Abstract
Background:
There currently exists no substantial evidence reporting the efficacy of peritoneal irrigation in reducing the incidence of postoperative intra-abdominal abscess in pediatric patients. The purpose of our study was to perform a meta-analysis to compare rates of intra-abdominal abscess after appendectomy between irrigation and suction alone groups.
Methods:
We identified studies by a systematic search in EMBASE, PubMed, Web of Science, and the Cochrane Library to recognize randomized controlled trials and case control studies from the 1950 to May 2019. We limited the English language studies. We checked the reference list of studies to recognize other potentially qualified trials. We analyzed the merged data with use of the Review Manager 5.3.
Results:
We identified 6 eligible papers enrolling a total of 1633 participants. We found no significant difference in the incidence of postoperative intraabdominal abscess, wound infection, and the length of hospitalization between 2 group, but duration of surgery is longer in irrigation group (MD = 6.76, 95% CI = 4.64 to 8.87, P < .001; heterogeneity, I2 = 25%, P = .26).
Conclusion:
Our meta-analysis did not provide strong evidence allowing definite conclusions to be drawn, but suggested that peritoneal irrigation during appendectomy did not decrease the incidence of postoperative IAA. This meta-analysis also indicated the need for more high-quality trials to identify methods to decrease the incidence of postoperative IAA in pediatric perforated appendicitis patients.
Trial registration number Standardization of endoscopic treatment of acute abdomen in children: 14RCGFSY00150
Keywords: meta-analysis, peritoneal irrigation, pediatric, suction
1. Introduction
In recent years, the incidence of perforated appendicitis in the general pediatric population has been reported to range from 15% to 20%.[1] Perforated appendicitis is a major risk factor for postoperative intra-abdominal abscess (IAA) formation, and approximately 5% to 10% of children with perforated appendicitis develop a postoperative IAA.[2–10] Postoperative IAA formation results in abdominal discomfort and may necessitate a drainage procedure or secondary surgery. In order to decrease the incidence of postoperative IAA, many surgeons have attempted to improve the surgical technique and perioperative management of pediatric appendectomy.
In 1906, Torek and his colleagues performed peritoneal irrigation (PI) with saline solution in pediatric patients with perforated appendicitis to decrease the incidence of postoperative IAA. Ever since, the use of PI has been accepted by the majority of surgeons.[11] Several articles have shown that the use of PI decreases the rate of postoperative IAA in adult perforated appendicitis patients. However, there currently exists no substantial evidence reporting the efficacy of PI in reducing the incidence of postoperative IAA in pediatric patients.[12,13] Rather, the use of PI is supported by its hypothesized underlying mechanism: if the bacterial load is diluted, the patient is less likely to develop peritonitis and the postoperative course will be milder. More recently, however, studies have suggested that the use of PI during appendectomy does not decrease the rate of postoperative IAA.[14–19] Indeed, recent meta-analyses have reported a similar incidence of postoperative IAA between PI and suction alone (SA) during appendectomy in both adult and pediatric patients.[20–22] PI may not wash off bacteria from the mesothelial surfaces, so the effect of reducing the population density of bacteria may be temporary.[14] Given this finding, it is important to identify whether it is clinically significant to perform PI during appendectomy in pediatric perforated appendicitis patients.
Thus, we performed this meta-analysis to compare the incidence of postoperative IAA in pediatric patients following appendectomy with PI and appendectomy with SA.
2. Methods
We conducted a meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards.[23]
2.1. Literature search strategy
Two researchers independently performed a systematic search of electronic databases including EMBASE, PubMed, Web of Science, and the Cochrane Library to identify randomized controlled trials (RCTs) and case control studies (CCSs) on the use of PI in appendectomy for pediatric perforated appendicitis published from 1950 to May 2019. A structured search strategy in combination with Boolean logic was employed: (children OR child OR pediatric) AND (ruptured appendicitis OR perforated appendicitis) AND (peritoneal irrigation OR peritoneal lavage). In addition, the reference list of each included study was reviewed to identify other relevant trials. The process was completed when no further studies could be identified. In cases where a study had multiple publications, the most recently published or most relevant study was included in this meta-analysis.
2.2. Inclusion and exclusion criteria
We included all RCTs and CCSs which met the following inclusion criteria:
-
(1)
population: children with acute perforated appendicitis who underwent appendectomy;
-
(2)
intervention: PI in appendectomy;
-
(3)
comparison intervention: SA in appendectomy;
-
(4)
outcomes (studies were required to include more than one of these): IAA incidence, wound infection incidence, length of hospitalization, and duration of surgery.
The following exclusion criteria were applied:
-
(1)
studies not published in English;
-
(2)
animal studies;
-
(3)
studies that involved adults and patients with other diseases, such as appendix tumors.
Two authors independently evaluated all the articles of all identified studies which met these criteria. The ethics committee review is not required.
2.3. Data extraction and risk of bias assessment
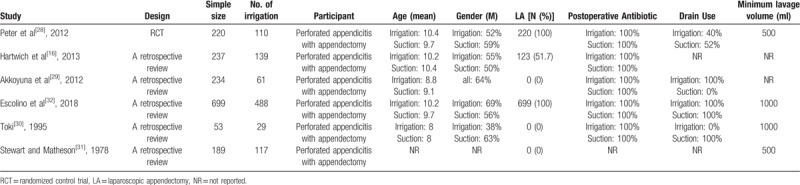
Two authors independently reviewed the full texts of all included studies. The following necessary information was extracted from each study: the first author's name, publication year, study design, and sample size. The other extracted information included demographic characteristics, diagnostic measurements, and treatment regimens. A standardized table was constructed, into which the extracted data were input. Two authors independently conducted data collection and disagreements were resolved by discussion. All extracted data are presented in Table 1.
Table 1.
Baseline characteristics of included studies and included population.
Two authors independently evaluated the methodological quality and risk of bias of the included RCTs and CCSs, using the Cochrane Tool[24] and the Newcastle-Ottawa Quality Assessment Scale (NOS),[25] respectively. The maximum total score of the NOS was 9 stars. Studies that scored 4 stars or less were considered low-quality, whereas those scoring 5 stars or more were considered high-quality. Any disagreements between the 2 authors were resolved by discussion.
2.4. Outcomes
The first treatment-related outcome of interest was the incidence of IAA, which we defined as the detection of an IAA by an abdominal ultrasound scan. Wound infection was defined by purulent discharge from the incision and the growth of microorganisms from the wound discharge.[26] The other treatment-related outcomes were length of hospital stay and duration of surgery.
2.5. Data synthesis and analysis
For continuous data (length of hospitalization and duration of surgery), mean differences (MDs) and 95% confidence interval (CIs) were calculated. For dichotomous outcome variables (IAA and wound infection incidences), odds ratios (ORs) and 95% CIs were calculated. All analyses were conducted using Review Manager 5.3. A P value <.05 was considered statistically significant. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0),[27] when there was evidence of significant heterogeneity (I2 > 50%), random-effects models were used; otherwise, fixed-effects models were used for analysis.
3. Results
3.1. Search results
We obtained a total of 199 records from the four databases (PubMed, n = 79; EMBASE, n = 47; Web of Science, n = 42; and the Cochrane Library, n = 31) for the first screening (Fig. 1). After screening the titles and abstracts of these articles, 13 articles fitting our criteria were identified. After the full texts of these 13 articles were reviewed, another 7 articles were excluded for various reasons (data unavailable, duplicate article, or patients with other relevant diseases). Finally, 6 trials were included in this meta-analysis.[16,28–32] These trials included a total of 1633 individuals: 944 underwent appendectomy with PI and 689 underwent appendectomy with SA. The general trial parameters, demographic characteristics, diagnostic measurements, treatment regimens, and outcomes of the included trials are shown in Table 1.
Figure 1.
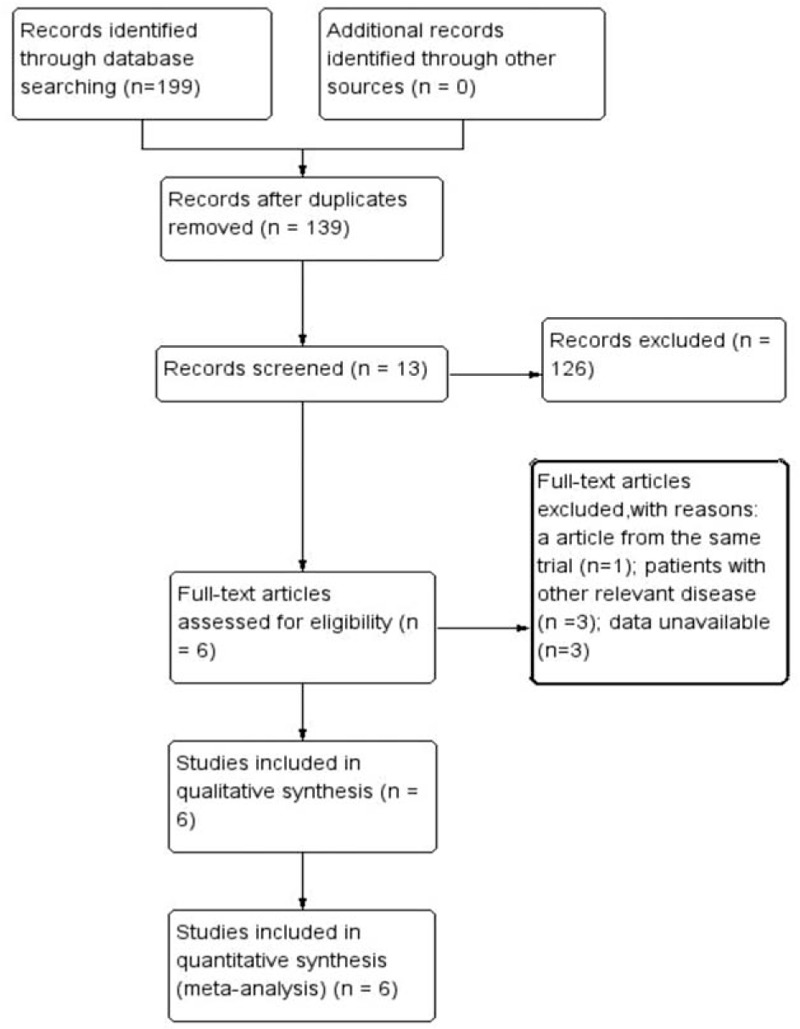
Flowchart of the study selection process.
3.2. Study quality and risk of bias
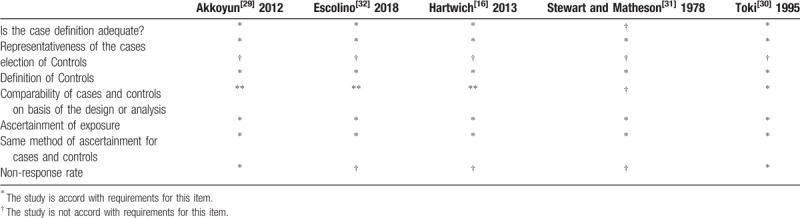
The quality of the included RCTs was evaluated by the Cochrane Tool, and the results are shown in Fig. 2. The CCSs were assessed by the NOS, and the calculated scores are summarized in Table 2. Each CCS scored more than 5 stars, so all CCSs included in this meta-analysis were considered to be of high quality.
Figure 2.
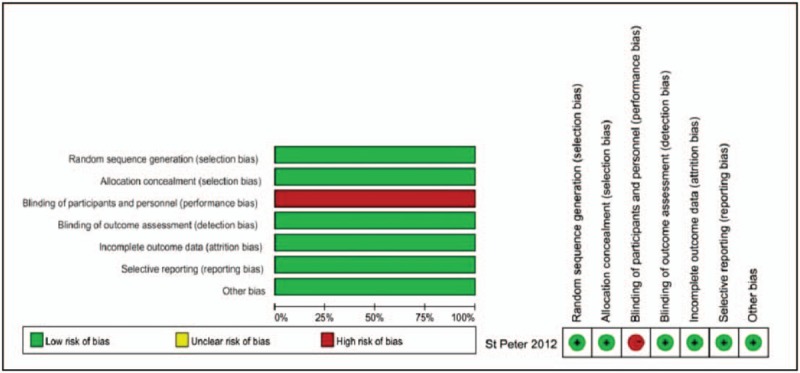
Risk of bias summary and graph for showing each risk of bias item for the randomization trial.
Table 2.
Quality assessment of case control studies.
3.3. Outcomes
3.3.1. Intraabdominal abscess incidence
All of the included publications reported outcomes related to the incidence of IAA (Fig. 3). Therefore, the incidence of IAA was compared between 944 patients in the PI group and 689 in the SA group. We found no statistical difference in the incidence of IAA between the 2 groups (PI:93/944, OA:77/689; OR = 1.03, 95% CI = 0.41–2.58, P = .96). High heterogeneity was noted, so a random-effects model was used (I2 = 80%, P < .05).
Figure 3.
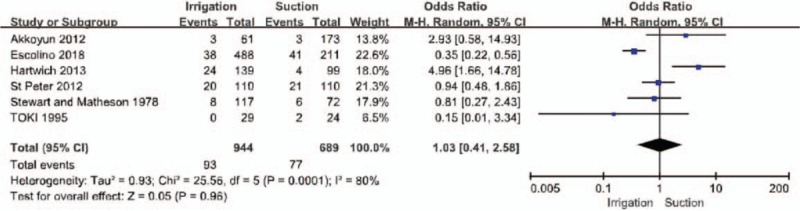
Forest plot regarding intra-abdominal abscess incidence among the groups of patients with irrigation and suctions vs those with suction alone.
3.3.2. Wound infection incidence
Five of the included publications reported outcomes related to the incidence of wound infection (Fig. 4).[16,29–32] Therefore, the incidence of wound infection was compared between 834 patients in the PI group and 579 in the SA group. We found no statistical difference in the incidence of wound infection between the 2 groups (PI:44/834, SA:35/579; OR = 0.97, 95% CI = 0.30–3.16, P = .96). High heterogeneity was noted, so a random-effects model was used (I2 = 71%, P < .05).
Figure 4.
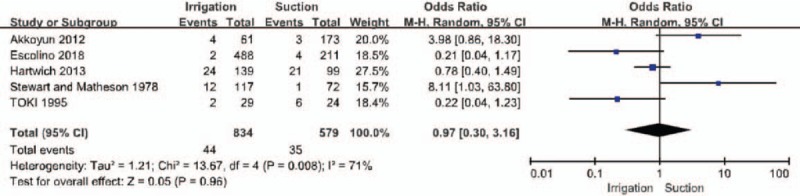
Forest plot regarding wound infection incidence among the groups of patients with irrigation and suctions vs those with suction alone.
3.3.3. Length of hospital stay
Five of the included publications reported outcomes related to length of hospital stay (Fig. 5).[28–32] There was no statistical difference in the length of hospitalization between the 2 groups (MD = −0.90, 95% CI = −3.28 to 1.48, P = .46). Significant heterogeneity was noted, so a random-effects model was used (I2 = 93%, P < .05).
Figure 5.
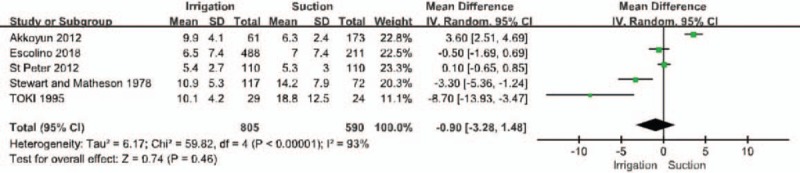
Forest plot regarding the length of hospital stay among the groups of patients with irrigation and suctions vs those with suction alone.
3.3.4. Duration of surgery
Three of the included publications reported outcomes related to duration of surgery (Fig. 6).[28,29,32] Duration of surgery was found to be significantly longer in the PI group than in the SA group (MD = 6.76, 95% CI = 4.64–8.87, P < .001). Obvious heterogeneity was noted, so a fixed-effects model was used (I2 = 25%, P = .26).
Figure 6.
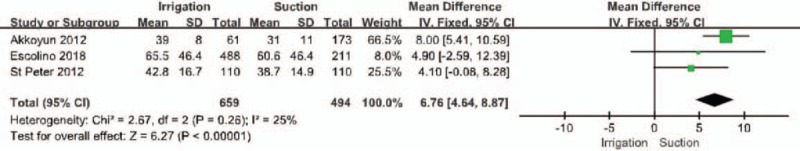
Forest plot regarding duration of surgery among the groups of patients with irrigation and suctions vs those with suction alone.
4. Discussion
Acute perforated appendicitis is the common surgical diagnosis that may lead to peritonitis in children. There is a high incidence of postoperative IAA in perforated appendicitis which could be influenced by differences in clinical management.[33,34] Postoperative IAA is mainly the most frequent and devastating complication.[15] It is associated with postoperative pain, slower intestinal function recovery, longer length of hospitalization and increasing cost even repeat intervention. A number of strategies are currently employed to avoid postoperative IAA formation, namely the perioperative administration of antibiotics, postoperative drainage, and intraoperative PI.
This analysis included 1 RCT and 5 retrospective observational trials involving a total of 1633 patients. This meta-analysis found no statistical differences between intraoperative PI and SA with respect to postoperative IAA incidence, postoperative wound infection incidence, or length of hospitalization. A significantly increased duration of surgery was noted in the PI group.
A similar postoperative IAA incidence was found between PI and SA in our study, consistent with articles published by Peter et al and Akkoyun et al on perforated appendicitis.[28,29] In contrast, Hartwich et al reported a postoperative IAA incidence of 17.2% in the PI group and 4% in the SA group, demonstrating a clear statistical difference.[16] In a survey of American Pediatric Surgical Association members, almost all surgeons reported that they perform intraoperative PI with saline solution during pediatric appendectomy.[35] This is because PI is cheap, easy to perform and may avoid postoperative IAA formation. However, our results suggest that in cases of perforated appendicitis, the use of PI did not decrease the incidence of postoperative IAA formation. PI may possibly spread the infected contents of the appendix and bacteria throughout the abdominal cavity, explaining our observed results. Unfortunately, the mechanisms underlying postoperative IAA formation remain unclear. In a 2013 article, Peter and Holcomb summarized why PI may not be effective:
-
(1)
bacteria adhere to the peritoneal mesothelial cells, so the micro-organism load on the peritoneum is not decreased by irrigation;
-
(2)
irrigation may cause diffuse or remote inoculation, thus spreading the pollution; and
-
(3)
irrigation may dilute mediators of phagocytosis (opsonic proteins and immunoglobulins).[14]
In contrast, however, in their international multicentric study, Escolino et al[32] demonstrated that the use of PI was associated with a lower incidence of IAA compared with SA in pediatric perforated appendicitis patients.
Additionally, Peter et al reported that irrigation with a moderate volume of saline did not improve the rate of postoperative IAA in perforated appendicitis patients.[28] They explained that while saline might lyse and dilute many of the intra-abdominal bacteria, it also displaces some bacteria and intestinal contents throughout the peritoneal cavity, potentially providing a nidus for future abscess development. Furthermore, Hartwich et al[16] suggested that saline could be more harmful to the patient's own defense mechanisms than to bacteria. Our results, along with those of other previously published articles, therefore cautiously suggest that the routine practice of intraoperative PI with saline should not be recommended.
We also found no statistically significant differences between PI and SA with respect to wound infection incidence and length of hospitalization, consistent with the findings of Peter et al[28], Akkoyun et al[29], and Escolino et al.[32] In contrast, a trial by Hartwich et al[16] reported that PI decreased the incidence of wound infection.
Optimizing surgery duration may have a dramatic impact on healthcare cost-effectiveness outcomes. In this meta-analysis, we reported a longer duration of surgery in patients treated by appendectomy with PI. An additional three studies have similarly reported longer operative times when PI is used in the treatment of perforated appendicitis.[28,29,32] However, a recent meta-analysis which separately analyzed pediatric patients failed to report a difference in operative time when PI was used.[20] Although the difference in operative time noted in this meta-analysis was quite small and thus has questionable clinical relevance, it may imply that the use of PI in appendectomy unnecessarily utilizes resources and increases the cost of care with no clinical benefit.
The volume of PI, drainage, and antibiotic treatment regimens are other clinical variables which may have influenced the incidence of postoperative IAA in the included studies. The volume of PI used varied greatly among the included studies, which may have influenced the results.[36] Unfortunately, we were unable to conduct a separate analysis of the data according to the volume of PI used In addition, some of the pediatric surgeons conducted postoperative drainage, which may also have influenced the incidence of postoperative IAA. Escolino et al reported that the use of drainage was not associated with an increased incidence of postoperative IAA.[32] However, other studies reported that drainage increased the incidence of postoperative IAA.[37–40] With respect to antibiotic treatment, different surgeons exhibited differences in the number and type of antibiotics used, in addition to the length of antibiotic therapy.[41] Many pediatric surgeons tailored the antibiotic treatment regimen to the individual patient. In this meta-analysis, we failed to standardize the utilization of antibiotics, although the demographics were comparable between the two groups. Henry et al conducted a case-control study and reported that the type and timing of preoperative antibiotics and the length of postoperative antibiotics were not associated with postoperative IAA formation.[42] Similarly, Snow et al assessed the influence of peri- and postoperative antibiotics on postoperative IAA development in children, and reported that neither the number and type of antibiotics nor the length of therapy had a significant impact on the incidence of postoperative IAA.[12] If possible, however, any study into postoperative IAA should control these clinically relevant variables.
Our study has a number of limitations:
-
(1)
this meta-analysis included only 6 eligible studies, of which 5 were non-randomized retrospective observational studies;
-
(2)
the number of patients in our analysis was relatively small; and
-
(3)
a high level of among-study heterogeneity was noted, which may have influenced the robustness of the results.
5. Conclusions
Our meta-analysis did not provide strong evidence allowing definite conclusions to be drawn, but suggested that PI during appendectomy did not decrease the incidence of postoperative IAA. This meta-analysis also indicated the need for more high-quality trials to identify methods to decrease the incidence of postoperative IAA in pediatric perforated appendicitis patients.
Acknowledgments
We wish to thank Tianjin Medical University for providing us with databases.
Author contributions
Conceptualization: Le-wee Bi, Bei-lei Yan.
Data curation: Le-wee Bi, Bei-lei Yan, Qian-Yu Yang.
Methodology: Le-wee Bi, Qian-Yu Yang, Hua-lei Cui.
Supervision: Bei-lei Yan, Hua-lei Cui.
Validation: Hua-lei Cui.
Writing – original draft: Le-wee Bi, Bei-lei Yan.
Writing – review & editing: Qian-Yu Yang, Hua-lei Cui.
Footnotes
Abbreviations: CCS = case control study, CI = confidence intervals, IAA = intra-abdominal abscess, MD = mean difference, NOS = Newcastle-Ottawa Quality Assessment Scale, OR = odds ratio, PI = peritoneal irrigation, RCT = randomized controlled trial, SA = suction alone.
How to cite this article: Bi LW, Yan BL, Yang QY, Cui HL. Peritoneal irrigation vs suction alone during pediatric appendectomy for perforated appendicitis: a meta-analysis. Medicine. 2019;98:50(e18047).
LWB and BLY contributed equally to this work.
The authors have no conflicts of interests to disclose.
References
- [1].Levin DE, Pegoli W., Jr Abscess after appendectomy: predisposing factors. Adv Surg 2015;49:263–80. [DOI] [PubMed] [Google Scholar]
- [2].Ingraham AM, Cohen ME, Bilimoria KY, et al. Comparison of outcomes after laparoscopic versus open appendectomy for acute appendicitis at 222 ACS NSQIP hospitals. Surgery 2010;148:625–37. [DOI] [PubMed] [Google Scholar]
- [3].Masoomi H, Nguyen NT, Dolich MO, et al. Laparoscopic appendectomy trends and outcomes in the United States: data from the Nationwide Inpatient Sample (NIS), 2004-2011. Am Surg 2014;80:1074–7. [PubMed] [Google Scholar]
- [4].Drake FT, Mottey NE, Farrokhi ET, et al. Time to appendectomy and risk of perforation in acute appendicitis. JAMA Surg 2014;149:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Markar SR, Blackburn S, Cobb R, et al. Laparoscopic versus open appendectomy for complicated and uncomplicated appendicitis in children. J Gastrointest Surg 2012;16:1993–2004. [DOI] [PubMed] [Google Scholar]
- [6].Katkhouda N, Friedlander MH, Grant SW, et al. Intraabdominal abscess rate after laparoscopic appendectomy. Am J Surg 2000;180:456–61. [DOI] [PubMed] [Google Scholar]
- [7].Cueto J, D’Allemagne B, Vázquez-Frias JA, et al. Morbidity of laparoscopic surgery for complicated appendicitis: an international study. Surg Endosc 2006;20:717–20. [DOI] [PubMed] [Google Scholar]
- [8].Kouwenhoven EA, Repelaer van Driel OJ, van Erp WFM. Fear for the intraabdominal abscess after laparoscopic appendectomy: not realistic. Surg Endosc 2005;19:923–6. [DOI] [PubMed] [Google Scholar]
- [9].Romano A, Parikh P, Byers P, et al. Simple acute appendicitis versus non-perforated gangrenous appendicitis: is there a difference in the rate of post-operative infectious complications? Surg Infect 2014;15:517–20. [DOI] [PubMed] [Google Scholar]
- [10].van Rossem CC, Schreinemacher MHF, Treskes K, et al. Duration of antibiotic treatment after appendicectomy for acute complicated appendicitis. Br J Surg 2014;101:715–9. [DOI] [PubMed] [Google Scholar]
- [11].Hotchkiss LWV. The treatment of diffuse suppurative peritonitis, following appendicitis. Ann Surg 1906;44:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Snow HA, Choi JM, Cheng MWH, et al. Irrigation versus suction alone during laparoscopic appendectomy; a randomized controlled equivalence trial. Int J Surg 2016;28:91–6. [DOI] [PubMed] [Google Scholar]
- [13].Sun F, Wang H, Zhang F, et al. Copious irrigation versus suction alone during laparoscopic appendectomy for complicated appendicitis in adults. J Investig Surg 2018;31:342–6. Aug. [DOI] [PubMed] [Google Scholar]
- [14].St Peter SD, Holcomb GW., 3rd Should peritoneal lavage be used with suction during laparoscopic appendectomy for perforated appendicitis? Adv Surg 2013;47:111–8. [DOI] [PubMed] [Google Scholar]
- [15].Gupta R, Sample C, Bamehriz F, et al. Infectious complications following laparoscopic appendectomy. Can J Surg 2006;49:397–400. [PMC free article] [PubMed] [Google Scholar]
- [16].Hartwich JE, Carter RF, Wolfe L, et al. The effects of irrigation on outcomes in cases of perforated appendicitis in children. J Surg Res 2013;180:222–5. [DOI] [PubMed] [Google Scholar]
- [17].Cho J, Park I, Lee D, et al. Risk factors for postoperative intra-abdominal abscess after laparoscopic appendectomy: analysis for consecutive 1,817 Experiences. Dig Surg 2015;32:375–81. [DOI] [PubMed] [Google Scholar]
- [18].Mogilner JG, Slijper N, Kandelis E, et al. The management of pediatric appendicitis: an opinion survey of Israeli pediatric surgeons. Harefuah 2007;146:414–503. [PubMed] [Google Scholar]
- [19].Moore CB, Smith RS, Herbertson R, et al. Does use of intraoperative irrigation with open or laparoscopic appendectomy reduce post-operative intra-abdominal abscess? Am Surg 2011;77:78–80. [PubMed] [Google Scholar]
- [20].Hajibandeh S, Hajibandeh S, Kelly A, et al. Irrigation versus suction alone in laparoscopic appendectomy: is dilution the solution to pollution? A systematic review and meta-Analysis. Surg Innov 2018;25:174–82. [DOI] [PubMed] [Google Scholar]
- [21].Gammeri E, Petrinic T, Bond-Smith G, et al. Meta-analysis of peritoneal lavage in appendicectomy. BJS Open 2019;3:24–30. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Siotos C, Stergios K, Prasath V, et al. Irrigation versus suction in laparoscopic appendectomy for complicated appendicitis: a meta-analysis. J Surg Res 2019;235:237–43. [DOI] [PubMed] [Google Scholar]
- [23].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [26].Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–8. Oct. [PubMed] [Google Scholar]
- [27]. Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane collaboration. https://training.cochrane.org/handbook. Accessed 20 July 2019. [Google Scholar]
- [28].St Peter SD, Adibe OO, Iqbal CW, et al. Irrigation versus suction alone during laparoscopic appendectomy for perforated appendicitis: a prospective randomized trial. Ann Surg 2012;256:581–5. [DOI] [PubMed] [Google Scholar]
- [29].Akkoyun I, Tuna AT. Advantages of abandoning abdominal cavity irrigation and drainage in operations performed on children with perforated appendicitis. J Pediatric Surg 2012;47:1886–90. [DOI] [PubMed] [Google Scholar]
- [30].Toki A, Ogura K, Horimi T, et al. Peritoneal lavage versus drainage for perforated appendicitis in children. Surg Today 1995;25:207–10. [DOI] [PubMed] [Google Scholar]
- [31].Stewart DJ, Matheson NA. Peritoneal lavage in appendicular peritonitis. Br J Surg 1978;65:54–6. Jan. [DOI] [PubMed] [Google Scholar]
- [32].Escolino M, Becmeur F, Saxena A, et al. Infectious complications after laparoscopic appendectomy in pediatric patients with perforated appendicitis: is there a difference in the outcome using irrigation and suction versus suction only? Results of a multicentric International retrospective study. J Laparoendosc Adv Surg Tech 2018;28:1266–70. Oct. [DOI] [PubMed] [Google Scholar]
- [33].Fishman SJ, Pelosi L, Klavon SL, et al. Perforated appendicitis: prospective outcome analysis for 150 children. J Pediatric Surg 2000;35:923–6. [DOI] [PubMed] [Google Scholar]
- [34].Morrow SE, Newman KD. Current management of appendicitis. Semin Pediatric Surg 2007;16:34–40. [DOI] [PubMed] [Google Scholar]
- [35].Muehlstedt SG, Pham TQ, Schmeling DJ. The management of pediatric appendicitis: a survey of North American Pediatric Surgeons. J Pediatric Surg 2004;39:875–9. [DOI] [PubMed] [Google Scholar]
- [36].Platell C, Papadimitriou JM, Hall JC. The influence of lavage on peritonitis. J Am Coll Surg 2000;191:672–80. [DOI] [PubMed] [Google Scholar]
- [37].Castagnetti M, Cimador M, De Grazia E. Duodenal perforation due to an abdominal drain placed after appendectomy in a child. Med Surg Pediatrics 2008;30:99–101. Mar-Apr. [PubMed] [Google Scholar]
- [38].Carneiro HA, Mavrakis A, Mylonakis E. Candida peritonitis: an update on the latest research and treatments. World J Surg 2011;35:2650–9. [DOI] [PubMed] [Google Scholar]
- [39].Narci A, Karaman I, Karaman A, et al. Is peritoneal drainage necessary in childhood perforated appendicitis? -- A comparative study. J Pediatric Surg 2007;42:1864–8. [DOI] [PubMed] [Google Scholar]
- [40].Ezer A, Törer N, Calişkan K, et al. Perfore apandisit ameliyatinda dren kullaniminin komplikasyonlara etkisi. Ulusal travma ve acil cerrahi dergisi = Turk J Trauma Emerg Surg 2010;16:427–32. [PubMed] [Google Scholar]
- [41].St Peter SD, Tsao K, Spilde TL, et al. Single daily dosing ceftriaxone and metronidazole vs standard triple antibiotic regimen for perforated appendicitis in children: a prospective randomized trial. J Pediatric Surg 2008;43:981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Henry MCW, Walker A, Silverman BL, et al. Risk factors for the development of abdominal abscess following operation for perforated appendicitis in children: a multicenter case-control study. Arch Surg (Chicago, Ill : 1960) 2007;142:236–41. [DOI] [PubMed] [Google Scholar]


