Abstract
We aimed to investigate the association between excess body mass index (BMI) and papillary thyroid cancer (PTC) in an operative population, and the impact of higher BMI on clinicopathological aggressiveness of PTC.
Charts of 10,844 consecutive patients with thyroid nodules undergoing partial or total thyroidectomy between 1993 and 2015 were reviewed. Patients diagnosed with PTC were stratified in 4 groups: BMI < 18.5 (underweight), 18.5 ≤ BMI < 24 (normal-weight), 24 ≤ BMI < 28 (overweight) and BMI ≥ 28(obese). The impacts of high BMI on prevalence and clinicopathological parameters of PTC were retrospectively analyzed in both univariate and multivariate binary logistic regression analysis.
For every 5-unit increase in body mass, the odds of risk-adjusted malignance increased by 36.6%. The individuals who were obese and overweight were associated with high risk of thyroid cancer [odds ratio (OR)= 1.982, P < .001; OR= 1.377, P < .001; respectively] compared to normal weight patients, and this positive association was found in both genders. Obesity was independent predictors for tumors larger than 1 cm (OR = 1.562, P < .001) and multifocality (OR = 1.616, P < .001). However, there was no difference in cervical lymph node (LN) metastasis among BMI groups. Crude analysis showed BMI was associated with advanced tumor-node-metastasis (TNM) stage (relative risk, approximately 1.23 per 5 BMI units, P < .001), but this association disappeared after adjusting for confounding factors.
Obesity was significantly associated with the risk of PTC in a large, operative population. Higher BMI was significantly associated with larger tumor size and multifocal tumor.
Keywords: body mass index, cancer, obesity, thyroid cancer
1. Introduction
Thyroid cancer incidence increased substantially in many countries over the last decade, driven largely by increases in papillary thyroid cancer (PTC). In the United States, the incidence of thyroid cancer has tripled over the past 30 years and increased 3% annually from 1974 to 2013.[1] In China, the incidence increased by 181.86% from 1990 to 2013.[2] The precise mechanism remains incompletely defined. Some reported that the widespread use of ultrasonography and ultrasound-guided biopsy was likely to have a significant impact on increasing detection of smaller tumors. Nevertheless, this does not sufficiently clarify the soaring incidence as larger-size tumors also have increased in incidence. The only clearly identifiable contributor hitherto is radiation exposure.[3] It has been demonstrated that obesity contributes to an increase risk of many carcinomas, such as esophageal adenocarcinoma, colon and rectal cancer, pancreatic, kidney cancer and endometrial cancer, breast cancer.[4] Obesity has been a worldwide health concern as the prevalence tripled (from 3.2% to 10.8%) in men and doubled in women (from 6.4% to 14.9%) between 1975 and 2014 according to a meta-analysis with 19.2 million participants.[5] In China, the prevalence of overweight and obesity increased by 38.6% (70 million) and 80.6% (30 million) respectively during the period of 1992 to 2002.[6] Epidemiology studies have reported the positive association between obesity and thyroid cancer risk,[7–11] and body mass index (BMI) was used as a major measurement in most studies. In addition to cancer risk, obesity has also been associated with aggressive tumor pathological features and unfavorable outcomes, especially in breast cancer.[12] Although such passively impact of obesity on PTC is inconclusive, increasing number of researches have been reported. Obesity has been shown to be associated with many pathological features of thyroid cancer, such as larger tumor size,[10] extra-thyroidal invasion, advanced TNM stage,[13] lymph node (LN) metastasis and tumor multiplicity.[14] Against this background, we sought to clarify the association between obesity and the risk of PTC in an operative population, and the impact of higher BMI on aggressiveness of PTC.
2. Materials and methods
2.1. Study population
A total of 10,844 consecutive patients who underwent partial or total thyroidectomy for thyroid nodules in the General Hospital of People's Liberation Army between 1993 and 2015 were retrospectively reviewed in this study. This study was limited to the adults with PTC. Therefore, 84 patients aged under 18 and 257 individuals with follicular carcinoma were excluded. We also excluded 10 patients with indeterminate pathology, 663 patients with missing BMI data and 65 patients without pathologically confirmed maximal nodule diameter. Finally, a total of 9765 subjects (6949 females and 2861 males) were analyzed, including 5114 (52.4%) patients with PTC. Medical records and pathology reports of each patients were reviewed. If the patient received 2 or more operations, only the first one was included in the analysis. All subjects signed an informed consent form. The study was approved by the Ethical Review Committee of the Chinese PLA General Hospital.
2.2. BMI calculations and exposure measurements
Height and weight data were measured by trained nurses with standardized instruments during the first hospitalization. BMI was calculated as the weight in kilograms divided by the height in meters squared (kg/m2). Patients were stratified in 4 BMI groups according to criterion in the “Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults” as follows: BMI < 18.5 (underweight), 18.5 ≤ BMI < 24 (normal-weight), 24 ≤ BMI < 28 (overweight) and BMI ≥ 28 (obese).
Fasting blood glucose (FBG) were measured in consideration of relationship between obesity and impaired fasting glucose. We also measured the serum thyroid stimulating hormone (TSH) concentrations at the time of thyroidectomy, as subclinical hypothyroidism is reported frequently with obesity.[15] The maximal nodule diameters in this study were pathologically confirmed. If the patient had several nodules including both the benign and the malignant, the calculation of nodule size was confined to the malignant ones. TNM stage was based on the UICC/AJCC 7th edition.[16]
2.3. Statistic analyses
SPSS version 16.0 was used for all the statistical analysis. The results are reported as median (Inter-quartile range, IQR) or mean ± SD. In the first part of analysis, the relationship between BMI and PTC diagnosis was investigated in the overall operative population. In the second part of analysis, we focused on the patients with PTC and analyzed the impact of higher BMI on the aggressiveness of PTC. When comparing clinicopathological characteristics of patients with benign or malignant thyroid nodules and analyzing the relationship between BMI and categorical clinicopathological factors, comparisons of categorical variables were performed by Pearson Chi-square test or Fisher exact test and comparisons of continuous variables were performed by Student t test, ANOVA test or Mann–Whitney test. In addition, a multivariate binary logistic regression analysis was performed to calculate BMI odds ratios (OR) with 95% confidence intervals (CI) to estimate the impact of BMI on the risk of PTC and more aggressive pathological features of PTC. The risk of PTC was also analyzed in models stratified by gender. BMI was analyzed both as a continuous and a categorical exposure. Gender, age, serum TSH level, FBG level, maximal nodule diameter and multifocality were used as confounding variables. All tests were 2-sided, and all P values <.05 were considered to be statistically significant.
3. Results
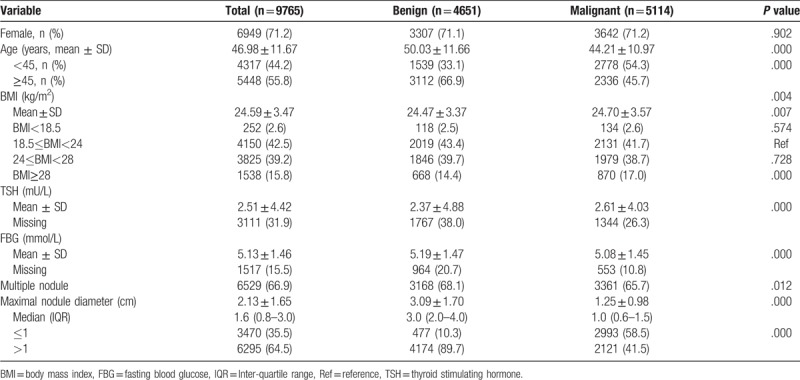
3.1. Baseline characteristic of patients with benign or malignant thyroid nodules
A total of 9765 patients (4651 benign and 5114 malignant cases) were studied, Figure 1 shows the schematic diagram of the study design. The Comparison of clinicopathological characteristics of patients with benign or malignant thyroid nodules showed significant differences in all variables except gender (Table 1). The mean age was 47 ± 11.7 years (range, 18–85 years) and 55.8% (5448/9765) were older than 45 years of age for all patients. The mean age was lower in the malignant group than that in the benign group (44.21 ± 10.97 years vs 50.03 ± 11.66 years; P < .001). The proportion of female was similar in the 2 group (71.1% vs 72.2%, P > .05). The mean BMI was 24.59 ± 3.47 kg/m2 for all patients, 24.47 ± 3.37 kg/m2 for the benign group, and 24.70 ± 3.57 kg/m2 for the malignant group (P = .007). Of the 9765 patients, 3528 (39.2%) were overweight (24 ≤ BMI < 28), and 1538 (15.8%) were obese (BMI ≥ 28). The rate of obese subjects was greater in the malignant group than that in the benign group (17.0% vs 14.4%, P < .001). In addition, mean TSH level was significantly higher (2.61 ± 4.03 mU/L vs 2.37 ± 4.88 mU/L, P < .001), while average FBG level and mean maximal nodule diameter were significantly lower (5.08 ± 1.45 mmol/L vs 5.19 ± 1.47 mmol/L, P < .001; 1.25 ± 0.98 vs 3.09 ± 1.70, P < .001; respectively) in the malignant group than that in the benign group.
Figure 1.
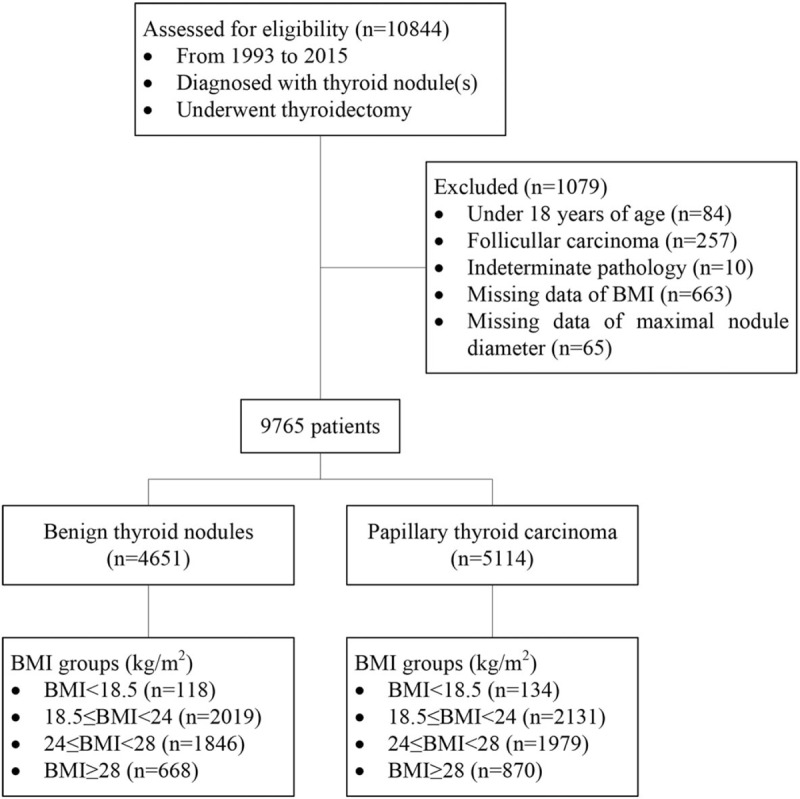
The schematic diagram of the study design, a total of 10,844 patients who were diagnose with thyroid nodule(s) and underwent thyroidectomy were reviewed in this study. The 1079 patients were excluded for several reasons. Finally, 9765 patients were enrolled and divided into 2 groups according to pathologic diagnosis.
Table 1.
Comparison of clinicopathological characteristics of patients with benign or malignant thyroid nodules (1993–2015).
3.2. Risk factors for PTC patients
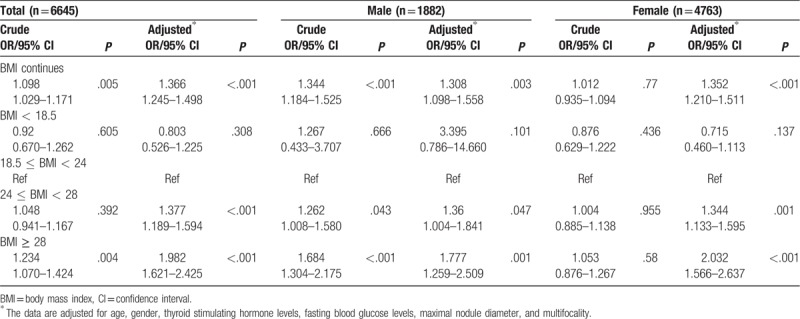
We examined the potential risk factors for thyroid cancer patients: age, gender, BMI, TSH level, FBG level, multifocality and maximal nodule diameter, to analyze their associations with PTC (Fig. 2). A total of 6645 patients were included in risk factor analysis (3767 patients with pathologically confirmed PTC), because 3120 patients with missing TSH or FBG data. In the univariate analysis, risk factors for malignancy were younger age, higher level of BMI and TSH, lower level of FBG, smaller nodule diameter and multiple nodule but not gender (Table 1). In multivariate binary logistic regression analysis, younger age, higher BMI, smaller nodule diameter and multiple nodule were still significantly associated with existence of PTC, but the associations between TSH or FBG level and risk of PTC were disappeared. Such results were consistent between male and female (data was not provided). In the population as a whole, a marginally and inversely significant association was shown between female and PTC (OR = 0.866, CI 0.744–1.009, P = .066) (Fig. 2). The malignant risk of thyroid nodules was also explored according to BMI category (Table 2). Before adjusting confound factors, the prevalence of PTC (OR = 1.344, CI 1.184–1.525, P < .001) was associated with high BMI (modeled continuously, per 5 kg/m2) in men, but high BMI did not significantly correlate with prevalence of PTC in women (OR = 1.012, CI 0.935–1.094, P = .770). However, when adjusting merely for age, BMI (per 5 kg/m2 increase) became a significant predictor of thyroid cancer (OR = 1.186, CI 1.090–1.290, P < .001) in women. Furthermore, after adjusting for gender, age, serum TSH level, FBG level, maximal nodule diameter and multifocality in all the patients, individuals who were obese and overweight had a significantly greater risk of having a malignant nodule (OR = 1.982, CI 1.621–2.425, P < .001; OR = 1.377, CI 1.189–1.594, P < .001; respectively) compared to normal weight patients, and this positive association between increased BMI and the risk of thyroid cancer was found in both gender.
Figure 2.
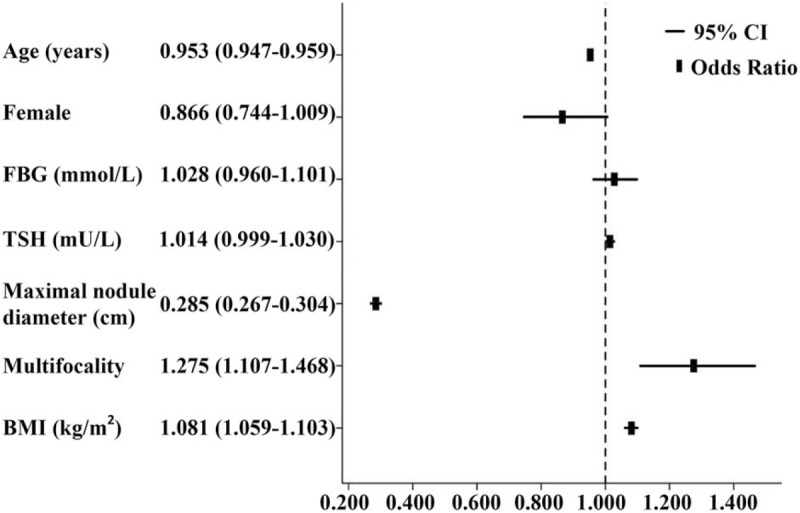
Adjusted odds ratio (95% CI) of risk factors for papillary thyroid cancer.
Table 2.
Adjusted odds ratios (OR) (with 95% CI) for papillary thyroid cancer by BMI groups according gender.
3.3. Baseline clinicopathological characteristics in patients with PTC
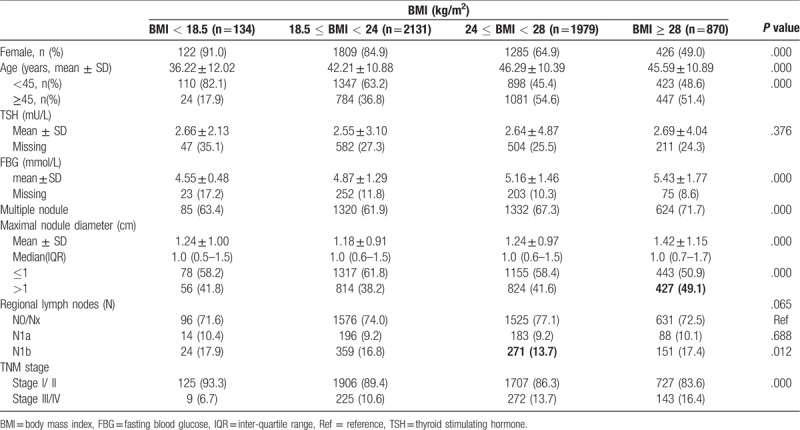
For the patients with PTC, the clinicopathological characteristics were evaluated according to BMI quartile categories (Table 3). The mean age, FBG and advanced TNM stage (stage III/IV) showed a significant increase across BMI quartiles (P < .001). In addition, multiple nodules were the least common and the mean maximal nodule diameter was the smallest in normal weight group than other groups (P < .001). However, there were no significant associations for gender, serum TSH level and LN metastasis according to BMI quartiles.
Table 3.
Clinicopathological characteristics of patients with papillary thyroid cancer according to BMI groups.
3.4. Associations of BMI with clinicopathological features of PTC
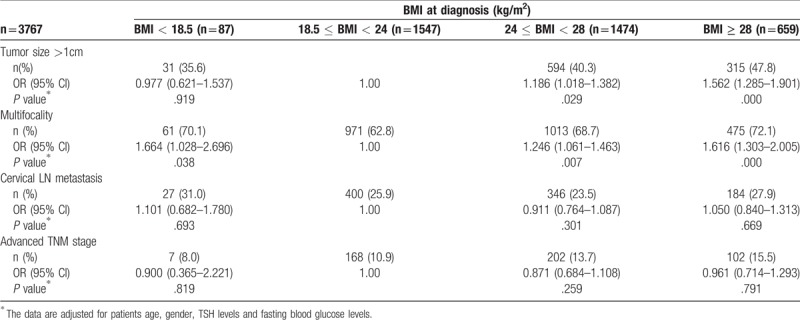
A total of 3767 cases with PTC were analyzed for risk of more aggressive pathological features analysis. OR for disease severity indicators per 5-point increase in BMI were shown in Table 4. A 5 kg/m2 increase in BMI was associated with multifocality (OR = 1.253, CI 1.136–1.383, P < .001). Risk ratio for tumor size >1 cm per 5 kg/m2 increase BMI was 1.137 (P = .006). However, the association of BMI with advanced TNM stage (relative risk, approximately 1.297 per 5 BMI units, P < .001) were disappeared after adjusting for gender, age, TSH and FBG levels. Patients who were overweight and obese had a significantly greater risk of having a tumor larger than 1 cm (OR = 1.186, CI 1.018–1.382, P = .029; OR = 1.562, CI 1.285–1.901, P < .001, respectively) and multifocal nodules (OR = 1.246, CI 1.061–1.463, P = .007; OR = 1.616, CI 1.303–2.005, P < .001, respectively) compared to normal weight patients (Table 5).
Table 4.
Odds ratios (OR) (with 95% CI) for disease severity indicators per 5-point increase in BMI.
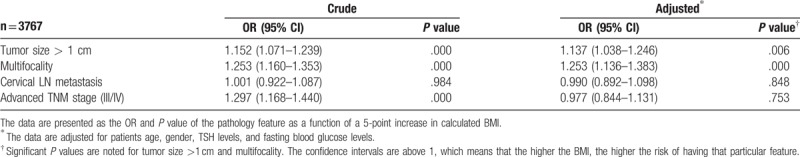
Table 5.
Risk of more aggressive pathological features of patients with papillary thyroid cancer according to BMI groups.
4. Discussion
This study did not only observe that high BMI, regardless of gender, was significantly associated with the risk of PTC in a large, operative population, but also showed positive association between excess body weight and aggressive pathological features of PTC, with larger tumor size and multifocality occurring in the patients with a higher BMI.
In the last decade, the incidence of thyroid cancer has increased along with obesity rate, which aroused researchers to investigate the link between obesity and thyroid cancer. Both series case-control studies[17,18] and pooled analyses[19–21] suggested that higher BMI was associated with an increased risk of PTC. However, few researches focused on the operative population and some drew the inverse conclusion. A Canadian study[22] investigated the relationship between BMI and thyroid cancer in patients with indeterminate cytology on fine needle aspiration biopsy (FNAB). Their results showed that higher BMI correlated with lower rates of thyroid malignancy except women older than 45 years in whom a positive association was found. An Italian study[23] reported that obesity did not modify the risk of differentiated thyroid cancer in patients underwent FNAB. A French study[24] recruited 6684 consecutive postoperative patients, and suggested that overweight and obese patients were not at greater risk of thyroid cancer, whereas they argued that BMI was risk factor for postoperative locoregional events in the macro-PTC patients. Their study defined obesity as BMI ≥ 30 kg/m2 and the malignant rate was notably lower than this in our study (18.2% vs 52.4%), which might account for the discrepancy.
Besides higher BMI, we found that younger age, smaller nodule diameter and multiple nodule were also independent risk factors for PTC patients. There was no gender difference between benign and malignant groups, but a marginally significant association was shown between female and PTC (OR = 0.866, CI 0.744–1.009, P = .066). The level of TSH was significantly higher, while the level of FBG was significantly lower in malignant group, but this relationship disappeared in multivariate analysis. Interestingly, our study showed that the mean maximal nodule diameter was significantly lower in the malignant group than that in the benign group. It is mainly due to more patients with malignant nodules ≤1 cm underwent surgery in our hospital.
Few studies simultaneously analyzed the impact of obesity on risk of thyroid cancer and its aggressiveness. This study included large, homogenous, operate population with PTC to extend the results of such researches. We observed that a 13.7% increase in risk of tumor size >1 cm and 25.3% increase in risk of multifocality for every 5-point increase in BMI. The PTC-patient with greater BMI were older, more frequently men, and more likely to have multiple nodules, larger tumor, higher FBG level and advanced TNM stage. However, there were no differences of TSH level or regional LN in the four BMI groups. Although some studies have investigated the relationship between obesity and clinicopathological features of thyroid cancer, the results of previous researches were still controversial.[10,13,14] An American study[25] reported that no positive associations were identified between BMI and T, N, or M stage, vascular invasion, or recurrent or persistent disease. Moreover, inverse association between BMI and nodal metastasis and tumor invasion was discovered. Their study enrolled 259 patients, and 25 of whom (10%) had follicular thyroid cancer (FTC) and 93% were Caucasian. Therefore, the discrepancies may be explained by various sample size, racial diversity, obesity criterion, and methodologies.
Possible mechanisms underlying the relationship between obesity and cancer is complex and involves multiple factors both at the systemic and cellular level. Disruptions in insulin metabolism, adipokines, inflammation, and sex hormones all contribute to the effects of obesity in cancer development and progression.[26] Obesity is often characterized by insulin resistance. Insulin shares structural homology with insulin-like growth factor-1 (IGF-1), which binds to the IGF-1 receptor and functions as a potent growth factor with key roles in malignant transformation, tumor progression and metastasis.[27] The TSH also play a major role in the carcinogenesis and several studies have reported an association between TSH and thyroid cancer. TSH cooperates with insulin and IGF-1 signaling to activate downstream MAPK and PI3K pathways that are central in thyroid carcinogenesis.[28,29] Additionally, the main signaling pathways linking obesity and cancer is the PI3K/Akt/mammalian target of rapamycin (mTOR) cascade, which regulates cell growth, proliferation and apoptosis.[30] TSH improves insulin sensitivity in skeletal muscle by increasing insulin receptor substrate (Irs)-1 gene expression, and this regulatory effect is mediated by a protein kinase A- cAMP response element binding protein (PKA-CREB) dependent pathway.[31] Our study showed that the positive relationship between TSH levels and PTC disappeared after adjusting age, gender, FBG levels, maximal nodule diameter and multifocality. Hence, we suggested that higher TSH level is related, but not independently, to the risk of PTC.
This retrospective study had limitations as well. First, BMI is a good measurement for the total mass of body, whereas it is limited in reflecting adiposity distribution and percent body fat. Second, some confounding factors related to lifestyle were not considered because of data deficiencies, such as smoking, alcohol consumption and degree of physical activity. Third, even though we enrolled as much participates as possible, this is a single-center study, we are preparing the multi-center study to further analyzing the association of BMI and clinicopathological features of thyroid cancer.
5. Conclusions
In summary, this single-center study indicates that patients with excess BMI are associated with higher risk of PTC in a population who are suggested to have thyroid operation, and they are more likely to get pathological outcome with larger tumor size and multifocality. Further studies to clarify the mechanisms behind the association of obesity with thyroid cancer are deserved.
Author contributions
Conceptualization: Sitong Zhao, Yajing Wang, Zhaohui Lyu.
Data curation: Sitong Zhao, Xiaomeng Jia, Xiaojing Fan, Ling Zhao, Ping Pang, Yajing Wang, Yukun Luo, Fulin Wang, Guoqing Yang, Xianling Wang, Weijun Gu, Li Zang, Jianming Ba, Jingtao Dou.
Formal analysis: Xiaomeng Jia, Ling Zhao, Ping Pang, Yukun Luo, Fulin Wang, Xianling Wang.
Funding acquisition: Zhaohui Lyu.
Investigation: Xiaomeng Jia, Ling Zhao.
Methodology: Xiaomeng Jia, Xiaojing Fan, Ling Zhao, Yukun Luo, Weijun Gu, Yu Pei, Jin Du.
Resources: Sitong Zhao, Yajing Wang, Yukun Luo, Xianling Wang, Weijun Gu, Jianming Ba, Jingtao Dou, Yiming Mu.
Software: Xiaojing Fan, Ping Pang, Yajing Wang, Fulin Wang, Guoqing Yang, Xianling Wang, Weijun Gu, Li Zang, Yu Pei, Jin Du, Jianming Ba, Jingtao Dou, Yiming Mu.
Supervision: Yajing Wang, Li Zang, Yu Pei, Jin Du, Jianming Ba, Yiming Mu, Zhaohui Lyu.
Validation: Xiaojing Fan, Ping Pang, Yajing Wang, Fulin Wang, Guoqing Yang, Xianling Wang, Li Zang, Yu Pei, Jin Du, Jingtao Dou.
Writing – original draft: Sitong Zhao, Zhaohui Lyu.
Writing – review & editing: Sitong Zhao, Yiming Mu, Zhaohui Lyu.
Footnotes
Abbreviations: BMI = body mass index, FBG = fasting blood glucose, FNAB = fine needle aspiration biopsy, IGF-1 = insulin-like growth factor-1, LN = lymph node, OR = odds ratio, PTC = papillary thyroid cancer, TNM = tumor-node-metastasis.
How to cite this article: Zhao S, Jia X, Fan X, Zhao L, Pang P, Wang Y, Luo Y, Wang F, Yang G, Wang X, Gu W, Zang L, Pei Y, Du J, Ba J, Dou J, Mu Y, Lyu Z. Association of obesity with the clinicopathological features of thyroid cancer in a large, operative population: a retrospective case-control study. Medicine. 2019;98:50(e18213).
This research was supported by the Medical Science and Technology Foundation of Military “Twelve-Five” Program (CWS11J063).
The authors declare that they have no competing interests.
References
- [1].Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the united states, 1974–2013. JAMA 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cong S, Fang LW, Bao HL, et al. Disease burden of thyroid cancer in the Chinese population, in 1990 and 2013. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:773–7. [DOI] [PubMed] [Google Scholar]
- [3].Davies L, Morris LG, Haymart M, et al. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract 2015;21:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Park Y, Colditz GA. Fresh evidence links adiposity with multiple cancers. BMJ 2017;356:j908. [DOI] [PubMed] [Google Scholar]
- [5].NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ma GS, Li YP, Wu YF, et al. The prevalence of body overweight and obesity and its changes among Chinese people during 1992 to 2002. Zhonghua Yu Fang Yi Xue Za Zhi 2005;39:311–5. [PubMed] [Google Scholar]
- [7].Han JM, Kim TY, Jeon MJ, et al. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol 2013;168:879–86. [DOI] [PubMed] [Google Scholar]
- [8].Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014;384:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arduc A, Dogan BA, Tuna MM, et al. Higher body mass index and larger waist circumference may be predictors of thyroid carcinoma in patients with Hurthle-cell lesion/neoplasm fine-needle aspiration diagnosis. Clin Endocrinol (Oxf) 2015;83:405–11. [DOI] [PubMed] [Google Scholar]
- [10].Dieringer P, Klass EM, Caine B, et al. Associations between body mass and papillary thyroid cancer stage and tumor size: a population-based study. J Cancer Res Clin Oncol 2015;141:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shin HY, Jee YH, Cho ER. Body mass index and incidence of thyroid cancer in Korea: the Korean Cancer Prevention Study-II. J Cancer Res Clin Oncol 2017;143:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer–viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim HJ, Kim NK, Choi JH, et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol (Oxf) 2013;78:134–40. [DOI] [PubMed] [Google Scholar]
- [14].Kim SH, Park HS, Kim KH, et al. Correlation between obesity and clinicopathological factors in patients with papillary thyroid cancer. Surg Today 2015;45:723–9. [DOI] [PubMed] [Google Scholar]
- [15].Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010;95:3614–7. [DOI] [PubMed] [Google Scholar]
- [16].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [17].Oberman B, Khaku A, Camacho F, et al. Relationship between obesity, diabetes and the risk of thyroid cancer. Am J Otolaryngol 2015;36:535–41. [DOI] [PubMed] [Google Scholar]
- [18].Myung SK, Lee CW, Lee J, et al. Risk factors for thyroid cancer: a hospital-based case-control study in Korean adults. Cancer Res Treat 2017;49:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu L, Port M, Landi S, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid 2014;24:966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ma J, Huang M, Wang L, et al. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monit 2015;21:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schmid D, Ricci C, Behrens G, et al. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 2015;16:1042–54. [DOI] [PubMed] [Google Scholar]
- [22].Mijovic T, How J, Pakdaman M, et al. Body mass index in the evaluation of thyroid cancer risk. Thyroid 2009;19:467–72. [DOI] [PubMed] [Google Scholar]
- [23].Rotondi M, Castagna MG, Cappelli C, et al. Obesity does not modify the risk of differentiated thyroid cancer in a cytological series of thyroid nodules. Eur Thyroid J 2016;5:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tresallet C, Seman M, Tissier F, et al. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery 2014;156:1145–52. [DOI] [PubMed] [Google Scholar]
- [25].Paes JE, Hua K, Nagy R, et al. The relationship between body mass index and thyroid cancer pathology features and outcomes: a clinicopathological cohort study. J Clin Endocrinol Metab 2010;95:4244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lohmann AE, Goodwin PJ, Chlebowski RT, et al. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol 2016;34:4249–55. [DOI] [PubMed] [Google Scholar]
- [27].Bae MJ, Kim SS, Kim WJ, et al. High prevalence of papillary thyroid cancer in Korean women with insulin resistance. Head Neck 2016;38:66–71. [DOI] [PubMed] [Google Scholar]
- [28].Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jiao J, Zhou Y. Relationship between serum thyroxin-stimulating hormone and papillary thyroid micrcarcinoma in nodular thyroid disease. Zhonghua Yi Xue Za Zhi 2015;95:908–11. [PubMed] [Google Scholar]
- [30].Moon HS. Chemopreventive effects of alpha lipoic acid on obesity-related cancers. Ann Nutr Metab 2016;68:137–44. [DOI] [PubMed] [Google Scholar]
- [31].Moon MK, Kang GH, Kim HH, et al. Thyroid-stimulating hormone improves insulin sensitivity in skeletal muscle cells via cAMP/PKA/CREB pathway-dependent upregulation of insulin receptor substrate-1 expression. Mol Cell Endocrinol 2016;436:50–8. [DOI] [PubMed] [Google Scholar]


