Abstract
Tenofovir disoproxil fumarate (TDF) is thought to cause varying degrees of hypophosphatemia in patients with chronic hepatitis B (CHB). Therefore, we investigated factors that cause hypophosphatemia in patients treated with TDF and methods to increase serum phosphorus concentrations in clinical practice.
We completed a retrospective review of patients with CHB treated with TDF initially at Kosin University Gospel Hospital, Busan, Korea from January 2012 to January 2017. Subclinical hypophosphatemia and hypophosphatemia were defined as serum phosphorus below 3.0 mg/dL and 2.5 mg/dL, respectively.
We screened 206 patients with CHB treated with TDF, among which 135 were excluded for the following reasons: baseline malignancy (59), limited data (50), co-administered other antivirals (14), hypophosphatemia at baseline (7), and other reasons (5). The final study population comprised 71 patients. Subclinical hypophosphatemia developed in 43 (60.5%) patients. Hypophosphatemia occurred in 18 patients (25.3%). Liver cirrhosis was the most significant predictor of hypophosphatemia (P = .038, OR = 3.440, CI = 1.082–10.937) Patients diagnosed with subclinical hypophosphatemia were encouraged to increase their intake of nuts and dairy products (25 patients) or reduce their alcohol intake (2), dose reduction of TDF (4) or placed under observation (4). Among patients with subclinical hypophosphatemia, serum phosphorus concentrations were elevated (>3.0 mg/dL) in 23 of 36 patients (63.8%). Increased nut and dairy intake increased phosphorus concentrations to more than 3.0 mg/dl in 16 of 25 patients (64.0%).
Entecavir or tenofovir alafenamide fumarate (TAF) should be considered rather than TDF in patients with liver cirrhosis because of the risk of hypophosphatemia. Instead of stopping TDF treatment, encouraging increased intake of phosphorus-rich foods could increase serum phosphorus concentrations in clinical practice.
Keywords: hepatitis B virus, hypophosphatemia, liver cirrhosis, tenofovir
1. Introduction
Entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide fumarate (TAF) are recommended as first-line treatments for HBeAg-positive or -negative chronic hepatitis B (CHB).[1] Both ETV and TDF have proven long-term antiviral efficacy and safety. In addition, TDF has been shown to be an effective rescue therapy in patients with multidrug-resistant CHB and is widely used in clinical practice.[2,3] Moreover, less than 2% of patients taking long-term TDF have been reported to have adverse effects associated with renal function.[4] However, later studies have suggested that renal dysfunction may occur in patients with CHB who receive TDF,[5] especially in patients with eGFR < 60.[6]
Prolonged administration of TDF may damage mitochondria and proximal renal tubules in the kidneys.[7] Damage to the proximal renal tubules may lead to excessive excretion of bicarbonate, uric acid, phosphate, potassium, glucose and other constituents of urine, with consequent acidosis, hypouricemia, hypophosphatemia, hypokalemia, and glucosuria. These clinical features are called Fanconi syndrome. Hypophosphatemia may affect bone metabolism, and if persistent, may lead to osteomalacia,[8] osteopenia, or osteoporosis.[9]
Recent guidelines have recommended the use of TAF instead of TDF in patients over 60 years of age, bone disease, chronic kidney disease (CKD) and hypophosphatemia (<2.5 mg/dL).[1,10] TAF has similar antiviral effects to those of TDF, but is prescribed at a lower dose, systemic drug exposure is reduced in patients taking TAF and renal toxicity and alterations in bone metabolism can be reduced[10]; however, the characteristics of patients who develop hypophosphatemia when administered TDF have not been clearly established.
The aim of this study was to investigate the incidence of and risk factors for hypophosphatemia in patients receiving TDF. We also assessed the factors involved in the correction of TDF-induced hypophosphatemia and methods to increase serum phosphorus concentrations in clinical practice.
2. Materials and methods
2.1. Study design
From January 2012 to January 2017, patients with CHB who were initially prescribed TDF at Kosin University Gospel Hospital (KUGH), Busan, Korea were analyzed retrospectively. At the KUGH, clinical hypophosphatemia is defined as serum phosphorus concentrations below 3.0 mg/dl. For this study subclinical hypophosphatemia and hypophosphatemia were defined as serum phosphorus concentrations below 3.0 mg/dL and 2.5 mg/dL, respectively.
Newly developed hypophosphatemia after TDF administration was defined using comparisons between baseline phosphorus and follow-up phosphorus concentrations. The baseline serum phosphorus concentration was checked within 2 months from the first administration of TDF, and follow-up serum phosphorus levels were consecutively reviewed during continuous administration of TDF. The follow-up duration was the period from the day of TDF initiation to last serum phosphorus measurement.
The exclusion criteria were co-administration of other anti-viral agents and underlying disease, including CKD and malignancy. This study design was approved by the Institutional Review Board/Ethics Committee of Kosin University Gospel Hospital (KUGH 2019-05-003). Informed consent was waived because of the retrospective study design.
2.2. Study population
A total of 206 patients with CHB were initially prescribed TDF, among which 135 were excluded for the following reasons: 59 patients had malignancy; 50 patients had limited medical records (36 without baseline phosphorus measurements and 14 without follow-up data); 14 patients had been co-administered other antiviral agents; 7 patients were hypophosphatemic at baseline; and 5 patients had special medical conditions (hemodialysis, Stage 4 CKD, kidney transplantation or liver transplantation). Overall, 71 patients were included in the final study population (Fig. 1).
Figure 1.
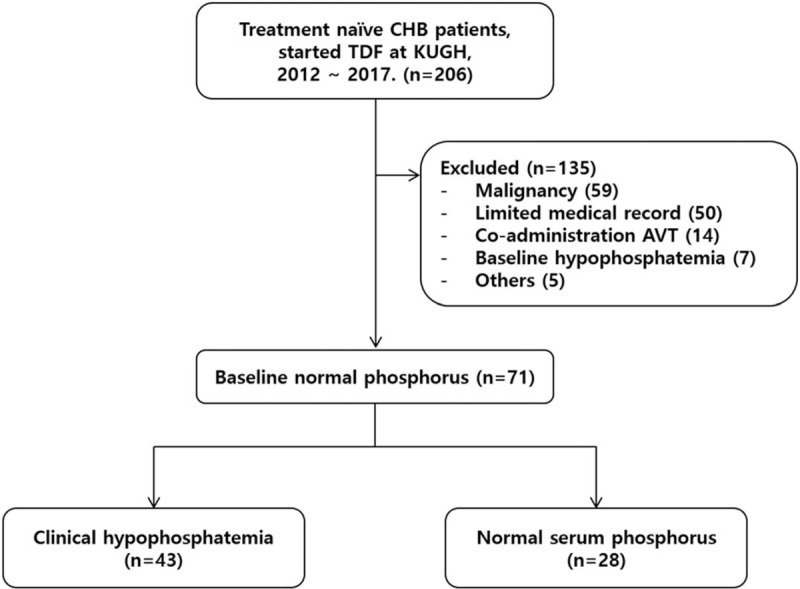
Flow chart of study population. A total 206 patients with CHB were initially prescribed TDF. Of these, 135 were excluded for the following reasons: 59 patients had malignancy; 50 had limited medical records (36 without baseline phosphorus measurements, 14 without follow-up data); 14 were co-administered other antiviral agents; 7 patients have hypophosphatemia at baseline; and 5 patients had special medical conditions (hemodialysis, CKD stage 4, kidney transplantation and liver transplantation). We enrolled 71 patients in the study.
Each patient's medical records were reviewed to obtain information regarding demographic parameters, co-morbidities (including hypertension [HTN], diabetes [DM], liver cirrhosis) and medications (diuretics, angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARB]).
Liver cirrhosis was diagnosed if one of the following were identified: evidence of cirrhosis in imaging studies (computed tomography or sonography); esophageal or gastric varices on endoscopy; laboratory data suggesting liver cirrhosis; or patient history of clinical symptoms (ascites, hepatic encephalopathy, variceal bleeding). Decompensated liver cirrhosis was diagnosed in patients with jaundice, ascites, variceal bleeding, and hepatic encephalopathy.
2.3. Statistical analyses
According to the result of normality test, the mean and standard deviation were calculated for continuous variables, and an independent sample t test was performed to compare the mean between groups. Categorical variables were expressed as percentages and the Chi square-test was used to compare these groups. Multivariate logistic regression analyses were performed to identify risk factors for hypophosphatemia. Statistical significance was determined with P < .05 using SPSS software version 23 (IBM Corp., Armonk, NY).
3. Results
3.1. Baseline characteristics
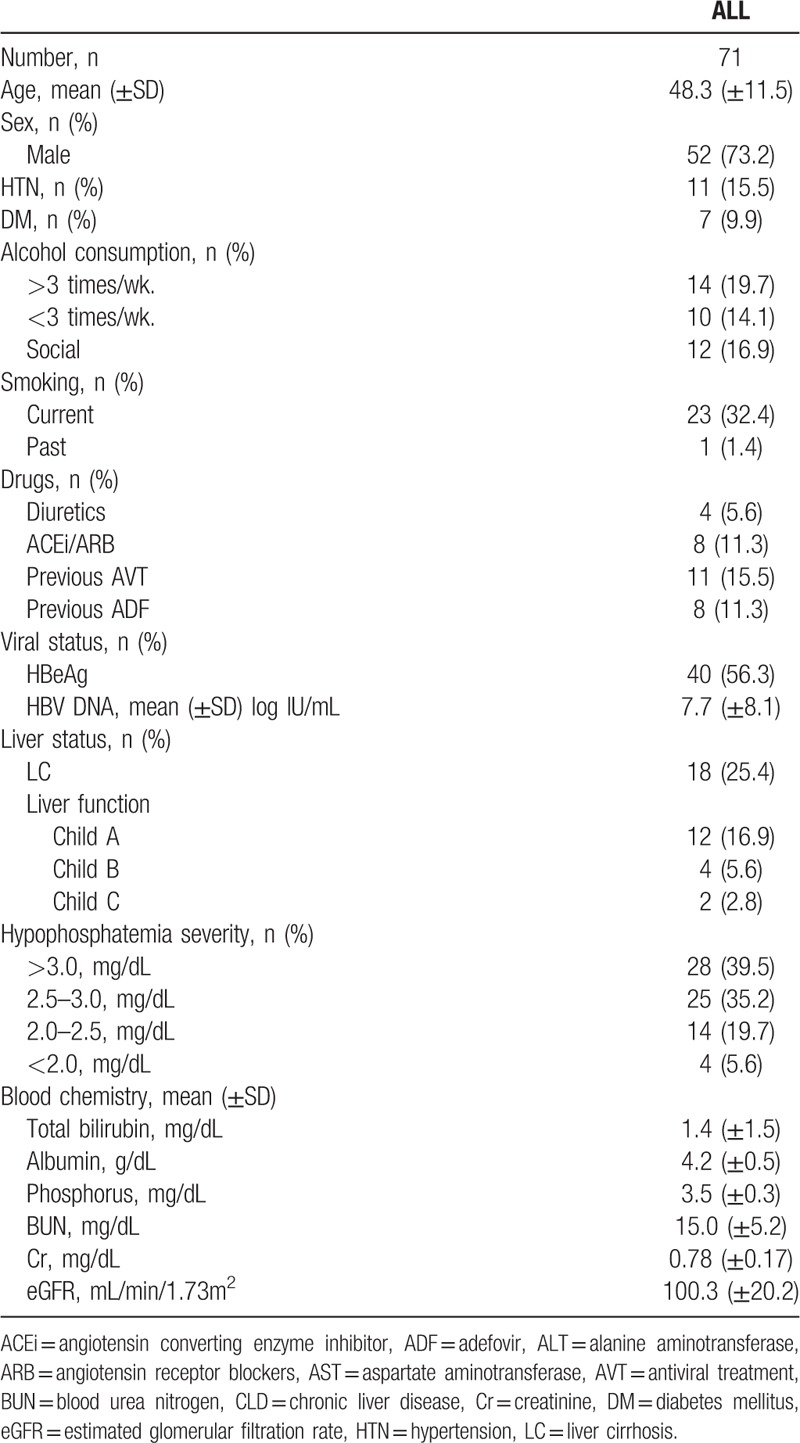
The data from 71 TDF-treated patients with CHB were analyzed. The mean age was 48.3 years in females and 52 in males (73.2%). The co-morbidities were HTN in 11 (15.5%) and diabetes in 7 (9.9%) patients. Current patient medications included diuretics in 4 patients (4.5%), and ACEi or ARB to control blood pressure in 8 patients (11.3%); 11 patients had previously received antiviral medication, 8 of which had been administered adefovir (ADF).
Hepatitis B status was assessed in the 71 patients: 40 (56.3%) were HBeAg-positive and the mean viral load (copies of HBV DNA) was 7.7 log IU/mL. There were 18 patients (25.4%) with liver cirrhosis, among which 12 (16.9%) were Child-Pugh Class A, 4 (5.6%) were Class B and 2 (2.8%) were Class C. At baseline, mean serum creatinine was 0.78 mg/dL, eGFR was 100.3 mL/min/1.73 m2, mean total serum bilirubin was 1.4 mg/dL, albumin was 4.2 g/dL and phosphorus was 3.5 mg/dL (Table 1). The median duration of treatment for all patients was 735 days.
Table 1.
Patient baseline characteristics.
3.2. Hypophosphatemia after TDF administration
Of the 71 patients, 43 (60.5%) had serum phosphorus concentrations below 3.0 mg/dL. The median follow-up period from the baseline phosphorus measurement to detection of the lowest phosphorus concentration was 370 days. Serum phosphorus concentrations were reduced to less than 2.5 mg/dL in 18 (26%), and less than 2.0 mg/dL in 4 (6%) patients (Fig. 2).
Figure 2.
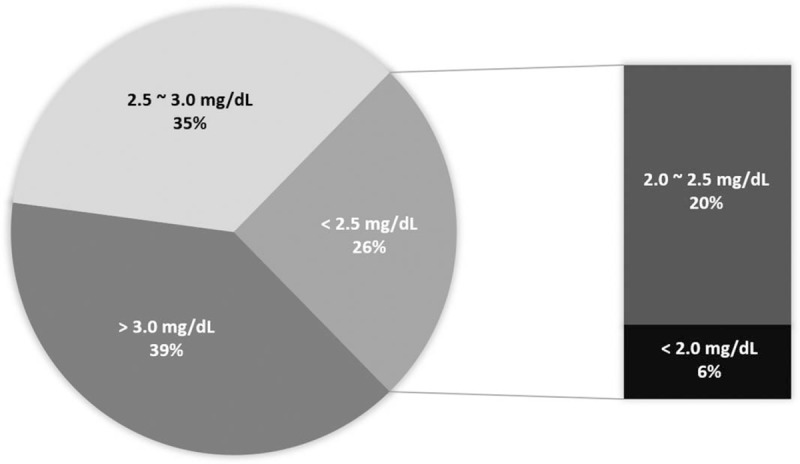
Severity of hypophosphatemia. Of the 71 patients, serum phosphorus concentrations were reduced to less than 3.0 mg/dL in 43 (60.5%), below 2.5 mg/ dL in 18 (26%) and below 2.0 mg/dL in 4 (6%).
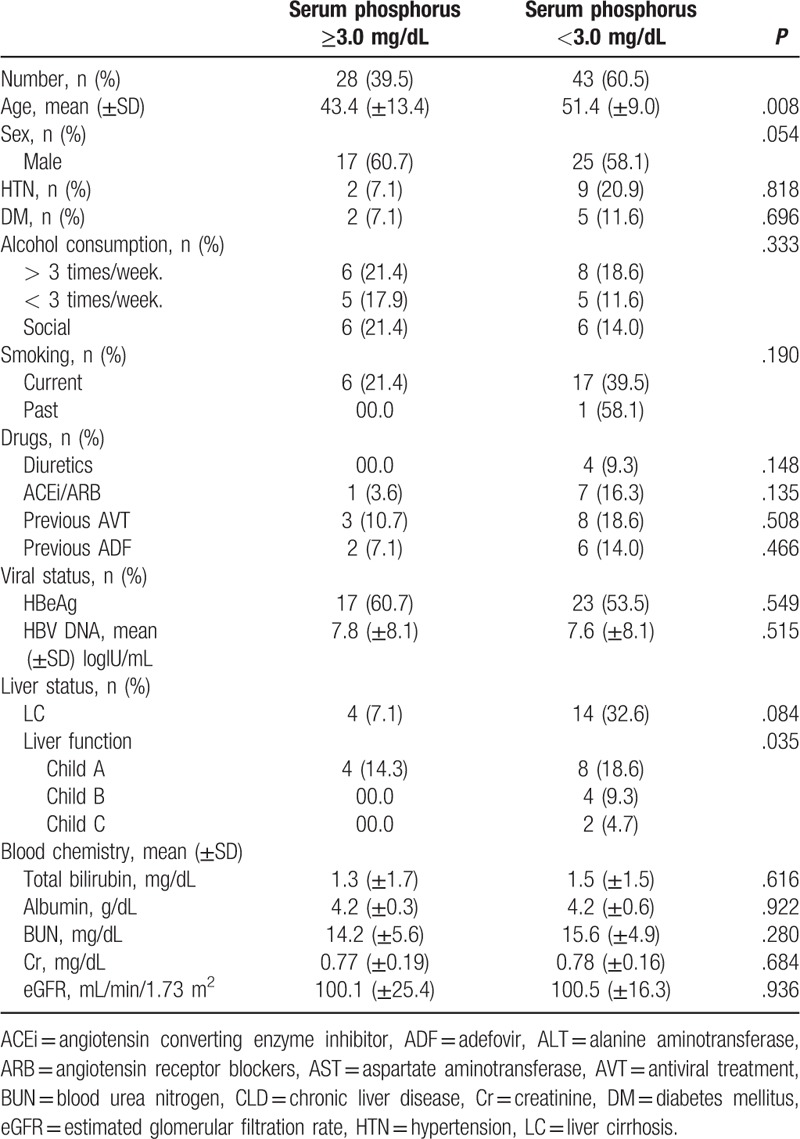
Subclinical hypophosphatemia, defined as serum phosphorus concentrations below 3.0 mg/dL, occurred in 43 patients (60.5%). The mean age of the subclinical hypophosphatemia group was 51.4 years, and 25 of these patients were men (58.1%). Nine of these patients were diagnosed with HTN (20.9%), 5 were diabetics (11.6%) and 14 had liver cirrhosis (32.2%). Four patients (9.3%) were taking diuretics and 7 (16.3%) were taking antihypertensive drugs, including ACEi or ARB. Eight patients (18.6%) had received antiviral drugs previously, among them 6 (14.0%) received ADF. There was a statistically significant difference between the 2 groups (subclinical hypophosphatemia vs normal) in age (P = .008) and liver function (P = .035; Table 2). Regression analyses revealed age as a significant predictor of serum phosphorus concentrations <3.0 mg/dL (P = .006, OR = 0.934, CI = 0.890–0.981)
Table 2.
Characteristics of patients who developed subclinical hypophosphatemia (<3.0 mg/dL) after TDF administration.
Hypophosphatemia, defined as serum phosphorus concentrations below 2.5 mg/dL, developed in 18 patients (25.4%). Their mean age was 51.3 years and 16 patients were male (88.9%). Four of these patients were diagnosed with HTN (22.2%) and 3 with diabetes mellitus (16.7%). Two patients (11.1%) had previously received antiviral therapy and none had been treated with ADF. Three patients (16.7%) were taking diuretics and 4 (22.2%) were taking ACEi or ARB. Eight patients (44.4%) were diagnosed with liver cirrhosis: 2 were Child-Pugh Class B (11.1%) and another 2 patients (11.1%) were Child-Pugh Class C. Reduction of serum phosphorus to less than 2.5 mg/dL was significantly associated with use of diuretics (P = .048) and reduced liver function (P = .005; Table 3). Univariate and multivariate logistic regression analyses showed that liver cirrhosis was the most significant predictor of serum phosphorus concentrations <2.5 mg/dL (P = .038, OR = 3.440, CI = 1.082–10.937; Table 4).
Table 3.
Characteristics of patients who developed hypophosphatemia (<2.5 mg/dL) after TDF administration.
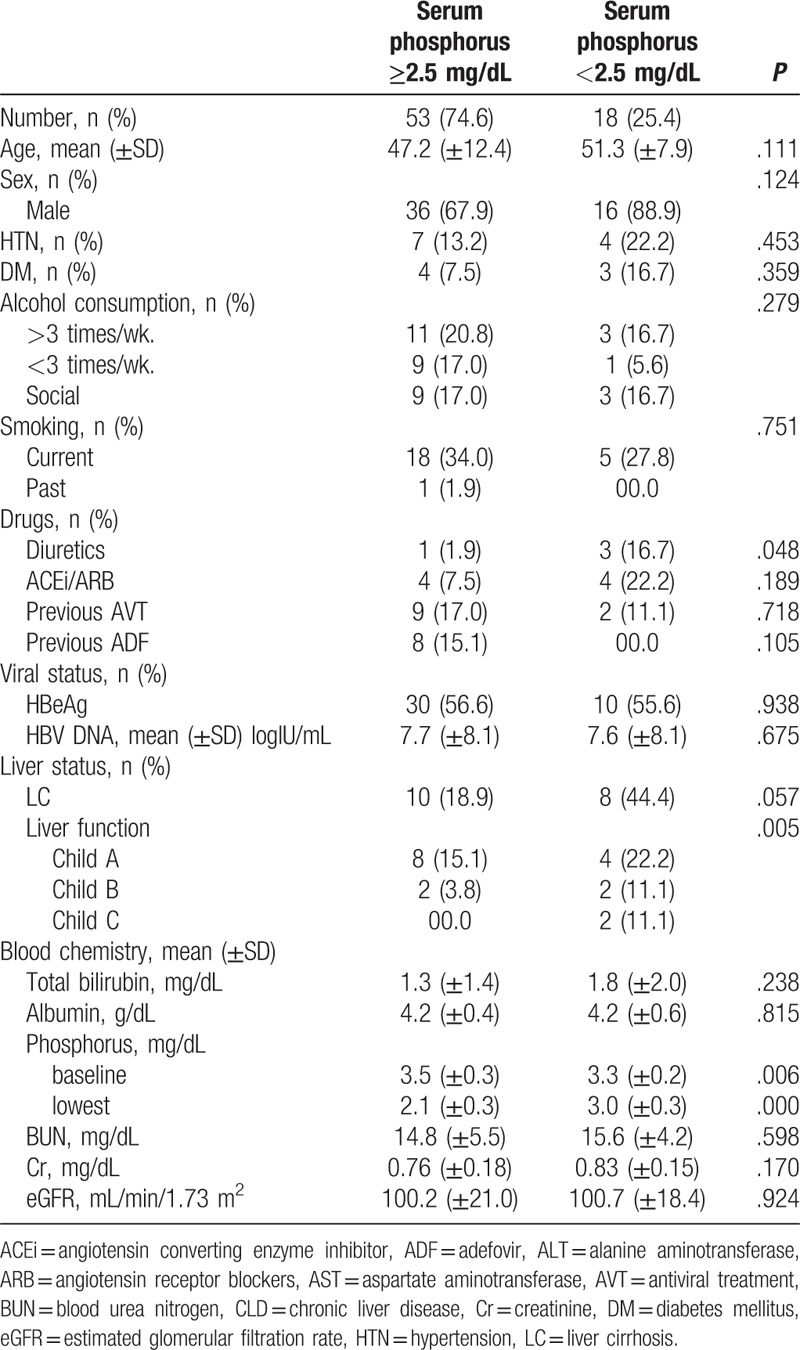
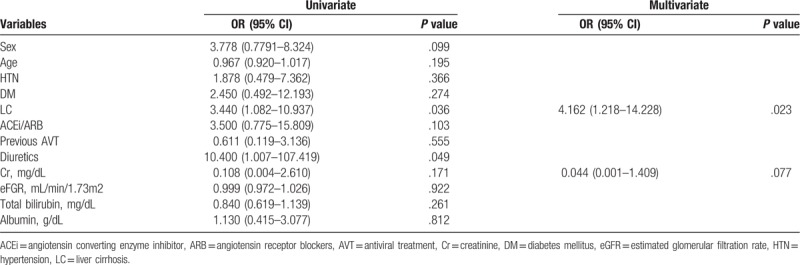
Table 4.
Univariate and multivariate analyses of risk factors for hypophosphatemia (<2.5 mg/dL).
Four male patients (4/71, 5.6%) had serum phosphorus lower than 2.0 mg/dl: 2 (50%) were diagnosed with liver cirrhosis and 1 (25%) was taking diuretics. There were no statistically significant differences between these 4 patients and the others.
3.3. Recovery from hypophosphatemia
Of the patients who were diagnosed with subclinical hypophosphatemia (P < 3.0 mg/dL), 36 were followed for more than 2 months to detect changes in serum phosphorus. In order to increase serum phosphorus concentrations, 25 patients were encouraged to increase their intake of nuts and dairy products, the dose of TDF was reduced (every other day) in 4 patients, and 2 patients were instructed to stop drinking completely (that means abstinence). The remaining 5 patients were monitored without any treatment.
The mean follow-up period in the 36 patients was 473.9 days. In 23 patients (74.6%), serum phosphorus concentrations increased to more than 3.0 mg/dL. Lifestyle modification, such as increasing intake of nuts or dairy products and alcohol withdrawal may increase the patient's serum phosphorus. In the 4 patients, TDF was administered every other day. Serum phosphorus increased with no change of antiviral effect. There were no statistical differences in the serum phosphorus correction according to the type of treatment (P = .954; Fig. 3).
Figure 3.
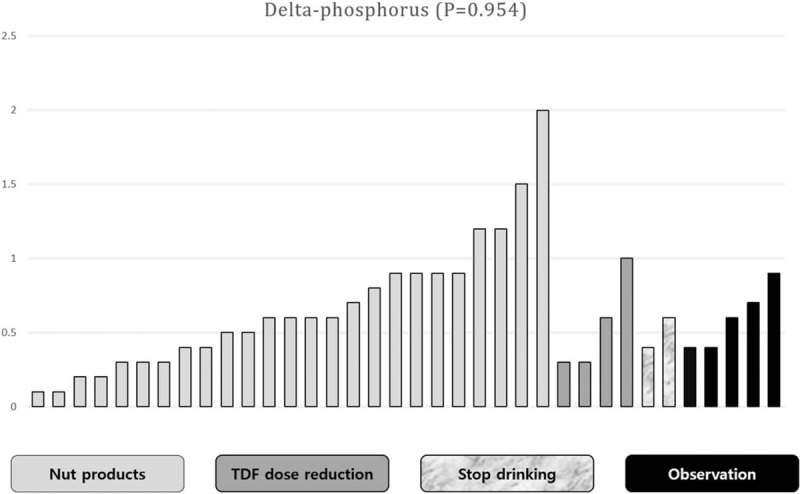
Improvement of hypophosphatemia. Of the patients who were diagnosed with subclinical hypophosphatemia (P < 3.0 mg/dL), 36 patients were followed for more than 2 months to detect changes in serum phosphorus concentrations. In order to increase serum phosphorus, 25 patients were encouraged to increase their intake of nuts and dairy products, the dose of TDF was reduced in 4, and 2 patients were instructed to refrain from drinking alcohol. The remaining 5 patients were followed up without any treatment. There were no significant differences in the serum phosphorus correction according to the type of treatment (P = .954).
A total of 25 patients were encouraged to increase their intake of nuts or dairy products, and were followed up for more than 1 year: serum phosphorus concentrations normalized (to above 3.0 mg/dL) in 16 patients, but remained low in the remaining 9. Factors that were significantly different between the 2 groups were liver cirrhosis and decompensation (Table 5). Multivariate analyses found that liver cirrhosis was a statistically significant inhibitor of phosphorus recovery (P = .007).
Table 5.
Recovery of serum phosphorus (>3.0 mg/dL) with taking nuts or dairy products.
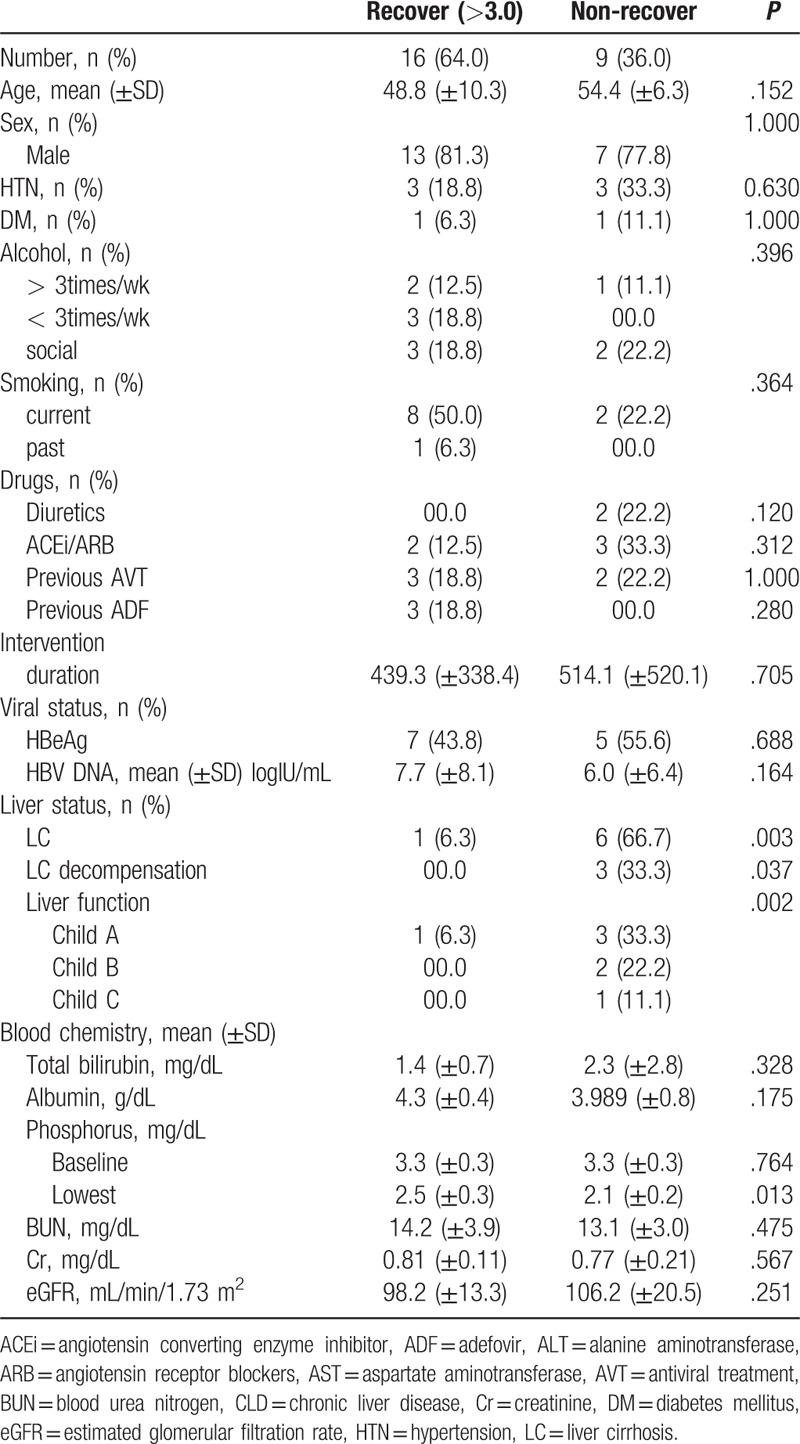
Thirteen patients (25.4%) had not recovered serum phosphorus level more than 3.0 mg/dL. The mean age of this group was 55.3 years old. The mean age of patients with improved serum phosphorus more than 3.0 mg/dL was 50.4 years old. However, age was not statistically significant (P = .108). In addition, taking diuretics (P = .124), ADF exposure (P = .634), and dose reduction of TDF (P = .124) had no statistical significance. Eight of the 13 patients were diagnosed with cirrhosis (61.5%), of which 4 patients were diagnosed with decompensated cirrhosis (30.8%), including 2 patients with Child-Pugh Class B (15.4%) and 2 patients with Child-Pugh Class C (15.4%; Table 6).
Table 6.
Recovery of serum phosphorus (>3.0 mg/dL) in patients diagnosed with subclinical hypophosphatemia.
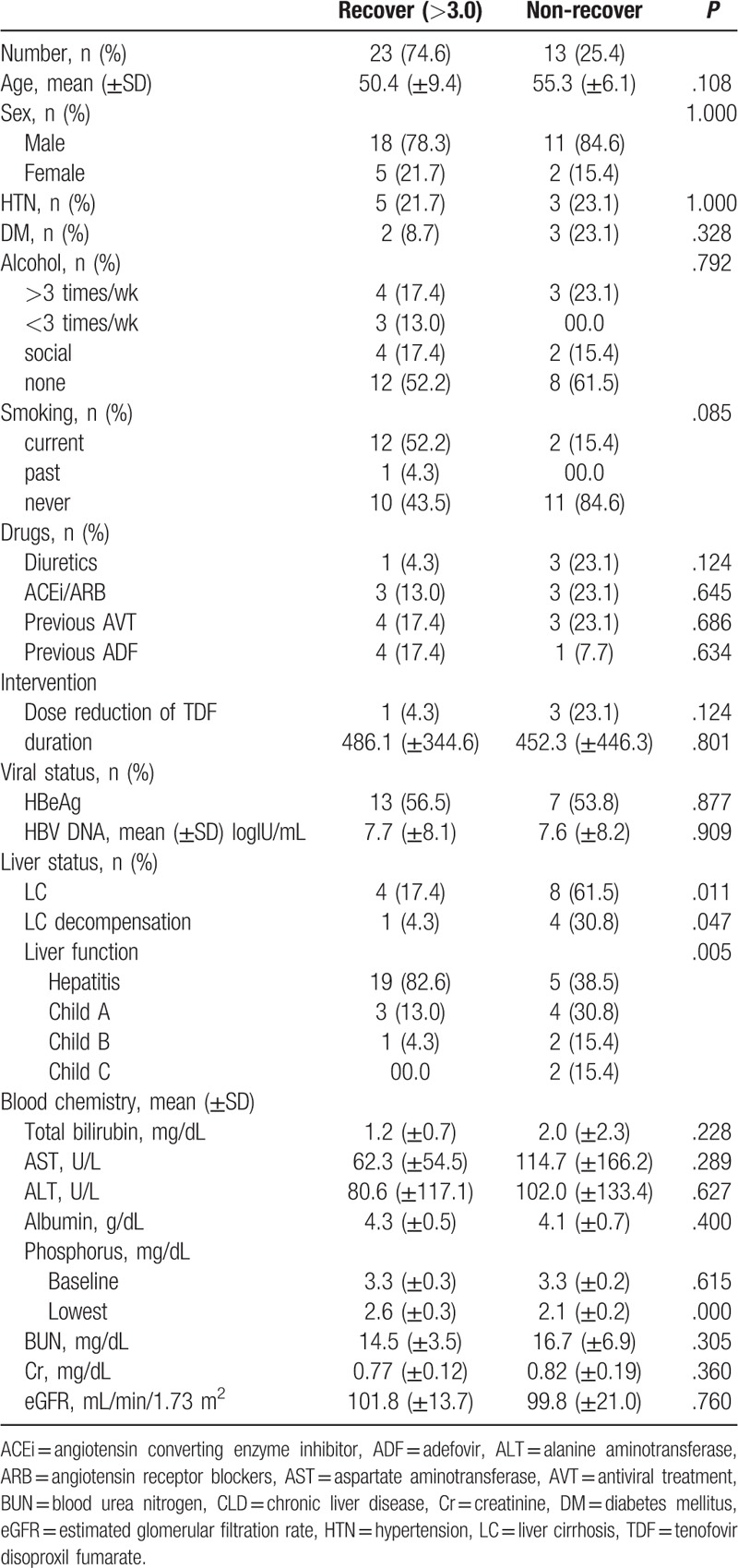
In comparisons to the recovered group (defined as patients having serum phosphorus elevated above 3.0 mg/dL) and the unrecovered group, univariate analyses indicated that liver cirrhosis (P = .011), decompensated liver cirrhosis (P = .047), and liver function (P = .005) were significantly different between the 2 groups. Multivariate analyses indicated that cirrhosis was a factor associated with failure to recover normal serum phosphorus concentrations (P = .010, OR = 0.132, CI = 0.028–0.622).
Serum phosphorus decreased to less than 2.5 mg/dL in 18 patients. Of these, 16 patients were followed for more than 2 months. Only one patient, a 62-year-old man with decompensated liver cirrhosis (Child-Pugh Class B) did not recover. His serum phosphorus concentration was 3.1 mg/dL at baseline. The follow-up serum phosphorus at 643 days after initiation of TDF was 2.0 mg/dL. After 212 days of increased intake of nuts and dairy products, his serum phosphorus concentrations increased to 2.2 mg/dL. Previously, this patient had been switched from ETV to TDF due to viral resistance. He was encouraged to supplement his diet with additional nuts and dairy products, but he continued to drink alcohol more than 3 times a week, smoked constantly, and took diuretics.
4. Discussion
Advanced age, diabetes, and hyperbilirubinemia are known to be factors contributing to the impairment of kidney function in CHB patients prescribed TDF.[11] Particularly, patients with lower baseline renal function are more likely to experience exacerbation of renal dysfunction when taking TDF compared to other antiviral drugs.[5,12] When cirrhotic patients are taking TDF, renal function may decline more rapidly if baseline eGFR is low, the patient has co-morbid conditions, and/or diuretics are taken.[13] The main mechanism of renal injury is proximal tubular dysfunction, and when this site is damaged a Fanconi-like renal tubular acidosis may be observed clinically.[14] Traditional Fanconi syndrome is known to be genetic in origin, or to occur secondary to heavy metal poisoning or metabolic disease. Recently, antiretroviral agents have been shown to be an important cause of Fanconi syndrome in clinical practice as a result of increased renal damage.[8]
Administration of TDF to patients with CHB or decompensated liver cirrhosis is known to cause hypophosphatemia 4 times more frequently than ETV.[15] In addition, TDF administration in patients with vitamin D deficiency is known to increase the risk of hypophosphatemia.[16] Other factors that may cause hypophosphatemia include advanced age, co-infection with HIV or HCV, and co-administration of other nephrotoxic agents; however, most of the risk factors have been studied in HIV patients.[17]
This study has shown the degree to which serum phosphorus is reduced in patients with CHB taking TDF and has identified several factors associated with newly developed hypophosphatemia after TDF and with recovery from hypophosphatemia. About 1 year after initiation of administration of TDF, serum phosphorus was reduced to less than 2.5 mg/dL in 26% of patients with CHB. Diuretics and liver function have been shown to influence the incidence of hypophosphatemia, and the risk of hypophosphatemia in patients with cirrhosis is about 3.4-fold greater than in patients with normal liver function. In patients with subclinical hypophosphatemia (serum phosphorus concentrations below 3.0 mg/dL), liver cirrhosis was significantly associated with failure to elevate serum phosphorus above 3.0 mg/dL. The results of this study suggest that administration of TDF in patients with cirrhosis may create a greater risk of hypophosphatemia, and these patients may have a limited response to efforts to normalize serum phosphorus concentrations while receiving TDF.
In our study, none of the patients with hypophosphatemia had a serum creatinine elevation. Our findings suggest that serum phosphorus may be a useful marker for kidney injury due to antiviral agents. Essig et al also suggested serum phosphorus was a sensitive method for prediction of kidney injury in patients taking antiviral drugs.[9] However, the use of hypophosphatemia as a predictor of kidney injury has yet to be established. Additional investigations of elevation of serum phosphorus concentrations as a predictor of creatinine elevation in patients taking antiviral drugs are needed.
Clinically, not all patients receiving TDF are able to switch to TAF or ETV. Instead, normalization of serum phosphorus concentrations may be achieved by increasing phosphorus intake or reducing dose of TDF. In this study, patients were encouraged to increase phosphorus intake by increasing their intake of nuts or dairy products, which resulted in recovery of serum phosphorous concentrations to over 3.0 mg/dL in 64% of CHB. Additional factors influencing phosphorus recovery in this group were liver cirrhosis and decompensation: recovery from TDF-induced hypophosphatemia through increased ingestion of nuts or dairy products was more frequent in patients without cirrhosis.
However, this was a retrospective study undertaken at a single center, and it is unreasonable to make generalizations based on the results. This study has inevitable selection bias because only patients with serum phosphorus measurements were enrolled. In addition, according to the clinical judgment of the attending physician, only patients with risk factors for kidney problems were examined. As a result, other studies have suggested that patients prescribed ADF had a risk of hypophosphatemia, but not in our study. This result may be due to the small study population. Moreover, nut and dairy product intakes were very subjective and could not be quantified. Even in a few patients, TDF dose reduction (every other day) was performed with no change in antiviral effects. Of course, this was an empirical method with the consent of the patient. However, this method cannot be officially recommended. Nevertheless, this study revealed the prevalence of and risk factors for hypophosphatemia in patients with CHB receiving TDF in Korea. It is clinically meaningful in that it provides evidence for considering TAF or ETV in patients with CHB combined with cirrhosis or decompensation instead of TDF. In addition, when hypophosphatemia occurs in patients receiving TDF, instead of stopping or changing medication, making a lifestyle modification such as taking nuts and dairy products may help non-cirrhotic patients to increase serum phosphorus concentrations, which may be useful in clinical practice.
In conclusion, hypophosphatemia occurred in about 26% of patients with CHB taking TDF. An important factor in reducing serum phosphorus to less than 2.5 mg/dL is co-administration of diuretics or impaired liver function. Significant factors determining the recovery of normal serum phosphorus concentrations were liver function and cirrhosis. Therefore, ETV or TAF should be considered for patients with CHB with liver cirrhosis or decompensation, rather than TDF because of the potential risk of hypophosphatemia. In non-cirrhotic patients, increasing nuts and dairy product intakes for more than 1 year may help normalize serum phosphorus concentrations without requiring cessation of TDF treatment.
Author contributions
Conceptualization: Byung Cheol Yun, Kwang Il Seo.
Data curation: Dohyeong Lee, Kwang Il Seo, Sang Uk Lee.
Formal analysis: Kwang Il Seo.
Investigation: Dohyeong Lee, Byung Cheol Yun, Kwang Il Seo, Sang Uk Lee, Eun Taek Park, Jin Wook Lee, Joonho Jeong.
Methodology: Byung Cheol Yun, Kwang Il Seo.
Resources: Byung Cheol Yun, Byung Hoon Han.
Supervision: Byung Cheol Yun, Kwang Il Seo, Byung Hoon Han, Sang Uk Lee, Eun Taek Park.
Writing – original draft: Dohyeong Lee, Kwang Il Seo.
Writing – review & editing: Byung Cheol Yun, Kwang Il Seo.
Kwang Il Seo orcid: 0000-0001-8854-5205.
Footnotes
Abbreviations: ACEi = angiotensin converting enzyme inhibitor, ADF = adefovir, ALT = alanine aminotransferase, ARB = angiotensin receptor blocker, AST = aspartate aminotransferase, CHB = chronic hepatitis B, CKD = chronic kidney disease, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, ETV = entecavir, HBeAg = e antigen, HBsAb = B surface antibody, HBV = hepatitis B virus, HTN = hypertension, NUC = nucleo(t)ide, TAF = tenofovir alafenamide fumarate, TDF = tenofovir disoproxil fumarate.
How to cite this article: Lee D, Yun BC, Seo KI, Han BH, Lee SU, Park ET, Lee JW, Jeong J. Risk factors associated with hypophosphatemia in chronic Hepatitis B patients treated with tenofovir disoproxil fumarate. Medicine. 2019;98:50(e18351).
DL and BCY equally contributed to this work.
The authors have no funding and conflicts of interests to disclose.
References
- [1].European Association for the Study of the Liver, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- [2].Kim HJ, Cho JY, Kim YJ, et al. Long-term efficacy of tenofovir disoproxil fumarate therapy after multiple nucleos(t)ide analogue failure in chronic hepatitis B patients. Korean J Intern Med 2015;30:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jeon HJ, Jung SW, Park NH, et al. Efficacy of tenofovir-based rescue therapy for chronic hepatitis B patients with resistance to lamivudine and entecavir. Clin Mol Hepatol 2017;23:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci 2015;60:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wong GL, Chan HL, Tse YK, et al. Chronic kidney disease progression in patients with chronic hepatitis B on tenofovir, entecavir, or no treatment. Aliment Pharmacol Ther 2018;48:984–92. [DOI] [PubMed] [Google Scholar]
- [6].Trinh S, Le AK, Chang ET, et al. Changes in renal function in patients with chronic HBV infection treated with tenofovir disoproxil fumarate vs entecavir. Clin Gastroenterol Hepatol 2019;17:948–56. [DOI] [PubMed] [Google Scholar]
- [7].Duarte-Rojo A, Heathcote EJ. Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B. Therap Adv Gastroenterol 2010;3:107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS 2008;22:99–103. [DOI] [PubMed] [Google Scholar]
- [9].Essig M, Duval X, Kaied FA, et al. Is phosphatemia the best tool to monitor renal tenofovir toxicity? J Acquir Immune Defic Syndr 2007;46:256–8. [DOI] [PubMed] [Google Scholar]
- [10].Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jung WJ, Jang JY, Park WY, et al. Effect of tenofovir on renal function in patients with chronic hepatitis B. Medicine (Baltimore) 2018;97:e9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pradat P, Le Pogam MA, Okon JB, et al. Evolution of glomerular filtration rate in HIV-infected, HIV-HBV-coinfected and HBV-infected patients receiving tenofovir disoproxil fumarate. J Viral Hepat 2013;20:650–7. [DOI] [PubMed] [Google Scholar]
- [13].Park J, Jung KS, Lee HW, et al. Effects of entecavir and tenofovir on renal function in patients with hepatitis B virus-related compensated and decompensated cirrhosis. Gut Liver 2017;11:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009;49: 5 Suppl: S185–195. [DOI] [PubMed] [Google Scholar]
- [15].Han Y, Zeng A, Liao H, et al. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: a systematic review and Meta-analysis. Int Immunopharmacol 2017;42:168–75. [DOI] [PubMed] [Google Scholar]
- [16].Canale D, de Braganca AC, Goncalves JG, et al. Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: role of oxidative stress and renin-angiotensin system. PLoS One 2014;9:e103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Waheed S, Attia D, Estrella MM, et al. Proximal tubular dysfunction and kidney injury associated with tenofovir in HIV patients: a case series. Clin Kidney J 2015;8:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


