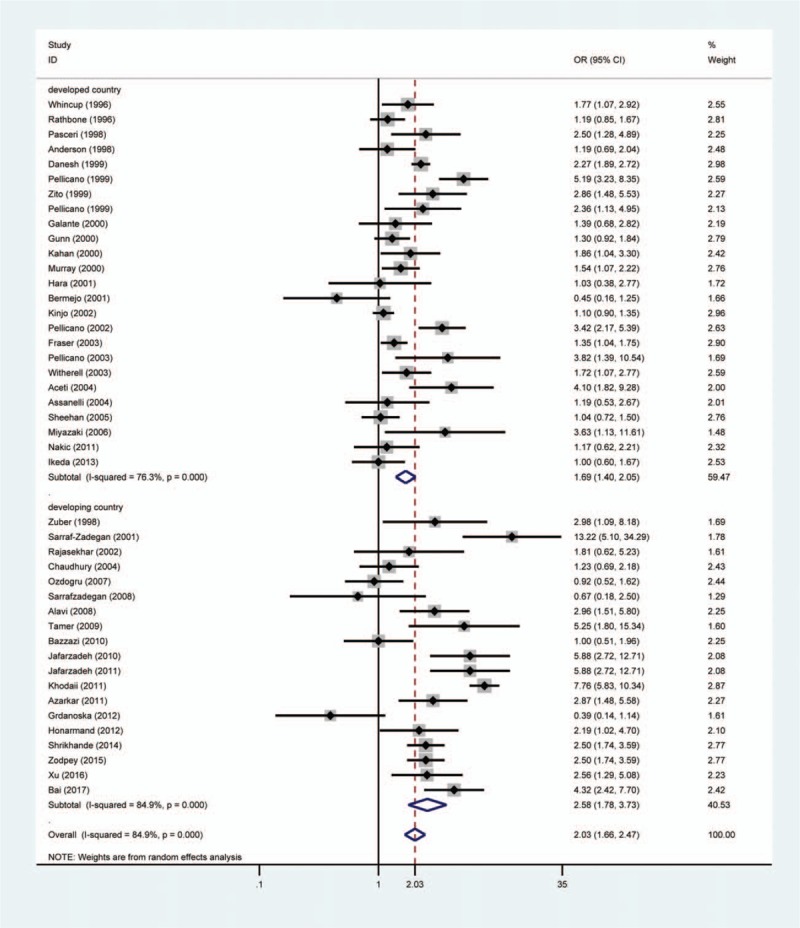
Forest plot of the association between Helicobacter pylori infection and acute coronary syndrome in the subgroup of human development index. The size of the black square represents the weight of the study in the meta-analysis. The rhombus represents the combined OR. OR = odds ratio.
In 8 included studies, researchers focused on the association between CagA-positive strains of H pylori and ACS. A positive relationship was observed in 6 studies; however, 2 other studies revealed that there was no significant relationship between CagA strains infection and ACS. The random pooled result suggested an OR of 2.39 (95% CI 1.21–4.74, PH = .000).
3.4. Sensitivity analysis and publication bias
We detected the effect of individual studies on the pooled OR by sensitivity analysis. The pooled OR was not significantly altered after excluding 1 study at a time. Publication bias was assessed by the Begg funnel plot and Egger test. The results showed that no publication bias could be detected in the meta-analysis (P-value of Egger test:.560, P-value of Begg test:.317). Figure 6 shows the Begg funnel plot.
Figure 6.
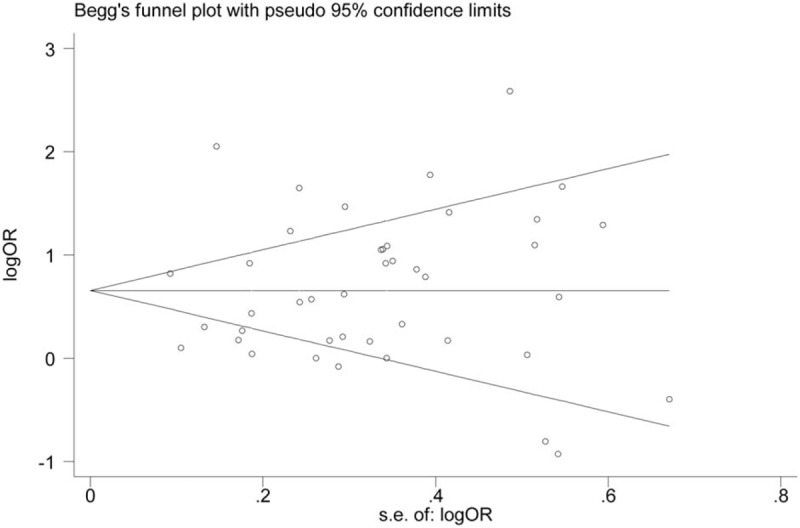
Begg funnel plot showing the publication bias analysis.
4. Discussion
H pylori has been proven to be the main pathopoiesis factor of various gastrointestinal diseases and the prevalence rate of H pylori infection was almost 48.5% around the world. Nowadays, many studies focus on the relationship between H pylori and ACS. However, the relationship of H pylori infection with ACS is still controversial. Our article including 15,833 participants is the first published meta-analysis to identify the association between H pylori and ACS. Based on the results from our meta-analysis, a significant higher prevalence of H pylori infection was found in ACS individuals than controls (64.49% vs 48.03%), which indicated a possible link between ACS and H pylori. The pooled result showed that individuals with H pylori infection have twice the risk of developing ACS. Sensitivity analysis demonstrated that any single study could not significantly influence the pooled result. Thus, all included studies had a good homogeneity and the pooled result was stable. No publication bias was found in this meta-analysis, which indicating that the pooled result was more reliable.
A positive association between H pylori and ACS was found in the high quality subgroup and low-quality subgroup, which was similar to the pooled result. In addition, 77.27% of included studies achieved satisfactory scores in the quality score assessment (≥6 scores) and more than half of studies were high-quality study. Therefore, the meta-analysis gave us reliable results, especially in the high quality subgroup. The meta-analysis demonstrated that the relationship between H pylori and ACS was stronger in developing countries than in developed countries (OR = 2.71 vs OR = 1.65). On one hand, the early poverty life was an important predicting factor of H pylori infection and the global prevalence rate of H pylori is 44.3%, ranging from 50.8% in developing countries to 34.7% in developed countries.[49] One the other hand, H pylori may contribute to the progression of ACS in many ways, such as promoting inflammation reaction, increasing LDL-C levels and so on. Developed countries, with better living standards and medical care, could take effective intervention to reduce H pylori-associated adverse reactions, such as using lipid-lowering medicine, anti-inflammatory drug, and so on, which may weaken the role of H pylori in the progress of ACS. Moreover, the prevalence of H pylori was lower in Europe than in Asia from 1970 to 2006.[50] The subgroup analysis of area showed that the link between ACS and H pylori was weaker in Europe than in Asia. All above further confirmed the positive correlation between H pylori and ACS. The result of breath test and H pylori DNA determination in stool might be affected by the proton pump inhibitors use and antibiotic use. However, the ELISA was used as a diagnosing test for H pylori infection in almost all included studies (95%, 42/44). Thus, we minimized these potential biases and the result of ELISA subgroup was consistent with the pooled result.
It is well known that many potential confounders are considered as risk factors for ACS and H pylori infection, such as age, gender, hypertension, smoking, socio-economic status, and so on. In the meta-analysis, 39 included studies considered the influences of these potential confounders. Thus, another meta-analysis including studies with matched variables was performed. The pooled result also showed a positive association between ACS and H pylori. It was suggested that these potential confounders were equally distributed between ACS patients and control individuals. Therefore, it was indicated that the pooled result was not affected by these confounding factors.
The meta-analysis also proved that the significantly increased risk of ACS could be caused by the CagA-positive strains of H pylori. The bacteria with a cytotoxin-associated protein could become more pathogenic and stimulated a heightened inflammatory response.[51] CagA-positive H pylori could produce more cytokines which were transported in the blood-stream and enhance an inflammatory response in arteries. Furthermore, systemic markers of inflammation and acute phase reactants have been shown to be associated prospectively with ACS risk, and part of the beneficial effects of aspirin on cardiovascular disease risk appeared to be directly related to its anti-inflammatory.[52] Thus, it is possible that CagA-positive H pylori infection may increase the risk of ACS through the induction of a more severe inflammatory response.
H pylori, the only microbial species surviving in the human stomach, is a gram-negative bacteria. H pylori infection is commonly acquired in early age and lasted a life-time. Nowadays, H pylori was identified as a major infectious agent as a vital player in the pathogenesis of ACS and could increase risk of ACS through multiple mechanisms. It is well known that chronic infections are accompanied by a persistent inflammatory response. Several studies reported that H pylori infection could increase serum levels of fibrinogen and inflammatory cytokines, which led to a systemic inflammatory response.[53,54] On one hand, The inflammatory cytokines may contribute to the instability of the plaque by increasing the production of oxide free radical and proteolytic enzymes. On the other hand, the inflammatory cytokines could also promote an inflammatory response in arteries and damage vascular walls.[55,56] The systematic inflammatory response, accompanying with an increase in plasma fibrinogen and inflammatory cytokines, could accelerate the atherosclerotic plaque instability and plaque disruption.[54] In addition, H pylori also could induce lipid peroxidation and oxidized LDL, which played an important role in both of early development and late evolution of atherosclerotic lesions.[57,58]
In the meta-analysis, we could more accurately clarify the relationship between H pylori and ACS with a larger number of samples. We not only improved the inclusion and exclusion criteria, but also evaluated the quality of all included studies. Sensitivity analysis and publication bias were conducted in the meta-analysis, which all suggested that pooled results were stable and reliable. Therefore, our results could most accurately describe the association between ACS and H pylori. However, several limitations should be considered in this meta-analysis. First, it is possible that we may omit several relevant publications though systematic search was performed. Second, Africa is the world's highest H pylori prevalence region (79.1%) in the world.[50] However, no African study was included in the meta-analysis, which may obscure any potential association. Finally, heterogeneity could not be eliminated in our meta-analysis. The diagnostic method in detecting H pylori infection could affect the accuracy and creditability of the data, which may be the source of heterogeneity. Ninety-five percent included studies detected H pylori infection by ELISA; however, different ELISA kits provided different sensitivity and specificity. In the meta-analysis, only 3 included studies used the same ELISA kit to detect H pylori infection.[14,25,48] The pooled result also showed a significant association between H pylori and ACS (OR = 2.27, 95% CI 1.72–4.31, PH = .410) in the same ELISA kit subgroup and the heterogeneity decreased markedly from 84.9% to 0%, which suggested that the detection method of H pylori contributed to the source of heterogeneity. In addition, the accuracy of serological testing for H pylori were descend by the increase of age.[59] Thus, H pylori infection should be detected by a unified method in the future, such as 13C-urea breath test, which may decrease the heterogeneity.
In conclusion, our meta-analysis suggested that H pylori infection was associated with an increased risk of ACS, especially in developing countries. H pylori is easily screened and can be treated with a wide range of drugs. Thus, more high-quality and well-designed studies are needed to confirm whether the treatment of H pylori is an effective way to reduce ACS risk.
Author contributions
Conceptualization: Yizhen Fang, Huabin Xie.
Data curation: Yizhen Fang, Chunming Fan, Huabin Xie.
Formal analysis: Yizhen Fang, Chunming Fan, Huabin Xie.
Investigation: Yizhen Fang, Chunming Fan, Huabin Xie.
Methodology: Yizhen Fang, Chunming Fan, Huabin Xie.
Project administration: Yizhen Fang, Chunming Fan.
Resources: Yizhen Fang, Chunming Fan, Huabin Xie.
Software: Yizhen Fang, Chunming Fan, Huabin Xie.
Supervision: Huabin Xie.
Validation: Huabin Xie.
Visualization: Chunming Fan, Huabin Xie.
Writing – original draft: Yizhen Fang.
Writing – review and editing: Yizhen Fang.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ACS = acute coronary syndrome, CagA = cytotoxin-associated antigen A, ELISA = enzyme-linked immunosorbent assay, Embase = Excerpta Medica Database, HDI = human development index, H pylori = Helicobacter pylori, ORs = odds ratios, UK = United Kingdom.
How to cite this article: Fang Y, Fan C, Xie H. Effect of Helicobacter pylori infection on the risk of acute coronary syndrome: A systematic review and meta-analysis. Medicine. 2019;98:50(e18348).
The authors have no conflicts of interest to disclose.
References
- [1].Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet 1997;350:430–6. [DOI] [PubMed] [Google Scholar]
- [2].Ossei-Gerning N, Moayyedi P, Smith S, et al. Helicobacter pylori infection is related to atheroma in patients undergoing coronary angiography. Cardiovasc Res 1997;35:120–4. [DOI] [PubMed] [Google Scholar]
- [3].Pasceri V, Cammarota G, Patti G, et al. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation 1998;97:1675–9. [DOI] [PubMed] [Google Scholar]
- [4].Aceti A, Mazzacurati G, Amendolea M, et al. Relation of C reactive protein to cardiovascular risk factors. H pylori and C pneumoniae infections may account for most acute coronary syndromes. BMJ 1996;313:428–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moher D, Liberati A, Tetzlaff J, et al. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009;89:873–80. [PubMed] [Google Scholar]
- [6].Sheehan J, Kearney PM, Sullivan SO, et al. Acute coronary syndrome and chronic infection in the Cork coronary care case-control study. Heart 2005;91:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pellicano R, Mazzarello MG, Morelloni S, et al. Acute myocardial infarction and Helicobacter pylori seropositivity. Int J Clin Lab Res 1999;29:141–4. [DOI] [PubMed] [Google Scholar]
- [8].Bai S, Hashmi SFA. Association between Helicobacter pylori infection and acute myocardial infarction (AMI). Pak Heart J 2017;50:33–8. [Google Scholar]
- [9].Jafarzadeh A, Nemati M, Tahmasbi M, et al. The association between infection burden in Iranian patients with acute myocardial infarction and unstable angina. Acta Med Indones 2011;43:105–11. [PubMed] [Google Scholar]
- [10].Khodaii Z, Vakili H, Ghaderiana SMH, et al. Association of Helicobacter pylori infection with acute myocardial infarction. Coron Artery Dis 2011;22:6–11. [DOI] [PubMed] [Google Scholar]
- [11].Shrikhande SN, Zodpey SP, Negandhi H. A case-control study examining association between infectious agents and acute myocardial infarction. Indian J Public Health 2014;58:106–9. [DOI] [PubMed] [Google Scholar]
- [12].Grdanoska T, Zafirovska P, Jaglikovski B, et al. Chlamydia pneumoniae and helicobacter pylori serology - importance in patients with coronary heart disease. Mater Sociomed 2012;24:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ikeda A, Iso H, Sasazuki S, et al. The combination of Helicobacter pylori- and cytotoxin-associated gene-A seropositivity in relation to the risk of myocardial infarction in middle-aged Japanese: the Japan Public Health Center-based study. Atherosclerosis 2013;230:67–72. [DOI] [PubMed] [Google Scholar]
- [14].Xu L, Li G, Chen X, et al. Correlation of Helicobacter pylori infection with serum inflammation reaction in patients with acute myocardial infarction. Prog Mod Biomed 2016;16:2127–9. [Google Scholar]
- [15].Witherell HL, Smith KL, Friedman GD, et al. C-reactive protein, Helicobacter pylori, Chlamydia pneumoniae, cytomegalovirus and risk for myocardial infarction. Ann Epidemiol 2003;13:170–7. [DOI] [PubMed] [Google Scholar]
- [16].Assanelli D, Bonanome A, Grassi M, et al. Determinants of early-onset cardiovascular disease: a case-control study of young myocardial infarction patients. Ital Heart J 2004;5:604–11. [PubMed] [Google Scholar]
- [17].Anderson JL, Carlquist JF, Muhlestein JB, et al. Evaluation of C-reactive protein, an inflammatory marker, and infectious serology as risk factors for coronary artery disease and myocardial infarction. J Am Coll Cardiol 1998;32:35–41. [DOI] [PubMed] [Google Scholar]
- [18].Hara K, Morita Y, Kamihata H, et al. Evidence for infection with Helicobacter pylori in patients with acute myocardial infarction. Clin Chim Acta 2001;313:87–94. [DOI] [PubMed] [Google Scholar]
- [19].Kahan T, Lundman P, Olsson G, et al. Greater than normal prevalence of seropositivity for Helicobacter pylori among patients who have suffered myocardial infarction. Coronary Artery Dis 2000;11:523–6. [DOI] [PubMed] [Google Scholar]
- [20].Aceti A, Are R, Sabino G, et al. Helicobacter pylori active infection in patients with acute coronary heart disease. J Infect 2004;49:8–12. [DOI] [PubMed] [Google Scholar]
- [21].Nakic D, Veev A, Jovic A, et al. Helicobacter Pylori infection and acute myocardial infarction. Collegium Antropologicum 2011;35:781–5. [PubMed] [Google Scholar]
- [22].Danesh J, Youngman L, Clark S, et al. Helicobacter pylori infection and early onset myocardial infarction: case-control and sibling pairs study. Br Med J 1999;319:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zito F, Di Castelnuovo A, D’Orazio A, et al. Helicobacter pylori infection and the risk of myocardial infarction: role of fibrinogen and its genetic control. Thromb Haemost 1999;82:14–8. [PubMed] [Google Scholar]
- [24].Sarraf-Zadegan N, Amiri M, Maghsoudloo S. Helicobacter pylori relation to acute myocardial infarction in an Iranian sample. Coronary Health Care 2001;5:202–7. [Google Scholar]
- [25].Tamer GS, Tengiz I, Ercan E, et al. Helicobacter pylori seropositivity in patients with acute coronary syndromes. Dig Dis Sci 2009;54:1253–6. [DOI] [PubMed] [Google Scholar]
- [26].Pellicano R, Mazzarello MG, Morelloni S, et al. Helicobacter pylori seropositivity in patients with unstable angina. J Cardiovasc Surg 2003;44:605–9. [PubMed] [Google Scholar]
- [27].Rathbone B, Martin D, Stephens J, et al. Helicobacter pylori seropositivity in subjects with acute myocardial infarction. Heart 1996;76:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fraser AG, Scragg RK, Cox B, et al. Helicobacter pylori, Chlamydia pneumoniae and myocardial infarction. Intern Med J 2003;33:267–72. [DOI] [PubMed] [Google Scholar]
- [29].Zuber BF, Shaikh SA, Shaikh WM, et al. IgG antibodies against Helicobacter pylori in patients of myocardial infarction. J Coll Phys Surg Pak 1998;8:249–51. [Google Scholar]
- [30].Pellicano R, Parravicini PP, Bigi R, et al. Infection by Helicobacter pylori and acute myocardial infarction. Do cytotoxic strains make a difference? New Microbiol 2002;25:315–21. [PubMed] [Google Scholar]
- [31].Galante A, Pietroiusti A, Carta S, et al. Infection with Helicobacter pylori and leukocyte response in patients with myocardial infarction. Eur J Clin Microbiol Infect Dis 2000;19:298–300. [DOI] [PubMed] [Google Scholar]
- [32].Murray LJ, Bamford KB, Kee F, et al. Infection with virulent strains of Helicobacter pylori is not associated with ischaemic heart disease: evidence from a population-based case-control study of myocardial infarction. Atherosclerosis 2000;149:379–85. [DOI] [PubMed] [Google Scholar]
- [33].Rajasekhar D, Subramanyam G, Latheef SA, et al. Infectious aetiology in acute coronary syndromes. Indian J Med Microbiol 2002;20:83–7. [PubMed] [Google Scholar]
- [34].Bermejo García J, Martínez Martínez P, Martín Rodríguez JF, et al. Inflammation and infection in stable coronary disease and acute coronary syndrome. Rev Esp Cardiol 2001;54:453–9. [DOI] [PubMed] [Google Scholar]
- [35].Miyazaki M, Babazono A, Kadowaki K, et al. Is Helicobacter pylori infection a risk factor for acute coronary syndromes? J Infect 2006;52:86–91. [DOI] [PubMed] [Google Scholar]
- [36].Pellicano R, Parravicini PP, Bigi R, et al. Patients with acute myocardial infarction in northern Italy are often infected by Helicobacter pylori. Panminerva Med 1999;41:279–82. [PubMed] [Google Scholar]
- [37].Kinjo K, Sato H, Sato H, et al. Prevalence of Helicobacter pylori infection and its link to coronary risk factors in Japanese patients with acute myocardial infarction. Cir J 2002;66:805–10. [DOI] [PubMed] [Google Scholar]
- [38].Sarrafzadegan N, Rezaporian P, Kaypour M, et al. Prognostic value of infection and inflammation markers for late cardiac events in an Iranian sample. Eastern Mediterranean Health J 2008;14:1246–56. [PubMed] [Google Scholar]
- [39].Whincup PH, Mendall MA, Perry IJ, et al. Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart 1996;75:568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ozdogru I, Kalay N, Dogan A, et al. The relationship between Helicobacter pylori IgG titre and coronary atherosclerosis. Acta Cardiol 2007;62:501–5. [DOI] [PubMed] [Google Scholar]
- [41].Azarkar Z, Jafarnejad M, Sharifzadeh G. The relationship between Helicobacter pylori infection and myocardial infarction. Caspian J Intern Med 2011;2:222–5. [PMC free article] [PubMed] [Google Scholar]
- [42].Alavi SM, Adel SMH, Rajabzadeh A. Relationship between the serologic status of helicobacter pylori with the presence of unstable angina. Pak J Med Sci 2008;24:29–32. [Google Scholar]
- [43].Zodpey SP, Shrikhande SN, Negandhi HN, et al. Risk factors for acute myocardial infarction in central India: a case-control study. Indian J Community Med 2015;40:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bazzazi H, Ghaemi EA, Ramezani MA. The seroepidemiology of the chronic infections in patients with myocardial infarction in North of Iran. J Res Med Sci 2010;15:116–9. [PMC free article] [PubMed] [Google Scholar]
- [45].Chaudhury A, Rajasekhar D, Latheef SA, et al. Seroprevalence of IgG antibodies to Chlamydia pneumoniae and Helicobacter pylori among coronary heart disease patients and normal individuals in South Indian population. Indian J Pathol Microbiol 2004;47:433–4. [PubMed] [Google Scholar]
- [46].Jafarzadeh A, Esmaeeli-Nadimi A, Nemati M, et al. Serum concentrations of Helicobacter pylori IgG and the virulence factor CagA in patients with ischaemic heart disease. Eastern Mediterranean Health J 2010;16:1039–44. [PubMed] [Google Scholar]
- [47].Gunn M, Stephens JC, Thompson JR, et al. Significant association of cagA positive Helicobacter pylori strains with risk of premature myocardial infarction. Heart 2000;84:267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Honarmand H, Mirzajani E, Mirbolouk F, et al. Study on persistent bacterial and viral infections as risk factors for myocardial infarction. Ann Biol Res 2012;3:5612–5. [Google Scholar]
- [49].Zamani M, Ebrahimtabar F. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–76. [DOI] [PubMed] [Google Scholar]
- [50].Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–9. [DOI] [PubMed] [Google Scholar]
- [51].Xiang Z, Censini S, Bayeli PF, et al. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun 1995;63:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9. [DOI] [PubMed] [Google Scholar]
- [53].Torgano G, Cosentini R, Mandelli C, et al. Treatment of Helicobacter pylori and Chlamydia pneumoniae infections decreases fibrinogen plasma level in patients with ischemic heart disease. Circulation 1999;99:1555–9. [DOI] [PubMed] [Google Scholar]
- [54].Patel P, Carrington D, Strachan DP, et al. Fibrinogen: a link between chronic infection and coronary heart disease. Lancet 1994;343:1634–5. [DOI] [PubMed] [Google Scholar]
- [55].Pober JS. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol 1988;133:426–33. [PMC free article] [PubMed] [Google Scholar]
- [56].Crabtree JE, Covacci A, Farmery SM, et al. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol 1995;48:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fan XG, Yakoob J, Chua A, et al. Helicobacter pylori-induced lipid peroxidation in peripheral blood lymphocytes. APMIS 1995;103:316–9. [PubMed] [Google Scholar]
- [58].Stemme S, Faber B, Holm J, et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A 1995;92:3893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schembri MA, Lin SK, Lambert JR. Comparison of commercial diagnostic tests for Helicobacter pylori antibodies. J Clin Microbiol 1993;31:2621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


