Abstract
T helper 17 (Th17) cells are related to the progression of aortic dissection. This study aimed to determine whether circulating Th17 levels are associated with the prognosis of acute Stanford type B aortic dissection (STBAD) after thoracic endovascular aortic repair (TEVAR).
A cohort study was performed and STBAD patients (n = 140) received TEVAR were enrolled, the circulating Th17 levels were measured and the patients were divided into low and high Th17 groups, and 36 months of follow-up was performed. The data for mortality, survival outcomes, heart structure and function changes, aortic regurgitation prevalence, and aortic remodeling outcomes were recorded.
Lower mortality and fewer complications were observed in the low Th17 group than in the high Th17 group in the third year of follow-up. In addition, the low Th17 group exhibited better cardiac remodeling and cardiac function when compared with that in the high Th17 group in the second to third year after TEVAR. Aortic reflux was improved in both groups but was more pronounced in the low Th17 group. During follow-up, the true lumen of the proximal thoracic aorta at the level of the celiac trunk in both the low and high Th17 groups continuously enlarged and was more pronounced in the low Th17 group.
Circulating Th17 cells were related to cardiac and aortic remodeling and prognosis during STBAD after TEVAR. Anti-inflammatory therapy may be useful for STBAD patients who have undergone TEVAR.
Keywords: aortic remodeling, Stanford type B aortic dissection, Th17 cells, thoracic endovascular aortic repair, ventricular remodeling
1. Introduction
Aortic dissection (AD) can be classified into 2 types according to whether the tear site involves ascending aorta, in which, the tear site is only involves the descending aorta and/or the arch, without the involvement of the ascending aorta, is called Stanford type B aortic dissection (STBAD). Acute STBAD is uncommon in clinical settings. The incidence rate in Sweden from 1987 to 2002 was approximately 3 to 4 per 100,000 people, and there was a significant increase in both men and women, particularly in men.[1] According to the recommendations of Clinical Practice Guidelines for Vascular Surgery, thoracic endovascular aortic repair (TEVAR) should be the first line intervention for patients who suffered from STBAD and has rapidly become recognized because this procedure can significantly reduce early mortality and improve prognosis.[2,3] TEVAR can lead to a variety of serious clinical complications, and the long-term prognosis is not better than that with conservative treatment, making this treatment controversial; this has brought concern to surgeons and considerable psychological pressure and economic burden to patients.[4]
According to the statistics of the large-sample EUROSTAR trial, the incidence of complications after TEVAR for STBAD reached 6% to 27%[5,6]; complications included internal leakage, systemic inflammatory response syndrome after implantation, ischemia of major organs, and paraplegia.[3,4,7] In addition, some patients experienced abdominal aortic aneurysms after TEVAR in long-term follow-up, and 10% to 30% of these patients required repeated TEVAR.[8] In patients with aneurysms associated with other serious complications, conversion to open surgery is often necessary; open surgery is more difficult and invasive, and the mortality rate can be as high as 10% to 20%.[9–11] A large number of domestic and foreign studies on the short-term and medium-term prognoses of patients after TEVAR have shown that postoperative complications of aortic surgery are mainly caused by persistence of the aortic lumen.[12–15] The duration of time a false lumen remains present varies widely from patient to patient, and although the exact mechanisms remain unknown, there has been evidence suggesting that it may be closely related to the inflammatory response.[16–18]
CD4+ T helper (Th) cells, including Th1, Th2, Th9, Th17, Th22, and regulatory T cells, are one of the most important types of immune cells and have been shown to be closely related to AD. Data from clinical experiments have demonstrated that all subsets of CD4+ Th cells and their functional cytokine levels are altered in AD patients.[19–21] In angiotensin II-induced animal AD model, Th17 levels were also reported to be increased in the aortic wall.[22] Th17 cells are an important subset of CD4+ Th cells and exert biological effects mainly by secreting the functional cytokine interleukin (IL)-17. A previous study found that Th17 cells/IL-17 were increased in acute AD patients and positively associated with AD occurrence.[20,21] In addition, IL-6 deletion reduced AD occurrence by inhibiting signal transducer and activator of transcription 3 (STAT3) phosphorylation and alleviating the Th17 immune response.[22] However, whether Th17 cells are associated with the prognosis of STBAD patients after TEVAR remains unknown. This study aimed to clarify the effects of circulating levels of Th17 cells on prognosis in STBAD patients after TEVAR.
2. Methods
2.1. Study subjects
Patients (n = 152) who were diagnosed with acute STBAD by clinically experienced physicians according to clinical symptoms and the results of computed tomography angiography (CTA) and underwent TEVAR at the Longhua Central Hospital Affiliated Guangdong Medical University, the People's Hospital of Guangxi Zhuang Autonomous Region and the Beijing Anzhen Hospital from October 2013 to October 2014 were enrolled in this study. The exclusion criteria were a history of coronary artery disease (CAD), valvular heart disease (VHD), peripheral arterial disease (PAD), chronic heart failure (CHF), previous heart surgery, Marfan syndrome, Ehlers Danlos syndrome, a bicuspid aortic valve, and other diseases which could affect blood vessel wall strength, and 12 patients were excluded from the study because of a history of CAD (n = 4), VHD (n = 3), PAD (n = 3), and CHF (n = 2). After diagnosis, blood samples from the remaining 140 acute STBAD patients were collected by clinically experienced nurses before drug treatment and TEVAR, and the circulating levels of Th17 cells in each patient were detected by flow cytometry according to the methods of our previous study.[21] Then, patients were divided into low and high Th17 groups based on median levels of Th17 cells (as shown in Fig. 1). The patients’ attending physicians informed the patients or their families about the use of the blood samples, and the patients themselves or their families provided informed consent. This study protocol was approved by the medical ethics committees of the 3 institutions above.
Figure 1.
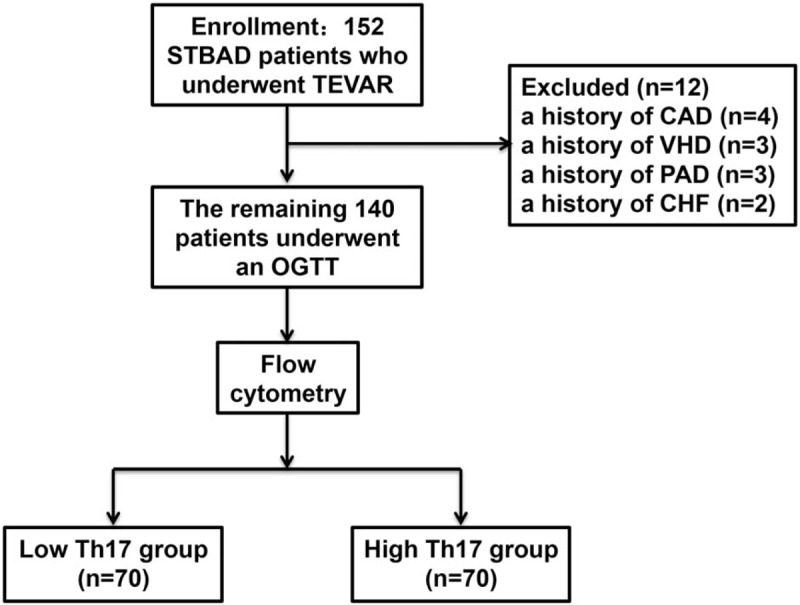
Inclusion and exclusion criteria for all patients. CAD = coronary artery diseases, CHF = chronic heart failure, CTA = computed tomography angiography, PAD = peripheral arterial disease, STBAD = Stanford type B aortic dissection, TEVAR = thoracic endovascular aortic repair, VHD = valvular heart disease.
2.2. Data collection
The basic clinical characteristics of each patient were collected from clinical records. Information on heart structure and function, including aortic reflux, left ventricular (LV) posterior wall thickness (PWT), LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV ejection fraction (LVEF), were obtained from echocardiographic results, and information regarding aortic structure, including the presence of a true lumen or a false lumen, were derived from the results of CTA.
2.3. Surgical techniques
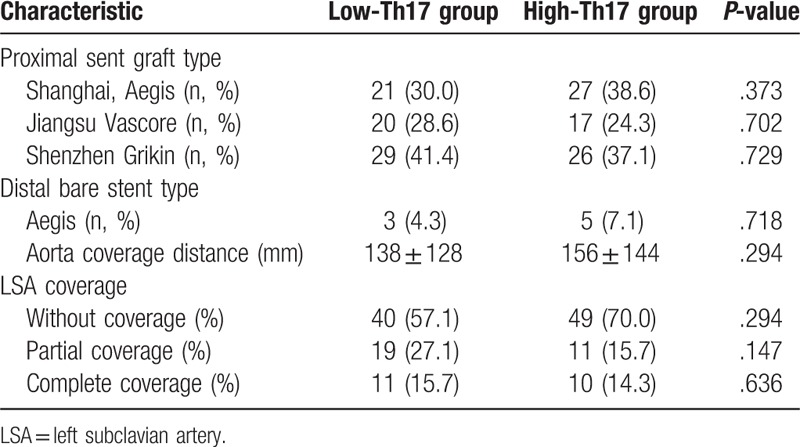
The patient lay flat on the operating table and received local anesthesia after skin disinfection. Puncture and catheterization of the left brachial artery were performed. Aortography was performed to comprehensively understand the lesion site, morphology and degree of involvement, and the diameter of the left subclavian artery (LSA) opening proximal to the aorta and the distance between the primary rupture and the LSA opening were measured. The diameter of the stent was 10% to 20% larger than that of the normal aorta at the proximal AD, and the length of the stent was 10 cm. One femoral artery was incised, and a membrane-covered stent was placed through the true lumen to block the primary rupture. If the distance from the original fracture to the LSA opening was more than 1.5 cm, the laminated part of the stent was localized below the LSA opening (n = 89), and if the distance from the primary fracture distance to the LSA opening was less than 1.5 cm, the coated part was used to seal the LSA opening (n = 51). The information on stent provisions and LSA coverage is listed in Table 1.
Table 1.
Preoperative characteristics between low-Th17 group and high-Th17 group.
2.4. Patient follow-up
All patients who underwent TEVAR were required to return to the hospital for re-examination once a month after they were discharged from the hospital. To ensure the smooth progression of follow-up, we also notified all patients by telephone 2 to 3 days before each follow-up time point. The re-interventions and survival outcomes of each patient at different follow-up time points were recorded. In addition, at 12, 24, and 36 months after discharge, the patients were required to be re-hospitalized, and transthoracic echocardiography and CTA were also performed. In the present study, follow-up time points 1 (T1), 2 (T2), and 3 (T3) were defined as 12 months, 24 months and 36 months, respectively, after STBAD patients had undergone TEVAR.
2.5. Statistical analyses
The clinical characteristics and circulating levels of Th17 cells are reported as the median (lower quartile to upper quartile) and were evaluated with the Mann–Whitney U test. Categorical variables are reported as counts (percentages) and were compared with the Chi-square test, and the Kaplan–Meier method and log-rank test were used to compute and analyze the survival rate. All data were analyzed with SPSS 22.0, and a P-value <.05 was considered significant.
3. Results
3.1. Comparisons between the low Th17 group and the high Th17 group
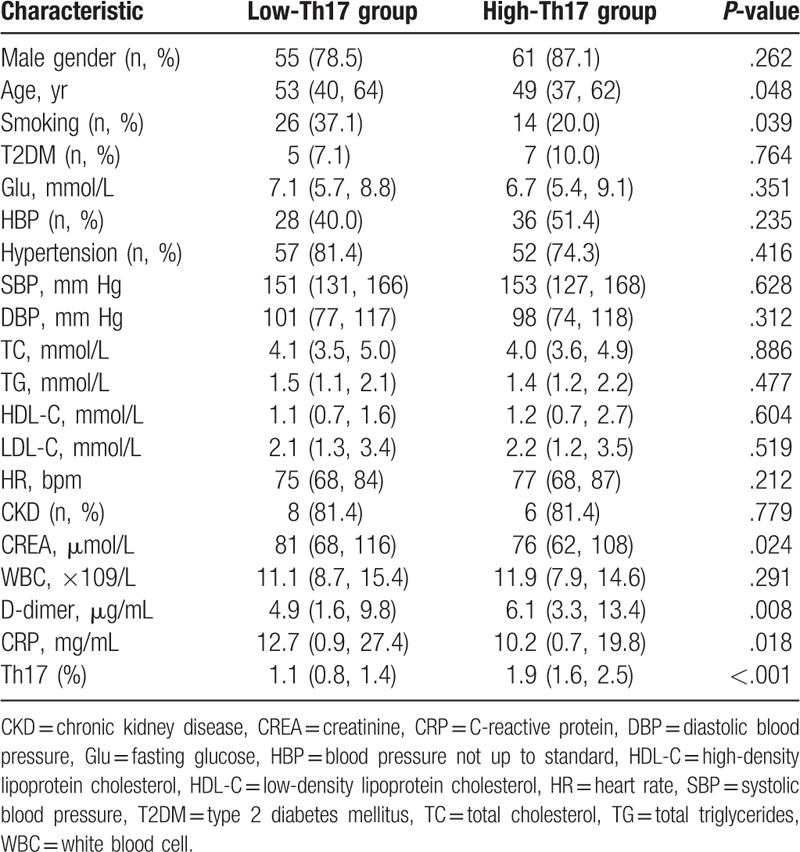
Among the 140 acute STBAD patients, increased D-dimer levels, and decreased age, smoking, creatinine, and C-reactive protein levels were found in the high-Th17 group compared with the low-Th17 group. No differences were observed between the 2 groups in other clinical characteristics, including gender, high blood pressure, type 2 diabetes mellitus proportion, hypertension proportion, fasting blood glucose, systolic blood pressure, diastolic blood pressure, lipids, white blood cell counts, heart rate, and chronic kidney disease proportion. The clinical characteristics of each group are listed in Table 2.
Table 2.
Clinical characteristics between low-Th17 group and high-Th17 group.
3.2. Increased survival rate in the low Th17 group
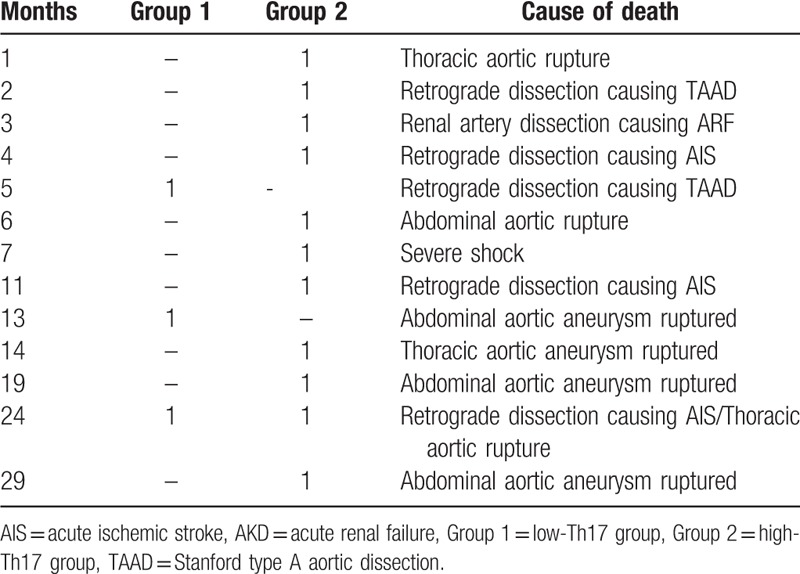
During the follow-up, 3 patients died in the low Th17 group, and the final survival rate was 95.7%. Eleven patients died in the high Th17 group, and the final survival rate was 84.3%. The final survival rate in the low Th17 group was lower than that in the high Th17 group (Fig. 2). The most common causes of death were retrograde dissection and abdominal aortic aneurysms that formed and ruptured after AD. The time and cause of each death patient are listed in Table 3.
Figure 2.
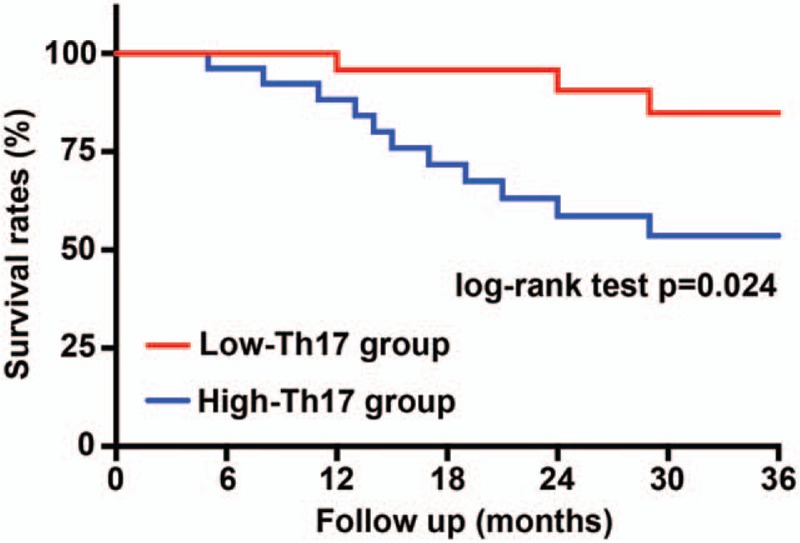
Kaplan–Meier curve of survival rates based on low and high circulating levels of Th17 cells. N = 70 for each group. The P-values are from the log-rank test.
Table 3.
Summary of death in low-Th17 group and high-Th17 group.
3.3. Fewer complications were observed in the low-Th17 group
Re-interventions were performed in 2 patients in the low Th17 group during follow-up. The reasons for re-interventions included proximal and distal stent graft-induced new entry (SINE) in 1 patient and distal SINE in 1 patient. In addition, 2 patients with endoleak and 1 patient with paraplegia were observed in the low Th17 group. In the high Th17 group, distal SINE occurred in 2 patients, and distal SINE occurred in 3 patients who received re-intervention. During follow-up, 5 patients had endoleak, 2 patients had paraplegia, 1 patient had lower extremity arterial thrombosis, and 1 patient had arterial embolization of the lower extremity.
3.4. LV remodeling reversed rapidly in the low Th17 group
Information on heart structure and function, including the PWT, LVEDD, LVESD, LVEDV, LVESV, and LVEF, was collected at different points for each patient. The results showed that there was no difference in the preoperative and postoperative PWT between the low Th17 group and high Th17 group, although the PWT was decreased postoperatively in both groups (Fig. 3A). From T1 to T3, the PWT still decreased progressively, but the decrease was more obvious in the low Th17 group (Fig. 3A). Similar trends to those observed for the PWT were obtained for the LVEDD, LVESD, LVEDV, and LVESV (Fig. 3B–E). In addition, there was no significant difference in the LVEF between the 2 groups before and after TEVAR, although the LVEF after TEVAR was higher than that before TEVAR (Fig. 3F). At T1, the LVEF in both groups was the highest but decreased at T2 and T3. However, from T1 to T3, the LVEF in the low Th17 group was higher than that in the high Th17 group (Fig. 3F).
Figure 3.
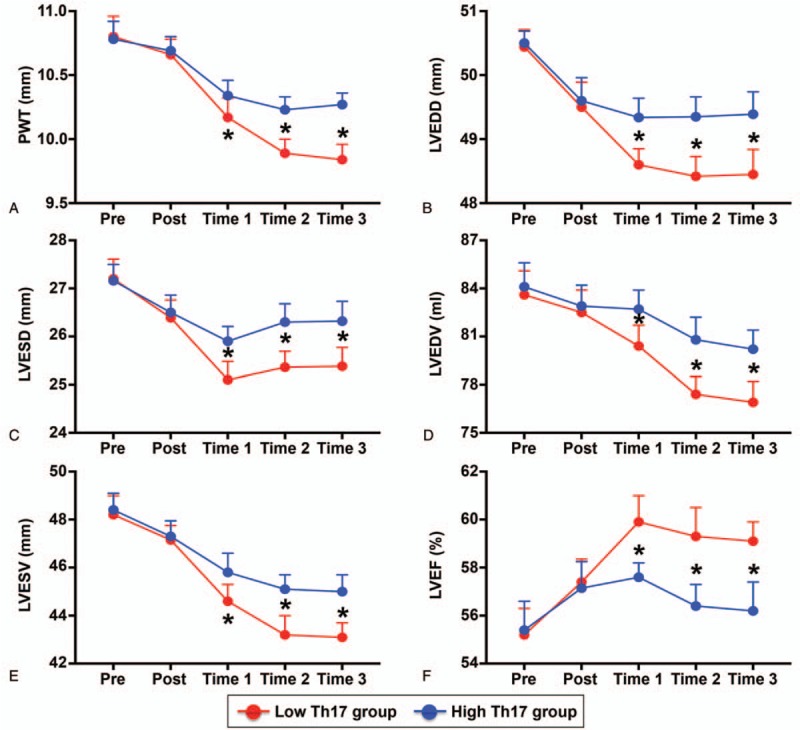
LV structure and function in each group. (A) PWT, (B) LVEDD, (C) LVESD, (D) LVEDV, (E) LVESV, and (F) LVEF in the low Th17 group and high Th17 group. ∗P < .05 versus low Th17 group. LVEDD = LV end-diastolic diameter, LVEDV = LV end-diastolic volume, LVEF = LV ejection fraction, LVESD = LV end-systolic diameter, LVESV = LV end-systolic volume, PWT = posterior wall thickness.
3.5. Less aortic reflux after TEVAR in the low Th17 group
Aortic reflux was mainly mild, and no difference was observed between the 2 groups (Fig. 4A and B). The proportion of patients without aortic drainage increased significantly after TEVAR; however, there was no difference between the 2 groups (Fig. 4A and B). From T1 to T3, the proportion of patients without aortic drainage was further increased in both groups, but the increase was more pronounced in the low Th17 group (Fig. 4A and B).
Figure 4.
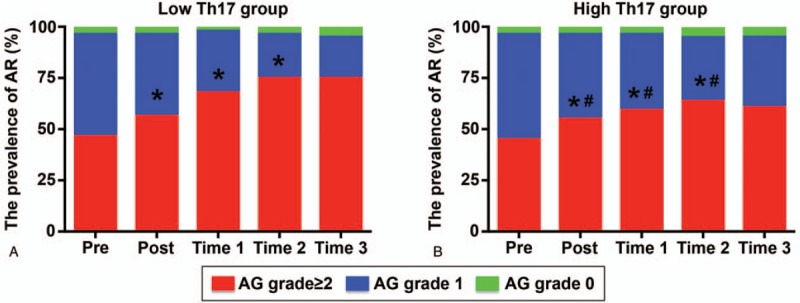
Aortic reflux in each group. (A) Aortic reflux in the low-Th17 group preoperatively (pre), postoperatively (post), and at T1, T2, and T3; (B) aortic reflux in the high-Th17 group measured at different time points. ∗P < .05 versus previous time points; #P < .05 versus low Th17 group.
3.6. Aortic remodeling recovered more significantly in the low-Th17 group
Regarding the true lumen area in the proximal thoracic aorta, no difference was observed between the low Th17 group and the high Th17 group before TEVAR, and the area gradually increased from T1 to T3. The true lumen area in the low Th17 group was larger than that in the high Th17 group at the same follow-up time points (Fig. 5A). Similar trends were observed for true lumen area in the celiac trunk (Fig. 5B). Regarding the false lumen area in the proximal thoracic aorta, no difference was found during the 2 groups before TEVAR. The false lumen area was decreased at T1 in both groups, but that in the low Th17 group decreased more dramatically (Fig. 5C). From T2 to T3, the false lumen area rapidly increased and became larger than that before TEVAR, and the increase was more pronounced in the high Th17 group (Fig. 5C). In both groups, the false lumen area in the celiac trunk gradually increased during follow-up, and the change in the false lumen area in the celiac trunk in the high Th17 group was more significant (Fig. 5D).
Figure 5.
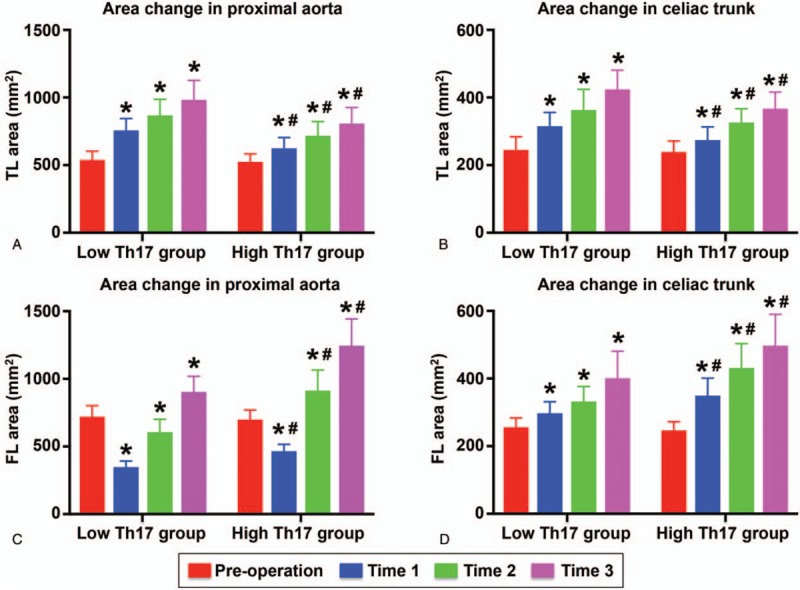
True lumen and false lumen areas in the 2 groups. (A, B) True lumen area in the proximal aorta and celiac trunk detected preoperatively, and at T1, T2, and T3; (C, D) false lumen areas in the proximal aorta and celiac trunk measured at different time points.
4. Discussion
In this paper, we found for the first time that immune Th17 cells were closely related to the prognosis of acute STBAD patients after TEVAR. The low Th17 group exhibited fewer deaths and clinical complications among acute STBAD patients after TEVAR. In addition, LV remodeling and mild aortic reflux reversed faster and LV function increased more significantly in this group. Acute STBAD patients with lower circulating levels of Th17 cells had faster recovery of the true lumen in the proximal thoracic aorta and celiac trunk after TEVAR and a delay in the increase in the false lumen area in the proximal thoracic aorta and celiac trunk. These data suggested that Th17 cells participated in the reversal of LV remodeling and aortic remodeling and affected the complication occurrence and prognosis in acute STBAD patients after TEVAR.
AD is a very fatal cardiovascular disease, and the incidence has been increasing gradually in recent years.[1] According to statistics, the mortality rate of untreated AD patients is 1% to 2% per hour, and the total mortality rate may be as high as 90%.[23] With decades of research and deepening of the understanding of AD, especially the emergence and progress of new treatment methods, the disease has been effectively controlled.[24,25] Among them, TEVAR was initiated in 1999 and has been used to treat STBAD for 20 years. TEVAR can significantly reduce the short-term mortality, of AD patients, but postoperative complications, especially aortic rupture, of which more than 50% of patients die, can still threaten their lives.[26] In addition, retrograde dissection can cause insufficient blood supply to the head, while continuous downward dissection can lead to renal, mesenteric, and lower limb ischemia. Ischemia of these vital organ is often difficult to correct and leads to an increased mortality rate even with prompt treatment.[26,27] In our current study, we found that during a 3-year follow-up period, 1 patient died of aortic rupture in the low Th17 group, and 6 patients died of aortic rupture in the high Th17 group. Aortic rupture accounted for 50% of all deaths, which was consistent the findings of with previous studies. In addition, 1 patient in the low Th17 group and 3 patients in the high Th17 group died of vital organ ischemia due to an increased aortic tear range. According to these results, more than 80% of deaths were closely associated with uncorrected or worsening aortic remodeling after dissection.
The occurrence of AD is actually the process of false lumen formation and true lumen area reduction after aortic endothelial dissection. Given the specific anatomic structure, the formation of a false lumen before TEVAR in STBAD patients is often accompanied by important hemodynamic changes, which often manifest as an increase in the pressure gradient between the ascending aorta and descending aorta and pressure overload, which can lead to LV remodeling and decreased cardiac function.[27] The increase in pressure overload can also lead to aortic reflux, and most patients experienced mild aortic reflux because the intimal flap did not involve the aortic valve.[28,29] Both aortic reflux and LV remodeling are associated with the severity of pressure overload, namely, with the false lumen area, and an increase in the false lumen area can aggravate the pressure overload and thus aortic reflux and LV remodeling.[30] In fact, TEVAR can significantly reduce the false lumen area, but TEVAR cannot completely eliminate the false lumen in all patients. Some STBAD patients still have a false lumen after TEVAR, which can lead to minor hemodynamic disorders and cause a continuous impact on the tear site; with the passage of time, this increases the false lumen area because of high pressure in the aorta.[28] However, TEVAR can significantly reduce the short-term mortality rate, but not the long-term mortality rate, which is consistent with the trends in the false lumen area after TEVAR.[29] In our present study, we found that the trends in aortic reflux and LV remodeling were similar to those in the aortic false lumen, although the true lumen area did not shrink but increased progressively. Combined with previous evidence, prevention of dilation of the false lumen area may be the most important treatment for STBAD patients after TEVAR and maybe more important than enlargement of the true lumen area.
Vascular smooth muscle cells (VSMCs) are an important part of the aortic structure, and VSMCs can continuously synthesize and expand the extracellular matrix, which is essential to maintain the normal structure of blood vessels.[31] When VSMCs excessively apoptotic and significantly few VSMCs are present due to external causes, the synthesis and decomposition of extracellular matrix are imbalanced, and the aortic structure is destroyed, which can lead to aortic aneurysm, AD, and even aortic rupture.[32,33] Although the causes of excessive loss of VSMCs are complex, there has been evidence suggesting that inflammatory responses play an important role in this process.[22] Furthermore, in STBAD patients, increased inflammation can also result in loss of VSMCs in sites other than the dissection site, leading to abdominal and retrograde dissection and an increased false lumen area.[16–18] In the present study, we determined whether circulating Th17 cells were related to the prognosis of STBAD patients after TEVAR. Our results showed that both survival mortality and complications were lower in the low Th17 group than in the high Th17 group. In addition, LV remodeling and the resolution of aortic reflux were more pronounced in the low Th17 group. Finally, patients in the low Th17 group exhibited a larger true lumen area and a smaller false lumen area at the end of follow-up. Considering that survival, LV remodeling, and aortic reflux are all related to the false lumen area, Th17 cells may affect the prognosis by affecting the lumen areas in STBAD patients after TEVAR, and more studies are needed to confirm this finding.
In conclusion, our study shows that circulating Th17 cells are closely related to LV remodeling, aortic remodeling, and the prognosis of STBAD patients after TEVAR. Anti-inflammatory treatment after TEVAR may be beneficial for the prognosis of STBAD patients. However, there are some limitations in our paper. First, although the patients were enrolled from 3 research centers, the sample size in this study was small, which may have affected the conclusion. Second, the follow-up time was relatively short and should be extended.
Author contributions
Conceptualization: Ling Liu.
Data curation: Tao Zeng.
Formal analysis: Ting Xiao.
Funding acquisition: Ying Huang, Ying Shi, Qingwei Ji, Yingzhong Lin.
Investigation: Hongtao Liu.
Methodology: Ying Huang, Yingzhong Lin.
Project administration: Ying Shi, Yingzhong Lin.
Software: Le Zhang.
Writing – original draft: Lei Shi, Tao Zeng.
Writing – review and editing: Qingwei Ji, Yingzhong Lin.
Footnotes
Abbreviations: AD = aortic dissection, CAD = coronary artery disease, CHF = chronic heart failure, CTA = computed tomography angiography, IL = interleukin, LSA = left subclavian artery, LV = left ventricular, LVEDD = LV end-diastolic diameter, LVEDV = LV end-diastolic volume, LVEF = LV ejection fraction, LVESD = LV end-systolic diameter, LVESV = LV end-systolic volume, PAD = peripheral arterial disease, PWT = posterior wall thickness, SINE = stent graft-induced new entry, STBAD = Stanford type B aortic dissection, TEVAR = thoracic endovascular aortic repair, Th17 = T helper 17, VHD = valvular heart disease, VSMCs = vascular smooth muscle cells.
How to cite this article: Liu H, Xiao T, Zhang L, Huang Y, Shi Y, Ji Q, Shi L, Zeng T, Lin Y, Liu L. Effects of circulating levels of Th17 cells on the outcomes of acute Stanford B aortic dissection patients after thoracic endovascular aortic repair: A 36-month follow-up study a cohort study. Medicine. 2019;98:50(e18241).
This work was supported by the National Natural Science Foundation of China (No. 81770472, 81460081, 81460061, and 81760051).
The authors have no conflicts of interest to disclose.
References
- [1].Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006;114:2611–8. [DOI] [PubMed] [Google Scholar]
- [2].Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340:1539–45. [DOI] [PubMed] [Google Scholar]
- [3].Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546–52. [DOI] [PubMed] [Google Scholar]
- [4].Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661–78. [DOI] [PubMed] [Google Scholar]
- [5].Laheij RJ, Buth J, Harris PL, et al. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry. Br J Surg 2000;87:1666–73. [DOI] [PubMed] [Google Scholar]
- [6].Maldonado TS, Gagne PJ. Controversies in the management of type II “branch” endoleaks following endovascular abdominal aortic aneurysm repair. Vasc Endovascular Surg 2003;37:1–2. [DOI] [PubMed] [Google Scholar]
- [7].Kawaharada N, Morishita K, Kurimoto Y, et al. Spinal cord ischemia after elective endovascular stent-graft repair of the thoracic aorta. Eur J Cardiothorac Surg 2007;31:998–1003. [DOI] [PubMed] [Google Scholar]
- [8].Piazza M, Gloviczki P, Huang Y, et al. Evolution in management and outcome after repair of abdominal aortic aneurysms in the pre- and post-EVAR era. Perspect Vasc Surg Endovasc Ther 2013;25:11–9. [DOI] [PubMed] [Google Scholar]
- [9].Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366–74. [DOI] [PubMed] [Google Scholar]
- [10].Kato T, Tamaki M, Tsunekawa T, et al. Health-related quality of life prospectively evaluated by the 8-item short form after endovascular repair versus open surgery for abdominal aortic aneurysms. Heart Vessels 2017;32:960–8. [DOI] [PubMed] [Google Scholar]
- [11].Krishnamoorthi H, Jeon-Slaughter H, Wall A, et al. Rate of secondary intervention after open versus endovascular abdominal aortic aneurysm repair. J Surg Res 2018;232:99–106. [DOI] [PubMed] [Google Scholar]
- [12].Thrumurthy SG1, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632–47. [DOI] [PubMed] [Google Scholar]
- [13].Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part I. Evaluation of causative factors in phantoms with pulsatile flow. Radiology 2000;214:87–98. [DOI] [PubMed] [Google Scholar]
- [14].Tsai TT, Schlicht MS, Khanafer K, et al. Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. J Vasc Surg 2008;47:844–51. [DOI] [PubMed] [Google Scholar]
- [15].Onitsuka S1, Akashi H, Tayama K, et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg 2004;78:1268–73. [DOI] [PubMed] [Google Scholar]
- [16].Hosaka A, Kato M, Motoki M, et al. Quantified aortic luminal irregularity as a predictor of complications and prognosis after endovascular aneurysm repair. Medicine (Baltimore) 2016;95:e2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen ZR, Huang B, Lu HS, et al. Admission white blood cell count predicts short-term clinical outcomes in patients with uncomplicated Stanford type B acute aortic dissection. J Geriatr Cardiol 2017;14:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yamada Y, Tanno J, Nakano S, et al. Clinical implications of pleural effusion in patients with acute type B aortic dissection. Eur Heart J Acute Cardiovasc Care 2016;5:72–81. [DOI] [PubMed] [Google Scholar]
- [19].Ye J, Wang M, Jiang H, et al. Increased levels of interleukin-22 in thoracic aorta and plasma from patients with acute thoracic aortic dissection. Clin Chim Acta 2018;486:395–401. [DOI] [PubMed] [Google Scholar]
- [20].Zeng T, Shi L, Ji Q, et al. Cytokines in aortic dissection. Clin Chim Acta 2018;486:177–82. [DOI] [PubMed] [Google Scholar]
- [21].Ye J, Wang Y, Wang Z, et al. Circulating Th1, Th2, Th9, Th17, Th22, and Treg Levels in aortic dissection patients. Mediators Inflamm 2018;2018:5697149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ju X, Ijaz T, Sun H, et al. Interleukin-6-signal transducer and activator of transcription-3 signaling mediates aortic dissections induced by angiotensin II via the T-helper lymphocyte 17-interleukin 17 axis in C57BL/6 mice. Arterioscler Thromb Vasc Biol 2013;33:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- [24].Estrera AL, Miller CC, Safi HJ, et al. Outcomes of medical management of acute type B aortic dissection. Circulation 2006;114: 1 Suppl: I384–9. [DOI] [PubMed] [Google Scholar]
- [25].Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J 2012;33:26–35. [DOI] [PubMed] [Google Scholar]
- [26].Qin YL, Deng G, Li TX, et al. Treatment of acute type-B aortic dissection: thoracic endovascular aortic repair or medical management alone? JACC Cardiovasc Interv 2013;6:185–91. [DOI] [PubMed] [Google Scholar]
- [27].Karmonik C, Müller-Eschner M, Partovi S, et al. Computational fluid dynamics investigation of chronic aortic dissection hemodynamics versus normal aorta. Vasc Endovascular Surg 2013;47:625–31. [DOI] [PubMed] [Google Scholar]
- [28].Cheng D, Martin J, Shennib H, et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol 2010;55:986–1001. [DOI] [PubMed] [Google Scholar]
- [29].Du Y, Aizezi M, Lin H, et al. Left ventricular remodeling in patients with acute type B aortic dissection after thoracic endovascular aortic repair: short- and mid-term outcomes. Int J Cardiol 2019;274:283–9. [DOI] [PubMed] [Google Scholar]
- [30].Alimohammadi M, Sherwood JM, Karimpour M, et al. Aortic dissection simulation models for clinical support: fluid-structure interaction vs. rigid wall models. Biomed Eng Online 2015;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol 2009;6:771–86. [DOI] [PubMed] [Google Scholar]
- [32].Allahverdian S, Chaabane C, Boukais K, et al. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res 2018;114:540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu R, Lo L, Lay AJ, et al. ARHGAP18 Protects against thoracic aortic aneurysm formation by mitigating the synthetic and pro-inflammatory smooth muscle cell phenotype. Circ Res 2017;121:512–24. [DOI] [PubMed] [Google Scholar]


